Abstract
The initial therapeutic approach to acute ischemic stroke consists of thrombolytic therapy and early initiation of supportive care, usually commenced prior to the determination of the underlying stroke etiology. Varying stroke mechanisms may call for specific, etiology-based treatment. The majority of strokes result from cardioembolism, large-vessel atherothromboembolism, and small-vessel occlusive disease. There are scant data to support the use of acute anticoagulation therapy over anti-platelet therapy in cardioembolic stroke and large-vessel atherosclerosis, although it may be reasonable in a certain subset of patients. However, augmentation of blood flow with early surgery, stenting, or induced hypertension, may play a role in patients with large artery stenosis. The less commonly identified stroke mechanisms may warrant special consideration in treatment. Controversy remains regarding the optimal anti-thrombotic treatment of arterial dissection. Reversible cerebral vasoconstriction syndrome may benefit from therapy with calcium channel blockers, high-dose steroids, or magnesium, although spontaneous recovery may occur. Inflammatory vasculopathies, such as isolated angiitis of the central nervous system and temporal arteritis, require prompt diagnosis as the mainstay of therapy is immunosuppression. Cerebral venous thrombosis is a rare cause of stroke, but one that needs early identification and treatment with anticoagulation. Rapid determination of stroke mechanism is essential for making these critical early treatment decisions.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-011-0041-5) contains supplementary material, which is available to authorized users.
Keywords: Nonthrombolytic stroke therapy, Cardioembolic stroke, Large-artery atherosclerosis, Arterial dissection, Inflammatory cerebral vasculopathy, Cerebral venous thrombosis
The initial therapeutic approach to acute stroke consists of thrombolytic therapy and early initiation of supportive care, as described in several accompanying articles in this issue. These interventions are typically undertaken prior to determining the pathophysiology of the stroke. Ischemic stroke causative mechanisms are difficult to distinguish in the first few hours, but they become critically important for subsequent therapeutic considerations. Ischemic strokes have been categorized as resulting from cardioembolism, large-vessel atherothromboembolism, small-vessel occlusive disease, other identified mechanisms, or cryptogenic (idiopathic) causes [1]. The first 3 causes account for approximately 70% of ischemic strokes, and their long-term management is described in the series of articles in this issue on secondary prevention. The “other identified mechanisms” are pathophysiologically, diagnostically, and therapeutically distinct from the others and require special consideration, whereas the cryptogenic stroke group remains poorly understood, but is typically medically treated like those with the common causes. This article aims to outline specific early treatments that are targeted toward the stroke pathophysiologic mechanisms.
Cardioembolism
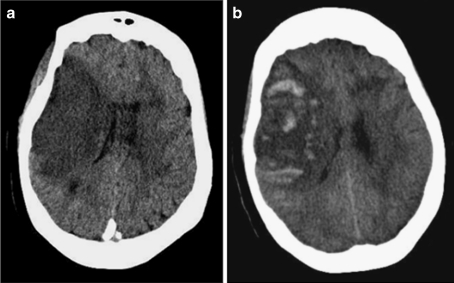
Cardioembolic stroke is a result of structural heart disease and/or arrhythmia. For secondary prevention of stroke, oral anticoagulation with warfarin is well established as effective for patients with substantial risk of recurrent cardioembolism, including those with atrial fibrillation, mechanical prosthetic heart valves, or mural thrombi, among others. Recently, the direct thrombin inhibitor dabigatran was also shown to be effective for prevention of stroke due to atrial fibrillation [2]. However, the role of anticoagulation in the early management of patients with cardioembolic stroke is much less clear. In trials involving a conglomerate of stroke mechanisms, anticoagulation has been repeatedly shown to have no net benefit, although there is a modest reduction in the risk of recurrent ischemic stroke in the short term that is completely offset by an increased risk of symptomatic hemorrhagic stroke [3] (Fig. 1a and b). Nevertheless, cardioembolism has been considered a special case where the benefit may outweigh the risks.
Fig. 1.
(a) Computed tomographic head scan without contrast showing right middle cerebral artery territory infarction. (b) Hemorrhagic conversion of the same infarct, with mass effect and midline shift
Early reports from the Cerebral Embolism Study Group suggested a possible benefit to acute anticoagulation in patients with cardioembolic stroke [4]. This small trial involved 45 patients who were randomized within 48 hours of the onset of cardioembolic ischemic stroke to either dose-adjusted intravenous heparin (partial thromblastin time, 1.5-2.5 times baseline) or a placebo for 10 days. None of the 24 patients randomized to heparin had recurrent events, whereas 2 of the 21 patients in the placebo group experienced recurrent embolization and 2 developed delayed hemorrhagic infarction. This study is limited by the extremely small sample size and the avoidance of all anti-thrombotic therapy (i.e., aspirin and low-dose subcutaneous heparin) in the placebo group. However, many authors have recommended the use of intravenous heparin in patients with cardioembolic stroke based on these very limited data.
The Heparin in Acute Embolic Stroke Trial (HAEST) was unique among heparin trials because a singular stroke mechanism was evaluated. This trial randomized 449 patients with acute ischemic stroke and known atrial fibrillation to either the low molecular weight dalteparin or aspirin within 30 hours of stroke onset [5]. During the initial 2 weeks following randomization, recurrent ischemic stroke occurred in 8.5% of the dalteparin group and 7.5% of the aspirin group (odds ratio, 1.13; 95% confidence interval 0.57–2.24). The proportions of patients with cerebral hemorrhage, stroke progression, and death was similar in both groups. No significant difference in functional outcomes or death was seen at 2 weeks or 3 months after enrollment.
Other trials addressed early heparin treatment in broader stroke populations, but analysis of the cardioembolic subgroup was possible. The International Stroke Trial (IST) was a major multicenter, randomized trial to evaluate heparin in acute ischemic stroke [3]. This study enrolled 19,435 patients within 48 hours of stroke onset and notably included a substantial number of subjects with atrial fibrillation. Patients received either 12,500 units subcutaneous heparin twice daily, 5000 units twice daily, or were assigned to “avoid heparin” for a course of 14 days or until hospital discharge, if sooner. This trial was performed simultaneously with a factorial analysis of acute aspirin therapy, so half the patients also received aspirin. Among the 3169 patients with known atrial fibrillation, the risk of recurrent ischemic stroke at 14 days in the patients given either dose of heparin was significantly reduced compared to no heparin (2.8% vs 4.9%), but the rate of hemorrhagic stroke was increased (2.1% vs 0.4%), yielding no significant net reduction in overall recurrent stroke (4.9% vs 5.3%). In contrast, aspirin in this population was associated with lower risks of recurrent ischemic stroke (3.3 vs 4.5%) without a substantial increase in hemorrhage (1.4% vs 1.1%), resulting in a modest net benefit (4.6% vs 5.6%) compared to no aspirin. Similarly, analysis of the 266 patients in the subgroup with cardioembolic stroke in the Trial of ORG-10172 in the Acute Stroke Trial showed no benefit to treatment with the heparinoid (danaparoid) [1]. In the Tinzaparin in Acute Ischemic Stroke Trial (TAIST), 368 subjects were classified as having cardioembolic strokes, and functional outcomes after treatment with another low-molecular-weight heparin tinzaparin vs aspirin were not different, although the point estimates favored aspirin [6].
In sum, the existing data supports aspirin anti-platelet therapy in comparison with acute anticoagulation with heparin for the vast majority of patients with acute cardioembolic stroke. There may be rare instances for which anticoagulation may be reasonable, such as patients with extremely high-risk cardioembolic source (e.g., left ventricular thrombus) who have recurrent strokes despite anti-platelet therapy. Furthermore, a prominent lingering uncertainty is the appropriate timing to begin anticoagulation for long-term secondary prevention after early anti-platelet therapy. This seems to be at least partly related to the size of the infarction, and we recommend waiting at least 48 hours for small infarcts, approximately 3 to 7 days for moderate infarcts, and 7 to 14 days for large infarcts or those with early minor hemorrhagic conversion while on anti-platelet therapy, although this approach has not been formally tested.
Large Artery Atherothromboembolism
Atherosclerotic stenosis or occlusion of a major cervicocerebral artery is likely the leading cause of stroke worldwide, and carotid artery disease in particular has been well-studied with respect to treatments for secondary stroke prevention [7]. Medical therapy for secondary prevention of stroke consists of anti-platelet therapy and atherosclerotic risk factor modification, although few studies have specifically addressed acute therapy. For ischemic stroke in general, aspirin 160 to 300 mg/day started within 48 hours of the ischemic stroke is of small significance, but it is still significantly beneficial and should be administered early to the majority of ischemic stroke patients; this is typically applied to the subgroup with large artery disease [3, 8]. The patient allergic to aspirin may be reasonably acutely treated with clopidogrel, given as a loading dose of 300 mg, followed by 75 mg daily, based on extrapolation from trials involving acute coronary syndromes, although the safety and efficacy in acute stroke remain uncertain [9]. There are limited data to suggest that acute combination anti-platelet therapy is beneficial after a transient ischemic attack (TIA) or minor stroke, and further investigations are ongoing [10].
Early anticoagulation has been considered for acute ischemic stroke due to large artery disease, but the weight of the evidence does not support this practice, although this may be an issue of patient identification and selection. The Fraxaparine in Ischemic Stroke Study (FISS) series of studies appears to be particularly relevant to large artery disease, as they included Asian populations with a high prevalence of intracranial atherosclerosis. The first FISS Trial randomized 312 patients to 1 of 3 treatments, all given subcutaneously: high-dose nadroparin, low-dose nadroparin, or placebo [11]. Patients were enrolled within 48 hours of symptom onset and received study medication for 10 days. A significant reduction in the percentage of patients dead or dependent at 6 months was found with nadroparin compared to a placebo (45% dead or dependent in high-dose nadroparin group, 52% in low-dose nadroparin group, and 65% in the placebo group; p = 0.005 for trend). FISS-bis was the follow-up study to FISS and was designed to confirm the efficacy of nadroparin in a larger sample size [12]. There were 727 patients who were randomized to begin treatment (similar to the treatment for the FISS studies) within 24 hours. There was no significant difference in the number of patients dead or disabled between the 3 groups. Nadroparin was associated with a lower incidence of pulmonary emboli, but a higher frequency of hemorrhage, including intracranial hemorrhage (symptomatic intracerebral hemorrhage (ICH) rate 2.8% placebo; 3.7% low-dose nadroparin; 6.1% high-dose nadroparin). FISS-tris aimed to demonstrate a benefit of nadroparin in acute stroke patients with large artery occlusive disease [13]. There were 353 patients who had demonstrable large artery disease (evidenced by carotid duplex scan, magnetic resonance angiography, or transcranial Doppler imaging) and were randomly assigned to receive either nadroparin or aspirin (160 mg daily) for 10 days. All patients then received aspirin (80 to 300 mg daily) for 6 months. The proportion of patients with good outcomes at 6 months (Barthel index ≥ 85) was 73% in the low molecular weight heparin (LMWH) group and 69% in the aspirin group (absolute risk reduction, 4%; 95% confidence interval −5–13), with similar rates of hemorrhagic transformation of infarcts and severe adverse events in both groups. The results do not show a significant benefit to the use of low molecular weight heparin in comparison to aspirin in patients with large artery occlusions.
Other trials addressed acute anticoagulation of large artery disease as a subgroup of a broader trial population. The Trial of ORG-10172 (danaparoid) in Acute Stroke Treatment (TOAST) randomized 1281 patients to treatment with dose-adjusted intravenous danaparoid or placebo within 24 hours of onset [1]. There was no difference in favorable outcomes at 3 months in the overall population (75.2% danaparoid vs 73.7% placebo; p = 0.49). However, post hoc analysis based on stroke subtype suggested a possible benefit in patients with large vessel stenosis (68.3% danaparoid vs 53.2% placebo; p = 0.02). Hemorrhagic complications were more common in the danaparoid-treated group, including symptomatic intracerebral hemorrhage (2.3% danaparoid; 0.8% placebo; p = 0.05). In the Tinzaparin in Acute Ischemic Stroke Trial (TAIST), which randomized 1486 patients to high-dose tinzaparin, medium-dose tinzaparin, or aspirin within 48 hours of stroke onset, outcome measures were similar at 6 months in all 3 treatment groups: 1) mortality, 2) disability, and 3) measures of neurologic deterioration [6]. Subgroup analysis of stroke subtype (including large vessel atherosclerosis), anterior vs posterior circulation events and treatment within or after 24 hours did not reveal a significant benefit to tinzaparin use for any specific stroke mechanism. The International Stroke Trial (IST) did not specifically evaluate the subgroup with larger artery atherosclerosis, but found no benefit to heparin in a broad stroke population in terms of death or nonfatal recurrent stroke at 14 days (11.7% heparin vs 12.0% avoid heparin), and no difference in number dead or dependent at 6 months (62.9% for both groups) [3]. Treatment with heparin resulted in a 9 per 1000 excess of transfused or fatal extracranial bleeding.
Overall, these studies do not show a favorable benefit-to-risk ratio for anticoagulation as an acute treatment of stroke due to large artery atherosclerosis. The vast majority of subjects should be treated with anti-platelet therapy. However, there is speculation that it could be useful in a select minority of subjects, and future research may be able to identify such patients.
Acute revascularization interventions for large artery stenosis or occlusion include carotid endarectomy (CEA), angioplasty and carotid artery stenting, and intracranial angioplasty and stenting. Early revascularization is believed to improve symptoms of the acute stroke, prevent worsening, and reduce long-term recurrent stroke risk. However, there are very sparse data supporting this concept. The timing of CEA has been the subject of debate, traditionally in favor of delaying surgery for 2 to 4 weeks after stroke, although most recent data support the efficacy of early (i.e, within 2 weeks) CEA for symptomatic carotid artery stenosis after an ischemic stroke or TIA [14–16]. Very early revascularization within the first 24 to 48 hours after onset is often considered after TIA (particularly crescendo TIA) or very minor stroke, but there are very limited data supporting acute revascularization in such patients [17]. Moreover, acute revascularization poses a risk of hyperperfusion syndrome, including edema and hemorrhagic transformation for larger strokes [18]. Furthermore, many patients with more severe strokes may be too unstable for major vascular surgery.
Endovascular therapy is an evolving technique for the treatment of symptomatic, acute carotid artery occlusions, and an increasing number of interventions are being performed [19–21]. Jovin et al. [22] published a cohort of 25 patients who underwent carotid artery stenting for acute carotid occlusions. Stenting was technically successful in 23 of these patients, and 15 of these patients were revascularized within 6 hours of acute stroke presentation, with the remainder of 8 being stented at a mean of 30 hours (range, 7–120 hours). Of these patients, 10 (43%) showed an improvement in their NIHSS > 4 at 24 hours. There were no hyperperfusion-related complications noted in this case series. This study does not provide evidence that acute carotid endovascular therapy is clinically beneficial, but it does suggest relative safety. However, larger clinical trials are needed to further assess the safety and benefit of acute carotid revascularization.
Low systemic blood pressure has been associated with worse outcomes in acute ischemic stroke. Typically, a conservative approach is used to achieve “permissive hypertension,” allowing the blood pressure to remain relatively high to maximize cerebral blood flow during a period of presumed impaired autoregulation. This approach includes the use of intravenous fluids for volume repletion, cessation of antihypertensive therapy, and keeping the head of the patient’s bed flat for a period of time [23]. If a patient had fluctuating neurological symptoms (especially if correlated with relatively hypotensive episodes), then induced hypertension may be considered. This may be particularly important in the presence of a large artery stenosis. Pharmacologically induced hypertension theoretically should increase cerebral blood flow to both normal brain parenchyma and the ischemic penumbra. Mistri et al. [24] published a review of induced hypertension, which included 12 studies and 319 patients. The most common pressor agent used was phenylephrine, and adverse events were not consistently reported in any of the studies reviewed. Induced hypertension appears to be well-tolerated, but larger clinical trials are needed to further elucidate its safety and clinical benefit.
Small Artery Occlusive Disease
Prolonged vascular injury from hypertension, diabetes mellitus, smoking, obesity, and hypercholesterolemia cause lipohyalinosis and arteriosclerosis in small intracranial artery branches. This may lead to eventual small vessel occlusion and cerebral infarction. Acute aspirin therapy has been shown to be of small, but significant benefit in preventing recurrent stroke, as demonstrated in the International Stroke Trial (IST) and Chinese Acute Stroke Trial (CAST). Combination anti-platelet therapy may play a beneficial role in minor strokes, as studied in the Fast Assessment of Stroke and Transient Ischemic Attack to Prevent Early Recurrence (FASTER) Trial [10]. Although many strokes, due to small-vessel disease, are associated with low-stroke severity scores, and are therefore minor, this combination approach has not been studied specifically in patients with small artery disease, so the results of FASTER may or may not be applicable. The FASTER trail randomized 392 patients with TIA or minor stroke to clopidogrel (300 mg loading dose, then 75 mg daily) or placebo, and simvastatin (40 mg daily) or placebo. All patients were also given aspirin (162 mg loading dose then 81 mg daily). There were 14 patients (7.1%) on clopidogrel who had a stroke within 90 days compared with 21 patients (10.8%) on placebo (risk ratio, 0.7 [95% confidence interval, 0.3–1.2]; absolute risk reduction −3.8% [95% CI, -9.4 to 1.9]; p = 0.19). There were 21 patients (10.6%) on simvastatin had a stroke within 90 days compared with 14 patients (7.3%) on placebo (risk ratio, 1.3 [0.7–2.4]; absolute risk increase 3.3% [−2.3 to 8.9]; p = 0.25). The interaction between clopidogrel and simvastatin was not significant (p = 0.64). Two patients on clopidogrel had intracranial hemorrhage compared with none on placebo (absolute risk increase, 1.0% [−0.4–2.4]; p = 0.5). There was no difference between groups for the simvastatin safety outcomes. The trial was stopped early due to the failure to meet pre-specified enrollment rates. These data suggest that the increased stroke risk after a TIA or minor stroke may be reduced by early initiation of combination anti-platelet therapy, although confirmation of these findings is needed. Early administration of statins does not appear to improve the outlook for TIA or minor stroke.
Other Determined Causes of Stroke
The less common or atypical “other determined mechanisms” of stroke are a myriad, but are not frequently encountered in clinical practice. Suspicion must be maintained and these causes must be properly and promptly diagnosed, as they have distinct treatments from the more common causes previously described. A discussion of all of these causes is well beyond the scope of this review, but we focus on those with implications for early treatment.
Arterial Dissection
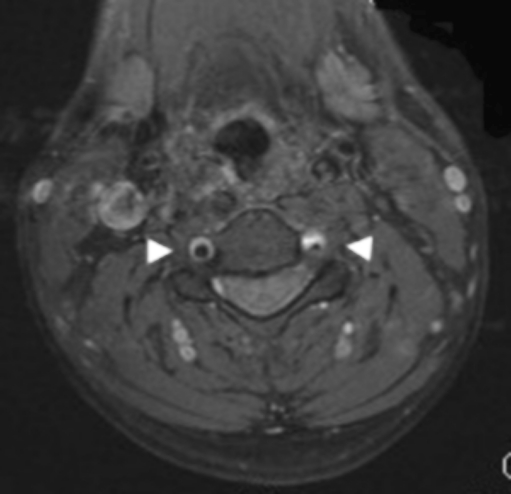
Dissection of the internal carotid and vertebral arteries may be caused by underlying connective tissue disease or trauma, or they may occur spontaneously, and they may cause ischemic strokes as a result of thromboembolism or hemodynamic impairment. (Figure 2). Thrombolysis theoretically presents a risk of bleeding within the torn arterial wall, but there is no evidence to support this conjecture [25, 26]. Therefore, standard approaches to thrombolytic therapy in the hyperacute setting should be considered.
Fig. 2.
Magnetic resonance image with contrast demonstrating bilateral vertebral artery dissections with wall hematomas and stenosis of the left vertebral artery
Early prevention of stroke in arterial dissection has been the subject of much debate. As the most common cause of stroke from dissection is thromboembolism, and because the risk of stroke seems to be greatest in the first day after the onset of the vascular injury, early anticoagulation has long been considered the first-line treatment [27–31]. However, only observational data exist and they are conflicting.
Lyrer and Engelter [32] reported a Cochrane systematic review of 327 patients with carotid dissection in 26 studies, all of which were case series. There was no statistically significant difference in death or disability between anti-platelet and anticoagulant therapy (23.7% anti-platelet vs 14.3% anticoagulant; odds ratio, 1.94; 95% confidence interval, 0.76 to 4.91). Recurrent stroke was observed in 1.7% with anticoagulation, 3.8% with anti-platelet therapy, and 3.3% with no therapy. Menon et al. [33] performed a systematic review that included 762 patients with carotid or vertebral artery dissection from 34 studies. This review showed no significant difference in risk of death (anti-platelet 5 of 268 [1.8%], anticoagulation 9 of 494 [1.8%]; p = 0.88); stroke (anti-platelet 5 of 268 [1.9%], anticoagulant 10 of 494 [2.0%]; p = 0.66), or stroke and death [33]. Subsequently, three large cohort studies examined the risk of stroke associated with dissection, yielding disparate results. Touze et al. [34] showed an annual stroke incidence to be only 0.3% in a historical cohort of 459 patients with carotid and vertebral dissections, but included both symptomatic and asymptomatic dissections. Similarly, in a prospective cohort of 298 patients, Georgiadis et al. [35] found a very a low overall risk of stroke (0.3% within 3 months) with extracranial carotid dissections, regardless of treatment with aspirin or anticoagulation. However, recurrent TIAs and retinal ischemic events may have been less frequent in patients treated with anticoagulation compared with those treated with aspirin. Recurrent ischemic events of all types were more common in patients who presented with initial ischemic symptoms compared with those who only had local symptoms. A potential limitation of this study was the initial exclusion of 8 patients who received early revascularization therapies. On the other hand, in a prospective cohort of 250 patients, all of whom presented with a clinical neurovascular event, Weimar et al. [36] found the risk to be as high as 5.2% during hospitalization and 10.7% within the first year. The conflicting data from these studies underscores the controversy regarding both the prognosis and treatment of dissection. The growing body of evidence hinders the recommendation of anticoagulation as the mainstay of early treatment. The American Heart Association and American Stroke Association guidelines note that early anticoagulation does not lower the risk of early recurrent stroke or early neurologic worsening, and that the efficacy of urgent anticoagulation is not established for treatment of patients with arterial dissdections [37].
Hemodynamic compromise is the other, more uncommon, cause of ischemic stroke from dissection, and revascularization procedures have been performed in patients with symptomatic stenosis as a result of dissection. In these select cases, angioplasty and/or stenting can be used to exclude a false lumen, relieve hemodynamically significant stenosis, and restore the true lumen to a more normal size, thereby increasing flow [38, 39]. In a small cohort of 26 patients undergoing carotid angioplasty and stenting for cervical carotid artery dissection, endovascular interventions were found to be effective in reducing stenosis and had low rates of ischemic complications [40].
Reversible Cerebral Vasoconstriction Syndrome
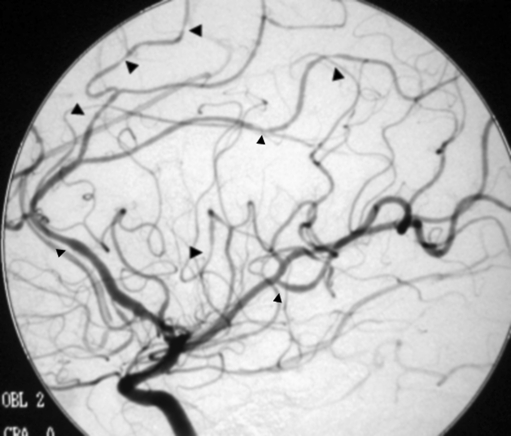
Reversible cerebral vasoconstriction syndromes (also known as Call-Fleming syndrome, benign angiopathy of the CNS, and other names) is manifested by dysregulation of cerebral vascular tone, leading to vasoconstriction of medium and large intracranial arteries [41] (Fig. 3). Clinical symptoms include a thunderclap headache, ischemic stroke, subarachnoid headache, or intracerebral hemorrhage. Postulated causes include vasoactive drugs, neurosurgical procedures, postpartum-induced hypercalcemia, and porphyria [41]. Some cases may recover spontaneously [42]. Optimal treatment has not been clearly established; however, agents used have included calcium channel blockers to treat vasospasm (particularly nimodipine and verapamil), magnesium (in the postpartum period) and high-dose steroids [42–46]. Patients should have follow-up vascular imaging to determine the evolution of their vasoconstriction.
Fig. 3.
Conventional angiogram demonstrating segmental narrowing in reversible cerebral vasoconstriction syndrome
Isolated Angiitis of the Central Nervous System
Isolated angiitis of the central nervous system is an inflammatory vasculopathy that affects only the brain and spinal cord. Patients may develop headaches, seizures, stroke, and encephalopathy, usually with a progressive course. Isolated angiitis is commonly diagnosed by angiographic demonstration of segmental narrowing of small and/or medium-sized vessels and evidence of inflammation in cerebrospinal fluid. Patients do not display systemic manifestations of vasculitis, and as such, systemic markers of inflammation (e.g., erythrocyte sedimentation rate [ESR]) may be normal. The mainstay of treatment is immunosuppression and combination therapy with prednisone and cyclophosphamide has been shown to have a benefit [47, 48].
In patients with large artery vasculitis causing hemodynamic compromise, angioplasty and stenting have been rarely attempted. However, results may be poor, with a high rate of re-occlusion after initially successful treatment [48].
Temporal (Giant Cell) Arteritis
Temporal arteritis is a systemic inflammatory vasculopathy affecting an older age group, usually >50 [49]. Patients may present with headache, temporal artery tenderness, jaw claudication during chewing or speaking, polymyalgia rheumatic, and ischemic stroke, and the ESR is typically elevated. If temporal arteritis is suspected, a temporal artery biopsy should be performed. Immunosuppression can be achieved by the use of steroids and should be initiated early if there is a strong suspicion of temporal arteritis. Steroids can be administered for as many as 10 to 14 days before a temporal artery biopsy, without affecting the biopsy results [49]. Oral prednisone has been the mainstay of treatment and the use of intravenous steroids has conflicting data to support the use, but it may be reasonable in severe cases [50–52]. The goal of maintenance prednisone therapy is to prevent recurrent ischemic events and the patient’s clinical response, and the ESR and C-reactive protein should be followed.
Sickle Cell Disease
Strokes occur in 10 to 20% of patients with sickle cell disease and rarely in those with sickle cell trait [53, 54]. Sickle cell disease causes both a progressive nonatherosclerotic vasculopathy, as well as an increase in blood viscosity, which may lead to direct vascular occlusion and cause subsequent ischemia and infarction [55, 56]. Apart from generic supportive measures, the mainstay of prevention and treatment of acute stroke in sickle cell patients is transfusion (both simple and exchange) with a goal to reduce hemoglobin S to < 30% [57]. The Stroke Prevention Trial in sickle cell anemia (STOP) was a randomized clinical trial that tested whether long-term transfusion therapy could reduce the risk of stroke [58]. The trial enrolled 130 children with the disease who were identified as high risk, namely if transcranial Doppler ultrasonography revealed velocities exceeding 200 cm/s in the intracranial internal carotid artery or the middle cerebral artery. Transfusions were performed to maintain the hemoglobin S concentration less than 30% of the total hemoglobin. Only 1 of 63 children treated with transfusion therapy developed a stroke compared to 11 of 67 who received standard care (p < 0.001). This demonstrated a 92% reduction in the risk of stroke and this vast benefit led to premature termination of the trial. In the setting of acute ischemic stroke due to sickle cell disease, transfusion is strongly recommended as the first-line therapy based on extrapolation from the previously mentioned data regarding prevention and its established use in other forms of sickle cell crisis.
Cerebral Venous Thrombosis
Cerebral venous thrombosis (CVT) is an uncommon cause of stroke, which often presents with atypical symptoms. However, early recognition is critical, as it requires specific treatment. Common causes are pregnancy and the puerperium, infection of the head and neck (mostly seen in children), severe dehydration, hypercoaguable states (including from the use of oral contraceptives), malignancy, myeloproliferative disorders and inflammatory bowel disease [59, 60]. If an underlying cause is identified, it must be treated to maximally prevent recurrent events. Patients may present with headache, seizures, visual abnormalities, and ischemic strokes. Strokes may be demonstrated on magnetic resonance imaging and are in a venous pattern (i.e., they do not follow any certain arterial distribution). Severe cases can lead to cerebral edema and increased intracranial pressure.
Anticoagulation has long been the mainstay of treatment and is believed to prevent thrombus propagation and venous infarctions. However, venous infarcts are particularly susceptible to hemorrhagic conversion, and there has been concern that anticoagulation use may increase this bleeding risk. Einhaupl et al. [61] performed a randomized controlled trial on patients with CVT. Patients were randomly assigned to receive either intravenous heparin (adjusted to a partial thromboplastin time of 80–100 s) vs placebo saline infusion. Although the plan was to study 60 patients, the study was halted prematurely after only 20 patients because of the dramatic and statistically significant benefit of heparin at 3 months (80% of the heparin-treated patients vs 10% of the placebo group had complete recovery and 20% of the heparin-treated group had slight residual deficits compared to 60% of the placebo group: p < 0.01). Moreover, the benefit of heparin was demonstrable after only 3 days of treatment. Einhaupl et al. [61] also performed a retrospective analysis to evaluate the role of heparin in CVT with hemorrhagic venous infarction, and found that of 43 patients, the mortality was 15% among the patients treated with heparin and 69% among those not treated with heparin. A clinical trial of nadroparin was performed in 60 patients with CVT and demonstrated a nonsignificant reduction in poor outcomes: 13% of the treated group and 21% in the placebo group, without an increase in new symptomatic intracerebral hemorrhages in the nadroparin group [62]. As a result of these trials (in spite of the small sample size), acute anticoagulation is recommended for the majority of patients with CVT, even in the presence of hemorrhagic infarction. The International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) was a multicenter, prospective, observational study, and more than 80% of patients were treated with acute anticoagulation in clinical practice [63].
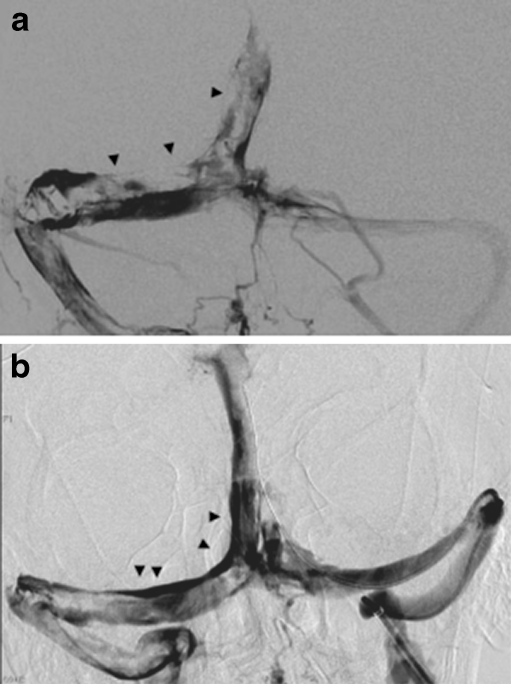
Thrombolysis of a CVT may be achieved by local infusion of urokinase or tPA into dural sinus thrombosis or through a combination of pharmacologic and mechanical means (Fig. 4a and b). The AngioJet catheter is a rheolytic thrombectomy catheter, which works by creating a vacuum to fragment and suction the thrombus through the catheter. The Merci clot retrieval device and/or the Penumbra device has also been reported to have achieved successful thrombectomy [64]. There have been no randomized controlled trials to support the use of mechanical catheters in this entity, but case series have been reported [65, 66]. In the absence of randomized controlled data, intravenous heparin should remain the first-line therapy. Mechanical and/or pharmacological thrombolysis may be considered in patients with thrombus propagation or worsening clinical status, despite adequate anticoagulation.
Fig. 4.
(a) Venous angiogram showing large filling defects and extensive thrombus in the right transverse sinus, torcula, and superior sagittal sinus. (b) Image taken after mechanical thrombolysis demonstrating partial vessel recanalization, with improved flow and decreased clot burden
Electronic supplementary material
(PDF 510 kb)
Acknowledgment
Full conflict of interest disclosure is available in the electronic supplementary material for this article.
References
- 1.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 3.The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 1997;349:1569–1581. [PubMed]
- 4.Immediate anticoagulation of embolic stroke: a randomized trial. Cerebral Embolism Study Group. Stroke 1983;14:668–676. [DOI] [PubMed]
- 5.Berge E, Abdelnoor M, Nakstad PH, Sandset PM. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205–1210. doi: 10.1016/S0140-6736(00)02085-7. [DOI] [PubMed] [Google Scholar]
- 6.Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358:702–710. doi: 10.1016/S0140-6736(01)05837-8. [DOI] [PubMed] [Google Scholar]
- 7.Gorelick PB. Cerebrovascular disease. Pathophysiology and diagnosis. Nurs Clin North Am. 1986;21:275–288. [PubMed] [Google Scholar]
- 8.CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 1997;349:1641–1649. [PubMed]
- 9.Mehta SR, Yusuf S. The Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial programme; rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular disease. Eur Heart J. 2000;21:2033–2041. doi: 10.1053/euhj.2000.2474. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961–969. doi: 10.1016/S1474-4422(07)70250-8. [DOI] [PubMed] [Google Scholar]
- 11.Kay R, Wong KS, Yu YL, et al. Low-molecular-weight heparin for the treatment of acute ischemic stroke. N Engl J Med. 1995;333:1588–1593. doi: 10.1056/NEJM199512143332402. [DOI] [PubMed] [Google Scholar]
- 12.Group HMftF-bI. Fraxiparine in Ischemic Stroke Study (FISS bis) Cerebrovasc Dis 1998;8.
- 13.Wong KS, Chen C, Ng PW, et al. Low-molecular-weight heparin compared with aspirin for the treatment of acute ischaemic stroke in Asian patients with large artery occlusive disease: a randomised study. Lancet Neurol. 2007;6:407–413. doi: 10.1016/S1474-4422(07)70079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–24. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 15.Ferrero E, Ferri M, Viazzo A, et al. Early carotid surgery in patients after acute ischemic stroke: is it safe? A retrospective analysis in a single center between early and delayed/deferred carotid surgery on 285 patients. Ann Vasc Surg. 2010;24:890–899. doi: 10.1016/j.avsg.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Baron EM, Baty DE, Loftus CM. The timing of carotid endarterectomy post stroke. Neurol Clin. 2006;24:669–680. doi: 10.1016/j.ncl.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Dorigo W, Pulli R, Nesi M, et al. Urgent Carotid Endarterectomy in Patients with Recent/Crescendo Transient Ischaemic Attacks or Acute Stroke. Eur J Vasc Endovasc Surg 2010;xx:xx. [DOI] [PubMed]
- 18.Abou-Chebl A, Yadav JS, Reginelli JP, Bajzer C, Bhatt D, Krieger DW. Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting: risk factors, prevention, and treatment. J Am Coll Cardiol. 2004;43:1596–1601. doi: 10.1016/j.jacc.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Fesl G, Wiesmann M, Patzig M, et al. Endovascular mechanical recanalisation of acute carotid-t occlusions: a single-center retrospective analysis. Cardiovasc Intervent Radiol 2010;xx:xx. [DOI] [PubMed]
- 20.Leong S, Abbas S, Galvin L, Moroney J, Brennan P, Thornton J. Emergency stenting of an acute internal carotid artery occlusion from spontaneous dissection. Interv Neuroradiol. 2008;14:69–72. doi: 10.1177/159101990801400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin R, Vora N, Zaidi S, et al. Mechanical approaches combined with intra-arterial pharmacological therapy are associated with higher recanalization rates than either intervention alone in revascularization of acute carotid terminus occlusion. Stroke. 2009;40:2092–2097. doi: 10.1161/STROKEAHA.108.544783. [DOI] [PubMed] [Google Scholar]
- 22.Jovin TG, Gupta R, Uchino K, et al. Emergent stenting of extracranial internal carotid artery occlusion in acute stroke has a high revascularization rate. Stroke. 2005;36:2426–2430. doi: 10.1161/01.STR.0000185924.22918.51. [DOI] [PubMed] [Google Scholar]
- 23.Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: flat positioning improves blood flow velocity in acute ischemic stroke. Neurology. 2005;64:1354–1357. doi: 10.1212/01.WNL.0000158284.41705.A5. [DOI] [PubMed] [Google Scholar]
- 24.Mistri AK, Robinson TG, Potter JF. Pressor therapy in acute ischemic stroke: systematic review. Stroke. 2006;37:1565–1571. doi: 10.1161/01.STR.0000222002.57530.05. [DOI] [PubMed] [Google Scholar]
- 25.Derex L, Nighoghossian N, Turjman F, et al. Intravenous tPA in acute ischemic stroke related to internal carotid artery dissection. Neurology. 2000;54:2159–2161. doi: 10.1212/wnl.54.11.2159. [DOI] [PubMed] [Google Scholar]
- 26.Georgiadis D, Lanczik O, Schwab S, et al. IV thrombolysis in patients with acute stroke due to spontaneous carotid dissection. Neurology. 2005;64:1612–1614. doi: 10.1212/01.WNL.0000159548.45013.C1. [DOI] [PubMed] [Google Scholar]
- 27.Leys D, Lucas C, Gobert M, Deklunder G, Pruvo JP. Cervical artery dissections. Eur Neurol. 1997;37:3–12. doi: 10.1159/000117396. [DOI] [PubMed] [Google Scholar]
- 28.Hart RG, Easton JD, Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1:155–182. [PubMed] [Google Scholar]
- 29.Sturzenegger M. Spontaneous internal carotid artery dissection: early diagnosis and management in 44 patients. J Neurol. 1995;242:231–238. doi: 10.1007/BF00919596. [DOI] [PubMed] [Google Scholar]
- 30.Hill MD, Hwa G, Perry JR. Extracranial cervical artery dissection. Stroke. 2000;31:799. doi: 10.1161/01.str.31.3.791-f. [DOI] [PubMed] [Google Scholar]
- 31.Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29:2646–2648. doi: 10.1161/01.STR.29.12.2646. [DOI] [PubMed] [Google Scholar]
- 32.Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev 2003:CD000255. [DOI] [PubMed]
- 33.Menon R, Kerry S, Norris JW, Markus HS. Treatment of cervical artery dissection: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2008;79:1122–1127. doi: 10.1136/jnnp.2007.138800. [DOI] [PubMed] [Google Scholar]
- 34.Touze E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003;61:1347–1351. doi: 10.1212/01.wnl.0000094325.95097.86. [DOI] [PubMed] [Google Scholar]
- 35.Georgiadis D, Arnold M, Buedingen HC, et al. Aspirin vs anticoagulation in carotid artery dissection: a study of 298 patients. Neurology. 2009;72:1810–1815. doi: 10.1212/WNL.0b013e3181a2a50a. [DOI] [PubMed] [Google Scholar]
- 36.Weimar C, Kraywinkel K, Hagemeister C, et al. Recurrent stroke after cervical artery dissection. J Neurol Neurosurg Psychiatry. 2010;81:869–873. doi: 10.1136/jnnp.2009.192153. [DOI] [PubMed] [Google Scholar]
- 37.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the Prevention of Stroke in Patients With Stroke or Transient Ischemic Attack. A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2010;xx:xx.
- 38.Cohen JE, Gomori JM, Umansky F. Endovascular management of symptomatic vertebral artery dissection achieved using stent angioplasty and emboli protection device. Neurol Res. 2003;25:418–422. doi: 10.1179/016164103101201599. [DOI] [PubMed] [Google Scholar]
- 39.Fateri F, Groebli Y, Rufenacht DA. Intraarterial thrombolysis and stent placement in the acute phase of blunt internal carotid artery trauma with subocclusive dissection and thromboembolic complication: case report and review of the literature. Ann Vasc Surg. 2005;19:434–437. doi: 10.1007/s10016-005-0023-3. [DOI] [PubMed] [Google Scholar]
- 40.Kadkhodayan Y, Jeck DT, Moran CJ, Derdeyn CP, Cross DT., 3rd Angioplasty and stenting in carotid dissection with or without associated pseudoaneurysm. AJNR Am J Neuroradiol. 2005;26:2328–2335. [PMC free article] [PubMed] [Google Scholar]
- 41.Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146:34–44. doi: 10.7326/0003-4819-146-1-200701020-00007. [DOI] [PubMed] [Google Scholar]
- 42.Hajj-Ali RA, Furlan A, Abou-Chebel A, Calabrese LH. Benign angiopathy of the central nervous system: cohort of 16 patients with clinical course and long-term followup. Arthritis Rheum. 2002;47:662–669. doi: 10.1002/art.10797. [DOI] [PubMed] [Google Scholar]
- 43.Nowak DA, Rodiek SO, Henneken S, et al. Reversible segmental cerebral vasoconstriction (Call-Fleming syndrome): are calcium channel inhibitors a potential treatment option? Cephalalgia. 2003;23:218–222. doi: 10.1046/j.1468-2982.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- 44.Dodick DW. Reversible segmental cerebral vasoconstriction (Call-Fleming syndrome): the role of calcium antagonists. Cephalalgia. 2003;23:163–165. doi: 10.1046/j.1468-2982.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen D, Nishizawa S, Yokota N, Ohta S, Yokoyama T, Namba H. High-dose methylprednisolone prevents vasospasm after subarachnoid hemorrhage through inhibition of protein kinase C activation. Neurol Res. 2002;24:215–222. doi: 10.1179/016164102101199639. [DOI] [PubMed] [Google Scholar]
- 46.Singhal AB. Postpartum angiopathy with reversible posterior leukoencephalopathy. Arch Neurol. 2004;61:411–416. doi: 10.1001/archneur.61.3.411. [DOI] [PubMed] [Google Scholar]
- 47.Calabrese LH, Mallek JA. Primary angiitis of the central nervous system. Report of 8 new cases, review of the literature, and proposal for diagnostic criteria. Medicine (Baltimore) 1988;67:20–39. doi: 10.1097/00005792-198801000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Salvarani C, Brown RD, Jr, Calamia KT, et al. Primary central nervous system vasculitis: analysis of 101 patients. Ann Neurol. 2007;62:442–451. doi: 10.1002/ana.21226. [DOI] [PubMed] [Google Scholar]
- 49.Lee AG, Brazis PW. Temporal arteritis: a clinical approach. J Am Geriatr Soc. 1999;47:1364–1370. doi: 10.1111/j.1532-5415.1999.tb07442.x. [DOI] [PubMed] [Google Scholar]
- 50.Swannell AJ. Polymyalgia rheumatica and temporal arteritis: diagnosis and management. BMJ. 1997;314:1329–1332. doi: 10.1136/bmj.314.7090.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sailler L, Carreiro M, Ollier S, et al. [Non-complicated Horton's disease: initial treatment with methylprednisolone 500 mg/day bolus for three days followed by 20 mg/day prednisone-equivalent. Evaluation of 15 patients] Rev Med Interne. 2001;22:1032–1038. doi: 10.1016/S0248-8663(01)00468-4. [DOI] [PubMed] [Google Scholar]
- 52.Hayreh SS, Zimmerman B. Management of giant cell arteritis. Our 27-year clinical study: new light on old controversies. Ophthalmologica. 2003;217:239–259. doi: 10.1159/000070631. [DOI] [PubMed] [Google Scholar]
- 53.Earley CJ, Kittner SJ, Feeser BR, et al. Stroke in children and sickle-cell disease: Baltimore-Washington Cooperative Young Stroke Study. Neurology. 1998;51:169–176. doi: 10.1212/wnl.51.1.169. [DOI] [PubMed] [Google Scholar]
- 54.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 55.Jeffries BF, Lipper MH, Kishore PR. Major intracerebral arterial involvement in sickle cell disease. Surg Neurol. 1980;14:291–295. [PubMed] [Google Scholar]
- 56.Gerald B, Sebes JI, Langston JW. Cerebral infarction secondary to sickle cell disease: arteriographic findings. AJR Am J Roentgenol. 1980;134:1209–1212. doi: 10.2214/ajr.134.6.1209. [DOI] [PubMed] [Google Scholar]
- 57.Adams RJ. Stroke prevention and treatment in sickle cell disease. Arch Neurol. 2001;58:565–568. doi: 10.1001/archneur.58.4.565. [DOI] [PubMed] [Google Scholar]
- 58.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 59.Bousser MG, Chiras J, Bories J, Castaigne P. Cerebral venous thrombosis: a review of 38 cases. Stroke. 1985;16:199–213. doi: 10.1161/01.STR.16.2.199. [DOI] [PubMed] [Google Scholar]
- 60.Martinelli I, Sacchi E, Landi G, Taioli E, Duca F, Mannucci PM. High risk of cerebral-vein thrombosis in carriers of a prothrombin-gene mutation and in users of oral contraceptives. N Engl J Med. 1998;338:1793–1797. doi: 10.1056/NEJM199806183382502. [DOI] [PubMed] [Google Scholar]
- 61.Einhaupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338:597–600. doi: 10.1016/0140-6736(91)90607-Q. [DOI] [PubMed] [Google Scholar]
- 62.Bruijn SF, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30:484–488. doi: 10.1161/01.STR.30.3.484. [DOI] [PubMed] [Google Scholar]
- 63.Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664–670. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 64.Newman CB, Pakbaz RS, Nguyen AD, Kerber CW. Endovascular treatment of extensive cerebral sinus thrombosis. J Neurosurg. 2009;110:442–445. doi: 10.3171/2008.4.17491. [DOI] [PubMed] [Google Scholar]
- 65.Hocker SE, Dafer RM, Hacein-Bey L. Successful delayed thrombolysis for cerebral venous and dural sinus thrombosis: a case report and review of the literature. J Stroke Cerebrovasc Dis. 2008;17:429–432. doi: 10.1016/j.jstrokecerebrovasdis.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Chow K, Gobin YP, Saver J, Kidwell C, Dong P, Vinuela F. Endovascular treatment of dural sinus thrombosis with rheolytic thrombectomy and intra-arterial thrombolysis. Stroke. 2000;31:1420–1425. doi: 10.1161/01.STR.31.6.1420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)