Abstract
During the G2–M transition, the highly organized Golgi apparatus undergoes reversible fragmentation through unstacking of the cisternal ribbon and disassembly into radially dispersed vesicles and tubules. These Golgi-derived fragments redistribute randomly within the cytoplasm, partition stochastically, and in telophase coalesce to generate a functionally and structurally intact Golgi complex. Here we identified a novel step in postmitotic Golgi reassembly that requires the clathrin heavy chain (CHC). We used siRNA-mediated CHC knockdown, biochemistry, and morphological analysis and showed that the spindle- and spindle pole-associated clathrin pools are membrane-bound and required for postmitotic Golgi reassembly. The results presented here show that clathrin remains associated with the spindle poles throughout mitosis and that this clathrin pool is distinct from the previously characterized spindle-associated population. We suggest that clathrin may provide a template for postmitotic Golgi reassembly and cisternal remodeling. In absence of the CHC, the Golgi apparatus remained disconnected and disordered and failed to regain its characteristic perinuclear, lace-like morphology. Our findings build on previous independent reports that clathrin is required for Golgi reassembly following disruption with pharmacological agents and for mitotic chromosome congression.—Radulescu, A. E., Shields, D. Clathrin is required for postmitotic Golgi reassembly.
Keywords: mitosis, remodeling, spindle poles, centrosome
Postmitotic Golgi reassembly is a complex process that includes early heterotypic fusion of mitotic clusters to form short cisternae, mediated by the interaction between p115, giantin, and GM130 (1–3), followed by growth (4, 5) and stacking (6, 7) via GRASP65- and GM130-mediated lateral fusion of cisternae (8). COPII-mediated ER exit as well as the direct interaction between the Golgi-associated protein p115 and the β-COP subunit of the COPI coat (9) have been implicated in maintaining Golgi homeostasis and overall organization. From these studies, however, it is unclear how postmitotic Golgi reassembly is initiated at spindle poles and how its organization is generated from the random, heterogeneous population of mitotic vesicles.
Clathrin has well-described functions in receptor-mediated endocytosis (10, 11) and vesicle budding from the TGN and transport to the plasma membrane (12), as well as in protein and lipid transfer between intracellular membranes (13, 14). The clathrin triskelion associates with membranes in a highly coordinated process to locally bend the membrane and generate clathrin coated vesicles (CCVs; ref. 11). During mitosis, vesicle transport from the Golgi apparatus is inhibited (15); however, clathrin-mediated endocytosis remains active and is required for plasma membrane retrieval, which controls cell surface area and mitotic roundup (16, 17). Indeed, the integrity and function of the mitotic spindle appear to depend on mitotic cell shape (16).
A pool of clathrin heavy chains (CHCs) and clathrin light chains (CLCs) localizes to the mitotic spindle of multiple cell lines (18, 19) and early mouse embryonic cells (20). The clathrin triskelion was implicated in stabilizing the kinetochore fibers of the mitotic spindle through a nonmembranous, lattice-like conformation that was proposed to cross-link microtubules (21). Clathrin-associated proteins, such as cyclin G-associated kinase (GAK; ref. 22) and autosomal recessive hypercholesterolemia (ARH; ref. 23), have also been shown to play roles in centrosome and spindle integrity, and their involvement suggests a function for membrane-associated clathrin in these processes. While there is agreement that clathrin plays several mitotic functions, its role and mechanism of action in mitosis remain to be established.
Pharmacological agents can mimic mechanisms of physiological Golgi disassembly and reassembly (24), and our laboratory demonstrated that clathrin is required for reformation of the fragmented Golgi apparatus after its disruption by several drugs (25). These results are consistent with the hypothesis that during Golgi reassembly, subsequent to recruitment of Golgi derived vesicles and tubules to the juxtanuclear region, clathrin and/or CCVs function in sorting Golgi resident proteins to their respective compartments. As an extension of these studies, we hypothesized that clathrin might have a similar function in postmitotic reassembly of the Golgi apparatus. We have now tested this idea and here present evidence suggesting that the membrane-associated CHC is required for postmitotic Golgi reassembly and cisternal remodeling.
MATERIALS AND METHODS
Cell culture and siRNA transfection
NRK and 293T cells were grown in DMEM supplemented with 10% FBS, glutamine, and penicillin/streptomycin at 37°C in 5% CO2. The CLC-dsRed plasmid was a kind gift from Dr. James Keen (Thomas Jefferson University, Philadelphia, PA, USA) and was used to generate stable NRK lines by G418 selection and fluorescence activated cell sorting (FACS).
For CHC knockdown studies in NRK cells, we used the rchc2 sequence (25), which is equivalent to a siRNA designed against the human CHC sequence (26). The nonsilencing siRNA was purchased from Ambion (Austin, TX, USA). Cells were transfected using oligofectamine (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions and incubated with the siRNA for 4 d to maximize CHC knockdown.
Antibodies
The rabbit anti-clathrin antibody was a gift from Dr. Silvia Corvera (University of Massachusetts Medical School, Worcester, MA, USA); the rabbit antibody to recombinant GRASP65 was a gift of Dr. Yanzhuang Wang (University of Michigan, Ann Arbor, MI, USA); and the rabbit anti-HRS antibody was from Dr. Sylvie Urbe (University of Liverpool, School of Biomedical Sciences, Liverpool, UK). Mouse monoclonal antibodies to CHC and α-tubulin were purchased from BD Biosciences (San Jose, CA, USA); the monoclonal anticlathrin antibody X22 was from Affinity Bioreagents (Rockford, IL, USA); the monoclonal antibody against γ-tubulin (clone GTU-88) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Microscopy and image analysis
Immunofluorescence microscopy
Cells grown on etched or polylysine-coated coverslips were fixed with 3% paraformaldehyde and processed for immunofluorescence as described previously (25). The coverslips were examined on a Nikon TE300 equipped with a DS camera, using the NIS Elements imaging software (Nikon, Melville, NY, USA) or a Leica AOBS laser-scanning confocal microscope (Leica Microsystems, Bannockburn, IL, USA).
Image analysis
For particle analysis, Z series were acquired using the Leica Confocal software (Leica Microsystems) through the depth of cells with a step size of 0.3 μm and projected using maximum pixel projection (ref. 25; ImageJ; http://rsb.info.nih.gov/ij/). Regions of interest were generated by thresholding GRASP65 staining and analyzed with the particle analysis algorithm using ImageJ (8).
Electron microscopy
NRK cells were grown on polylysine-coated coverslips, synchronized by treatment with 2.5 μg/ml aphidicolin for 14 h, and released into fresh medium for various lengths of time, between 6 and 8 h. The glass coverslips were fixed with 2.5% glutaraldehyde and postfixed with 1% osmium tetroxide followed by 1% uranyl acetate (25).
Spindle and centrosome isolation
NRK cells were synchronized by treatment with 2.5 μg/ml aphidicolin for 14 h followed by incubation for 6 h in fresh DMEM medium. Mitotic spindles were prepared according to Kuriyama et al. (27) in the presence of 0.25% TritonX-100 or 0.1%, 0.25% CHAPS. In the absence of detergent, cells were mechanically disrupted using a ball bearing homogenizer. The pellet was adsorbed onto polylysine-coated coverslips, fixed with 3% PFA or −20°C methanol, and processed for immunofluorescence microscopy. Both the TritonX-100/CHAPS soluble and insoluble fractions were also analyzed on 7.5% SDS-PAGE gels. Centrosomes were isolated from both NRK and 293T cells (28). In an alternate protocol, cells were lysed using a Dounce homogenizer, in the absence of Nonidet P-40 (NP-40).
RESULTS
Clathrin remains at spindle poles throughout mitosis
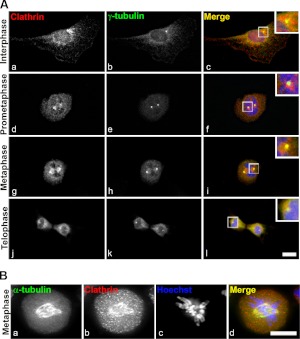
Previous results from our laboratory showed that clathrin is required for the reformation of the Golgi apparatus from dispersed fragments generated by treating cells with various pharmacological agents (i.e., brefeldinA and 1-butanol; ref. 25). In the absence of the CHC, the Golgi apparatus fails to regain its typical lace-like ribbon morphology and instead maintains an intermediate tight compact morphology consisting of heterogeneous vesicles and tubules. We and others have made the observation that CHCs and CLCs are present at spindle poles and on mitotic spindles (18, 19, 21) throughout mitosis (Fig. 1Ac, f, i, l and Supplemental Fig. S1A), while Golgi fragments distribute stochastically in the cytoplasm of dividing cells (ref. 29 and Supplemental Fig. S1B). Indeed, we found that several pools of clathrin were evident: one that colocalized with γ-tubulin at centrosomes (Fig. 1Ag–i), and another that distributed with α-tubulin on mitotic spindles (Fig. 1Ag, Ba, b, d) as well as peripheral membrane structures characterized by punctate staining (Fig. 1Aa, d, g, j, Bb). While clathrin was proposed to function in maintaining the mitotic spindle integrity (21), a distinction between the various mitotic clathrin populations has not been made.
Figure 1.
Clathrin is recruited to centrosomes and mitotic spindles during mitosis. A) Analysis of clathrin distribution in cycling NRK cells. Clathrin (red; a, d, g, j) segregates with the γ-tubulin-positive centrosomes (green; b, e, h, k) throughout mitosis. Punctate clathrin staining corresponds to peripheral membrane structures (a, d, g, j). Merged images show colocalization (c, f, i, l); insets show enlarged view of boxed areas. B) Synchronized NRK cells were attached to coverslips by cytospin, probed with antibodies against clathrin (red; a) and α-tubulin (green; b), and DNA or chromosomes stained with Hoechst (i); merged image shows colocalization (d). Images are single sections. Scale bar = 10 μm.
A large fraction of the spindle-associated clathrin population is membrane-bound
At present, the mechanism of clathrin localization to the mitotic spindle and poles is unclear, although Royle et al. (21) suggested that the CHC interacts with the spindle via its N terminus and implicated this interaction in the localization of the CHC to mitotic spindles. In this study, the spindle-associated clathrin pool was proposed to play a role in stabilizing the mitotic spindle, and this clathrin population was found to not be membrane associated (21). More recently, Booth et al. (30) showed that clathrin cannot bind microtubules and that it may be recruited to the spindle by other proteins.
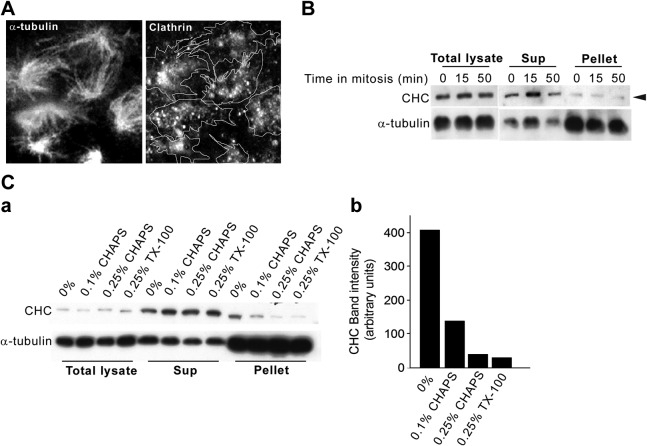
We further analyzed this CHC population by isolating spindles from cells at different stages of mitosis and showed a biochemical association between clathrin and mitotic spindles (ref. 27 and Fig. 2). As expected from the immunolocalization data in whole cells, there was significant localization of CHC-positive structures on the isolated spindle preparations (Fig. 2A, spindle traces), although a CHC fraction did dissociate from the spindles (Fig. 2A, B; see below). Consistent with this, a low level of CHC was present in the fraction corresponding to isolated spindles in the immunoblot analysis (Fig. 2B, pellet, arrowhead). To determine whether the low recovery of clathrin in the pellet fraction was due to labile interactions, we isolated mitotic spindles in the absence or presence of detergents at various stringencies (Fig. 2C). Significantly, in the absence of detergent (0%), CHC was enriched in the spindle pellet (Fig. 2C), whereas following treatment with 0.25% Triton X-100, it was released into the low-speed supernatant (Fig. 2B, C). Consistent with these results, treatment of the isolated spindles with low concentrations of the detergent CHAPS gave identical results in that CHC was quantitatively released into the low-speed supernatant fraction (Fig. 2Ca, pellet; compare 0.1 and 0.25% CHAPS), whereas α-tubulin remained in the pellet (Fig. 2C). Quantitation of the recovered CHC in the spindle fractions from the various detergent treatments (Fig. 2Cb) shows an 8-fold CHC enrichment in the absence of detergent (0%) over the highest used concentration (0.25% CHAPS/TX-100). This quantitative detergent sensitivity suggests that a majority of the spindle-associated CHC is membrane bound.
Figure 2.
A large fraction of the spindle-associated clathrin population is membrane bound. A) Mitotic spindles from synchronized NRK cells were prepared in the presence of 0.25% TritonX-100 and were adsorbed onto coverslips and probed with antibodies against α-tubulin and clathrin, followed by immunofluorescence microscopy. Traces in right panel represent outlines of the α-tubulin positive spindles in the left panel. B) Mitotic spindles from synchronized NRK cells were prepared in the presence of 0.25% TritonX-100 and analyzed by Western blotting. Total cell lysates, supernatants (sup), and low-speed pellets (4000 g) from 3 mitotic time points were analyzed (0 min, prometaphase; 15 min, metaphase; 50 min, telophase). C) a) Mitotic spindles were prepared as in panel B, in the absence (0%) or presence of the indicated detergent concentrations (0.1% CHAPS, 0.25% CHAPS, 0.25% TritonX-100) and analyzed by Western blotting with antibodies against CHC and α-tubulin. b) Relative levels of CHC recovered in the α-tubulin pellet in the presence of various detergent concentrations were quantified by densitometric analysis.
Clathrin populations associated with the mitotic spindle and the spindle poles are distinct
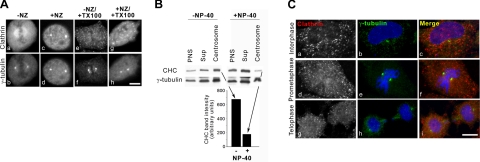
To determine whether the centrosome-associated clathrin population is distinct from the spindle pool, metaphase cells were treated with the microtubule depolymerizing drug nocodazole, which, as previously shown, disrupts the mitotic spindle (Fig. 3 and data not shown). Significantly, the metaphase spindle-associated clathrin population (compare Fig. 3Aa, c) was dispersed by nocodazole treatment, consistent with data showing clathrin association with spindle microtubules (21), whereas, in contrast, the centrosomal pool of CHC was unaffected by the nocodazole treatment (Fig. 3Ac, d). Surprisingly, when cells were treated with Triton X-100, the centrosome-associated CHC population was also dispersed regardless of whether the cells were also treated with nocodazole (Fig. 3Ae–h). Furthermore, when centrosomes isolated from nocodazole-treated cells (28) were incubated with detergent (Fig. 3B, +NP-40), CHC was released from the centrosome fraction, whereas in the absence of detergent, ∼4 times more CHC remained associated with the centrosomes (Fig. 3B, −NP-40 and graph). Significantly, centrosomes were efficiently isolated under both conditions (Fig. 3B). It is important to note here that the cytoskeleton was depolymerized by treatment with cytochalasin B and nocodazole prior to cell lysis, and therefore these centrosomes are expected to be free of cytoskeletal elements (i.e., α-tubulin; ref. 28). Taken together, our data suggest that large CHC populations associate with mitotic spindles and the centrosomes via membrane-bound structures. In support of this finding, CHC colocalization with γ-tubulin at other cell cycle stages (i.e., prometaphase and telophase) was abolished in nocodazole- and detergent-treated cells (Fig. 3C, Di). As expected, the pericentrosomal localization of clathrin-coated vesicles in interphase cells was abrogated by detergent treatment (Fig. 3Ca). These results suggest that whereas the CHC localization to mitotic spindles and centrosomes may involve different tubulin isoforms, both associations are likely to occur via membrane-associated clathrin structures.
Figure 3.
Centrosome-associated clathrin is membrane bound and distinct from the spindle-associated pool. A) Metaphase NRK cells were either untreated (a, b, e, f) or treated with 30 μM nocodazole (NZ) for 90 min (c, d, g, h). Some cells were also treated with 0.1% TritonX-100 (e–h). All cells were prepared for immunofluorescence microscopy as described previously (25). B) Centrosomes were prepared from 293T cells in the presence (+) or absence (−) of NP-40 and analyzed by Western blotting. Note that cytoskeletal components were disrupted prior to cell lysis by treatment with cytochalasin B and nocodazole. Amount of CHC isolated in the centrosome prep was quantified by densitometric analysis. C) Interphase (a–c) and mitotically synchronized (prometaphase, d–f; telophase, g–i) NRK cells were treated with nocodazole, followed by treatment with 0.1% TritonX-100, and probed with antibodies against clathrin (a, d, g) and γ-tubulin (b, e, h) for immunofluorescence microscopy; merge panels show colocalizaton (c, f, i). As a result of TX-100 treatment, CHC structures are displaced from all cellular compartments where they normally localize. Scale bars = 10 μm.
CHC is required for postmitotic Golgi reassembly
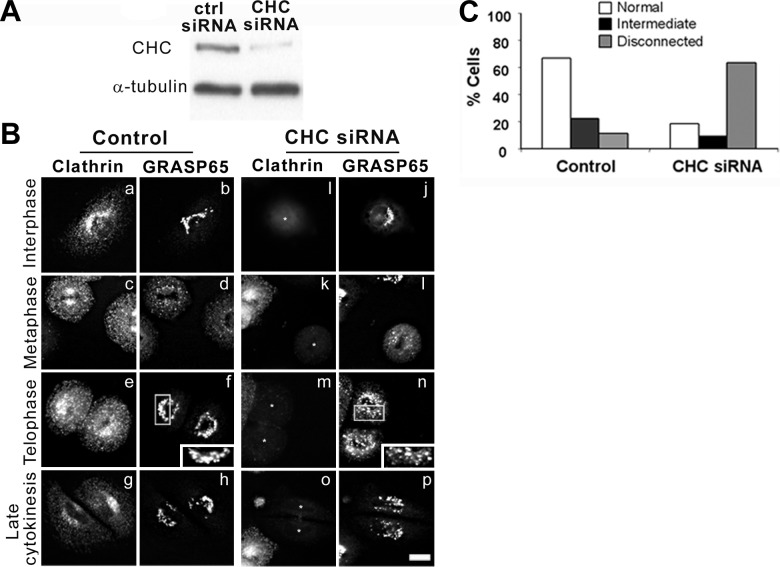
To address the requirement for CHC in postmitotic Golgi reassembly, CHC was depleted from NRK cells with a specific, previously characterized siRNA sequence (Fig. 4A). Consistent with published results (19, 21), knockdown of CHC did not alter the kinetics of mitosis progression (Fig. 4B). In control cells transfected with a nonsilencing siRNA, the fragmented Golgi apparatus assembled into a connected perinuclear structure in telophase (Fig. 4Be, f), which was remodeled into the classic ribbon-like structure during the later stages of cytokinesis (Fig. 4Bg, h). In CHC-knockdown cells, GRASP65 as well as other cis- and medial-Golgi markers (ref. 25 and data not shown) displayed a normal perinuclear distribution during interphase (Fig. 4Bi, j) and the expected fragmentation in metaphase (Fig. 4Bk, l). In contrast, during telophase and cytokinesis, the Golgi apparatus maintained a punctate, noncentrosomal, disconnected distribution with little evidence of cisternal formation (compare Fig. 4Bf, n; insets). Significantly, our data show that both control and CHC siRNA-treated cells proceed through mitosis with similar kinetics (compare Fig. 4Bg, h; o, p) and that at 8 h following release from the aphidicolin block, cells are in the final stages of cytokinesis. To compare the structures that occur during Golgi reassembly in CHC-knockdown and control cells, 3 morphological criteria (normal, intermediate, and disconnected) were defined and quantified by eye (Fig. 4C). In late cytokinesis, ∼70% of cells transfected with control siRNA regained a normal Golgi morphology consisting of interconnected tubules located in the pericentrosomal region of the cell, as determined by light microscopy. In contrast, only ∼20% of CHC-knockdown cells exhibited this morphology, whereas nearly 70% of these cells retained a fragmented and disconnected appearance. Significantly, in the absence of CHC, Golgi clusters failed to localize pericentrosomally and instead remained dispersed in the cytoplasm, implicating the CHC in a recruiting mechanism during postmitotic Golgi reassembly. These data are consistent with a model suggesting that efficient Golgi reassembly during the telophase and cytokinesis stages of mitosis requires the CHC.
Figure 4.
Clathrin is required for postmitotic Golgi reformation. A) NRK cells were treated with either nonsilencing (ctrl) or CHC siRNA for 4 d, at which point total cell lysates were prepared and resolved by Western blotting with antibodies to CHC and α-tubulin (loading control). B) siRNA-treated cells (asterisks) were synchronized in mitosis (metaphase, c, d, k, l; telophase, e, f, m, n) and released for 8 h to complete cytokinesis (g, h, o, p), after which they were prepared for immunofluorescence microscopy using antibodies against clathrin (a, c, e, g, i, k, m, o) and GRASP65 (b, d, f, h, j, l, n, p). Scale bar = 10 μm. C) Quantitation of Golgi morphologies. To analyze the effect of depleting CHC levels on Golgi structure, 3 morphological criteria (normal, intermediate, and disconnected) were defined. Number of cells exhibiting each morphological phenotype was determined by counting ∼100 cells.
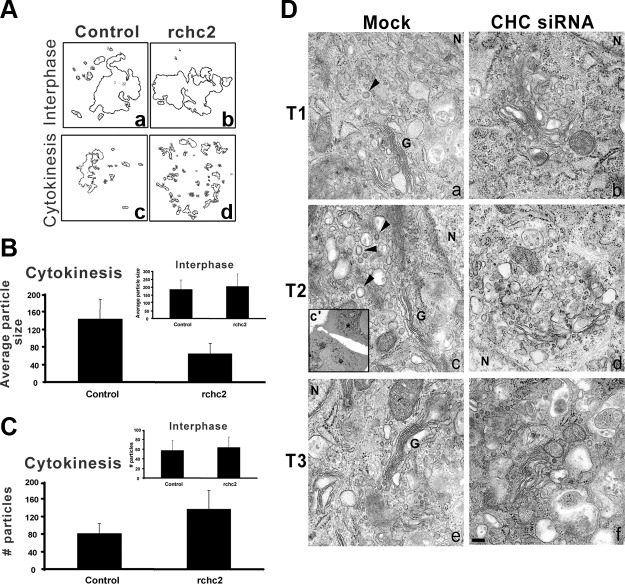
If such a model were correct, we predicted that in cytokinesis, the size of Golgi-derived structures, which presumably correspond to reforming cisternae, would be decreased in cells lacking CHC, whereas the number of Golgi “particles” (i.e., incompletely reformed cisternae) would increase in these cells. To test this idea, morphometric analysis was performed using algorithms (8) previously used to measure the formation of lateral Golgi cisternae (Fig. 5A). Strikingly, while in interphase the average particle size was similar in control and CHC siRNA-treated cells (Fig. 5B, inset), in cytokinesis, the Golgi particles of CHC-knockdown cells were ∼50% smaller than those of control cells (Fig. 5B). Consistent with our model, in CHC-knockdown cells the number of Golgi particles in cytokinesis was nearly 2-fold higher than in control-transfected cells (Fig. 5C). As expected, there was no significant difference in the total particle area of the Golgi in control and CHC-knockdown cells either during interphase or cytokinesis (Supplemental Fig. S1C). In addition, electron microscopy analysis of Golgi structures during cytokinesis confirmed the disconnected morphology (Fig. 5D). While mock-treated cells recovered normal Golgi stacks early in cytokinesis (Fig. 5Da, G), CHC siRNA-treated cells failed to acquire a normal Golgi apparatus even at late time points, when cytokinesis is completed (Figs. 4B and 5Df). Instead, the Golgi-derived structures remained dilated and fragmented (Fig. 5Db, d, f). Interestingly, the emerging Golgi stacks in control-transfected cells were accompanied by a heterogenous population of membranous structures that displayed the classic clathrin-coated appearance (Fig. 5D, arrowheads).
Figure 5.
Clathrin knockdown results in aberrant Golgi reformation on mitotic exit. A) Outlines of Golgi structures thresholded and analyzed using the particle analysis algorithm (see Materials and Methods and ref. 9) in interphase (a, b) and cytokinesis (c, d). B, C) Particle analysis generated measurements for particle size (B) and particle number (C) in interphase (insets) and cytokinetic cells. All values are averages from 30 cells. D) Electron micrographs of mock- and CHC siRNA-treated NRK cells at 3 time points following release from mitotic arrest (i.e., cytokinesis): T1 = 6 h 20 min (a, b), T2 = 7 h (c, d), T3 = 7 h 30 min (e, f). All images are from cytokinetic cells, as determined by cell pairs connected by a cytokinetic bridge (inset, c′). Arrowheads indicate heterogeneous clathrin structures. G, Golgi stack; N, nucleus. Scale bar = 200 nm.
Taken together, our data suggest that membrane-associated CHC plays a major role in the efficient reassembly of the Golgi apparatus under physiological conditions (i.e., mitosis) and that in its absence, cisternal reformation is aberrant.
DISCUSSION
Mitotic Golgi segregation has been proposed to be stochastic; however, the mechanisms whereby Golgi components are targeted to the centrosomal region of the cell and how postmitotic cisternal stacks are generated from seemingly random vesicles are not well understood, although several components have been implicated in this pathway (1, 31–34). The Golgi matrix was proposed to segregate in a somewhat regulated fashion (35) in order to provide a template for the reassembly of the Golgi apparatus as a whole. Conversely, golgins (i.e., GRASP65, GRASP 55, and giantin) appear to segregate randomly, and there is evidence that the Golgi apparatus may assemble de novo in the absence of a template (29). Here we characterize the CHC population that segregates with the spindle poles and suggest that these clathrin structures act as a pericentrosomal recruiting site for Golgi derived fragments as well as a remodeling mechanism for Golgi cisternae. In light of our previous findings that juxtanuclear clathrin plays a role in Golgi reassembly during recovery from drug treatment, it is tempting to propose that the spindle pole-associated clathrin pool may play a similar role in postmitotic Golgi reformation (25). However, further studies will be necessary to distinguish between a role for the spindle-associated clathrin and the spindle pole-associated pool in postmitotic Golgi reassembly.
The clathrin triskelion has been previously implicated in the stabilization of the mitotic spindle by Royle et al. (21). From deletion studies, these researchers suggest that the N-terminal domain of CHC mediates the localization of clathrin to the spindle. Moreover, based on the absence of membrane labeling in the spindle by staining with the styryl dye FM4–64, the researchers conclude that the spindle localized clathrin is not vesicle associated. While our biochemical and immunolocalization data agree that significant clathrin pools are present on mitotic spindles and centrosomes, we show that a large pool of clathrin is membrane-associated due to sensitivity to mild treatment with nonionic detergents. In support of this finding, clathrin-coated vesicle components, such as GAK (22) and ARH (23), have been shown to play important roles in centrosome and spindle integrity. Therefore, we assumed that the membrane bound clathrin structures might correspond to clathrin-coated vesicles and expected to find adaptor complexes such as those associated with the TGN or plasma membrane, i.e., GGAs, AP-1, AP-3, or AP-2, respectively. However, we and others (18, 21), were unable to detect any of these proteins in the centrosomal region of mitotic cells or in isolated spindle and centrosome preparations (data not shown). This suggested that clathrin was associated with centrosomes by a novel mechanism that may be different from that of its binding to coated pits or recycling endosomes. In this context, the association of clathrin with centrosomes may be analogous to its binding to microdomains on early endosomes, where, through interaction with the C-terminal region of the HRS protein, it acts as an organizer for the initial binding of the ESCRT complex to these membranes (36, 37). The planar clathrin lattices observed on early endosomes have been implicated in clustering selective cargo molecules to different regions of the endosome (38). In support of this idea, our data suggest that HRS-immunoreactive material colocalized with centrosomes and clathrin in mitotic cells (Supplemental Fig. S2). While the nature of the clathrin-membrane association at the centrosome remains to be determined, it is possible that the centrosomal pool of clathrin could function similarly to the endosomal planar lattices. Recently, ESCRTIII and Vps4 were proposed to play a role in centrosome and spindle maintenance (39). We envision a model whereby a membranous clathrin-associated lattice that is maintained at the centrosomes throughout mitosis acts as a scaffold for Golgi reassembly and cisternal remodeling by selectively concentrating Golgi proteins. Moreover, clathrin and endosomal components have been implicated in cytokinesis and the resolution of the cytokinetic bridge in various organisms (23, 40, 41). In this scenario, the spindle poles may act as sequestration sites during early mitosis until these components are needed in cytokinesis.
Evidence suggests that Golgi reassembly may be a sequential process in which the formation of a golgin-positive acceptor compartment, which is incompetent for cargo transport, precedes the accumulation of a glycosyltransferase into Golgi structures (42). We speculate that clathrin may act downstream of vesicle fusion and cisternal assembly and that this would ensure the correct partitioning of Golgi enzymes to restore cargo transport, particularly from the late Golgi or TGN (25). Most significantly, while our results do not differentiate between a role for the mitotic spindle- vs. centrosome-associated clathrin pools in Golgi reformation, our data suggest a novel and unexpected function for clathrin, namely its requirement as a key component in mediating the assembly and remodeling of the postmitotic Golgi apparatus.
Supplementary Material
Acknowledgments
This article is dedicated to the memory of Dr. Dennis Shields (1948–2008). The authors thank Drs. Silvia Corvera (University of Massachusetts Medical School, Worcester, MA, USA), Yanzhuang Wang (University of Michigan, Ann Arbor, MI, USA), and Sylvie Urbe (University of Liverpool, School of Biomedical Sciences, Liverpool, UK) for very generous gifts of antibodies and Dr. James Keen (Thomas Jefferson University, Philadelphia, PA, USA) for the CLCdsRed plasmid. The authors thank Dr. Shaeri Mukherjee for helpful suggestions with the manuscript.
This work was supported by U.S. National Institutes of Health grant DK21860 to D.S.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Shorter J., Warren G. (2002) Golgi architecture and inheritance. Annu. Rev. Cell Dev. Biol. 18, 379–420 [DOI] [PubMed] [Google Scholar]
- 2. Shorter J., Warren G. (1999) A role for the vesicle tethering protein, p115, in the post-mitotic stacking of reassembling Golgi cisternae in a cell-free system. J. Cell Biol. 146, 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shorter J., Beard M. B., Seemann J., Dirac-Svejstrup A. B., Warren G. (2002) Sequential tethering of golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J. Cell Biol. 157, 45–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diao A., Rahman D., Pappin D. J., Lucocq J., Lowe M. (2003) The coiled-coil membrane protein golgin-84 is a novel rab effector required for Golgi ribbon formation. J. Cell Biol. 160, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rabouille C., Misteli T., Watson R., Warren G. (1995) Reassembly of Golgi stacks from mitotic Golgi fragments in a cell-free system. J. Cell Biol. 129, 605–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Short B., Haas A., Barr F. A. (2005) Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim. Biophys. Acta 1744, 383–395 [DOI] [PubMed] [Google Scholar]
- 7. Wang Y., Seemann J., Pypaert M., Shorter J., Warren G. (2003) A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. EMBO J. 22, 3279–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puthenveedu M. A., Bachert C., Puri S., Lanni F., Linstedt A. D. (2006) GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat. Cell Biol. 8, 238–248 [DOI] [PubMed] [Google Scholar]
- 9. Guo Y., Punj V., Sengupta D., Linstedt A. D. (2008) Coat-tether interaction in Golgi organization. Mol. Biol. Cell 19, 2830–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirchhausen T. (1999) Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol. 15, 705–732 [DOI] [PubMed] [Google Scholar]
- 11. Kirchhausen T. (2000) Clathrin. Annu. Rev. Biochem. 69, 699–727 [DOI] [PubMed] [Google Scholar]
- 12. Bard F., Malhotra V. (2006) The formation of TGN-to-plasma-membrane transport carriers. Annu. Rev. Cell Dev. Biol. 22, 439–455 [DOI] [PubMed] [Google Scholar]
- 13. McNiven M. A., Thompson H. M. (2006) Vesicle formation at the plasma membrane and trans-Golgi network: the same but different. Science 313, 1591–1594 [DOI] [PubMed] [Google Scholar]
- 14. Di Paolo G., De Camilli P. (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 [DOI] [PubMed] [Google Scholar]
- 15. Lowe M., Nakamura N., Warren G. (1998) Golgi division and membrane traffic. Trends Cell Biol. 8, 40–44 [DOI] [PubMed] [Google Scholar]
- 16. Boucrot E., Kirchhausen T. (2007) Endosomal recycling controls plasma membrane area during mitosis. Proc. Natl. Acad. Sci. U. S. A. 104, 7939–7944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Z., Zheng Y. (2009) A requirement for epsin in mitotic membrane and spindle organization. J. Cell Biol. 186, 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okamoto C. T., McKinney J., Jeng Y. Y. (2000) Clathrin in mitotic spindles. Am. J. Physiol. Cell Physiol. 279, C369–C374 [DOI] [PubMed] [Google Scholar]
- 19. Borlido J., Veltri G., Jackson A. P., Mills I. G. (2008) Clathrin is spindle-associated but not essential for mitosis. PLoS One 3, e3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maro B., Johnson M. H., Pickering S. J., Louvard D. (1985) Changes in the distribution of membranous organelles during mouse early development. J. Embryol. Exp. Morphol. 90, 287–309 [PubMed] [Google Scholar]
- 21. Royle S. J., Bright N. A., Lagnado L. (2005) Clathrin is required for the function of the mitotic spindle. Nature 434, 1152–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shimizu H., Nagamori I., Yabuta N., Nojima H. (2009) GAK, a regulator of clathrin-mediated membrane traffic, also controls centrosome integrity and chromosome congression. J. Cell Sci. 122, 3145–3152 [DOI] [PubMed] [Google Scholar]
- 23. Lehtonen S., Shah M., Nielsen R., Iino N., Ryan J. J., Zhou H., Farquhar M. G. (2008) The endocytic adaptor protein ARH associates with motor and centrosomal proteins and is involved in centrosome assembly and cytokinesis. Mol. Biol. Cell 19, 2949–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dinter A., Berger E. G. (1998) Golgi-disturbing agents. Histochem. Cell Biol. 109, 571–590 [DOI] [PubMed] [Google Scholar]
- 25. Radulescu A. E., Siddhanta A., Shields D. (2007) A role for clathrin in reassembly of the Golgi apparatus. Mol. Biol. Cell 18, 94–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Motley A., Bright N. A., Seaman M. N., Robinson M. S. (2003) Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162, 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuriyama R., Keryer G., Borisy G. G. (1984) The mitotic spindle of Chinese hamster ovary cells isolated in taxol-containing medium. J. Cell Sci. 66, 265–275 [DOI] [PubMed] [Google Scholar]
- 28. Blomberg-Wirschell M., Doxsey S. J. (1998) Rapid isolation of centrosomes. Methods Enzymol. 298, 228–238 [DOI] [PubMed] [Google Scholar]
- 29. Puri S., Telfer H., Velliste M., Murphy R. F., Linstedt A. D. (2004) Dispersal of Golgi matrix proteins during mitotic Golgi disassembly. J. Cell Sci. 117, 451–456 [DOI] [PubMed] [Google Scholar]
- 30. Booth D. G., Hood F. E., Prior I. A., Royle S. J. A TACC3/ch-TOG/clathrin complex stabilises kinetochore fibres by inter-microtubule bridging. EMBO J. 30, 906–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Misteli T. (1996) Molecular mechanisms in the disassembly and reassembly of the mammalian Golgi apparatus during M-phase. FEBS Lett. 389, 66–69 [DOI] [PubMed] [Google Scholar]
- 32. Lowe M., Barr F. A. (2007) Inheritance and biogenesis of organelles in the secretory pathway. Nat. Rev. Mol. Cell. Biol. 8, 429–439 [DOI] [PubMed] [Google Scholar]
- 33. Corthesy-Theulaz I., Pauloin A., Pfeffer S. R. (1992) Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J. Cell Biol. 118, 1333–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marra P., Salvatore L., Mironov A., Jr., Di Campli A., Di Tullio G., Trucco A., Beznoussenko G., Mironov A., De Matteis M. A. (2007) The biogenesis of the Golgi ribbon: the roles of membrane input from the ER and of GM130. Mol. Biol. Cell 18, 1595–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seemann J., Pypaert M., Taguchi T., Malsam J., Warren G. (2002) Partitioning of the matrix fraction of the Golgi apparatus during mitosis in animal cells. Science 295, 848–851 [DOI] [PubMed] [Google Scholar]
- 36. Raiborg C., Bache K. G., Gillooly D. J., Madshus I. H., Stang E., Stenmark H. (2002) Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 4, 394–398 [DOI] [PubMed] [Google Scholar]
- 37. Williams R. L., Urbe S. (2007) The emerging shape of the ESCRT machinery. Nat. Rev. Mol. Cell. Biol. 8, 355–368 [DOI] [PubMed] [Google Scholar]
- 38. Sachse M., Urbe S., Oorschot V., Strous G. J., Klumperman J. (2002) Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell 13, 1313–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morita E., Colf L. A., Karren M. A., Sandrin V., Rodesch C. K., Sundquist W. I. Human ESCRT-III and VPS4 proteins are required for centrosome and spindle maintenance. Proc. Natl. Acad. Sci. U. S. A. 107, 12889–12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gerald N. J., Damer C. K., O'Halloran T. J., De Lozanne A. (2001) Cytokinesis failure in clathrin-minus cells is caused by cleavage furrow instability. Cell Motil. Cytoskeleton 48, 213–223 [DOI] [PubMed] [Google Scholar]
- 41. Warner A. K., Keen J. H., Wang Y. L. (2006) Dynamics of membrane clathrin-coated structures during cytokinesis. Traffic 7, 205–215 [DOI] [PubMed] [Google Scholar]
- 42. Jiang S., Rhee S. W., Gleeson P. A., Storrie B. (2006) Capacity of the Golgi apparatus for cargo transport prior to complete assembly. Mol. Biol. Cell 17, 4105–4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.