Abstract
Tri-iodo-l-thyronine (T3) suppresses the proliferation of near-term serum-stimulated fetal ovine cardiomyocytes in vitro. Thus, we hypothesized that T3 is a major stimulant of cardiomyocyte maturation in vivo. We studied 3 groups of sheep fetuses on gestational days 125–130 (term ∼145 d): a T3-infusion group, to mimic fetal term levels (plasma T3 levels increased from ∼0.1 to ∼1.0 ng/ml; t1/2∼24 h); a thyroidectomized group, to produce low thyroid hormone levels; and a vehicle-infusion group, to serve as intact controls. At 130 d of gestation, sections of left ventricular freewall were harvested, and the remaining myocardium was enzymatically dissociated. Proteins involved in cell cycle regulation (p21, cyclin D1), proliferation (ERK), and hypertrophy (mTOR) were measured in left ventricular tissue. Evidence that elevated T3 augmented the maturation rate of cardiomyocytes included 14% increased width, 31% increase in binucleation, 39% reduction in proliferation, 150% reduction in cyclin D1 protein, and 500% increase in p21 protein. Increased expression of phospho-mTOR, ANP, and SERCA2a also suggests that T3 promotes maturation and hypertrophy of fetal cardiomyocytes. Thyroidectomized fetuses had reduced cell cycle activity and binucleation. These findings support the hypothesis that T3 is a prime driver of prenatal cardiomyocyte maturation.—Chattergoon, N. N., Giraud, G. D., Louey, S., Stork, P., Fowden, A. L., Thornburg, K. L. Thyroid hormone drives fetal cardiomyocyte maturation
Keywords: proliferation, sheep fetus, p21
Thyroid hormones (THs) are phylogenetically ancient and known to regulate diverse developmental processes among lower vertebrates, as exemplified by their fundamental role in the transformation of aquatic tadpoles into terrestrial frogs (1). In humans, abnormal TH levels in the fetus can lead to a range of complications, including decreased cardiac output, growth restriction, tachycardia, neuropathologies, and even fetal demise (2–4). Nevertheless, the influence of TH on cardiomyocyte development in mammals has been studied very little. This gap in knowledge remains a serious detriment to our understanding of potential mechanisms by which fetal hearts may be harmed when TH levels fall outside the normal range.
The primary form of TH secreted by the mammalian thyroid gland is 3,3′,5,5′tetra-iodo-l-thyronine (thyroxine or T4; ref. 5). T4 levels increase during gestation as the human fetal thyroid gland becomes active at around 15 wk, but fetal plasma levels are also determined by maternal T4 transported across the placenta (6, 7). In the ovine fetus, T4 levels in plasma gradually decrease toward term as T4 is converted to the more active form of TH, 3,3′,5 tri-iodo-l-thyronine (T3; ref. 8). The T4 to T3 conversion occurs through the activity of deiodinases whose expression is regulated by cortisol (9). As term approaches and the hypothalamic-pituitary-adrenal axis becomes activated (10), increased circulating cortisol concentrations stimulate an increase in expression of monodeiodinases, including the D2 form, which transforms T4 to T3. Thus, total T3 levels increase from ∼0.3 to ∼1.5 nM in fetal sheep during the last weeks before delivery (8). This prepartum rise in T3 promotes maturational processes in lung, liver, and kidney (11–13), and presumably in other organs as well.
The developing ovine myocardium is ideal for investigating the maturational role of THs because like human hearts—but unlike rodent hearts—most ovine cardiomyocytes go through their terminal maturation phase before birth (14–16). One sign of maturation in sheep and rat cardiomyocytes is the generation of a second nucleus and terminal differentiation, at which time cardiomyocytes permanently exit the cell cycle. However, the timing between rodents and large mammals is different. The myocyte population in the ovine myocardium is ∼80% binucleated at birth (16), but only ∼7% of cardiomyocytes in the rodent heart. By 2 wk of age, >95% of rodent cardiomyocytes have become binucleated (17).
The ovine heart grows primarily by increasing cardiomyocyte numbers (hyperplasia) over the first two-thirds of gestation, but in the last few weeks, cardiomyocyte volume expansion (hypertrophy) becomes increasingly important in myocardial growth (16, 18). In fetal sheep, the maturation of cardiomyocytes over the last third of gestation is characterized by binucleation, suppression of mitotic activity, and cardiomyocyte enlargement (16, 19). While the roles of angiotensin II, cortisol, and insulin-like growth factor 1 are known to regulate the hyperplastic response (15, 20, 21), the chemical signals that regulate these aforementioned “macromaturational” processes have not been discovered.
The features that characterize the maturational process in cardiomyocytes have been described in several experimental animals, including sheep (18, 22–24). In mammals, the maturing ventricular myocyte expands via physiological hypertrophy, packs the myocyte interior with contractile elements, establishes the t-tubule system to expedite excitation-contraction coupling, and switches to an internal Ca2+ cycling system through development of the internal Ca2+ storage compartment, the sarcoplasmic reticulum (SR) (14, 25, 26). Cardiac maturation is normally associated with increased expression of SR calcium ATPase (SERCA2a; ref. 27). In rats, SERCA2a is a known target of T3 action (28).
Atrial natriuretic peptide (ANP) is made by ventricular and atrial cardiomyocytes in the fetal heart, and its expression increases as gestational age proceeds. In normal adults, ANP is not expressed in the cardiac ventricles but is reexpressed in response to stress (29). ANP appears to be important as a protector of excessive myocardial growth (30–33). Hypothyroid conditions in the ovine fetus decrease ANP production (34) and decrease cardiac output (2). Thus, ANP is another molecule that is associated with myocardial maturation.
Mitogen-activated protein kinase (MAPK) and phosphoinositol-3 kinase (PI3K)-related cascades are important in the regulation of growth and maturation of the heart, including the hypertrophic growth of the postnatal heart (35–37). However, roles of T3 in regulating specific branches of the MAPK and PI3K cascades involved in cardiomyocyte growth and maturation, including the extracellular signal-related kinase (ERK) and the mammalian target of rapamycin (mTOR) protein, are not known.
In clinical studies, low maternal plasma TH levels during pregnancy are also associated with neuropathologies in offspring and increased rates of fetal demise (38–40). Conversely, fetal hyperthyroxinemia leads to fetal complications that include growth restriction, tachycardia, and suppression of the hypothalamic-pituitary-thyroid axis (3). Thus, it appears that adverse outcomes occur with TH levels outside a normal concentration window. Whether abnormal concentrations of TH affect the pattern of fetal heart development remains unknown.
To understand the role of T3 in regulating the maturation of fetal cardiomyocytes, we hypothesized that T3 is the primary driver of cardiomyocyte maturation in fetal sheep in vivo. We studied 3 groups of fetuses with widely different circulating T3 levels to characterize the normal macro features of cardiomyocyte maturation under these conditions as judged by 3 criteria: stimulation of binucleation, suppression of proliferation, and stimulation of cardiomyocyte enlargement among binucleated myocytes. We tested whether elevated T3 would activate target signaling proteins, ERK and mTOR, and alter cell cycle regulatory genes, including the cell cycle stimulant protein cyclin D1, the cell cycle inhibitor protein p21, and the antiproliferative peptide ANP. T3 was infused into the first group of preterm fetuses; a second group was thyroidectomized to produce lower than normal T3 levels; and a third group served as normal intact vehicle-infused controls.
MATERIALS AND METHODS
Animals
Sheep (Ovis aries, mixed western breed) were studied in accordance with the Institutional Animal Care and Use Committee at Oregon Health and Science University. The fetal sheep model was used rather than a rodent model because the size and growth rate of the heart is similar to that of the human; large numbers of cardiomyocytes can be dissociated from a single heart from midgestation onward; ovine fetal cardiomyocytes go through terminal differentiation and become binucleated during fetal life; and fetal hemodynamic factors, including heart rate and blood pressure, can be monitored continuously in the unanesthetized preparation from midgestation onward.
Surgery
Twelve time-mated ewes, carrying twin fetuses, underwent sterile surgery at 120 ± 1 d gestational age (dGA; term ∼145 d). Surgeries were performed on both of the twin fetuses. Polyvinyl catheters placed in the fetal carotid artery and jugular vein were used for continuous hemodynamic recordings, infusion of T3 or vehicle (i.v.), and blood sampling; a catheter attached to the fetal skin was used to measure amniotic fluid pressure (15). A subset of fetuses was thyroidectomized at the time of surgery prior to implanting catheters (41). Ewes were allowed 4 d recovery from surgery before they were moved to stanchions for the duration of the experiment. Infusions started 5 d after surgery.
Infusion studies
Fetuses (n=8/group) were assigned randomly to one of three groups: group 1, nonthyroidectomized (intact) T3-infusion (high T3) group; group 2, thyroidectomized (TX; low T3) group; or group 3, intact vehicle-infusion (normal T3) group. Each twin of a pair was treated similarly to negate any communication of treatments between the two fetuses. In group 1, T3 (Sigma, St. Louis, MO, USA) was infused at a rate of 54 μg/d i.v., for a period of 5 d (from 125 to 130 dGA). A target concentration of T3 in plasma of 1.0 ng/ml was chosen for the infusion group because it represents the circulating prepartum concentration (∼2 wk later than the age we are studying; ref. 8). Other investigators have used a similar regimen in fetal sheep (8, 42). In blood, >99% of total T4 and T3 is transported bound to thyroxine-binding globulin and other plasma proteins. Only the free unbound form of the hormone is available to stimulate receptors within individual cells. TH actions occur through their nuclear receptors TRα and TRβ (43–46) and through separate nongenomic mechanisms (47–49). TH receptors have affinities for T3 that are some 12 times higher than for T4 (48), thus explaining the greater potency of T3 vs. T4. Hence, we chose to infuse T3 rather than T4 directly into the sheep fetus in order to better control the target T3 concentration over the course of the study. Control animals were infused with a vehicle solution (54 μl 1M NaOH/d in lactated Ringers solution, 38.4 ml/d i.v.) at the same rate and duration as for T3. TX fetuses were infused with sterile lactated Ringers solution as vehicle. Fetal arterial blood was collected before the study and then daily to determine fetal pH, blood gases, and hematocrit (Radiometer ABL 720; Radiometer A/S, Copenhagen, Denmark; values corrected to 39°C); plasma samples were used to measure free and total T3.
Hemodynamic measurements
Fetal aortic pressure, right atrial pressure, amniotic pressure, and heart rate were continuously monitored and recorded throughout the study. Pressures were measured with Abbott Transpac pressure transducers (Abbott, Abbott Park, IL, USA) and a calibrated computerized recording system (Powerlab, ADInstruments, Colorado Springs, CO, USA; Apple, Cupertino, CA, USA). The system was calibrated against a mercury manometer and rezeroed for drift before each measurement. All fetal intravascular pressures were referred to amniotic fluid pressure as 0. Zeroes were checked every morning, after which the data were taken over the next 60-min period, averaged, and recorded for later analysis. Heart rates were derived from arterial pressure measurements.
Plasma hormone concentrations
Daily plasma samples were collected from each fetus and stored at −20°C until analysis. Plasma total T3 and free T3 were measured using respective Coat-a-Count radioimmunoassays (Siemens Healthcare Diagnostics, Inc., Los Angeles, CA, USA). The lower limits of detection were 0.07 ng/ml for total T3 and 0.2 pg/ml for free T3. The interassay coefficients of variation were 8% for total T3 and 7.8% for free T3.
Cardiac myocyte isolation
At the end of the study (130±1 dGA), ewes were euthanized by intravenous injection of a commercial solution of sodium pentobarbital (SomnaSol, ∼80 mg/kg; Butler Schein Animal Health, Dublin, OH, USA). A bolus dose of heparin (10,000 U) followed by 10 ml of saturated potassium chloride (KCl) was injected into the umbilical vein to arrest the fetal heart in diastole. Prior to dissociation of the heart to isolate cardiomyocytes, a 0.5-cm2 section of the left ventricle (midwall, full thickness) was removed and flash-frozen for molecular analyses. The cut edges of the hole in the ventricular wall were lightly cauterized before the myocyte dissociation procedure so that dissociation solutions would not leak.
Hearts were enzymatically dissociated as described previously by our laboratory (50). Briefly, hearts were perfused retrogradely with 3 gassed (95% O2 and 5% CO2 at 39°C) solutions: Tyrode's buffer for 5–10 min (140 mM NaCl, 5 mM KCl, 1 mM MgCl2·6H2O, 10 mM glucose, and 10 mM HEPES; pH adjusted to 7.35 with NaOH; no calcium added; components from Sigma); a collagenase/protease solution for 5–10 min consisting of type II collagenase (160 U/ml; Worthington Biochemical Corp., Lakewood, NJ, USA) and type XIV protease (0.78 U/ml; Sigma,) in Tyrode's buffer; and a calcium-free Kraftbrühe (KB) solution (74 mM glutamic acid, 30 mM KCl, 30 mM KH2PO4, 20 mM taurine, 3 mM MgSO4, 0.5 mM EGTA, 10 mM HEPES, and 10 mM glucose; pH adjusted to 7.37 using KOH; components from Sigma). Following the perfusion, the left ventricular (LV) and right ventricular (RV) free walls were dissected from the heart and placed in separate tubes containing KB solution. The tissue was gently swirled to release the myocytes. The cell slurry rested at room temperature for 30 min. Myocytes were fixed with an equal volume of 2% paraformaldehyde for measurement of maturational state (binucleation), cell cycle activity, and myocyte size.
Myocyte size
These measurements were performed as described previously in our laboratory (15). Cardiac myocyte lengths and maximal widths were measured from photomicrographs of methylene blue-stained wet mounts at ×400 view (Zeiss Axiophot; Bartels and Stout, Bellevue, WA, USA). Cardiac myocyte lengths and widths were measured using calibrated imaging software (Image-Pro Plus 6.2 for PC; Media Cybernetics, Bethesda, MD, USA). Myocyte volume was calculated as described previously by correcting from the volume of a cylinder with a height equal to the length of the myocyte and a diameter equal to the myocyte width (14). At least 50 mononucleated and binucleated myocytes were measured per ventricle per fetus. For all analyses of dissociated cardiac myocytes, cells were selected by nonrepeating, random sampling across the microscope slide.
Binucleation
Methylene blue staining allowed the number of nuclei per cardiac myocyte to be determined. At least 300 myocytes per ventricle per animal were counted to determine percentage binucleation, separately of the cells measured for size.
Cell cycle activity
Cell cycle activity was estimated by determining the fraction of cardiomyocytes that were positive for Ki-67 staining using methods previously described by our laboratory (16). The Ki-67 protein is present in all phases of the cell cycle; it disappears in cells in G0. Isolated fixed myocytes (50,000) were dried and postfixed onto slides (SuperFrost; Fisher, Pittsburgh, PA, USA) by immersion in acetone for 30 min at 4°C. Cells were permeabilized for antigen retrieval in sodium citrate (0.01 M, pH 6.0) for 6 min at 85°C. Nonspecific staining and endogenous peroxidase activities were blocked with 0.3% hydrogen peroxide. Slides were incubated overnight at 4°C with the Ki-67 antibody (1:200 dilution, mouse monoclonal MIB-1; DakoCytomation, Carpinteria, CA, USA). Samples were incubated with the biotinylated secondary antibody (1:200; Vectastain ABC Kit, Mouse IgG; VectorLabs, Burlingame, CA, USA). Positive nuclei were stained with 3,3′-diaminobenzidine chromagen (DAB) in substrate buffer (DakoCytomation) for 1–5 min, and cells were counterstained with 0.1% methylene blue. A minimum of 500 myocytes were counted per ventricle per fetus to determine the number of Ki-67-positive mononucleated myocytes (51). Results are expressed as the percentage of Ki-67-positive mononucleated myocytes ÷ total number of mononucleated myocytes. Phospho-histone 3 (pH 3, 1:200 dilution; Cell Signaling, Danvers, MA, USA) was used to compare the percentage of cells in mitosis at the end of the infusion period to those that are in the cell cycle. Histone 3 phosphorylation is tightly correlated with chromosome condensation in mitotic prophase. The staining protocol and analysis is the same as that described for Ki-67.
Western blot
Protein extracted from frozen LV tissue [lysis solution: 5 mM Tris-HCl, 5 mM EGTA, 5 mM EDTA, 0.06% SDS (RIPA buffer; Upstate, Temecula, CA, USA); protease inhibitor Complete Mini EDTA-free tablet (Roche, Indianapolis, IN, USA); and inhibitors against alkaline, serine/threonine, and tyrosine phosphatases (Sigma)] was quantified by BCA assay (Pierce, Rockford, IL, USA). Cytosolic protein from each ventricle (20 μg) was separated by SDS-PAGE and transferred onto reinforced nitrocellulose membranes (Optitran BA-S 83; Whatman-GE, Piscataway, NJ, USA) before incubation with primary antibodies (listed below). Protein was visualized by chemiluminescence (SuperSignal; Pierce) and digitally quantified (ImageJ 1.43; U.S. National Institutes of Health, Bethesda, MD, USA) (50).
Antibodies for Western blot analysis
The following antibodies were obtained from Cell Signaling. Phospho-p44/42 MAPK (ERK1/2) rabbit polyclonal antibody detects endogenous levels of p44 and p42 MAP kinase (ERK1 and ERK2) when phosphorylated either individually or dually at Thr202 and Tyr204. Phospho-AKT (Ser473; D9E) XP rabbit monoclonal antibody detects endogenous levels of AKT only when phosphorylated at Ser473. Phospho-p70 S6 kinase (Ser371) rabbit monoclonal antibody detects endogenous levels of p70 S6 kinase (p70S6K) only when phosphorylated at Ser371. AKT rabbit polyclonal antibody detects endogenous levels of total AKT1, AKT2, and AKT3 proteins. Rabbit p70S6K antibody detects endogenous levels of total p70S6K protein. Rabbit polyclonal Phospho-mTOR (Ser2448) antibody detects endogenous levels of mTOR only when phosphorylated at Ser2448, and rabbit polyclonal total mTOR detects endogenous mTOR. Mouse monoclonal p21 Waf1/Cip1 (DCS60) antibody detects endogenous levels of total p21 protein. The antibody does not cross-react with other cdk inhibitors and recognizes the amino-terminal portion of p21. Rabbit monoclonal α-tubulin detects endogenous levels of total α-tubulin protein, and does not cross-react with recombinant β-tubulin. Mouse monoclonal cyclin D1 recognizes full-length peptide (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Horseradish peroxidase-linked secondary antibodies (goat anti-rabbit IgG and horse anti-mouse IgG) were obtained from Cell Signaling.
Quantitative polymerase chain reaction (PCR) assays for hypertrophic genes
The following primers were used for PCR.
ANP: forward 5′-CCCGTGTATGGCTCTGTGTC-3′, reverse 5′-GAGGGCACAGCCTCATCTTC-3′ (195 bp).
SERCA2a: forward 5′-TGACAGGTGTACCCACATTC-3′, reverse 5′-AAGTTGGCAGAGTCCTCAAG-3′ (186 bp).
β-Actin: forward 5′-CTGCACCACCAACTGCTTAG-3′, reverse 5′-CAGTGGATGCAGGGATGATG-3′ (150 bp).
RNA was extracted from 50 mg of frozen fetal heart tissue using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized from 1 μg RNA from each sample using the GeneAmp RNA PCR kit (Applied Biosystems, Foster City, CA, USA). The size of each PCR product was verified on a 1% agarose gel with a 1 kb+ Ladder (Invitrogen). In subsequent runs, melting curve analysis (where temperature is raised from 65 to 95°C at a rate of 0.1°C/s) was used to verify correct production of product. PCR reactions were performed using ABI Power SYBR Green (Applied Biosystems) in the Stratagene MX-3005P PCR instrument (Stratagene, La Jolla, CA, USA). Optimal cycling conditions were determined for each primer pair. Quantification values were generated from samples with only a single product that melts at the expected temperature. For each unknown sample, the concentration of the amplicon was calculated based on the equation derived from the standard curve. These concentrations were normalized to the concentration of the housekeeping gene, β-actin, for the correlating sample. β-Actin was chosen for normalization after determining that it was not affected by changing experimental conditions.
Statistics
The in vivo effect of T3 on fetal hemodynamics was analyzed by 2-way analysis of variance (ANOVA) with post hoc Bonferroni's multiple comparison test against d 0 values. Myocyte parameters (cell sizes, cell cycle activity, and binucleation), protein expression, and immunohistochemistry (Ki-67 and pH3) from the 3 experimental groups were compared by 1-way ANOVA with post hoc Tukey's multiple comparison test. Western blot densitometry was quantified over the linear range using NIH ImageJ 1.4. Phosphoproteins analyzed by Western blot were normalized to their respective total unphosphorylated protein equivalent. Remaining protein analysis was normalized to α-tubulin, which served as the loading control. While the same myocyte analysis was performed on both ventricles, data from the right ventricle, except for cell size, are not shown, as the trends were the same as for the left ventricle. No tissue was collected for molecular studies from right ventricles. All statistics were performed using GraphPad Prism 4.03 for Windows (GraphPad, San Diego, CA, USA). Values of P < 0.05 were accepted as significant. Graphs display all three groups of animals per respective measurement. Data are expressed as means ± se.
RESULTS
Hemodynamic measurements
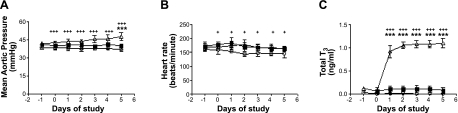
Fetal blood-gas values were unchanged among groups over the course of the study (Table 1). Mean fetal arterial pressure was elevated significantly but only on d 5 in the T3-infused group when compared to control animals (Fig. 1A). Fetal arterial pressure was not changed across the 5 experimental days in any group. However, pressures were slightly higher in the high-T3 group than in TX fetuses. Fetal heart rates were unchanged in control fetuses or in fetuses receiving T3, though a decrease in heart rate was found in TX fetuses compared to control fetuses across the 5-d experimental period (Fig. 1B).
Table 1.
Hemodynamic measurements with reference to thyroid status
| Measurement | Thyroid status |
||
|---|---|---|---|
| TX | Control | T3 infusion | |
| Arterial pressure (mmHg) | 37.1 ± 0.7+++ | 40.1 ± 1.0 | 47.6 ± 1.2*** |
| Heart rate (bpm) | 146.2 ± 5.4+ | 162.9 ± 3.3 | 163.2 ± 3.3 |
| Blood gas | |||
| Po2 (mmHg) | 23.5 | 20.9 | 22.8 |
| Pco2 (mmHg) | 50.1 | 52.1 | 48.6 |
| pH | 7.34 | 7.33 | 7.35 |
Measurements on final day of study. Data are means ± se; n = 8/group. Significant differences between groups are indicated.
P < 0.001 vs. control;
P < 0.05,
P < 0.001 vs. T3.
Figure 1.
Hemodynamic measurements with changes in thyroid status. A) Mean aortic pressure did not differ between control (solid squares) and TX (open circles), but pressure was significantly increased on d 5 in T3-infused fetuses (open triangles). Mean aortic pressure was depressed in TX compared to T3-infused fetuses throughout the study period. B) Heart rate was decreased significantly in the TX group. C) Total T3 levels were unchanged in control fetuses, while T3 infusion increased concentrations to those found near term. T3 was undetectable in TX fetuses. Data are means ± se; n = 8/group. ***P < 0.001 vs. control; +P < 0.05, +++P < 0.001 vs. TX.
TH concentrations
As expected, the plasma concentrations of total T3 in control fetuses and in the pre-T3-infusion animals were similar at ∼0.1 ± 0.1 ng/ml. The target concentration of T3 in plasma of 1.0 ng/ml was approximated within the first day of T3 infusion, reached 1.1 ± 0.3 ng/ml by d 2, and was maintained for the duration of the study. Free T3 levels reached 2.2 ± 0.8 pg/ml in the T3-infusion group and 0.1 ± 0.1 pg/ml in the control group; total and free T3 levels in the TX group were not detectable throughout the study period.
Organ weights
Unadjusted organ weights (g), as well as organ weights adjusted for body weight (g/kg), are summarized for heart, brain, liver, combined kidney, combined adrenal, and combined thyroid gland (control and T3 groups only) in Table 2. Unadjusted heart weight was unchanged by thyroid status, but heart weight to body weight ratio was significantly lower in TX fetuses. Kidney weights (g and g/kg body weight) were increased in TX fetuses compared to control fetuses and compared to T3-infused fetuses. No effect of thyroid status was found on brain, liver, adrenal glands, or thyroid gland weights.
Table 2.
Organ weights
| Organ | Thyroid status |
|||||
|---|---|---|---|---|---|---|
| TX |
Control |
T3 infusion |
||||
| Raw (g) | Ratio (g/kg) | Raw (g) | Ratio (g/kg) | Raw (g) | Ratio (g/kg) | |
| Body (kg) | 3.5 ± 0.1 | 3.2 ± 0.2 | 3.3 ± 0.2 | |||
| Heart | 21.1 ± 1.2 | 5.9 ± 0.2*,+ | 21.6 ± 0.9 | 6.8 ± 0.3 | 22.3 ± 1.0 | 6.8 ± 0.3 |
| Brain | 41.4 ± 1.3 | 11.7 ± 0.1+ | 40.1 ± 1.5 | 12.6 ± 0.5 | 42.4 ± 1.4 | 13 ± 0.5 |
| Liver | 74.5 ± 7.2 | 20.8 ± 1.3 | 58.8 ± 0.0 | 18.2 ± 0.6 | 65.7 ± 4.3 | 20 ± 1 |
| Kidney | 22.6 ± 1.6* | 6.4 ± 0.3**,++ | 17.3 ± 1.4 | 5.3 ± 0.2 | 17.7 ± 1 | 5.4 ± 0.2 |
| Adrenal | 0.3 ± 0.02 | 0.1 ± 0.01 | 0.3 ± 0.03 | 0.1 ± 0.01 | 0.3 ± 0.03 | 0.1 ± 0.01 |
| Thyroid | NA | NA | 0.6 ± 0.05 | 0.2 ± 0.02 | 0.7 ± 0.05 | 0.2 ± 0.01 |
Data are means ± se; n = 8/group. Ratio, organ to fetal body weight; NA, tissue not available. Significant differences between groups are indicated.
P < 0.05,
P < 0.01 vs. control;
P < 0.05,
P < 0.01 vs. T3.
Effect of T3 on maturational state of fetal cardiomyocytes (LV only; similar trends in RV)
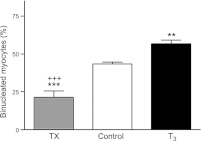
One index of cardiomyocyte maturation, cardiomyocyte binucleation, was related to the concentration of circulating T3 levels in the fetal groups (Fig. 2). In the left ventricle, 21.4 ± 4.1% of cardiomyocytes from TX animals were binucleated compared to 42.6 ± 1.1% in vehicle-infused animals (P<0.001) and 56.8 ± 2.4% in T3 infused fetuses (P<0.001). Binucleation was increased significantly in the left ventricles of T3-infused fetuses compared to vehicle-infused fetuses (P<0.01). Cardiomyocyte binucleation in right ventricles showed a similar trend (TX, 20.6±8.6%; control, 45.8±9%; and T3, 55.8±8.5%).
Figure 2.
Increased circulating T3 promotes terminal differentiation of fetal sheep cardiomyocytes. Elevated T3 concentration increased the portion of LV myocytes that were binucleated. Data are means ± se; n = 8/group. **P < 0.01, ***P < 0.001 vs. control; +++P < 0.001 vs. T3.
Proliferative markers in cardiomyocytes (LV only reported; similar trends in RV)
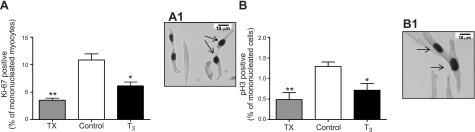
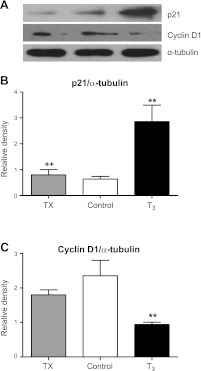
The percentage of mononucleated myocytes that stained positive for Ki-67 (Fig. 3A) was lower in fetuses exposed to elevated levels of T3 compared to control fetuses (6.1±0.7 vs. 11.4±1.1%, P<0.01) and even lower in fetuses with depressed T3 levels (3.1±0.6%, P<0.001 vs. control; Fig. 3A). The proportion of mononucleated myocytes positive for Ki-67 did not differ between T3 and TX fetuses. The percentage of phospho-histone 3-positive (Fig. 3B) mononucleated myocytes followed the same trend. In T3-infused fetuses, 0.8 ± 0.2% of mononucleated cardiomyocytes were phospho-histone 3 positive vs. 1.2 ± 0.1% in controls (P<0.05), and in thyroidectomized fetuses 0.4 ± 0.2% were positive (P<0.01). Consistent with this change, p21 protein levels were increased and cyclin D1 protein decreased in hearts from T3-infused fetuses compared to control and TX (P<0.01; Fig. 4). However, no difference was found in protein levels for either p21 or cyclin D1 in TX hearts compared to control.
Figure 3.
A) Cell cycle activity, measured by Ki-67, decreased in both T3-infused and TX fetuses. A1) Image at ×400. B) Mitotic activity of myocytes, measured by phospho-histone 3 expression, was decreased in both T3-infused and TX fetuses. B1) Image at ×400. Arrows indicate positively labeled cardiomyocytes. Data are means ± se; n = 8/group. *P < 0.05; **P < 0.01 vs. control.
Figure 4.
A) Immunoblots of cell cycle proteins in cardiomyocytes from T3-infused and TX fetuses compared to controls. B) Cell cycle suppressant p21 was markedly elevated in T3-infused compared to control and TX fetuses. C) Expression of cell cycle promoter cyclin D1 is diminished in T3-infused compared to control and TX fetuses. Data are means ± se; n = 8/group. **P < 0.05 vs. control; ++P < 0.01 vs. T3.
Cardiomyocyte size
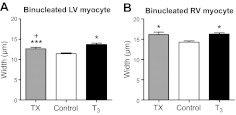
LV data are shown in Table 3. Cell measurements in the RV were similar to LV measurements and are not included in the table. No differences were found in the lengths of mononucleated or binucleated cardiomyocytes among the three groups (Table 3). However, the widths of both mononucleated and binucleated LV myocytes from T3-treated fetuses were larger than those from control fetuses (P<0.05 and P<0.001, respectively; Table 3). T3-infused fetuses also had wider mononucleated RV myocytes compared to control (12.9±0.3 vs. 11.5±0.1 μm, P<0.05), while thyroidectomy had no effect on mononucleated RV cardiomyocyte width. Binucleated cardiomyocytes from both ventricles of T3-infused and TX fetuses were wider than control myocytes (Fig. 5). Binucleated LV myocytes were also wider in T3-infused fetuses compared to TX fetuses (P<0.05; Table 3).
Table 3.
Myocyte measurements with reference to thyroid status
| Measurement | Thyroid status |
||
|---|---|---|---|
| TX | Control | T3 infusion | |
| Mononucleated LV myocytes | |||
| Ki67+ (%) | 3.1 ± 0.6*** | 11.4 ± 1.1 | 6.1 ± 0.7** |
| Phospho-histone 3+ (%) | 0.4 ± 0.2** | 1.2 ± 0.1 | 0.8 ± 0.2* |
| Dimensions | |||
| Length (μm) | 73.0 ± 0.7 | 70.7 ± 0.9 | 72.3 ± 1.2 |
| Width (μm) | 10 ± 0.2* | 9.9 ± 0.2 | 10.8 ± 0.2* |
| Volume (μm3) | 5685 ± 309* | 4608 ± 284 | 5725 ± 279* |
| Binucleated LV myocytes | |||
| Percentage | 21.4 ± 4.1***,+++ | 42.6 ± 1.1 | 56.8 ± 2.4** |
| Dimensions | |||
| Length (μm) | 91.3 ± 1.0 | 88.5 ± 1.6 | 91.1 ± 1.4 |
| Width (μm) | 12.6 ± 0.3*,+ | 11.6 ± 0.2 | 13.7 ± 0.3*** |
| Volume (μm3) | 10,000 ± 635*** | 8052 ± 31 | 11,330 ± 507* |
Data are means ± se; n = 8/group. Data from left ventricle are shown; trends are the same in the right ventricle. Significant differences between groups are indicated.
P < 0.05,
P < 0.01,
P < 0.001 vs. control;
P < 0.05,
P < 0.001 vs. T3.
Figure 5.
T3 promotes cardiomyocyte hypertrophy. Binucleated cardiomyocyte width was increased in left (A) and right (B) ventricles from fetuses that were either deficient or had elevated circulating T3. Both elevated T3 levels and T3 deprivation led to widening of ventricular binucleated myocytes. Data are means ± se; n = 8/group. *P < 0.05, ***P < 0.001 vs. control; +P < 0.05 vs. T3.
Molecular markers of cardiomyocyte maturation and hypertrophy
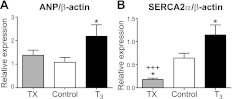
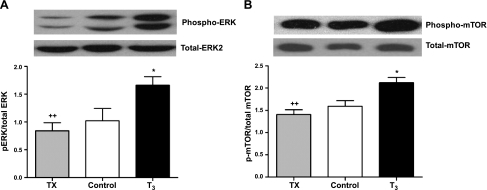
Figures 6 and 7 show expression levels of specific molecular markers in 3 fetal groups. In the high-T3 group, the expression level of ANP was increased significantly compared to control (P<0.05; Fig. 6A), while no difference was found between TX and control fetuses. SERCA2a expression was related to the concentration of circulating T3 across the groups. Compared to control hearts, SERCA2a expression was increased markedly in T3 fetuses (P<0.001; Fig. 6B) and decreased in TX fetuses (P<0.05 vs. control; P<0.01 vs. T3). Figure 7 shows that the phosphorylated form of ERK and mTOR protein increased with increasing levels of T3 (P<0.05 vs. control, P<0.01 TX vs. T3) with no significant differences between TX and control fetuses. No significant difference was found among the treatment groups for pAkt and p-p70S6K (data not shown).
Figure 6.
T3 promotes molecular markers of cardiomyocyte maturation. A) Expression of ANP was increased in the left ventricles of fetuses receiving exogenous T3, but thyroidectomy did not alter ventricular ANP. B) SERCA2a expression levels were lower in TX fetal hearts and higher in T3-infused hearts compared to controls. Data are means ± se; n = 8/group. *P < 0.05 vs. control; +++P < 0.001 vs. T3.
Figure 7.
T3 activates ERK and mTOR. Immunoblots showed elevated levels of phosphorylated ERK (A) and mTOR (B) in fetal hearts exposed to elevated levels T3, but phosphoprotein levels were unchanged in hearts from TX fetuses. Data are means ± se; n = 8/group. *P < 0.05 vs. control; ++P < 0.01 vs. T3.
DISCUSSION
We sought to determine the degree to which varying plasma levels of T3 affect the maturation of fetal ovine cardiomyocytes in vivo because we suspected the importance of T3 in regulating myocardial growth. In one group of fetuses, we increased the plasma levels of T3 by 10-fold for 5 d. Fetuses in this group were 125–130 dGA, ∼2 wk before they would experience the normal-term T3 surge. The concentration range achieved in this group was similar to that reached naturally in fetuses at term during the normal TH surge. In a second group of fetuses, we removed the fetal thyroid gland at a comparable gestational age, and T3 levels dropped to immeasurable levels within 4 d of surgery. A third group of intact fetuses received a vehicle infusion, had normal T3 levels, and served as a control group to both the treated groups. We compared indices of cardiomyocyte maturation among groups.
Cardiomyocyte maturation in a high-TH environment
We hypothesized that elevated levels of T3 in the fetal sheep would stimulate the maturation of the myocardium and would mimic the normal changes that ordinarily begin some 15 d later in gestation. We compared 3 features of cardiomyocyte maturation as a primary test of the hypothesis: increased cardiomyocyte binucleation, suppression of cardiomyocyte proliferation, and enlargement of cardiomyocytes. All three of our criteria for maturation changed in the directions the hypothesis predicted. The left ventricles of fetuses whose plasma T3 levels were elevated had a higher fraction of binucleated cardiomyocytes in the myocardium of 56.8 ± 2.4 vs. 42.6 ± 1.1% in age-matched controls; a cardiomyocyte cell cycle activity index that diminished by 46%, as shown by decreased levels of Ki-67; and an increase by some 14% in the width of cardiomyocytes that contained 2 nuclei. Cardiomyocytes from the right ventricle exhibited similar trends. The two cell cycle regulatory proteins that were analyzed also changed as predicted: protein levels of the cell cycle promoter cyclin D1 were depressed by 150%, and the cell cycle suppressor protein p21was elevated 5-fold.
We posit that cardiomyocytes from the high-T3 animals enlarged not because of mechanical stimulation in the form of wall stress but because cardiomyocytes were stimulated by the hypertrophic actions of T3. While the hormonal effect may have been bolstered slightly by the elevated mean arterial pressure of ∼2.0 mmHg on d 5 in the T3 infusion animals compared to the other groups, the load stimulus would likely have been small. Thus, it is likely that the hypertrophic response that we observed in the first group over 5 d of load was due to a direct effect of T3 on cardiomyocytes, as shown in adult cardiomyocytes (52–54). The expression of ANP was increased in response to elevated levels of T3. The peptide is expressed normally in all four chambers during fetal life but increases with maturation and/or with elevated loading conditions. Thus, the elevated levels in the ventricles may represent elevated maturation as well as increased loading, since mean arterial pressure was slightly elevated on the last day of T3 infusion.
In the immature mononucleated myocyte, the sarcoplasmic ATPase SERCA2a removes Ca2+ from the cytoplasm during the diastolic period of each beat. As cardiomyocytes become enlarged during the maturation process in utero, they develop an extensive SR in concert with a mature t-tubule system (55, 56). Gradually, the source of activator calcium ion switches from an extracellular fluid to the internal SR. However, as the myocytes enlarge during the maturation process, new SERCA2a protein is manufactured and installed on the internal SR membrane to rapidly remove Ca2+ from the cytoplasm during the diastolic phase of the cardiac cycle. This study took advantage of the fact that SERCA2a expression is T3 sensitive (23), as it normally rises toward term. In these experiments, SERCA2a expression was increased in the myocardium of fetuses exposed to elevated T3 and was suppressed in hearts not exposed to circulating T3.
Cardiomyocyte maturation in a low-TH environment
In thyroidectomized fetuses, plasma levels of T3 decreased to values that were undetectable by the assay. In this group, the percentage of cardiomyocytes with 2 nuclei (21.4±4.1%) was significantly lower than in both vehicle (42.6±1.1%) and T3 (56.8±2.4%) groups. This finding is in keeping with our maturation hypothesis and suggests that hearts growing in a low-TH environment had suppressed maturation. The hearts from thyroidectomized animals had fewer myocytes that labeled positive for Ki-67 or phospho-histone 3 than did control hearts, indicating that cardiomyocyte proliferation was depressed. We had reasoned that lower than normal levels of T3 would remove the T3-induced brake on proliferation and would allow it to increase. This was not the case. The explanation for this finding is uncertain. However, THs are known to be required for basal cell cycle activity in several cell types, including adult neural stem cells and hematopoietic progenitors (57, 58). Thus, one explanation for the suppression of proliferation in the absence of TH is that fetal cardiomyocytes require a minimal level of TH to maintain normal mitotic activity. Note that unlike hearts exposed to high T3 levels, depressed mitotic activity in the absence of TH occurred without apparent changes in the expression of the cyclin D1 or p21 proteins. This finding indicates that the suppression of mitotic activity in the absence of TH involves a signaling process different from the one used to suppress mitotic activity when T3 levels are elevated.
In the studies reported here, the widths of fetal binucleated cardiomyocytes increased in two very different hormonal environments, high plasma levels of T3 and low T3 levels. The potential explanation requires speculation. As noted above, the hearts from thyroidectomized fetuses had the lowest percentage of cardiomyocytes that were proliferating and that were binucleated. Thus, it is likely that the myocardium in a thyroid deficient fetus would be underendowed. If thyroidectomy led to a thinning of the ventricular free wall, its wall stress would have increased.
According to the Laplace relationship (59):
where Sw is wall stress, Pw is transmural pressure, r is meridional radius of curvature, and h is average free wall thickness. From this relationship, one can see that, with a constant transmural pressure and radius, wall stress will increase if wall thickness decreases. If wall stress did increase, cardiomyocyte hypertrophy would surely follow (14, 60). Wall thickness was not measured in these hearts, so this speculation awaits further experimental evidence. We have noted in past experiments that binucleated cardiomyocytes are more likely to enlarge than mononucleated cells would when wall stresses are elevated (14), but in these experiments both mono- and binucleated cells were larger following T3 deprivation and/or supplementation.
Signaling markers of maturation under differing T3 conditions
TH is known to stimulate cardiomyocyte hypertrophy in adult humans and in neonatal rodent models (61–63). Activation of PI3K, Akt, and mTOR is known to result in hypertrophy in neonatal rat hearts following T3 or T4 administration both in vitro and in vivo (63, 64). The MAP kinase cascade and ERKs have been implicated in many aspects of cardiac growth, including hyperplastic growth during the proliferative stage of heart development and hypertrophic growth that characterizes the growth of terminally differentiated cardiomyocytes (65–67). In this study, T3 was shown to stimulate phosphorylation of ERK and mTOR in the myocardium. Coupled with increases in myocyte size, these data suggest that T3 promotes hypertrophic growth in fetal cardiomyocytes through recognized signaling pathways.
As discussed above, p21Cip1/Waf1 is a member of a family of transcriptionally regulated cell-cycle inhibitors whose loss is often associated with increased proliferation and cancer (68). The relationship of ERK activation and p21 expression has not been examined in fetal cardiac cells. The Ras/ERK signals that are required for proliferation have been implicated in cell cycle arrest via p21 (69) and cardiomyocyte hypertrophy (65, 70, 71). Therefore, Ras/ERK signals can have both proliferative and antiproliferative actions (72), even though their proproliferative actions are better described and better known. In some cell types, the ERK dependency for both opposing pathways can be explained by the differential requirement for transient vs. sustained ERK activation (73). In other cell types, sequestration of ERK signaling streams via scaffold proteins may allow for the concurrent activation of distinct ERK signaling cascades within the same cells that achieve distinct outcomes (74–76). However, it is not known whether elevations in p-ERK are related to p21 regulation in ovine fetal cardiomyocytes.
ANP and SERCA 2a are sensitive to T3 levels
ANP is a well-accepted marker of cardiomyocyte hypertrophy in adult myocardium (56). The relative expression of the ANP gene in the heart was increased by T3 but unchanged in TX fetuses. As mentioned above, the increase was most likely a normal response to cardiomyocyte maturation. We have recently shown that cardiomyocyte proliferation is suppressed following the generation of intracellular cGMP in response to the binding of the transmembrane guanylyl cyclase, ANP receptor (NPRA; ref. 33). Thus, the elevated levels of ANP in hearts exposed to high levels of T3 would have suppressed the proliferation of cardiomyocytes along with the actions of T3. The fact that ANP did not increase in the low-TH environment further suggests that the cardiomyocytes in those hearts remained in an immature state.
Clinical application
Studies in animals and humans associate low maternal and fetal TH levels with suppressed neurodevelopment in offspring (39, 77). The fact that both hyper- and hypothyroid conditions lead to detrimental fetal outcomes suggests that an optimal range of TH levels must support the appropriate growth and maturation of the myocardium. If the maturation process is completed before the appropriate number of cardiomyocytes is generated, the heart could suffer a deficit in cardiomyocyte endowment at birth. That the ovine fetal heart can be underendowed has been demonstrated in placental insufficiency studies (78, 79) and experimental reduction in systolic pressure (80). Hearts with fewer than normal numbers of myocytes are expected to be more vulnerable to heart failure or other detrimental outcomes in adult life (81, 82). Data from experiments in rats suggest that cardiomyocyte numbers cannot recover during postnatal life following episodes of hypoxic stress (83, 84) or vitamin D deficiency in the womb (85).
Maternal and fetal TH levels
In normal pregnancies, TH molecules in the fetal plasma during the last trimester come from both the fetal thyroid gland and the mother. In humans, but not in sheep, elevated maternal plasma levels of TH lead to elevated fetal TH levels (86, 87). Thus, maternal hyperthyroidism leads to fetal hyperthyroidism. The monocarboxylate transporter, which transports T3 and T4, has been identified in human placentas (88) and appears to be the primary placental transporter for THs. We studied the effects of doses of T3 that led to plasma concentrations normally found in human and sheep fetuses in a later stage of their development. Maternal levels of circulating total T4 (∼110 ng/ml) and circulating total T3 (∼2.0 ng/ml) are elevated during normal pregnancy (89, 90). Normal human umbilical artery levels of T4 (90.5±0.02 ng/ml) and T3 (1.9±0.96 ng/ml) at birth are similar to fetal sheep levels (8, 91).
CONCLUSIONS
The present study suggests that the final maturation stages of the myocardium are regulated by conserved hormonal systems that are known to be phylogenetically ancient. When preterm cardiomyocytes were exposed to term levels of circulating T3 for several days, they developed phenotypic features of maturity ordinarily seen in cardiomyocytes some 2 wk later. On the one hand, our data are consistent with the conclusion that T3 serves as the primary driver of fetal cardiomyocyte maturation and powerfully reduces the generative capacity of the cardiomyocyte population near the time of birth. On the other hand, when circulating T3 levels are severely reduced, several features of cardiomyocyte growth and maturation suffered. These studies show for the first time that the normal development of the near-term fetal heart requires T3 concentrations to be maintained within a defined range. Over the past decade, it has become clear that gestational adversities may reduce heart cell numbers for life and increase vulnerability for chronic disease. Consequently, human hearts exposed to elevated T3 concentrations before birth might be compromised for life if they respond to T3 in a similar fashion to sheep.
Acknowledgments
The authors thank Mr. Robert Webber and Ms. Loni Socha for excellent technical assistance. The authors appreciate support from the M. Lowell Edwards Endowment.
This study was supported by funds from U.S. National Institute of Child Health and Human Development grant P01 HD 34430, National Heart, Lung, and Blood Institute (NHLBI) grant R21 HL093617, and National Institutes of Health grant R01 HL102763. N.N.C. was supported by NHLBI training grant T32HL094294.
REFERENCES
- 1. Brown D. D., Cai L. (2007) Amphibian metamorphosis. Dev. Biol. 306, 20–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Breall J. A., Rudolph A. M., Heymann M. A. (1984) Role of thyroid hormone in postnatal circulatory and metabolic adjustments. J. Clin. Invest. 73, 1418–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fisher D. A. (1997) Fetal thyroid function: diagnosis and management of fetal thyroid disorders. Clin. Obstet. Gynecol. 40, 16–31 [DOI] [PubMed] [Google Scholar]
- 4. Macchia P. E. (2000) Recent advances in understanding the molecular basis of primary congenital hypothyroidism. Mol. Med. Today. 6, 36–42 [DOI] [PubMed] [Google Scholar]
- 5. Bianco A. C., Kim B. W. (2006) Deiodinases: implications of the local control of thyroid hormone action. J. Clin. Invest. 116, 2571–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burrow G. N., Fisher D. A., Larsen P. R. (1994) Maternal and fetal thyroid function. N. Engl. J. Med. 331, 1072–1078 [DOI] [PubMed] [Google Scholar]
- 7. Chan S., Kachilele S., Hobbs E., Bulmer J. N., Boelaert K., McCabe C. J., Driver P. M., Bradwell A. R., Kester M., Visser T. J., Franklyn J. A., Kilby M. D. (2003) Placental iodothyronine deiodinase expression in normal and growth-restricted human pregnancies. J. Clin. Endocrinol. Metab. 88, 4488–4495 [DOI] [PubMed] [Google Scholar]
- 8. Polk D. H. (1995) Thyroid hormone metabolism during development. Reprod. Fertil. Dev. 7, 469–477 [DOI] [PubMed] [Google Scholar]
- 9. Forhead A. J., Curtis K., Kaptein E., Visser T. J., Fowden A. L. (2006) Developmental control of iodothyronine deiodinases by cortisol in the ovine fetus and placenta near term. Endocrinology 147, 5988–5994 [DOI] [PubMed] [Google Scholar]
- 10. Schellenberg J. C., Liggins G. C. (1987) New approaches to hormonal acceleration of fetal lung maturation. J. Perinat. Med. 15, 447–452 [DOI] [PubMed] [Google Scholar]
- 11. Forhead A. J., Fowden A. L. (2002) Effects of thyroid hormones on pulmonary and renal angiotensin-converting enzyme concentrations in fetal sheep near term. J. Endocrinol. 173, 143–150 [DOI] [PubMed] [Google Scholar]
- 12. Forhead A. J., Poore K. R., Mapstone J., Fowden A. L. (2003) Developmental regulation of hepatic and renal gluconeogenic enzymes by thyroid hormones in fetal sheep during late gestation. J. Physiol. 548, 941–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Tuyl M., Blommaart P. E., de Boer P. A., Wert S. E., Ruijter J. M., Islam S., Schnitzer J., Ellison A. R., Tibboel D., Moorman A. F., Lamers W. H. (2004) Prenatal exposure to thyroid hormone is necessary for normal postnatal development of murine heart and lungs. Dev. Biol. 272, 104–117 [DOI] [PubMed] [Google Scholar]
- 14. Barbera A., Giraud G. D., Reller M. D., Maylie J., Morton M. J., Thornburg K. L. (2000) Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am. J. Physiol. 279, R1157–R1164 [DOI] [PubMed] [Google Scholar]
- 15. Giraud G. D., Louey S., Jonker S., Schultz J., Thornburg K. L. (2006) Cortisol stimulates cell cycle activity in the cardiomyocyte of the sheep fetus. Endocrinology 147, 3643–3649 [DOI] [PubMed] [Google Scholar]
- 16. Jonker S. S., Zhang L., Louey S., Giraud G. D., Thornburg K. L., Faber J. J. (2007) Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J. Appl. Physiol. 102, 1130–1142 [DOI] [PubMed] [Google Scholar]
- 17. Soonpaa M. H., Kim K. K., Pajak L., Franklin M., Field L. J. (1996) Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 271, H2183–H2189 [DOI] [PubMed] [Google Scholar]
- 18. Smolich J. J., Walker A. M., Campbell G. R., Adamson T. M. (1989) Left and right ventricular myocardial morphometry in fetal, neonatal, and adult sheep. Am. J. Physiol. 257, H1–H9 [DOI] [PubMed] [Google Scholar]
- 19. Burrell J. H., Boyn A. M., Kumarasamy V., Hsieh A., Head S. I., Lumbers E. R. (2003) Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat. Rec. 274A, 952–961 [DOI] [PubMed] [Google Scholar]
- 20. Sundgren N. C., Giraud G. D., Schultz J. M., Lasarev M. R., Stork P. J., Thornburg K. L. (2003) Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am. J. Physiol. 285, R1481–R1489 [DOI] [PubMed] [Google Scholar]
- 21. Sundgren N. C., Giraud G. D., Stork P. J., Maylie J. G., Thornburg K. L. (2003) Angiotensin II stimulates hyperplasia but not hypertrophy in immature ovine cardiomyocytes. J. Physiol. 548, 881–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anversa P., Loud A. V., Giacomelli F., Wiener J. (1978) Absolute morphometric study of myocardial hypertrophy in experimental hypertension. II. Ultrastructure of myocytes and interstitium. Lab. Invest. 38, 597–609 [PubMed] [Google Scholar]
- 23. Anversa P., Olivetti G., Loud A. V. (1980) Morphometric study of early postnatal development in the left and right ventricular myocardium of the rat. I. Hypertrophy, hyperplasia, and binucleation of myocytes. Circ. Res. 46, 495–502 [DOI] [PubMed] [Google Scholar]
- 24. Hirakow R., Gotoh T., Watanabe T. (1980) Quantitative studies on the ultrastructural differentiation and growth of mammalian cardiac muscle cells. I. The atria and ventricles of the rat. Acta Anat. (Basel) 108, 144–152 [DOI] [PubMed] [Google Scholar]
- 25. Rakusan K. (1984) Cardiac growth, maturation, and aging. In Growth of the Heart in Health and Disease (Zak R., ed) pp. 25–40, Raven Press, New York [Google Scholar]
- 26. Thornburg K. L., Shaut C. A. E. (2009) Development of the cardiovascular system. In Fetal Medicine (Rodeck C., Whittle M., eds), Harcourt, Brace & Co., San Diego, CA, USA [Google Scholar]
- 27. Kahaly G. J., Dillmann W. H. (2005) Thyroid hormone action in the heart. Endocr. Rev. 26, 704–728 [DOI] [PubMed] [Google Scholar]
- 28. Cernohorsky J., Kolar F., Pelouch V., Korecky B., Vetter R. (1998) Thyroid control of sarcolemmal Na+/Ca2+ exchanger and SR Ca2+-ATPase in developing rat heart. Am. J. Physiol. 275, H264–H273 [DOI] [PubMed] [Google Scholar]
- 29. Gardner D. G. (2003) Natriuretic peptides: markers or modulators of cardiac hypertrophy? Trends Endocrinol. Metab. 14, 411–416 [DOI] [PubMed] [Google Scholar]
- 30. Cheung C. Y., Gibbs D. M., Brace R. A. (1987) Atrial natriuretic factor in maternal and fetal sheep. Am. J. Physiol. 252, E279–E282 [DOI] [PubMed] [Google Scholar]
- 31. Cheung C. Y., Roberts V. J. (1993) Developmental changes in atrial natriuretic factor content and localization of its messenger ribonucleic acid in ovine fetal heart. Am. J. Obstet. Gynecol. 169, 1345–1351 [DOI] [PubMed] [Google Scholar]
- 32. Knowles J. W., Esposito G., Mao L., Hagaman J. R., Fox J. E., Smithies O., Rockman H. A., Maeda N. (2001) Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J. Clin. Invest. 107, 975–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Tierney P. F., Chattergoon N. N., Louey S., Giraud G. D., Thornburg K. L. (2010) Atrial natriuretic peptide inhibits angiotensin II-stimulated proliferation in fetal cardiomyocytes. J. Physiol. 588, 2879–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castro R., Polk D. H., Lam R. W., Leake R. D., Fisher D. A. (1988) Atrial natriuretic factor: Effect of thyroidectomy on concentration in fetal sheep atria and ventricles. Pediatr. Res. 23, 274A [Google Scholar]
- 35. Boluyt M. O., Li Z. B., Loyd A. M., Scalia A. F., Cirrincione G. M., Jackson R. R. (2004) The mTOR/p70S6K signal transduction pathway plays a role in cardiac hypertrophy and influences expression of myosin heavy chain genes in vivo. Cardiovasc. Drugs Ther. 18, 257–267 [DOI] [PubMed] [Google Scholar]
- 36. Rommel C., Clarke B. A., Zimmermann S., Nunez L., Rossman R., Reid K., Moelling K., Yancopoulos G. D., Glass D. J. (1999) Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286, 1738–1741 [DOI] [PubMed] [Google Scholar]
- 37. Wang L., Gout I., Proud C. G. (2001) Cross-talk between the ERK and p70 S6 kinase (S6K) signaling pathways. MEK-dependent activation of S6K2 in cardiomyocytes. J. Biol. Chem. 276, 32670–32677 [DOI] [PubMed] [Google Scholar]
- 38. Casey B. M., Dashe J. S., Wells C. E., McIntire D. D., Byrd W., Leveno K. J., Cunningham F. G. (2005) Subclinical hypothyroidism and pregnancy outcomes. Obstet. Gynecol. 105, 239–245 [DOI] [PubMed] [Google Scholar]
- 39. Glinoer D., Delange F. (2000) The potential repercussions of maternal, fetal, and neonatal hypothyroxinemia on the progeny. Thyroid 10, 871–887 [DOI] [PubMed] [Google Scholar]
- 40. Radetti G., Zavallone A., Gentili L., Beck-Peccoz P., Bona G. (2002) Foetal and neonatal thyroid disorders. Minerva Pediatr. 54, 383–400 [PubMed] [Google Scholar]
- 41. Hopkins P. S., Thorburn G. D. (1972) The effects of foetal thyroidectomy on the development of the ovine foetus. J. Endocrinol. 54, 55–66 [DOI] [PubMed] [Google Scholar]
- 42. Forhead A. J., Li J., Gilmour R. S., Dauncey M. J., Fowden A. L. (2002) Thyroid hormones and the mRNA of the GH receptor and IGFs in skeletal muscle of fetal sheep. Am. J. Physiol. Endocrinol. Metab. 282, E80–E86 [DOI] [PubMed] [Google Scholar]
- 43. Dillmann W. H. (2002) Cellular action of thyroid hormone on the heart. Thyroid 12, 447–452 [DOI] [PubMed] [Google Scholar]
- 44. Flamant F., Samarut J. (2003) Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol. Metab. 14, 85–90 [DOI] [PubMed] [Google Scholar]
- 45. Moriscot A. S., Sayen M. R., Hartong R., Wu P., Dillmann W. H. (1997) Transcription of the rat sarcoplasmic reticulum Ca2+ adenosine triphosphatase gene is increased by 3,5,3′-triiodothyronine receptor isoform-specific interactions with the myocyte-specific enhancer factor-2a. Endocrinology 138, 26–32 [DOI] [PubMed] [Google Scholar]
- 46. Oppenheimer J. H., Dillmann W. H. (1978) Molecular mechanisms at the tissue level in hyperthyroidism. Clin. Endocrinol. Metab. 7, 145–165 [DOI] [PubMed] [Google Scholar]
- 47. Bergh J. J., Lin H. Y., Lansing L., Mohamed S. N., Davis F. B., Mousa S., Davis P. J. (2005) Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146, 2864–2871 [DOI] [PubMed] [Google Scholar]
- 48. Chopra I. J., Carlson H. E., Solomon D. H. (1978) Comparison of inhibitory effects of 3,5,3′-triiodothyronine (T3), thyroxine (T4), 3,3,′,5′-triiodothyronine (rT3), and 3,3′-diiodothyronine (T2) on thyrotropin-releasing hormone-induced release of thyrotropin in the rat in vitro. Endocrinology 103, 393–402 [DOI] [PubMed] [Google Scholar]
- 49. Davis P. J., Davis F. B. (2002) Nongenomic actions of thyroid hormone on the heart. Thyroid 12, 459–466 [DOI] [PubMed] [Google Scholar]
- 50. Chattergoon N. N., Giraud G. D., Thornburg K. L. (2007) Thyroid hormone inhibits proliferation of fetal cardiac myocytes in vitro. J. Endocrinol. 192, R1–8 [DOI] [PubMed] [Google Scholar]
- 51. Jonker S. S., Faber J. J., Anderson D. F., Thornburg K. L., Louey S., Giraud G. D. (2007) Sequential growth of fetal sheep cardiac myocytes in response to simultaneous arterial and venous hypertension. Am. J. Physiol. 292, R913–919 [DOI] [PubMed] [Google Scholar]
- 52. Dillmann W. H. (1985) Mechanism of action of thyroid hormones. Med. Clin. North Am. 69, 849–861 [DOI] [PubMed] [Google Scholar]
- 53. Goldenthal M. J., Ananthakrishnan R., Marin-Garcia J. (2005) Nuclear-mitochondrial cross-talk in cardiomyocyte T3 signaling: a time-course analysis. J. Mol. Cell. Cardiol. 39, 319–326 [DOI] [PubMed] [Google Scholar]
- 54. Thomas T. A., Kuzman J. A., Anderson B. E., Andersen S. M., Schlenker E. H., Holder M. S., Gerdes A. M. (2005) Thyroid hormones induce unique and potentially beneficial changes in cardiac myocyte shape in hypertensive rats near heart failure. Am. J. Physiol. Heart Circ. Physiol. 288, H2118–H2122 [DOI] [PubMed] [Google Scholar]
- 55. Kawamura Y., Ishiwata T., Takizawa M., Ishida H., Asano Y., Nonoyama S. (2010) Fetal and neonatal development of Ca2+ transients and functional sarcoplasmic reticulum in beating mouse hearts. Circ. J. 74, 1442–1450 [DOI] [PubMed] [Google Scholar]
- 56. Schaub M. C., Hefti M. A., Harder B. A., Eppenberger H. M. (1997) Various hypertrophic stimuli induce distinct phenotypes in cardiomyocytes. J. Mol. Med. 75, 901–920 [DOI] [PubMed] [Google Scholar]
- 57. Chen C., Zhou Z., Zhong M., Li M., Yang X., Zhang Y., Wang Y., Wei A., Qu M., Zhang L., Xu S., Chen S., Yu Z. (2011) Excess thyroid hormone inhibits embryonic neural stem/progenitor cells proliferation and maintenance through STAT3 signalling pathway. Neurotox. Res. 20, 15–25 [DOI] [PubMed] [Google Scholar]
- 58. Kawa M. P., Grymula K., Paczkowska E., Baskiewicz-Masiuk M., Dabkowska E., Koziolek M., Tarnowski M., Klos P., Dziedziejko V., Kucia M., Syrenicz A., Machalinski B. (2010) Clinical relevance of thyroid dysfunction in human haematopoiesis: biochemical and molecular studies. Eur. J. Endocrinol. 162, 295–305 [DOI] [PubMed] [Google Scholar]
- 59. Grossman W., Jones D., McLaurin L. P. (1975) Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Invest. 56, 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pinson C. W., Morton M. J., Thornburg K. L. (1991) Mild pressure loading alters right ventricular function in fetal sheep. Circ. Res. 68, 947–957 [DOI] [PubMed] [Google Scholar]
- 61. Ching G. W., Franklyn J. A., Stallard T. J., Daykin J., Sheppard M. C., Gammage M. D. (1996) Cardiac hypertrophy as a result of long-term thyroxine therapy and thyrotoxicosis. Heart 75, 363–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kenessey A., Ojamaa K. (2006) Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J. Biol. Chem. 281, 20666–20672 [DOI] [PubMed] [Google Scholar]
- 63. Kuzman J. A., Vogelsang K. A., Thomas T. A., Gerdes A. M. (2005) L-Thyroxine activates Akt signaling in the heart. J. Mol. Cell. Cardiol. 39, 251–258 [DOI] [PubMed] [Google Scholar]
- 64. Kuzman J. A., Gerdes A. M., Kobayashi S., Liang Q. (2005) Thyroid hormone activates Akt and prevents serum starvation-induced cell death in neonatal rat cardiomyocytes. J. Mol. Cell. Cardiol. 39, 841–844 [DOI] [PubMed] [Google Scholar]
- 65. Bueno O. F., De Windt L. J., Tymitz K. M., Witt S. A., Kimball T. R., Klevitsky R., Hewett T. E., Jones S. P., Lefer D. J., Peng C. F., Kitsis R. N., Molkentin J. D. (2000) The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 19, 6341–6350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ueyama T., Kawashima S., Sakoda T., Rikitake Y., Ishida T., Kawai M., Yamashita T., Ishido S., Hotta H., Yokoyama M. (2000) Requirement of activation of the extracellular signal-regulated kinase cascade in myocardial cell hypertrophy. J. Mol. Cell. Cardiol. 32, 947–960 [DOI] [PubMed] [Google Scholar]
- 67. Wang M. Y., Koyama K., Shimabukuro M., Newgard C. B., Unger R. H. (1998) OB-Rb gene transfer to leptin-resistant islets reverses diabetogenic phenotype. Proc. Natl. Acad. Sci. U. S. A. 95, 714–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thaler S., Hahnel P. S., Schad A., Dammann R., Schuler M. (2009) RASSF1A mediates p21Cip1/Waf1-dependent cell cycle arrest and senescence through modulation of the Raf-MEK-ERK pathway and inhibition of Akt. Cancer Res. 69, 1748–1757 [DOI] [PubMed] [Google Scholar]
- 69. Chen D., Heath V., O'Garra A., Johnston J., McMahon M. (1999) Sustained activation of the raf-MEK-ERK pathway elicits cytokine unresponsiveness in T cells. J. Immunol. 163, 5796–5805 [PubMed] [Google Scholar]
- 70. Muslin A. J. (2005) Role of raf proteins in cardiac hypertrophy and cardiomyocyte survival. Trends Cardiovasc. Med. 15, 225–229 [DOI] [PubMed] [Google Scholar]
- 71. Thorburn A. (1994) Ras activity is required for phenylephrine-induced activation of mitogen-activated protein kinase in cardiac muscle cells. Biochem. Biophys. Res. Commun. 205, 1417–1422 [DOI] [PubMed] [Google Scholar]
- 72. Chang F., Steelman L. S., McCubrey J. A. (2002) Raf-induced cell cycle progression in human TF-1 hematopoietic cells. Cell Cycle 1, 220–226 [PubMed] [Google Scholar]
- 73. Bottazzi M. E., Zhu X., Bohmer R. M., Assoian R. K. (1999) Regulation of p21(cip1) expression by growth factors and the extracellular matrix reveals a role for transient ERK activity in G1 phase. J. Cell Biol. 146, 1255–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chuderland D., Marmor G., Shainskaya A., Seger R. (2008) Calcium-mediated interactions regulate the subcellular localization of extracellular signal-regulated kinases. J. Biol. Chem. 283, 11176–11188 [DOI] [PubMed] [Google Scholar]
- 75. Chuderland D., Seger R. (2005) Protein-protein interactions in the regulation of the extracellular signal-regulated kinase. Mol. Biotechnol. 29, 57–74 [DOI] [PubMed] [Google Scholar]
- 76. Ramos J. W. (2008) The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int. J. Biochem. Cell Biol. 40, 2707–2719 [DOI] [PubMed] [Google Scholar]
- 77. Becks G. P., Burrow G. N. (1991) Thyroid disease and pregnancy. Med. Clin. North Am. 75, 121–150 [DOI] [PubMed] [Google Scholar]
- 78. Bubb K. J., Cock M. L., Black M. J., Dodic M., Boon W. M., Parkington H. C., Harding R., Tare M. (2007) Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J. Physiol. 578, 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Louey S., Jonker S. S., Giraud G. D., Thornburg K. L. (2007) Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. J. Physiol. 580, 639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. O'Tierney P. F., Anderson D. F., Faber J. J., Louey S., Thornburg K. L., Giraud G. D. (2010) Reduced systolic pressure load decreases cell-cycle activity in the fetal sheep heart. Am. J. Physiol. 299, R573–R578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Barker D. J., Gelow J., Thornburg K., Osmond C., Kajantie E., Eriksson J. G. (2010) The early origins of chronic heart failure: impaired placental growth and initiation of insulin resistance in childhood. Eur. J. Heart Fail. 12, 819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thornburg K., Jonker S., O'Tierney P., Chattergoon N., Louey S., Faber J., Giraud G. (2011) Regulation of the cardiomyocyte population in the developing heart. Prog. Biophys. Mol. Biol. 106, 289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li G., Bae S., Zhang L. (2004) Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am. J. Physiol. Heart Circ. Physiol. 286, H1712–H1719 [DOI] [PubMed] [Google Scholar]
- 84. Li G., Xiao Y., Estrella J. L., Ducsay C. A., Gilbert R. D., Zhang L. (2003) Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J. Soc. Gynecol. Investig. 10, 265–274 [DOI] [PubMed] [Google Scholar]
- 85. Gezmish O., Tare M., Parkington H. C., Morley R., Porrello E. R., Bubb K. J., Black M. J. (2010) Maternal vitamin D deficiency leads to cardiac hypertrophy in rat offspring. Reprod. Sci. 17, 168–176 [DOI] [PubMed] [Google Scholar]
- 86. Chan S. Y., Vasilopoulou E., Kilby M. D. (2009) The role of the placenta in thyroid hormone delivery to the fetus. Nat. Clin. Pract. Endocrinol. Metab. 5, 45–54 [DOI] [PubMed] [Google Scholar]
- 87. Higuchi R., Kumagai T., Kobayashi M., Minami T., Koyama H., Ishii Y. (2001) Short-term hyperthyroidism followed by transient pituitary hypothyroidism in a very low birth weight infant born to a mother with uncontrolled Graves' disease. Pediatrics 107, E57. [DOI] [PubMed] [Google Scholar]
- 88. Chan S. Y., Franklyn J. A., Pemberton H. N., Bulmer J. N., Visser T. J., McCabe C. J., Kilby M. D. (2006) Monocarboxylate transporter 8 expression in the human placenta: the effects of severe intrauterine growth restriction. J. Endocrinol. 189, 465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Niswander K. R., Gordon M. (1972) The collaborative perinatal study of the National Institute of Neurological Disease and Stroke: the women and their babies, pp. 246–249, W. B. Saunders, Philadelphia [Google Scholar]
- 90. Seely B. L., Burrow G. N. (1994) Thyroid disease and pregnancy. In Maternal-Fetal Medicine: Principles and Practice (Creasy R. K., Resnik R., eds) pp. 979–1003, W. B. Saunders, Philadelphia [Google Scholar]
- 91. Hume R., Simpson J., Delahunty C., van Toor H., Wu S. Y., Williams F. L., Visser T. J. (2004) Human fetal and cord serum thyroid hormones: developmental trends and interrelationships. J. Clin. Endocrinol. Metab. 89, 4097–4103 [DOI] [PubMed] [Google Scholar]