This study demonstrates HDP-CDV, a transport micelle form for use in a crystalline HDP-cCDV intravitreal delivery system, has a long-lasting, slow-release property that may be directly used in intravitreal therapy for cytomegalovirus retinitis.
Abstract
Purpose.
To evaluate the intraocular safety and pharmacokinetics of hexadecyloxypropyl-cidofovir (HDP-CDV), the hydrolysis product of HDP-cyclic-CDV, a long-lasting intravitreal cidofovir prodrug for cytomegalovirus (CMV) retinitis.
Methods.
HDP-cyclic-CDV was suspended in phosphate-buffered saline (PBS) at 37°C and formation of HDP-CDV was monitored by high-performance liquid chromatography (HPLC) analysis for 30 weeks. The safety and pharmacokinetics of HDP-CDV intravitreal injections were studied using New Zealand Red rabbits and 14C labeled HDP-CDV. Ocular tissues from five time points (1, 3, 7, 14, and 35 days) were analyzed by scintillation counting and HPLC to characterize the pharmacokinetics.
Results.
During the hydrolysis study, approximately 35% of the HDP-cyclic-CDV was converted to HDP-CDV. Evaluation of safety found no toxicity after intravitreal injection of HDP-CDV up to 28 μg/eye. Intravitreal pharmacokinetics of HDP-CDV in the retina, choroid, and vitreous followed a two-phase elimination process and elimination half-lives of 8.4 days (retina), 6.9 days (choroid), and 6.2 days (vitreous). In the retina, cidofovir and an unknown metabolite were detected in the first 2 weeks, and the maximum metabolite concentrations were present 48 hours after the maximum HDP-CDV concentration.
Conclusions.
HDP-cyclic CDV, under simulated physiologic conditions, slowly converts to HDP-CDV, another potent anti-CMV prodrug that may be taken up by retinal cells and metabolized further to the active antiviral metabolite, cidofovir diphosphate. Taken together, these observations help to explain the ability of a single intravitreal dose of HDP-cyclic-CDV to prevent viral retinitis for up to 68 days in a rabbit model.
Human cytomegalovirus (CMV) infection can cause sight-threatening retinitis in patients with acquired immune deficiency syndrome (AIDS). Even though highly active antiretroviral therapy (HAART) has lowered the incidence of CMV retinitis, some patients have manifested poor response to HAART, and some patients do not qualify for it.1,2 Currently, the clinical treatment of CMV retinitis includes systemic and local administration of antiviral medications, such as ganciclovir (GCV), foscarnet, and cidofovir (CDV), as well as surgical GCV implants. With the lengthened exposure of patients to the drugs in the systemic treatment modality, GCV and foscarnet-resistant cytomegalovirus are becoming a concern.3,4 Therefore, the development of CDV for intravitreal injection would be a helpful therapeutic alternative in patients with GCV and foscarnet-resistant CMV strains. Intravitreal injection of small molecules often requires frequent repeated injections, which increases the risk of devastating endophthalmitis. Frequent injections also affect the quality of the patient's life.1,2 Surgical intravitreal implants provide longer therapeutic duration but the side effects associated with surgery, such as vitreous hemorrhage and retinal detachment, are often sight-threatening complications.2 As a result, our efforts have been directed toward developing long-lasting intravitreal injectable drugs to circumvent these problems.5
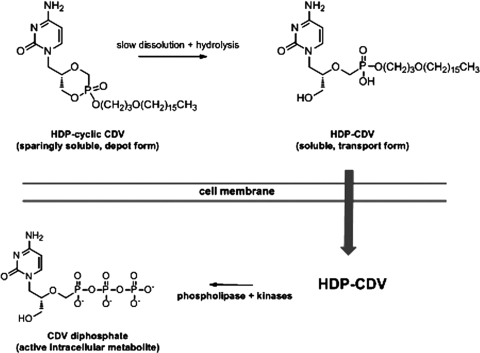
We previously identified lipid derivatized drugs that work well against both viral infections and proliferative diseases of the eye. For viral infections, these include hexadecyloxypropyl-phospho-ganciclovir and hexadecyloxypropyl-cyclic cidofovir (HDP-cCDV) and for proliferative vitreoretinopathy, hexadecyloxypropyl 5-fluoro-2′-deoxyuridine 3′,5′ cyclic monophosphate.5,6 In particular, HDP-cCDV, a long-lasting crystalline lipid prodrug of CDV that we have reported previously,5 shows a longer intraocular therapeutic effect than CDV in the experimental HSV retinitis of rabbit eye. Subsequent confirmatory evaluation of ocular toxicity in guinea pig eyes demonstrated that a dose of 18 μg HDP-cCDV/eye (equivalent to 100 μg/eye in the rabbit eye) is safe.7 Although the experimental treatment demonstrated efficacy, the ocular pharmacokinetics of this crystalline formulation has not been well characterized because of its low water solubility and low free drug concentration in the vitreous. Most recently, we performed an intraocular pharmacokinetic study of octadecyloxyethylcyclic-[2-14C]cidofovir (ODE-cCDV), which is a crystalline prodrug similar to HDP-cCDV, except that it has one more carbon in the alkoxyalkyl moiety.8 Monitor drug levels in various tissues with scintillation counting showed that drug was detectable during the whole study period (9 weeks). Interestingly, the drug in vitreous demonstrated a two-compartment model with a very long vitreous elimination half-life of 25 days. How these crystalline lipid prodrugs work in a living eye to inhibit viral replication is not completely clear. After intravitreal injection, the drug forms a depot in the vitreous due to its limited water solubility. It is possible that small quantities of HDP-cCDV leave the drug depot and migrate into the retina where HDP-cCDV is converted to CDV through phospholipase C cleavage of HDP and hydrolysis of cCDV to CDV. Another possibility is that, in the vitreous, HDP-cCDV is slowly hydrolyzed to HDP-CDV which can aggregate to form micelles, which are then transported into retinal cells (Fig. 1). If the latter is the case, study of ocular pharmacokinetics of HDP-CDV would help to better understand the crystalline intravitreal drug delivery system.
Figure 1.
Proposed mechanism for sustained release of HDP-CDV from a crystalline depot of HDP-cCDV in the vitreous.
In this study, we used 14C-labeled HDP-CDV to study the intraocular pharmacokinetics of this compound and attempted to detect its metabolites such as CDV, CDV monophosphate, and CDV diphosphate in retinal tissues.
Methods
Hydrolysis Study
HDP-cCDV was synthesized as previously reported.9 HDP-cCDV (1 mg) was suspended in phosphate-buffered saline (PBS) (2 mL) and placed in a conical tube. The tube was placed in a heated orbital shaker at 37°C and 220 rpm. Samples of the suspension (100 μL) were withdrawn at appropriate time intervals, placed into vials, and stored at −20°C until further analysis was performed.
Analysis was performed by high-performance liquid chromatography (HPLC) at the Department of Chemistry and Biochemistry, University of California San Diego (UCSD). Lyophilized samples were dissolved in 20% methanol and water. HPLC separation was performed on a commercial system (HP 1100 LC system [Agilent, Palo Alto, CA] and a Synergi Polar-RP column: 4.6 × 150 mm, 4 μm, 80Å [Phenomenex, Torrance, CA]) at a flow rate of 1.0 mL/min. Mobile phase A was 95% water/5% methanol, and mobile phase B was 95% methanol/5% water. Gradient elution was as follows: 10% B (0–3 minutes), 10% to 95% B (3–20 minutes), 95% B (20–25 minutes), and 95% to 10% B (25–34 minutes). Compounds were detected by ultraviolet light (UV) absorption at 274 nm. The percentage of remaining HDP-cCDV and the percentage of HDP-CDV produced were plotted versus time.
Animal Study
All procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Determining the HDP-CDV Dose for Pharmacokinetic Study.
According to our previous study, the CC50 of HDP-CDV for WI-38 cells (CCL-75; ATCC, Manassas, VA) was 15.6 μM,10 which is equivalent to a concentration of 8.7 μg/mL (MW 561.7). The concentration half-log lower is 2.8 μg/mL and half-log higher is 28 μg/mL. These were determined to be the three doses for safety testing to further identify the dose for pharmacokinetic evaluation.
Nine New Zealand Red rabbits were used in the toxicity studies and divided into three equal groups: high-dose, medium-dose, and low-dose. Baseline intraocular pressure (IOP), anterior chamber, and fundus examinations were documented with a handheld tonometer (Tonopen; Medtronic, Jacksonville, FL), a slit lamp, and an indirect ophthalmoscope. The rabbit eyes had a topical anesthetization with 1% proparacaine-HCl 1 minute before IOP measurement. One eye of every animal was intravitreally injected with HDP-CDV in 50 μL of 5% dextrose, and the fellow eye was injected with 50 μL of 5% dextrose alone as the control. For the intravitreal injection procedure, the rabbits were anesthetized with intramuscular injections of 20 mg/kg ketamine and 5 mg/kg xylazine. After eye preparations for sterilization and topical anesthetization with 1% proparacaine-HCl, 50 μL of drug or 5% dextrose solution was injected into the midvitreous cavity under direct view of a surgical microscope. A 1-mL syringe and a 27-gauge needle were used. The needle entry site was 2 mm from the limbus. After injection, eyes were monitored with the tonometer, slit lamp, indirect ophthalmoscope, and fundus camera at days 3, 7, and 14 and week 5. The electroretinograms (ERGs) of all animals were examined at week 5. After enucleation, the eye globes were embedded in paraffin and sectioned for light microscopy and TUNEL staining. Light microscopy helps to identify drug-related necrosis or inflammation in vitreous and retina, and TUNEL staining reveals more subtle adverse changes, such as apoptosis. Apoptosis has been reported in renal tubular cells in a renal biopsy from an acute renal failure due to CDV.11 In addition, CDV induces apoptosis in primary cultures of human proximal tubular cells in vitro.11
Intravitreal Pharmacokinetics Study.
Hexadecyloxypropyl 14C-cidofovir (14C-HDP-CDV) was synthesized by Moravek Biochemicals (Brea, CA) with initial specific activity of 55.1 mCi/mmol. 14C-HDP-CDV (20 μCi) and unlabeled HDP-CDV (1.1 mg) were added to 2 mL of 5% dextrose, and the mixture was homogenized to clarity with a bath sonicator, resulting in the formulation 28 μg drug/0.5 μCi/50 μL.
For the pharmacokinetic study, we chose the highest nontoxic dose (28 μg in 50 μL) of HDP-CDV for intravitreal injection based on the results of the toxicity study. Twenty New Zealand Red rabbits were used and divided into five groups. One eye of every rabbit was intravitreally injected with a 28-μg compound in 50 μL of 5% dextrose, and the fellow eye received 50 μL of 5% dextrose alone as the control. After injection, four rabbits at each time point (days 1, 3, 7, and 14 and week 5) were euthanized and the aqueous humor, iris, ciliary body, vitreous, retina, and choroid were sampled after eye globe enucleation, as we previously described.8,9 For retina and ciliary body, the samples were divided into two, one for total radioactivity counting with scintillation and the other for metabolite study by HPLC/MS. The vials were kept at −80°C until further analysis.
For metabolism experiments, rabbit eye tissues of retina and ciliary body were weighed. Replicates at each time point were pooled and homogenized with a polytron probe (Brinkmann Instruments, Westbury, NY) for 15 seconds. These were dried and rehydrated in a small volume of water. The samples were frozen and thawed two times and bath sonicated on ice for 5 minutes. Cold trichloroacetic acid was added to a final concentration of 8%, and the contents were vortexed and centrifuged at 4°C for 10 minutes. The supernatant was removed, counted to determine whether there was enough radioactivity, and immediately analyzed by HPLC (Partisil 10 SAX column, 4.6 × 15 cm with a SAX guard column; Whatman, Clifton, NJ). Metabolites were eluted at a flow rate of 1 mL/min with a potassium phosphate–buffered gradient of 20 to 700 mM (pH 5.8), beginning at 9 minutes for a duration of 20 minutes and held for 5 minutes. Fractions (1 mL/min) were collected, scintillation fluid (Ultima Flo; PerkinElmer Life and Analytical Sciences, Waltham, MA) was added, and the samples were analyzed by liquid scintillation counting.
The drug concentration in each type of ocular tissue was plotted against time. For pharmacokinetic data analysis, the concentration–time data were analyzed (Phoenix WinNonlin 6.0; Pharsight, St. Louis, MO) by fitting the data to an extravascular input model, and the noncompartmental parameters of the drug elimination were estimated and reported.
Results
Hydrolysis Study
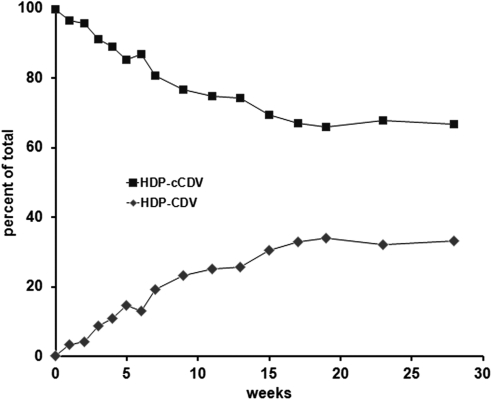
During the 30-week study, HDP-cCDV gradually decreased, whereas HDP-CDV gradually increased (Fig. 2). After approximately 20 weeks, the rate of conversion slowed. After 30 weeks, approximately 35% of the HDP-cCDV had been converted into HDP-CDV.
Figure 2.
Hydrolysis of HDP-cCDV in phosphate-buffered saline at 37°C. Data show HDP-cCDV and HDP-CDV as percentages of the total.
Intravitreal Safety Study to Determine the PK Dose
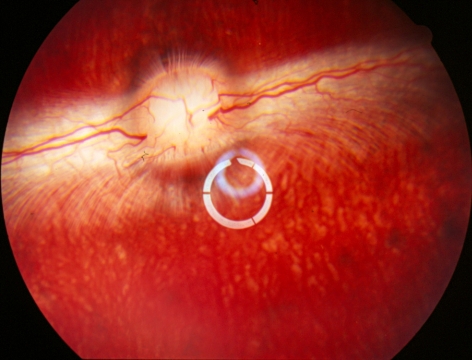
After injection, no toxic reaction was shown in any animals with all three doses during a 5-week observation. The vitreous was completely clear, and the retina appeared normal in indirect ophthalmoscopy (Fig. 3).
Figure 3.
Rabbit fundus at 5 weeks after high-dose HDP-CDV intravitreal injection. The date show normal retina and clear vitreous without the drug aggregate caused by micelle formulation. The whitish rings in the center were the light reflections from the preposing lens, which renders a wider view of vitreous and retina.
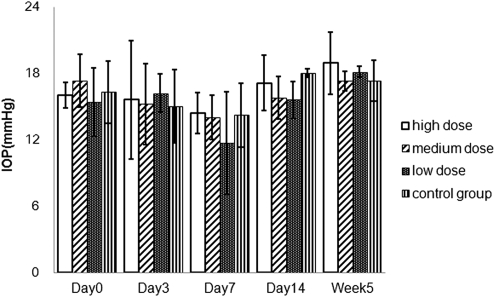
The IOP was measured with the handheld tonometer at days 0, 3, 7, and 14 and week 5. There was no statistically significant difference between the mean IOP of the drug-injected eyes and that of the control eyes (P = 0.76; Fig. 4).
Figure 4.
Mean IOPs with three doses of HDP-CDV and control at various time points after the injection.
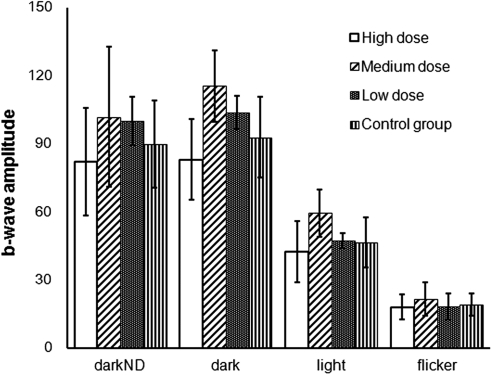
Dark-adapted, light-adapted, and flicker ERGs were performed on both eyes of all animals at week 5. There was no significant difference between the drug-injected and control eyes with respect to amplitude and implicit time, although the high-dose group trend was lower than that of the other groups in the rod-dominated responses (Fig. 5).
Figure 5.
The b-wave amplitude with three doses of HDP-CDV and control at week 5.
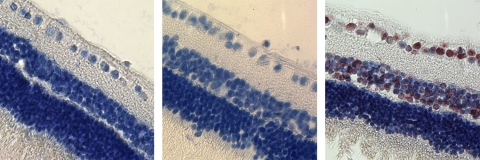
No inflammation, cell infiltration, or cell necrosis was observed in iris, ciliary body, vitreous, and retina and choroid in the hematoxylin and eosin (HE)–stained, paraffin-embedded sections. The normality of the study eyes was confirmed by light microscopy and TUNEL staining (Fig. 6).
Figure 6.
Histology micrographs with TUNEL staining as well as counterstaining with hematoxylin. Left: a retina from the eye injected with 28 μg of HDP-CDV; middle: a retina used as negative control without primary antibody incubation; and right: a retina that was treated with DNase and used as positive control.
Intravitreal Pharmacokinetics Study
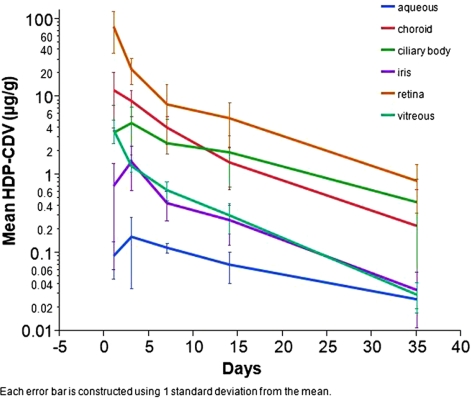
HDP-CDV kinetics in the anterior segment demonstrated an accumulation phase from the injection to postinjection day 3 at which the concentration reached the peak (Table 1, Fig. 7). From day 3 onward, drug in aqueous, iris, and ciliary body showed a steady elimination. In contrast, drug in vitreous, retina, and choroid showed peak concentrations at the first time point (day 1) and their elimination demonstrated a two-phase process, with a faster phase in the first week and a slower phase thereafter (Fig. 7). Retina had the maximum drug exposure, followed by choroid and ciliary body. Drug exposure was lowest in aqueous and then iris.
Table 1.
Pharmacokinetic Parameters of HDP-CDV in Ocular Tissues
| Parameters | Aqueous | Iris | Ciliary Body | Vitreous | Retina | Choroid |
|---|---|---|---|---|---|---|
| R2, adjusted | 0.99 | 0.99 | 0.98 | 0.99 | 0.98 | 0.97 |
| Points used | 3 | 3 | 3 | 3 | 3 | 3 |
| K, day−1 | 0.053 | 0.093 | 0.064 | 0.11 | 0.082 | 0.1 |
| Half-life, d | 13.1 | 7.4 | 10.8 | 6.2 | 8.4 | 6.9 |
| Tmax, d | 3 | 3 | 3 | 1 | 1 | 1 |
| Cmax, ng/mL | 163 | 1,493 | 4,666 | 3,812 | 80,377 | 12,330 |
| Tlast, d | 35 | 35 | 35 | 35 | 35 | 35 |
| AUClast, day · ng/mL | 2,550 | 12,082 | 65,703 | 17,789 | 317,971 | 90,585 |
| MRTINF, pred, d | 18.3 | 9.1 | 14.6 | 6.9 | 7.6 | 7.8 |
K, first-order rate constant of the drug clearance; Cmax, maximum concentration; Tmax, the time at which the concentration was maximum; MRTINF pred, extrapolated and predicted mean residence time; AUC, area under curve.
Figure 7.
HDP-cCDV concentration–time profiles for tissues in rabbit eyeballs.
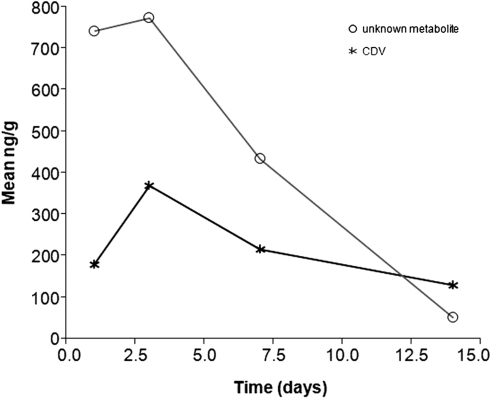
HPLC analysis of ciliary body revealed no metabolites at any time point. In retina, CDV and an unknown metabolite were detected in the first 2 weeks (Fig. 8). Unlike HDP-CDV, the maximum metabolite concentrations were detected 72 hours after injection, which was 48 hours after the peak concentration of HDP-CDV in retina. No CDV monophosphate or diphosphate was detected.
Figure 8.
CDV and metabolite concentration in retina.
Discussion
In previous studies, we showed that intravitreal injection of crystalline lipid prodrugs of small molecules, such as antiviral and antiproliferative nucleosides can be an effective strategy for the sustained delivery of drugs to the posterior segments of the eye.5,6,8 In a recent study, we found that hexadecyloxypropyl cytarabine cyclic 3′,5′-monophosphate can be hydrolyzed to its noncyclic form, hexadecyloxypropyl-phospho-cytarabine, which is water soluble and forms micelles (Cheng L, et al., unpublished data, 2011). We hypothesized that the cyclic form of HDP-cCDV could also be hydrolyzed in vitreous fluid, converting a significant portion of HDP-cCDV into HDP-CDV before reaching the retina. Therefore, the present study was an attempt to examine our hypothesis and study the pharmacokinetics of the converted form of the drug using HDP-CDV as a model drug to better understand our crystalline ocular drug delivery system.
The present study revealed that the HDP-cCDV was slowly converted into HDP-CDV in PBS and that the proportion of HDP-CDV steadily increased until 20 weeks after the experiment started. After 20 weeks, the conversion became minimal, which may have been caused by the high HDP-CDV micelle concentration in which the cyclic structure in HDP-cCDV was somehow masked. This question needs further investigation, but in a living eye, HDP-CDV micelle concentration will never reach this level, because of the constant vitreous fluid turnover.
The in vivo pharmacokinetic study demonstrated that lipid prodrug micelles in vitreous had a relatively long vitreous half-life of 6.2 days and a good retina exposure (AUC = 317,971 day · ng/mL), whereas CDV or cyclic CDV had only 42 hours of half-life in the vitreous and 66 to 77 hours in the retina.12 The peak concentration time (1 day) for the retina was the same as that for the vitreous, which indicates good penetration of HDP-CDV into the retina. Indeed, the drug concentration was consistently higher in the retina, which is the target for CMV treatment. Compared with ODE-cCDV, the half-lives in the retina were similar (8.4 days for HDP-CDV and 10.1 days for ODE-cCDV), whereas the half-life in vitreous for ODE-cCDV was much longer (25 days for ODE-cCDV and 6.2 days for HDP-CDV), due to the drug depot effect of ODE-cCDV. This also indicates that the cyclic form had been largely hydrolyzed into the noncyclic form, which formed micelle concentration in vitreous fluid, and the drug was transported in its noncyclic form into the retina. Hence, the retina half-lives were similar but not the vitreous half-lives. At the end of this study (week 5) after a single intravitreal injection of 28 μg per eye, HDP-CDV was still detected in the vitreous and the retina at concentrations of 28 ng/g (50 nM) and 848 ng/g (1.51 μM), respectively, which remained far above the EC50 (0.002–1 nM) of HDP-CDV against HCMV.13,14 As we expected, HDP-CDV accumulated in the retina due to its lipophilicity, as the drug concentration in the retina was consistently higher than that in the vitreous. We reasoned that the micelle formulation of HDP-CDV itself may be an effective, long-lasting system for CMV retinitis treatment.
It is known that CDV is metabolized into CDV monophosphate and CDV diphosphate through intracellular phosphorylation by host enzymes.15,16 Other metabolites have also been reported, such as CDV-phosphocholine.17 In our study, we found that the maximum metabolite concentration was detected at postinjection day 3, which was 2 days after the peak concentration of HDP-CDV. CDV was detected along with an unknown metabolite that could have been CDV-phosphocholine.17 The failure of detecting CDV monophosphate and diphosphate may be related to the detection limit and the low concentration of metabolites. Indeed, at day 3 the level of HDP-CDV in the retina was approximately 20,000 ng/g versus less than 400 ng/g of CDV. The other metabolites, such as CDV monophosphate, CDV diphosphate, and CDV-phosphocholine may be concurrently present. For example, a relatively high concentration of CDV-phosphocholine was detected. In our previous in vitro study, CDV-diphosphate was detected in cultured cells after application of HDP-CDV.15
It is noteworthy that HDP-CDV had a low concentration in the aqueous but a high one in the iris, which had a drug concentration similar to that of the vitreous. This finding may indicate that HDP-CDV binds to pigment as a storage site. Although the precise nature of binding of drugs to melanin has not been fully elucidated, hydrophobic and lipophilic drugs that bind to pigment have been reported,18–21 and HDP-CDV is a hydrophobic/lipophilic compound that forms micelles in water. The pigmented ocular tissues including iris, ciliary body, and choroid, all had relatively high concentrations of HDP-CDV. This pigment drug combination may serve a secondary drug depot to further extend the drug residence time. On the other hand, the data suggest that HDP-CDV was also cleared from the eye via the anterior route in addition to the route through the retina.
In conclusion, we previously found that a single intravitreal dose of HDP-cCDV (100 μg/eye) has the ability to prevent HSV retinitis for up to 68 days in a rabbit model.5 In an effort to explain the long-lasting antiviral effect, we showed herein that, under simulated physiologic conditions, suspended crystalline HDP-cCDV slowly converts to HDP-CDV, another potent anti-HSV/ CMV prodrug of CDV.14 HDP-CDV, a more soluble compound, may then be dispersed in the vitreous, taken up by retinal cells, and metabolized further to the active antiviral CDV diphosphate, consistent with its known metabolic fate in other types of cells.15 In addition, our pharmacokinetic studies suggest that HDP-CDV itself has a relatively long half-life after intravitreal injection and may be beneficial if used directly as an intravitreal therapeutic for CMV retinitis. We acknowledge that pharmacokinetic data from this study cannot be equally translated into human eyes, because the rabbit eye has a larger lens, smaller vitreous body, less vascularized retina, and more pigmented uveal tract. However, preclinical pharmacokinetic studies have been performed in rabbit eyes22,23 and the predicted drug half-life seems to be very close to the data obtained from a nonhuman primate eye study.24 A well-designed interspecies scaling study may shed more light on this topic.
Footnotes
Supported National Institutes of Health Grants EY 018589, EY 007366, and EY 020617-01A1 and Unrestricted Research Funds to the UCSD Jacobs Retina Center.
Disclosure: H. Wang, None; J. Chhablani, None; W.R. Freeman, None; J.R. Beadle, None; K.Y. Hostetler, None; K. Hartmann, None; L. Conner, None; K.A. Aldern, None; L. Pearson, None; L. Cheng, None
References
- 1. Jabs DA, Ahuja A, Van Natta M, Lyon A, Srivastava S, Gangaputra S. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology. 2010;117:2152–2161 e2151–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holland GN. AIDS and ophthalmology: the first quarter century. Am J Ophthalmol. 2008;145:397–408 [DOI] [PubMed] [Google Scholar]
- 3. Drew WL, Miner RC, Busch DF, et al. Prevalence of resistance in patients receiving ganciclovir for serious cytomegalovirus infection. J Infect Dis. 1991;163:716–719 [DOI] [PubMed] [Google Scholar]
- 4. Weinberg A, Jabs DA, Chou S, et al. Mutations conferring foscarnet resistance in a cohort of patients with acquired immunodeficiency syndrome and cytomegalovirus retinitis. J Infect Dis. 2003;187:777–784 [DOI] [PubMed] [Google Scholar]
- 5. Cheng L, Hostetler KY, Lee J, et al. Characterization of a novel intraocular drug-delivery system using crystalline lipid antiviral prodrugs of ganciclovir and cyclic cidofovir. Invest Ophthalmol Vis Sci. 2004;45:4138–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng L, Hostetler K, Valiaeva N, et al. Intravitreal crystalline drug delivery for intraocular proliferation diseases. Invest Ophthalmol Vis Sci. 2010;51:474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu S, Cheng L, Hostetler KY, et al. Intraocular properties of hexadecyloxypropyl-cyclic-cidofovir in Guinea pigs. J Ocul Pharmacol Ther. 2005;21:205–209 [DOI] [PubMed] [Google Scholar]
- 8. Cheng L, Beadle JR, Tammewar A, Hostetler KY, Hoh C, Freeman WR. Intraocular pharmacokinetics of a crystalline lipid prodrug, octadecyloxyethyl-cyclic-cidofovir, for cytomegalovirus retinitis. J Ocul Pharmacol Ther. 2011;27:157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng L, Hostetler KY, Lee J, et al. Characterization of a novel intraocular drug-delivery system using crystalline lipid antiviral prodrugs of ganciclovir and cyclic cidofovir. Invest Ophthalmol Vis Sci. 2004;45:4138–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Randhawa P, Farasati NA, Shapiro R, Hostetler KY. Ether lipid ester derivatives of cidofovir inhibit polyomavirus BK replication in vitro. Antimicrob Agents Chemother. 2006;50:1564–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ortiz A, Justo P, Sanz A, et al. Tubular cell apoptosis and cidofovir-induced acute renal failure. Antivir Ther. 2005;10:185–190 [PubMed] [Google Scholar]
- 12. Cundy KC, Lynch G, Shaw JP, Hitchcock MJ, Lee WA. Distribution and metabolism of intravitreal cidofovir and cyclic HPMPC in rabbits. Curr Eye Res. 1996;15:569–576 [DOI] [PubMed] [Google Scholar]
- 13. Wan WB, Beadle JR, Hartline C, et al. Comparison of the antiviral activities of alkoxyalkyl and alkyl esters of cidofovir against human and murine cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 2005;49:656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beadle JR, Hartline C, Aldern KA, et al. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob Agents Chemother. 2002;46:2381–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aldern KA, Ciesla SL, Winegarden KL, Hostetler KY. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-(14)C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol Pharmacol. 2003;63:678–681 [DOI] [PubMed] [Google Scholar]
- 16. Balzarini J. Metabolism and mechanism of antiretroviral action of purine and pyrimidine derivatives. Pharm World Sci. 1994;16:113–126 [DOI] [PubMed] [Google Scholar]
- 17. Eisenberg EJ, Lynch GR, Bidgood AM, Krishnamurty K, Cundy KC. Isolation and identification of a metabolite of cidofovir from rat kidney. J Pharm Biomed Anal. 1998;16:1349–1356 [DOI] [PubMed] [Google Scholar]
- 18. Cheruvu NP, Amrite AC, Kompella UB. Effect of eye pigmentation on transscleral drug delivery. Invest Ophthalmol Vis Sci. 2008;49:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nan K, Sun S, Li Y, et al. Characterisation of systemic and ocular drug level of triamcinolone acetonide following a single sub-Tenon injection. Br J Ophthalmol. 2010;94:654–658 [DOI] [PubMed] [Google Scholar]
- 20. Howells L, Godfrey M, Sauer MJ. Melanin as an adsorbent for drug residues. Analyst. 1994;119:2691–2693 [DOI] [PubMed] [Google Scholar]
- 21. Leblanc B, Jezequel S, Davies T, Hanton G, Taradach C. Binding of drugs to eye melanin is not predictive of ocular toxicity. Regul Toxicol Pharmacol. 1998;28:124–132 [DOI] [PubMed] [Google Scholar]
- 22. Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007;114:855–859 [DOI] [PubMed] [Google Scholar]
- 23. Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007;114:2179–2182 [DOI] [PubMed] [Google Scholar]
- 24. Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–733 [DOI] [PubMed] [Google Scholar]