Abstract
Huntington’s disease (HD) is an inherited, relentlessly progressive neurodegenerative disease with an invariably fatal outcome. HD is inherited in an autosomal dominant fashion, and is characterized pathologically by the loss of cortical and striatal neurons, and clinically by involuntary choreiform movements accompanied by progressive cognitive impairment and emotional lability. The disorder is caused by an expanded cystosine adenine guanine (CAG) tri-nucleotide repeat encoding polyglutamine (polyQ) in the first exon of the Huntingtin gene. There is a correlation between the number of CAG repeats and disease onset, such that in patients with CAG repeat lengths of 36 to 60, disease symptoms typically manifest after 35 years of age, whereas CAG repeat lengths >60 yield the more severe juvenile form of the disease. Even though mutant huntingtin is expressed throughout the brain, it is characterized by the selective degeneration of medium spiny neurons of the caudate and putamen, which heralds more widespread neuronal degeneration with disease progression. The mechanisms of cell dysfunction and death in HD have been the subjects of a number of studies, which have led to therapeutic strategies largely based on the amelioration of mutant huntingtin-related metabolic impairment and cellular toxicity. Each of these approaches has aimed to delay or stop the preferential degeneration of medium spiny neurons early in the disease course. Yet, in later stages of the disease, after cell death has become prominent, cell replacement therapy (whether by direct cell transplantation or by the mobilization of endogenous progenitors) may comprise a stronger potential avenue for therapy. In this review, we will consider recent progress in the transplantation of fetal striatal cells to the HD brain, as well as emerging alternative sources for human striatal progenitor cells. We will then consider the potential application of gene therapy toward the induction of striatal neurogenesis and neuronal recruitment, with an eye toward its potential therapeutic use in HD.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-011-0075-8) contains supplementary material, which is available to authorized users.
Keywords: Cell therapy, Neural transplants, Stem cells, Neurogenesis, Medium spiny neuron
Transplant-Based Therapy via Allogeneic Graft of Fetal Striatal Cells
The neostriatum is the region first and most affected by Huntington’s disease (HD). As such, the selective loss of medium spiny neurons, which comprise 95% of striatal neurons, is a neuropathological hallmark of the disease. Other striatal neurons, such as large cholinergic neurons and somatostatinergic neurons are relatively spared. Although neocortical and subthalamic neurons are also lost in the later stages of HD, the loss of these populations may, in part, reflect secondary degeneration following the loss of striatal efferents (for more detail see Peschanski et al. [1]). Given the predominance of medium spiny neuronal loss in HD, a number of investigators have proposed that allogeneic striatal cell grafts, intended to replace lost medium spiny neurons, might ameliorate disease progression. However, such efforts presumed that neuronal cell death in HD is cell-autonomous, an assumption that has recently been questioned by reports of the deficient provision of brain-derived neurotrophic factor (BDNF) to medium spiny neurons via corticostriatal transport, which have implicated the latter as causal in HD pathogenesis [2, 3].
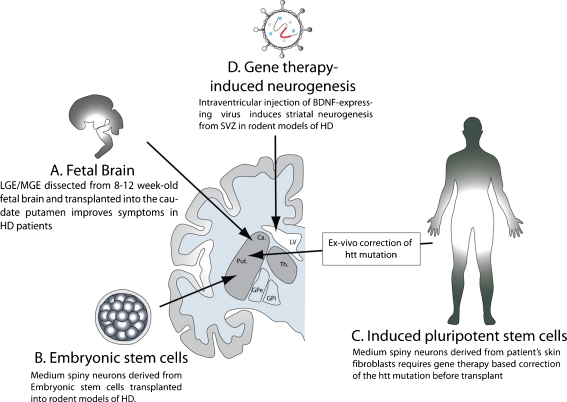
Several preclinical studies initially reported the efficacy of human fetal striatal tissue to provide functional recovery in a variety of rodent and nonhuman primate models of striatal neuronal loss [4–9]. On this basis, several clinical trials then assessed the potential of fetal neural transplants for the treatment of HD (Fig. 1). These trials all used fetal striatal primordium, although they differed in the age of the fetal tissue source and the methodology of graft preparation.
Fig. 1.
Cellular sources for neural transplants for the treatment of Huntington’s disease (HD). (A) The fetal brain tissue currently being used for cell transplantation in HD patients. Other currently tested cells, such as embryonic (B) or induced pluripotent stem cells (C) are presently being used in animal models of HD. (D) Gene therapy-induced neuronal replacement from endogenous neural stem cells is another method undergoing scientific evaluation. Viral over-expression of growth factors or neurotrophins into the lateral ventricle stimulates neurogenesis in the SVZ and triggers striatal recruitment of new medium spiny neurons in both rodent and nonhuman primates. BDNF = brain-derived neurotrophic factor; Ca = caudate; LGE/MGE = lateral ganglionic eminence/medial ganglionic eminence; SVZ = subventricular zone; LV = lateral ventricle; GPe = globus externalis; GPi = globus pallidus internalis; Put = putamen; TH = Thalamus
An initial uncontrolled trial was carried out in 3 moderately advanced HD patients [4]. These patients received bilateral grafts of 8- to 10-week-old human fetal striatal primordia, each pooled from 5 to 8 donors. The grafts were placed into the caudate nucleus (1 graft/hemisphere) and the putamen (4 grafts/hemisphere). The recipients succumbed to the disease at 74, 79, and 121 months after surgery; their autopsies revealed that the grafts grossly survived in 2 patients, with evidence of mature donor-derived neuronal differentiation. No evidence of neuronal intranuclear inclusions was identified in grafted neurons. Yet these grafts exhibited little integration with the host striatum. Although they appeared innervated by nigrostriatal dopaminergic afferents, there was little evidence otherwise of efferent target acquisition or even axogenesis by the grafted neurons; this likely accounted for the lack of clear clinical improvement noted in these patients. Besides the lack of benefit provided by these grafts, they proved potentially unsafe; in the single patient who survived 10 years, autopsy revealed residual, donor-derived fetal ependymal and periventricular cells, which were associated with multiple cysts and graft-derived mass lesions [4].
Another trial was reported 2 years later by Bachoud-Levi et al. [10], in which 5 HD patients received temporally staggered bilateral intrastriatal grafts, with a 1-year interval between the 2 procedures. Each graft was comprised of a single ganglionic eminence, dissected from 7.5- to 9.5-week-old human fetal brains. Positron-emission tomographic (PET) measurement of (18F)-fluorodeoxyglucose uptake revealed sustained metabolic activity following engraftment (EPs), whereas the median nerve somatosensory evoked potentials of transplant recipients exhibited less degradation of cortical EPs than did those of 22 untreated patients [10]. Serial PET imaging confirmed that cortical metabolism in 3 patients improved during the 2-year follow-up, concurrent with clinical stabilization, suggesting functional integration of the grafts [11]. Nonetheless, by 4 to 6 years after grafting, the early benefits of surgery had largely been lost. However, this decline did not affect all aspects of disease phenotype alike, although even the dystonia of graft recipients continued to worsen with time, their cognitive decline may have been mitigated. This is surprising in light of the striatal targets engrafted, perhaps emphasizing the importance of striatocortical afferents in higher cortical function [12].
In another trial, 7 moderately advanced HD patients were given intrastriatal grafts consisting of microdissected lateral ganglionic eminence from 8 to 12 human fetal brains [13]. Microdissection was performed to reduce the contaminant tissue that resulted in the development of cysts and lesions in the Kopyov clinical trial [4]. Despite the use of multiple donors and injection sites (lateral ganglionic eminence of 2 to 8 fetuses per recipient striatum, immunosuppressed for 6 months), only 8 to 10% of the recipient striata engrafted in 1 patient, who was autopsied 18 months after transplant. Furthermore, despite the apparent viable maintenance of the transplant, and the lack of histopathologically apparent immune response, no functional integration of the graft within the host striatum was noted. PET imaging failed to show any integration of the graft into the host parenchyma because the glucose and dopamine receptor binding was significantly reduced in HD striatum. This correlated with the lack of clinical improvement in these patients [14]. Autopsies showed that grafted cells survived in 2 of 3 patients a decade after transplantation. Importantly, the grafts showed a marked reduction in medium spiny neurons, whereas the donor-derived interneurons were relatively spared; these observations mirrored the pattern of striatal cellular loss typically noted in HD, thereby suggesting the role of noncell autonomous features of the disease environment in HD [15].
A 2-phase safety study was next undertaken by the European Network for CNS Transplantation and Repair (NECTAR) and the European Network for Striatal Transplantation in Huntington’s Disease (NEST-HD) in a United Kingdom-based multicenter trial. The first phase of this study addressed the safety of unilateral intrastriatal grafts, whereas the second phase addressed the safety of bilateral transplants [16]. Four patients with mild to moderate HD were transplanted during the course of the first phase of this study. They received unilateral grafts consisting of the equivalent of 2 to 2 whole striatal primordia. None of the patients suffered significant morbidity related to the transplant at 6 months; the only complications that arose were related to immunosuppression, which in all cases had been controlled by titration of drug dose. Magnetic resonance imaging showed no indication of uncontrolled or invasive growth of the implanted tissue in any of the 4 patients [16]. Two other patients were grafted, and the outcome of the transplantation has not been published. The trial itself has been discontinued [17].
Another pilot study assessing the usefulness of fetal cell grafts involving 2 patients with moderate HD was reported by Reuter et al. [18]. The patients had identical huntingtin mutations (with 43 cystosine adenine guanine (CAG) repeats); one had been symptomatic for 6 years, and the other for 4 years. Both patients received bilateral fetal striatal allografts of 2 to 3 ganglionic eminences. In 1 patient, graft function was confirmed by PET, which revealed increased striatal D2 receptor binding of [11C]-raclopride. This was associated with prolonged clinical motor improvement during a 5-year period, as assessed by Unified Huntington’s Disease Rating Scale (UHDRS). The other patient showed no evidence of either clinical improvement or of graft function; the patient’s motor decline was similar to that of 6 untreated controls.
It is difficult to compare these studies because of the heterogeneity in their design, lack of controls, unblended nature, and different methods used to assess clinical and motor outcome in each. Yet even in the best of cases, the reported improvements in these trials appear to have been modest. Although some transplanted patients may have exhibited brief symptomatic improvement following the transplants, in most cases disease progression continued unabated, with no significant survival increment. Given these divergent reports, predicated on trials involving different cell preparation and implantation protocols and invariably small sample sizes, there is a clear need for a larger scale, technically standardized, and well-controlled evaluation. To this end, there are currently 3 ongoing clinical trials, part of the Euro-HD Multicenter, Randomized Controlled Phase II study, which is intended to re-assess graft efficacy using improved tissue preparation and surgical protocols [19, 20].
Differences in both the age and source of transplants are also a major source of variation, which are not well addressed in any trial yet either performed or designed. The nature of the grafts and differences in ganglionic eminence dissection and preparation methods may have profound consequences on the effects of the transplantation. The biology and the ontogeny of the striatum, as well as the stage-dependent lineage potential of cells in the ganglionic eminence, have not been typically well-considered. Yet the ganglionic eminences comprise germinal, subependymal, and deep layers in 2 lateral folds that comprise the medial and lateral ganglionic eminences. In normal development, both striatal and nonstriatal precursors originate in the superficial layers and migrate ventrally and laterally to and through the underlying striatal primordium [21, 22]. Although the lateral ganglionic eminence gives rise to mostly striatal [23] medium spiny neurons, the medial ganglionic eminence gives rise to globus pallidus and cortical interneurons, which are cells that can share the expression of several nominally striatal markers [24]. Yet medial ganglionic eminence components are also important for both the proper development of striatal structures, and for their functional integration [25]. This may explain, at least in part, the lack of functional integration in the clinical trial reported by Hauser et al. [13], in which the grafts only consisted of the most lateral part of the lateral ganglionic eminence [13, 26].
The immune response is also another major concern in these clinical trials. The brain may enjoy some degree of immune privilege because of its restricted entry of peripheral immune cells, and its relative reliance on innate rather than acquired immune responses. Nonetheless, immune responses against the fetal tissue allografts were evident. In the study reported by Krystkowiak et al. [23], 5 of 13 patients manifested alloimmunization to donor antigens within a few months after cessation of pharmacological immunosuppression. In this study, 1 patient showed obvious clinical and radiological signs of rejection that was reversed by pharmacological immunosuppression. In this regard, the lack of transplant effectiveness in the Hauser et al. [13] study may be associated with its limited, 6-month course of postoperative immunosuppression.
Thus, the variable results across these studies may be due in part to differences in both tissue preparation and immunosuppression, as well as study design and outcomes assessment. Several studies have shown that the outcome of transplantation may depend of the physical nature of the graft, whether as dissociated cells, reformulations thereof, or minced tissue fragments [27, 28]. In 1 study that compared cell suspensions and minced tissue directly, the engrafted dispersed cells grew larger and included more medium spiny neurons, with better afferent and efferent connectivity, than did the grafts implanted as fragments of tissue [29]. Interestingly, similar intrastriatal suspension grafts derived from 6- to 9-week-old fetal midbrains were used in a trial of cell therapy for Parkinson’s disease. Postmortem analysis showed that the grafts added dopaminergic neurons to the recipient striata, and were able to survive for more than a decade [30, 31]. The patients received only 6 months of immunosuppression; nonetheless, the transplants appeared only mildly immunogenic to the host brain [32].
Despite the apparent advantages of dispersed cell suspension grafts, the heterogeneity of these otherwise unsorted preparations may be problematic. Cell suspensions consist of trypsinized fetal tissue, nominally disaggregated to the single cell stage, which can have the same assortment of phenotypes as minced fetal tissue. The capillaries in grafts, which may be donor-derived, can be extremely immunogenic because of their high levels of donor class I antigens [27, 33]. Thus, an enriched cell preparation containing only desired and lineage-restricted phenotypes may greatly reduce graft immunogenicity, while improving the likely functional outcome of transplantation [34]. For instance, neural and glial progenitors isolated on the basis of their expression of defined stem and progenitor cell markers, such as the intermediate filament nestin, the RNA binding protein Musashi, the transcription factor Sox2, or the glial progenitor marker A2B5, have all been shown to stably engraft for long periods of time in xenografted mice and rats, with minimal immunosuppression [35–39]. Future studies of striatal neuronal engraftment may profitably study the effect of pre-transplant phenotypic enrichment, as a means of mitigating rejection potential, and of thereby increasing graft acceptance and functional integration.
Embryonic Stem Cells as a Source of New Medium Spiny Neurons
The development of cell replacement therapies for HD has also been hindered by the availability of fetal donor cells, their heterogeneity, and the ethical concerns related to the use of tissue derived from abortions. As such, there is a great need for more practical sources of cells that can be used to generate medium spiny neurons.
In that context, human embryonic stem cells (hESCs) have been recently evaluated as a readily renewable source of potential medium spiny neurons and their progenitors. Aubry et al. [40] first described the differentiation of hESCs into medium spiny neurons in vitro, and their integration in vivo following xenotransplantation into adult rats. To achieve this goal, the authors adopted a 3-stage in vitro protocol for generating medium spiny neurons, using agents identified as critical for the in vivo development of these cells. To investigate the functional integration of these hESC-derived striatal neurons, the quinolinate-lesioned striata of immunocompetent rats were then injected with cells isolated at different stages of medium spiny phenotypic induction. Striata engrafted with committed neuroepithelial cells revealed clusters of hESC-derived medium spiny neurons, thus establishing the potential usefulness of hESC-derived medium spiny neurons (MSNs) in striatal neuronal addition (Fig. 1).
However, several issues may yet hinder the clinical use of hESC-derived MSNs, at least at our current level of technology. First, ES-derived grafts have been reported to induce formation of teratomas or neuroepithelial tumors [41]. The formation of these tumors is a function of the degree to which the cells are selectively enriched and differentiated prior to transplantation [40, 42]. However, embryoid bodies (EBs) from early stage of the culture can be contaminated with undifferentiated multipotent cells that escaped the differentiation process, permitting teratogenesis in the host [41–44]. Thus, the faster the hESCs are differentiated in vitro, the higher the risk may be of teratoma formation after transplantation [45]. A number of studies have reported the successful engraftment of hESC-derived cells within the brain, as treatment for Parkinson’s disease and HD, without the formation of tumors [46–48]. It is important to note that these studies were conducted in rodents, and did not include the long survival times needed to rigorously assess tumorigenic potential. Second, hESCs express low levels of human leukocyte antigen class I molecules in both the undifferentiated and differentiated state [49–51]. Thus, the expression of mismatched human leukocyte antigens by embryonic stem cell (ESC)-derived progeny might elicit an immune response, sufficient to induce a local immune response, if not frank rejection. In particular, activated microglia and lymphocytes triggered by engrafted cells can produce cytokines, such as interleukin 6, which can both suppress neuronal differentiation and promote astrocytic differentiation of engrafted neural precursor cells in vivo [52].
Induced Pluripotent Stem Cells as Sources of Engraftable Neurons
Pluripotent stem cells may be derived from genetically reprogrammed skin fibroblasts, potentially allowing the generation of patient-specific donor cells. Induced pluripotent stem (iPS) cells are typically derived from genetically reprogrammed fibroblasts and keratinocytes (although they may conceivably be generated from any mitotically competent phenotype) by the concurrent forced expression of a specific set of 4 to 6 transcription factors [53, 54]. iPS cells were first generated by introducing the relevant reprogramming genes using virally integrating vectors, such as retrovirus or lentivirus. However, because the insertion into the host genome may result in insertional mutagenesis and is potentially tumorigenic, alternative methods of generating iPS cells have been developed [55]. With in vitro expansion and selection, the cells regain an undifferentiated ground state that shares similarities with that of ESCs. Similarly, they can also be driven to differentiate into neuroepithelial and subsequently fully differentiated neurons [56]. Importantly, tissue-specified cells derived from these iPS cells would likely, although not certainly, evade rejection at autograft [57].
A variety of disease-specific iPS cells, including those for HD, have been generated and have already found uses in drug screening and in the investigation of disease-specific molecular pathways [58–61]. Yet these cells also comprise an important source of cells for phenotype-specific cell replacement and tissue repair. For instance, Wernig et al. [62] first used iPS cells derived from mouse fibroblasts to generate dopaminergic neurons, which proved both engraftable and functional. More recently, dopaminergic neurons have also been generated using iPS cells derived from a PD patient’s own somatic cells. After transplantation to rodents rendered Parkinsonian by intrastriatal 6-OHDA (6-hydroxydopamine) injection, these human iPS cell-derived dopaminergic neurons survived and were associated with functional benefit, as reflected in a significant reduction in amphetamine-induced rotations, 16 weeks post-transplantation [63].
Yet unlike PD (in which effectively normal dopaminergic neurons may be generated from a patient’s own cells), in HD, further genetic modification would be needed to permit autograft, given the underlying CAG repeat expansions of the donor cells. Several techniques have already been developed to achieve this end, all directed at the inactivation or blockade of mutant Huntingtin gene expression. Future studies of iPS cell derivatives as potential therapeutic vectors will need to address this issue by removing or inactivating the offending segment of poly-CAG expansion.
Aside from these issues, the tumorigenic behavior of transplanted cells remains a major safety concern that will need to be addressed before iPS cells can be clinically useful. Recent studies analyzing the genome of iPS cells showed evidence of mutations following reprogramming and subsequent expansion of the cells in vitro. These mutations frequently occur in regions prone to amplification, deletion, or point mutation, and seem to be enriched in genes involved in cell-cycle regulation and cancer [64–66]. Hargus et al. [63] reported no sign of tumors in rats grafted with neurons from PD patient-derived iPS cells for up to 16 weeks post-transplantation. Nonetheless, whether this will remain the case in the aged brain, and for how many years after grafting, remains unknown.
Induced Neurogenesis from Endogenous Neural Stem Cells as a Potential Treatment for HD
In adult vertebrates, the subependymal lining of the lateral ventricles harbors persistent neuroepithelial stem and progenitor cells, from which new neurons and glia may be derived. In mammals, neural stem cells of the subventricular zone (SVZ) continually generate transit-amplifying glial and neuronal daughters, at least some of which give rise to new neurons [67–70]; the latter are typically recruited anteriorly to the olfactory bulb as GABAergic interneurons of several phenotypes [68, 71–75]. Olfactory neuronal recruitment is rapid; in mice, it takes approximately 15 days for neuronally restricted progenitor cells of the SVZ to migrate through the rostral migratory stream to the olfactory bulb, traversing a distance of 3 to 5 mm, wherein they differentiate into olfactory interneurons [67, 76, 77] (for more detail see Whitman and Greer [78] and Abrous et al. [79]). In addition, some neural stem cells migrate posteriorly in development to form a separate germinal neuroepithelium, the subgranular zone of the hippocampal dentate gyrus [80–83]. The population of neural stem cells that give rise to the neuronal progenitor pool of the anterior SVZ, which gives rise to the rostral migratory stream, is the pool of greatest interest in regard to HD therapeutics, because it is this pool from which new striatal neurons may be recruited (Fig. 1).
Mitogens Can Trigger Adult Neurogenesis In vivo
Neural stem cells from the adult subventricular zone can be expanded and maintained in vitro under the influence of fibroblast growth factor 2 (FGF2) and epidermal growth factor (EGF); these observations suggested that FGF2 and EGF might also act in vivo [84–88]. Indeed, knockout mice for FGF2 or transforming growth factor-α, a ligand for EGF receptor, exhibit decreased neurogenesis in the SVZ, and fewer neurons recruited to the olfactory bulb [89, 90]. In the adult rodent, the majority of SVZ cells coexpress both FGF2 and EGF receptors [87, 91]. On that basis, several groups explored the possibility that intraventricular infusion of either growth factor might potentiate SVZ neurogenesis. Infusion of FGF2 into the lateral ventricle resulted in enhanced neurogenesis within the SVZ and a subsequent increase in the number of new neurons in the olfactory bulb, whereas similar infusion of EGF resulted in a decrease of new neurons in the olfactory bulb. The latter was associated with a significant increase of astrocytes in the neighboring striatum [84, 92]. These observations are in contrast with a previous report demonstrating an increase in neural recruitment to the olfactory bulb and a recruitment of new neurons to the striata following intraventricular infusion of EGF [93]. These new neurons were defined by their incorporation of the mitotic tracer 5-bromo-2'-deoxyuridine (BrdU) and expression of the mature neuronal marker Neuronal Nuclei (NeuN). However, this study did not provide any indication as to the exact phenotype of these neurons and whether they could be of any relevance to treating Huntington’s disease.
FGF2 also plays a prime role in the regulation of SVZ neurogenesis in vivo. Intracerebral injection of FGF2-neutralizing antibodies led to a decrease in neurogenesis, whereas subcutaneous injection of FGF2, which was reported to cross the blood brain barrier, stimulated neurogenesis in vivo [94, 95]. In a recent report, Jin et al. [96] investigated whether systemic injection of FGF2 could treat HD either by its neuroprotective mechanisms or by regenerating medium spiny neurons. In this study, the authors reported that R6/2 mice, a widely used mouse model of HD, treated with FGF2 showed enhanced neurogenesis and an improvement in the disease condition of the mice. The FGF2 treatment resulted in a roughly 150% increase in the BrdU index in the SVZ of R6/2 mice, almost fivefold greater than the FGF2-stimulated increase in SVZ cell proliferation noted in wild-type animals. This result is in accord with a previous report that indicated neurogenesis in the SVZ is enhanced in R6/2 mice [97, 98]. FGF2 treatment also resulted in the recruitment of new medium spiny neurons (as defined by their expression of DARPP-32) to the neostriata of the treated animals. Importantly, retrograde tracing experiments confirmed that these new neurons developed striatopallidal projections, which were accompanied by improved motor coordination, and an increased lifespan of roughly 20% in the treated R6/2 mice. In addition, the striatal neurons of treated mice had reduced intranuclear inclusions and restored expression of cannabinoid 1 receptor, which was lost in HD mice [99]. It is noteworthy that other previous studies have shown that conditional HD mice with prominent neurological dysfunction can recover normal motor function and aggregate clearance with the down-regulation of mutant Huntingtin expression [100]. Thus, it is not clear whether the fibroblast growth factor-associated improvement in neurological phenotype can be solely attributed to neurogenesis.
BDNF Induces a Neuronal Addition to the Adult Striatum
BDNF is a neurotrophin broadly expressed in the brain, wherein it supports a plethora of functions, including neuronal differentiation, maintenance, and survival. [101–107]. BDNF exerts its function by binding its high affinity TrkB receptor, either together with or apart from its low affinity p75 receptor. TrkB receptors exist in 3 isoforms, including full length and 2 truncated variants, which are missing the intracellular domains TKT1 and TKT2 [108, 109]. Several studies have linked BDNF to the etiology of HD, which is characterized by impaired BDNF expression and axonal transports. Notably, BDNF expression can be regulated by wild-type huntingtin at both transcriptional and post-transcriptional levels, suggesting that its production and availability might be limited in the HD brain [110, 111]. BDNF is normally present at high levels in the caudate and putamen, in which it is released from corticostriatal afferents [112]. This transport was investigated in HD and it has been shown that huntingtin controls the axonal transport of cortical BDNF to striatal targets, and such transport is also impaired in HD [3, 113]. Thus, BDNF impairment appears to be a key factor in the pathology of the HD. Indeed, comparative analysis of gene expression profiles of striatal tissues from BDNF knockout mice with those from a postmortem HD patient, and those from HD rodent models including 3-nitropropionic acid-lesioned or transgenic models showed that the BDNF knockout model profile is more comparable to human HD than the other murine HD profiles [114].
A number of studies have demonstrated that BDNF is a potent neuroprotective agent in excitotoxic quinolinic acid-lesioned or 3-nitropropionic acid-lesioned models of HD [115–118]. On that basis, the restoration of BDNF levels was actively targeted as a potential treatment for HD. In fact, a number of drugs proposed for HD treatment stimulate BDNF expression (e.g., riluzole [126], cystamine [120], memantine [121], ampakine [122], and CEP-1347 [123, 124]).
Besides its supportive and neuroprotective functions, BDNF strongly potentiates the neuronal differentiation and maturation of neuroblasts derived from the adult SVZ [125]. BDNF protein infused into the lateral ventricle of adult rat brains was the first noted to increase the number of newly generated neurons in the olfactory bulb [126], an observation that we confirmed using an adenoviral vector expressing BDNF [127], the intraventricular administration of which yielded an increase in the number of newly recruited olfactory bulb neurons. A similar observation was also noted 3 weeks post-treatment using a lentiviral vector expressing BDNF, in which olfactory neuronal addition was noted in response to BDNF over-expression [128]. Most interestingly, the striata of these animals exhibited substantial heterotopic neurogenesis following intraventricular adenoviral delivery of BDNF. New striatal neurons expressed markers of mature medium spiny neurons, including NeuN and DARPP-32 [127].
Interestingly, when high-dose infusions of BDNF were delivered to adult rats, heterotopic neuronal addition was noted to a variety of sites, which included the striatum and septum, as well as the thalamus and hypothalamus; each showed evidence of neuronal addition, as demonstrated by co-immunolabeling of BrdU and neuronal markers, such as microtubule-associated protein-2 and ß-III-tubulin [129]. This provocative observation remains largely unexplored.
Suppression of Bone Morphogenetic Proteins (BMPs) Can Be Used to Induce Striatal Neuronal Replacement
Previous studies have reported that neural progenitor cells of the adult SVZ can be driven to astrocytic phenotype by bone morphogenetic proteins (BMPs), in particular BMP-2 and BMP-4 [130–132]. High concentrations of these BMPs and their receptors have been identified in the SVZ of the adult rat brain [130, 132] and may dictate the glial bias of newly generated cells in the adult subependyma. Yet at the same time, ventricular ependymal cells can express Noggin, an endogenous antagonist of the BMP family, thereby defining local niches that may remain permissive for neuronal cell fate [133–136]. On the basis of these observations, we hypothesized that the induced over-expression of Noggin in the adult ventricular wall might inhibit glial differentiation, making more SVZ daughter cells available to respond to BDNF, and thereby potentiating BDNF-induced neuronal differentiation and striatal recruitment. Therefore, we delivered intraventricular injections of adenoviral brain-derived neurotrophic factor (AdBDNF) and adenoviral Noggin (AdNoggin) to adult rats, injected them for 3 weeks with the mitotic tracer BrdU, and analyzed their neostriata stereologically 3 and 8 weeks later. We found that rats treated with AdNoggin together with AdBDNF showed a significant increase in striatal neuronal addition, relative to those given AdBDNF alone [134]. At the same time, these animals generated significantly fewer subependymal astrocytes. Yet no significant increase in new neurons was found between AdNoggin and AdEGFP (null control adenovirus expressing Enhanced Green Fluorescent Protein) treated groups, suggesting that noggin alone exercised no effect on neuronal addition; its actions appeared limited to the suppression of gliogenesis and concurrent potentiation of progenitor responsiveness to BDNF [137, 138].
Importantly, a combination of BrdU tagging, retrograde backfills, and immunolabeling confirmed that these new striatal neurons matured as DARPP32+ GABAergic medium spiny neurons, which efficiently extended fibers to their normal developmental target, the globus pallidus (Fig. 2a). The latter point was demonstrated using intraventricular injection of AdBDNF/AdNoggin or infusion of BDNF/Noggin proteins, followed by a retrograde tracer injection into the globus pallidus 1 week before the animals were killed. In these animals, a majority of newly generated striatal neurons co-expressed BrdU and either neuronal NeuN or DARPP32, together with the retrograde tracer, indicating their maturation as striatopallidal projection neurons (Figs. 2d and 3b). Importantly, the newly generated neurons included roughly equal proportions of both enkephalinergic and Substance P-expressing cells, which respectively define the major indirect and direct pathway outputs of the neostriatum (Fig. 2b, c). Together, these data indicated that the coadministration of BDNF and Noggin induced the significant addition of new medium spiny neurons to the striata of wild-type rats, and their anatomic integration with existing striatopallidal outputs. Thus, these findings suggested the feasibility of induced neuronal addition as a treatment strategy in HD [137, 139].
Fig. 2.
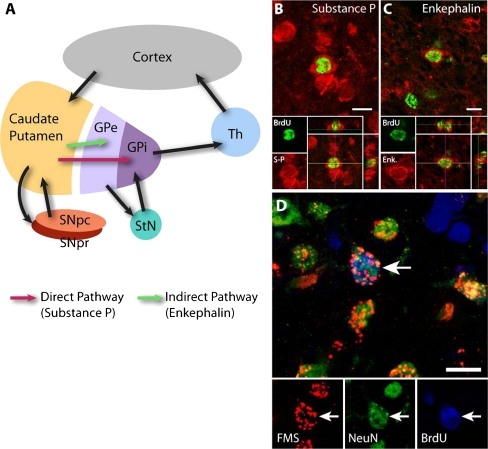
Newly generated medium spiny neurons integrate into the 2 major output pathways of the basal ganglia. (A) Schematic representation of the different neural pathways of the basal ganglia and related structures. The red arrow indicates Substance P-expressing medium spiny neurons of the direct pathway. The green arrow indicates Enkephalinergic medium spiny neurons of the indirect pathway. Following intraventricular injection of adeno-associated virus serotype 4 (AAV-4)-brain-derived neurotrophic factor/Noggin, the newly generated medium spiny neurons in the rat striatum expressed either Substance P (B) or Enkephalin (Enk.) (C) neurotransmitters. Thus, these neurons integrate as part of the direct or indirect pathway as shown in the schematic. (D) Intrapallidal injection of fluorescent microspheres (FMS), a retrograde tracer, allowed identification of newly generated neurons that successfully extended axonal fibers to the globus pallidus, the natural target of medium spiny neurons. BrdU = 5-bromo-2'-deoxyuridine; Enk = enkephalin; GPe = globus pallidus externalis; GPi = globus pallidus internalis; SNpc = substantia nigra pars compacta; SNpr = substantia nigra pars reticulata; S-P = substance P; StN = subthalamic nucleus; Th = Thalamus. Scale bar = 10 μm
Fig. 3.
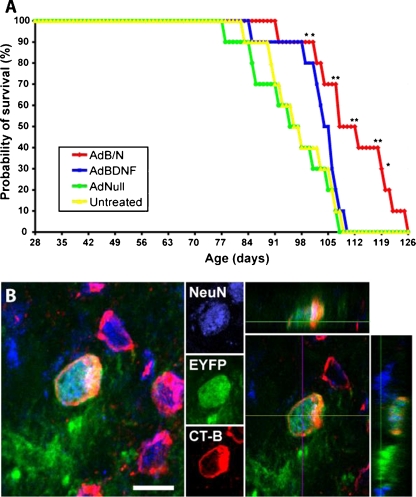
Induced striatal neurogenesis was associated with both anatomic integration and increased lifespan in R6/2 HD mice. (A) Adenoviral brain-derived neurotrophic factor (AdBDNF)/adenoviral Noggin (AdNoggin)-induced neurogenesis was associated with prolonged survival in the R6/2 mouse model of Huntington’s disease. Kaplan-Meier analysis revealed that the lifespan was significantly extended in R6/2 mice injected with AdBDNF/AdNoggin compared with R6/2 mice injected with AdBDNF only, or adenoviral Null (AdNull), or left untreated. *p < 0.05; **p < 0.01. (B) To trace the origins of the newly generated neurons, tamoxifen-treated Nestin-CreERT2/ROSA-EYFP bigenic mice (courtesy of Dr. Kageyama, [149]) received intraventricular infusion of brain-derived neurotrophic factor (BDNF) and Noggin proteins followed by a resting period of 2 weeks to allow for differentiation and maturation. The mice also received intrapallidal injection of the retrograde tracer Alexa Fluor 555-conjugated Cholera Toxin subunit B (CT-B). Newly-generated neurons expressing the enhanced yellow fluorescent protein also expressed the mature neuronal marker, Neuronal Nuclei (NeuN), and integrated into the striato-pallidal circuitry as revealed by their incorporation of the fluorescent CT-B retrograde tracer. AdB/N = AdBDNF/AdNoggin. Scale bar = 10 μm
Induced Neuronal Addition as a Treatment for Huntington’s Disease
To assess the therapeutic value of induced striatal neurogenesis as a potential treatment for HD, Cho et al. [98] evaluated the effects of AdBDNF/Noggin on disease phenotype and progression in R6/2 HD mice. R6/2 mice express a 144 CAG repeat expansion within the first exon of the huntingtin gene, which yields a relatively severe disease phenotype. Untreated R6/2 mice develop rapidly progressive neurological impairment, and typically die between 13 and 15 weeks of age [140, 141]. As in the rat, AdBDNF and AdNoggin treatment induced significant neuronal recruitment in both R6/2 mice and their wild-type controls [98]. Similarly, the newly generated neurons expressed markers of medium spiny neurons, including DARPP-32 and GAD-67, extending fibers to the globus pallidus, and were either enkephalin+ or Substance P+, suggesting their contributions to both the indirect and direct motor outputs of the basal ganglia. Thus, newly-recruited neurons in the HD brain developed into mature MSNs, and correctly projected to their pallidal targets [98].
Importantly, the BDNF and Noggin-induced anatomic reconstitution of the striatopallidal projection was associated with a diminished rate of motor deterioration, as assessed by Rota-Rod and open field activity, as well as with an increased lifespan (Fig. 3a). Treatment with AdBDNF/AdNoggin, therefore, induced medium spiny neuronal addition in HD mice, which was associated with delayed motor deterioration and prolonged survival. Importantly, this result was the specific product of neuronal addition rather than BDNF-associated neuroprotection. Treatment with mitotic inhibitor cytosine-β-D-arabinofuranoside abolished AdBDNF/Noggin-associated improvement of both motor and lifespan, suggesting that induced neurogenesis was solely responsible for the beneficial effects of BDNF and Noggin delivery [98].
Of note, adenoviral-delivered BDNF has also proven sufficient to induce the addition of GAD67+/DARPP32+ medium spiny neurons to the striata of nonhuman primates [142], although further work needs to be done to establish whether Noggin can potentiate this effect, and how quantitatively robust this process may be as a function of BDNF dose. Despite the potential of BDNF and noggin administration to regenerate new medium spiny neurons in the diseased R6/2 neostriatum, any such replacement strategy will require the tonic production of new neurons during extended periods of time to compensate for the chronic disease-related loss of functional neurons, whether in a mouse model or an HD patient. Yet, despite their value in providing a needed proof-of-principle, the effects of adenoviral-delivered BDNF and Noggin were limited by the use of adenovirus as a viral vector. Adenovirus expresses transiently and drives significant transgene expression for only a few weeks [143, 144]. The vector is also immunogenic, which effectively limits its use to a single injection [145]. To address these limitations, we have recently assessed the usefulness of adeno-associated virus serotype 4 (AAV-4), an ependymotrophic vector that expresses in a sustained fashion, and is relatively nonimmunogenic. Indeed, AAV has been shown to support expression for as much as 15 months in the mouse brain [146], 19 months in the rat brain [147], and for more than 6 years in the primate brain [148]. On that basis, we used intraventricular injections of AAV-4, which selectively transduce the ependymal cells of the ventricular wall to deliver BDNF and Noggin to the ependymal layer, which lies in close proximity to the adjacent subependymal cell population. We found that AAV-4-driven BDNF and Noggin delivered into the lateral ventricle of wild-type rats yielded high-level expression of both BDNF and Noggin proteins in the ventricular cerebrospinal fluid, and that this was associated with a sustained and quantitatively robust neuronal addition to the neostriatum. This effect was tonic, yielding high-level protein expression for least 4 months after viral delivery. Most importantly, the sustained expression of BDNF and Noggin supported ongoing neuronal addition to the neostriatum with no evident exhaustion, suggesting that an AAV-4-based strategy of sustained BDNF and Noggin delivery might be an effective means of eliciting neuronal replacement in disorders of striatal degeneration, including not only Huntington’s disease, but conceivably other conditions of striatal neuronal loss as well [139] and (unpublished data).
Future Direction and Potential Limitations
Despite almost 2 decades of assessment of fetal tissue transplants in Huntington’s disease, the usefulness of intrastriatal grafts of human striatal cellsremains uncertain, whether of tissue, ESC, or iPS cell derivation. Indeed, in light of the contribution of corticostriatal afferents toward maintaining the viability of medium spiny neurons, it remains unclear just how much therapeutic benefit may be achieved by a simple 1-time addition of new neurons to the diseased striatal neuronal pool. Nonetheless, hope persists that avenues toward more sustained medium spiny neuronal replacement may prove of benefit to HD patients. In that regard, besides transplant-based strategies, we may now consider the potential of gene therapeutic strategies for eliciting medium spiny neuronal addition from resident neural stem and progenitor cells.
Although the effectiveness of this strategy has been demonstrated in rodent models of HD, analogous studies in nonhuman primates are now required to assess the adult primate’s capacity for such sustained subependymal neuronal production and striatal recruitment. Nonetheless, should such studies prove successful, then this strategy of induced mobilization and phenotypically restricted neuronal differentiation from resident neural stem and progenitor cells may prove a viable and effective therapeutic option in clinical HD. More broadly, these studies promise to define the usefulness of endogenous neural stem cells as vehicles for both neuronal replacement and circuit reconstruction in an adult neurodegenerative disorder.
Electronic supplementary material
electronic supplementary material
(PDF 510 kb)
Acknowledgments
This work (as discussed in the Goldman and Benraiss laboratories) was supported by the National Institute of Neurological Disorders and Stroke (NINDS) R01NS52534 by CHDI, and by the New York State Stem Cell Research Program (NYSTEM).
Full conflict of interest disclosures is available in the electronic supplementary material for this article.
Contributor Information
Abdellatif Benraiss, Email: Abdellatif_Benraiss@URMC.Rochester.edu.
Steven A. Goldman, Email: Steven_Goldman@URMC.Rochester.edu
References
- 1.Peschanski M, Cesaro P, Hantraye P. Rationale for intrastriatal grafting of striatal neuroblasts in patients with Huntington's disease. Neuroscience. 1995;68:273–285. doi: 10.1016/0306-4522(95)00162-C. [DOI] [PubMed] [Google Scholar]
- 2.Traficante A, Riozzi B, Cannella M, Rampello L, Squitieri F, Battaglia G. Reduced activity of cortico-striatal fibres in the R6/2 mouse model of Huntington's disease. Neuroreport. 2007;18:1997–2000. doi: 10.1097/WNR.0b013e3282f262ca. [DOI] [PubMed] [Google Scholar]
- 3.Gauthier LR, Charrin BC, Borrell-Pages M, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Kopyov OV, Jacques S, Lieberman A, Duma CM, Eagle KS. Safety of intrastriatal neurotransplantation for Huntington's disease patients. Exp Neurol. 1998;149:97–108. doi: 10.1006/exnr.1997.6685. [DOI] [PubMed] [Google Scholar]
- 5.Pundt LL, Kondoh T, Conrad JA, Low WC. Transplantation of human striatal tissue into a rodent model of Huntington's disease: phenotypic expression of transplanted neurons and host-to-graft innervation. Brain Res Bull. 1996;39:23–32. doi: 10.1016/0361-9230(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 6.Borlongan CV, Koutouzis TK, Poulos SG, Saporta S, Sanberg PR. Bilateral fetal striatal grafts in the 3-nitropropionic acid-induced hypoactive model of Huntington's disease. Cell Transplant. 1998;7:131–135. doi: 10.1016/S0963-6897(97)00170-X. [DOI] [PubMed] [Google Scholar]
- 7.Hurelbrink CB, Armstrong RJ, Dunnett SB, Rosser AE, Barker RA. Neural cells from primary human striatal xenografts migrate extensively in the adult rat CNS. Eur J Neurosci. 2002;15:1255–1266. doi: 10.1046/j.1460-9568.2002.01959.x. [DOI] [PubMed] [Google Scholar]
- 8.Wictorin K, Ouimet CC, Bjorklund A. Intrinsic Organization and connectivity of intrastriatal striatal transplants in rats as revealed by DARPP-32 immunohistochemistry: specificity of connections with the lesioned host brain. Eur J Neurosci. 1989;1:690–701. doi: 10.1111/j.1460-9568.1989.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 9.Palfi S, Conde F, Riche D, et al. Fetal striatal allografts reverse cognitive deficits in a primate model of Huntington disease. Nat Med. 1998;4:963–966. doi: 10.1038/nm0898-963. [DOI] [PubMed] [Google Scholar]
- 10.Bachoud-Levi AC, Remy P, Nguyen JP, et al. Motor and cognitive improvements in patients with Huntington's disease after neural transplantation. Lancet. 2000;356:1975–1979. doi: 10.1016/S0140-6736(00)03310-9. [DOI] [PubMed] [Google Scholar]
- 11.Gaura V, Bachoud-Levi AC, Ribeiro MJ, et al. Striatal neural grafting improves cortical metabolism in Huntington's disease patients. Brain 2004;127(part 1):65–72. [DOI] [PubMed]
- 12.Bachoud-Levi AC, Gaura V, Brugieres P, et al. Effect of fetal neural transplants in patients with Huntington's disease 6 years after surgery: a long-term follow-up study. Lancet Neurol. 2006;5:303–309. doi: 10.1016/S1474-4422(06)70381-7. [DOI] [PubMed] [Google Scholar]
- 13.Hauser RA, Furtado S, Cimino CR, et al. Bilateral human fetal striatal transplantation in Huntington's disease. Neurology. 2002;58:687–695. doi: 10.1212/wnl.58.5.687. [DOI] [PubMed] [Google Scholar]
- 14.Furtado S, Sossi V, Hauser RA, et al. Positron emission tomography after fetal transplantation in Huntington's disease. Ann Neurol. 2005;58:331–337. doi: 10.1002/ana.20564. [DOI] [PubMed] [Google Scholar]
- 15.Cicchetti F, Soulet D, Freeman TB. Neuronal degeneration in striatal transplants and Huntington's disease: potential mechanisms and clinical implications. Brain 2011;134(part 3):641–652. [DOI] [PubMed]
- 16.Rosser AE, Barker RA, Harrower T, et al. Unilateral transplantation of human primary fetal tissue in four patients with Huntington's disease: NEST-UK safety report ISRCTN no 36485475. J Neurol Neurosurg Psychiatry. 2002;73:678–685. doi: 10.1136/jnnp.73.6.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunnett SB, Rosser AE. Cell transplantation for Huntington's disease Should we continue? Brain Res Bull. 2007;72:132–147. doi: 10.1016/j.brainresbull.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Reuter I, Tai YF, Pavese N, et al. Long-term clinical and positron emission tomography outcome of fetal striatal transplantation in Huntington's disease. J Neurol Neurosurg Psychiatry. 2008;79:948–951. doi: 10.1136/jnnp.2007.142380. [DOI] [PubMed] [Google Scholar]
- 19.Capetian P, Knoth R, Maciaczyk J, et al. Histological findings on fetal striatal grafts in a Huntington's disease patient early after transplantation. Neuroscience. 2009;160:661–675. doi: 10.1016/j.neuroscience.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 20.Gallina P, Paganini M, Lombardini L, et al. Human striatal neuroblasts develop and build a striatal-like structure into the brain of Huntington's disease patients after transplantation. Exp Neurol. 2010;222:30–41. doi: 10.1016/j.expneurol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci Res. 1997;27:1–8. doi: 10.1016/S0168-0102(96)01134-0. [DOI] [PubMed] [Google Scholar]
- 22.Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649:159–173. doi: 10.1016/0006-8993(94)91060-X. [DOI] [PubMed] [Google Scholar]
- 23.Krystkowiak P, Gaura V, Labalette M, et al. Alloimmunisation to donor antigens and immune rejection following foetal neural grafts to the brain in patients with Huntington's disease. PLoS One. 2007;2:e166. doi: 10.1371/journal.pone.0000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pakzaban P, Deacon TW, Burns LH, Isacson O. Increased proportion of acetylcholinesterase-rich zones and improved morphological integration in host striatum of fetal grafts derived from the lateral but not the medial ganglionic eminence. Exp Brain Res. 1993;97:13–22. doi: 10.1007/BF00228813. [DOI] [PubMed] [Google Scholar]
- 25.Watts C, Brasted PJ, Dunnett SB. Embryonic donor age and dissection influences striatal graft development and functional integration in a rodent model of Huntington's disease. Exp Neurol. 2000;163:85–97. doi: 10.1006/exnr.1999.7341. [DOI] [PubMed] [Google Scholar]
- 26.Cicchetti F, Saporta S, Hauser RA, et al. Neural transplants in patients with Huntington's disease undergo disease-like neuronal degeneration. Proc Natl Acad Sci U S A. 2009;106:12483–12488. doi: 10.1073/pnas.0904239106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broadwell RD, Charlton HM, Ebert P, Hickey WF, Villegas JC, Wolf AL. Angiogenesis and the blood-brain barrier in solid and dissociated cell grafts within the CNS. Prog Brain Res. 1990;82:95–101. doi: 10.1016/S0079-6123(08)62595-9. [DOI] [PubMed] [Google Scholar]
- 28.Kirik D, Bjorklund A. Histological analysis of fetal dopamine cell suspension grafts in two patients with Parkinson's disease gives promising results. Brain 2005;128(part 7):1478–1479. [DOI] [PubMed]
- 29.Watts C, Brasted PJ, Dunnett SB. The morphology, integration, and functional efficacy of striatal grafts differ between cell suspensions and tissue pieces. Cell Transplant. 2000;9:395–407. doi: 10.1177/096368970000900310. [DOI] [PubMed] [Google Scholar]
- 30.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 31.Isacson O, Deacon TW, Pakzaban P, Galpern WR, Dinsmore J, Burns LH. Transplanted xenogeneic neural cells in neurodegenerative disease models exhibit remarkable axonal target specificity and distinct growth patterns of glial and axonal fibres. Nat Med. 1995;1:1189–1194. doi: 10.1038/nm1195-1189. [DOI] [PubMed] [Google Scholar]
- 32.Mendez I, Sanchez-Pernaute R, Cooper O, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain 2005;128(part 7):1498–1510. [DOI] [PMC free article] [PubMed]
- 33.Baker-Cairns BJ, Sloan DJ, Broadwell RD, Puklavec M, Charlton HM. Contributions of donor and host blood vessels in CNS allografts. Exp Neurol. 1996;142:36–46. doi: 10.1006/exnr.1996.0177. [DOI] [PubMed] [Google Scholar]
- 34.Nakao N, Grasbon-Frodl EM, Widner H, Brundin P. DARPP-32-rich zones in grafts of lateral ganglionic eminence govern the extent of functional recovery in skilled paw reaching in an animal model of Huntington's disease. Neuroscience. 1996;74:959–970. doi: 10.1016/0306-4522(96)00238-2. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Roy NS, Benraiss A, Goldman SA. Promoter-based isolation and fluorescence-activated sorting of mitotic neuronal progenitor cells from the adult mammalian ependymal/subependymal zone. Dev Neurosci. 2000;22:167–176. doi: 10.1159/000017437. [DOI] [PubMed] [Google Scholar]
- 36.Keyoung HM, Roy NS, Benraiss A, et al. High-yield selection and extraction of two promoter-defined phenotypes of neural stem cells from the fetal human brain. Nat Biotechnol. 2001;19:843–850. doi: 10.1038/nbt0901-843. [DOI] [PubMed] [Google Scholar]
- 37.Wang S, Chandler-Militello D, Lu G, et al. Prospective identification, isolation, and profiling of a telomerase-expressing subpopulation of human neural stem cells, using sox2 enhancer-directed fluorescence-activated cell sorting. J Neurosci. 2010;30:14635–14648. doi: 10.1523/JNEUROSCI.1729-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Windrem MS, Nunes MC, Rashbaum WK, et al. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- 39.Windrem MS, Roy NS, Wang J, et al. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 2002;69:966–975. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- 40.Aubry L, Bugi A, Lefort N, Rousseau F, Peschanski M, Perrier AL. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc Natl Acad Sci USA. 2008;105:16707–16712. doi: 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 42.Bjorklund LM, Sanchez-Pernaute R, Chung S, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barberi T, Klivenyi P, Calingasan NY, et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- 44.Erdo F, Buhrle C, Blunk J. et al Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:780–785. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- 45.Brederlau A, Correia AS, Anisimov SV, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- 46.Ko JY, Lee HS, Park CH, Koh HC, Lee YS, Lee SH. Conditions for tumor-free and dopamine neuron-enriched grafts after transplanting human ES cell-derived neural precursor cells. Mol Ther. 2009;17:1761–1770. doi: 10.1038/mt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Hur T, Idelson M, Khaner H, et al. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22:1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez-Pernaute R, Studer L, Ferrari D, et al. Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. Stem Cells. 2005;23:914–922. doi: 10.1634/stemcells.2004-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Draper JS, Pigott C, Thomson JA, Andrews PW. Surface antigens of human embryonic stem cells: changes upon differentiation in culture. J Anat 2002;200(part 3):249–258. [DOI] [PMC free article] [PubMed]
- 50.Drukker M, Katz G, Urbach A, et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drukker M, Benvenisty N. The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol. 2004;22:136–141. doi: 10.1016/j.tibtech.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Ideguchi M, Shinoyama M, Gomi M, Hayashi H, Hashimoto N, Takahashi J. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell-derived neural precursor cells. J Neurosci Res. 2008;86:1936–1943. doi: 10.1002/jnr.21652. [DOI] [PubMed] [Google Scholar]
- 53.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 55.Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Germain N, Banda E, Grabel L. Embryonic stem cell neurogenesis and neural specification. J Cell Biochem. 2010;111:535–542. doi: 10.1002/jcb.22747. [DOI] [PubMed] [Google Scholar]
- 57.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature 2011;474:212–215. [DOI] [PubMed]
- 58.Beyene R, Boockvar JA. Disease-specific induced pluripotent stem cells. Neurosurgery. 2008;63:12. doi: 10.1227/01.NEU.0000313629.07947.66. [DOI] [PubMed] [Google Scholar]
- 59.Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang N, An MC, Montoro D, Ellerby LM. Characterization of Human huntington's disease cell model from induced pluripotent stem cells. PLoS Curr 2010;2:RRN1193. [DOI] [PMC free article] [PubMed]
- 61.Ebert AD, Svendsen CN. Stem cell model of spinal muscular atrophy. Arch Neurol 2010;67:665–669. [DOI] [PMC free article] [PubMed]
- 62.Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hargus G, Cooper O, Deleidi M, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci U S A. 2010;107:15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hussein SM, Batada NN, Vuoristo S, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 65.Gore A, Li Z, Fung HL, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lister R, Pelizzola M, Kida YS, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-U. [DOI] [PubMed] [Google Scholar]
- 68.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 69.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 70.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doetsch F. Garcia Verdugo JM, Alvarez Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain 1. J Neurosci. 1997;17:5046. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol. 1998;36:234–248. doi: 10.1002/(SICI)1097-4695(199808)36:2<234::AID-NEU10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 73.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/S0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 74.Doetsch F. The glial identity of neural stem cells. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 75.Goldman S. Glia as neural progenitor cells. Trends in Neurosci. 2003;26:590–596. doi: 10.1016/j.tins.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 76.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-D. [DOI] [PubMed] [Google Scholar]
- 78.Whitman MC, Greer CA. Adult neurogenesis and the olfactory system. Prog Neurobiol. 2009;89:162–175. doi: 10.1016/j.pneurobio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abrous DN, Koehl M, Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 80.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 81.Kato T, Yokouchi K, Fukushima N, Kawagishi K, Li Z, Moriizumi T. Continual replacement of newly-generated olfactory neurons in adult rats. Neurdosci Lett. 2001;307:17–20. doi: 10.1016/S0304-3940(01)01914-0. [DOI] [PubMed] [Google Scholar]
- 82.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 83.Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- 84.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seroogy KB, Gall CM, Lee DC, Kornblum HI. Proliferative zones of postnatal rat brain express epidermal growth factor receptor mRNA. Brain Res. 1995;670:157–164. doi: 10.1016/0006-8993(94)01300-7. [DOI] [PubMed] [Google Scholar]
- 87.Gritti A, Frolichsthal-Schoeller P, Galli R, et al. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J Neurosci. 1999;19:3287–3297. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kornblum HI, Hussain RJ, Bronstein JM, Gall CM, Lee DC, Seroogy KB. Prenatal ontogeny of the epidermal growth factor receptor and its ligand, transforming growth factor alpha, in the rat brain. J Comp Neurol. 1997;380:243–261. doi: 10.1002/(SICI)1096-9861(19970407)380:2<243::AID-CNE7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 89.Tropepe V, Craig CG, Morshead CM, Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci. 2004;26:181–196. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]
- 91.Jin K, Sun Y, Xie L, et al. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 92.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/S0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 93.Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tao Y, Black IB, DiCicco-Bloom E. In vivo neurogenesis is inhibited by neutralizing antibodies to basic fibroblast growth factor. J Neurobiol. 1997;33:289–296. doi: 10.1002/(SICI)1097-4695(199709)33:3<289::AID-NEU7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 95.Tao Y, Black IB, DiCicco-Bloom E. Neurogenesis in neonatal rat brain is regulated by peripheral injection of basic fibroblast growth factor (bFGF) J Comp Neurol. 1996;376:653–663. doi: 10.1002/(SICI)1096-9861(19961223)376:4<653::AID-CNE11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 96.Jin K, LaFevre-Bernt M, Sun Y, et al. FGF2 promotes neurogenesis and neuroprotection in a transgenic mouse model of Huntington's disease. Proc Natl Acad Sci U S A. 2005;102:18189–18194. doi: 10.1073/pnas.0506375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Batista CM, Kippin TE, Willaime-Morawek S, Shimabukuro MK, Akamatsu W, Kooy D. A progressive and cell non-autonomous increase in striatal neural stem cells in the Huntington's disease R6/2 mouse. J Neurosci. 2006;26:10452–10460. doi: 10.1523/JNEUROSCI.2850-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cho SR, Benraiss A, Chmielnicki E, Samdani A, Economides A, Goldman SA. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J Clin Invest. 2007;117:2889–2902. doi: 10.1172/JCI31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glass M, Dragunow M, Faull RL. The pattern of neurodegeneration in Huntington's disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington's disease. Neuroscience. 2000;97:505–519. doi: 10.1016/S0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 100.Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- 101.Pastrana E, Moreno-Flores MT, Avila J, Wandosell F, Minichiello L, Diaz-Nido J. BDNF production by olfactory ensheathing cells contributes to axonal regeneration of cultured adult CNS neurons. Neurochem Int. 2007;50:491–498. doi: 10.1016/j.neuint.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 102.Donovan MH, Yamaguchi M, Eisch AJ. Dynamic expression of TrkB receptor protein on proliferating and maturing cells in the adult mouse dentate gyrus. Hippocampus. 2008;18:435–439. doi: 10.1002/hipo.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mizuno K, Carnahan J, Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol. 1994;165:243–256. doi: 10.1006/dbio.1994.1250. [DOI] [PubMed] [Google Scholar]
- 104.Ivkovic S, Ehrlich ME. Expression of the striatal DARPP-32/ARPP-21 phenotype in GABAergic neurons requires neurotrophins in vivo and in vitro. J Neurosci. 1999;19:5409–5419. doi: 10.1523/JNEUROSCI.19-13-05409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ivkovic S, Polonskaia O, Farinas I, Ehrlich ME. Brain-derived neurotrophic factor regulates maturation of the DARPP-32 phenotype in striatal medium spiny neurons: studies in vivo and in vitro. Neuroscience. 1997;79:509–516. doi: 10.1016/S0306-4522(96)00684-7. [DOI] [PubMed] [Google Scholar]
- 106.Ventimiglia R, Mather PE, Jones BE, Lindsay RM. The neurotrophins BDNF, NT-3 and NT-4/5 promote survival and morphological and biochemical differentiation of striatal neurons in vitro. Eur J Neurosci. 1995;7:213–222. doi: 10.1111/j.1460-9568.1995.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 107.Nakao N, Brundin P, Funa K, Lindvall O, Odin P. Trophic and protective actions of brain-derived neurotrophic factor on striatal DARPP-32-containing neurons in vitro. Brain Res Dev Brain Res. 1995;90:92–101. doi: 10.1016/0165-3806(96)83489-4. [DOI] [PubMed] [Google Scholar]
- 108.Klein R, Conway D, Parada LF, Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61:647–656. doi: 10.1016/0092-8674(90)90476-U. [DOI] [PubMed] [Google Scholar]
- 109.Middlemas DS, Lindberg RA, Hunter T. TrkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11:143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma B, Culver BP, Baj G, Tongiorgi E, Chao MV, Tanese N. Localization of BDNF mRNA with the Huntington's disease protein in rat brain. Mol Neurodegener 2010;5:22. [DOI] [PMC free article] [PubMed]
- 111.Zuccato C, Ciammola A, Rigamonti D, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- 112.Altar CA, Cai N, Bliven T, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 113.Colin E, Zala D, Liot G, et al. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Strand AD, Baquet ZC, Aragaki AK, et al. Expression profiling of Huntington's disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J Neurosci. 2007;27:11758–11768. doi: 10.1523/JNEUROSCI.2461-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu CL, Hwang CS, Chen SD, Yin JH, Yang DI. Neuroprotective mechanisms of brain-derived neurotrophic factor against 3-nitropropionic acid toxicity: therapeutic implications for Huntington's disease. Ann N Y Acad Sci 1201:8–12. [DOI] [PubMed]
- 116.Kells AP, Fong DM, Dragunow M, During MJ, Young D, Connor B. AAV-mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol Ther. 2004;9:682–688. doi: 10.1016/j.ymthe.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 117.Perez-Navarro E, Gavalda N, Gratacos E, Alberch J. Brain-derived neurotrophic factor prevents changes in Bcl-2 family members and caspase-3 activation induced by excitotoxicity in the striatum. J Neurochem. 2005;92:678–691. doi: 10.1111/j.1471-4159.2004.02904.x. [DOI] [PubMed] [Google Scholar]
- 118.Gratacos E, Perez-Navarro E, Tolosa E, Arenas E, Alberch J. Neuroprotection of striatal neurons against kainate excitotoxicity by neurotrophins and GDNF family members. J Neurochem. 2001;78:1287–1296. doi: 10.1046/j.1471-4159.2001.00538.x. [DOI] [PubMed] [Google Scholar]
- 119.Katoh-Semba R, Asano T, Ueda H, et al. Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. FASEB J. 2002;16:1328–1330. doi: 10.1096/fj.02-0143fje. [DOI] [PubMed] [Google Scholar]
- 120.Borrell-Pages M, Canals JM, Cordelieres FP, et al. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J Clin Invest. 2006;116:1410–1424. doi: 10.1172/JCI27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meisner F, Scheller C, Kneitz S, et al. Memantine upregulates BDNF and prevents dopamine deficits in SIV-infected macaques: a novel pharmacological action of memantine. Neuropsychopharmacology. 2008;33:2228–2236. doi: 10.1038/sj.npp.1301615. [DOI] [PubMed] [Google Scholar]
- 122.Simmons DA, Rex CS, Palmer L, et al. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice. Proc Natl Acad Sci U S A. 2009;106:4906–4911. doi: 10.1073/pnas.0811228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Apostol BL, Simmons DA, Zuccato C, et al. CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice. Mol Cell Neurosci. 2008;39:8–20. doi: 10.1016/j.mcn.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 124.Conforti P, Ramos C, Apostol BL, et al. Blood level of brain-derived neurotrophic factor mRNA is progressively reduced in rodent models of Huntington's disease: restoration by the neuroprotective compound CEP-1347. Mol Cell Neurosci. 2008;39:1–7. doi: 10.1016/j.mcn.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 125.Kirschenbaum B, Goldman SA. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc Natl Acad Sci. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]
- 127.Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reumers V, Deroose CM, Krylyshkina O, et al. Noninvasive and quantitative monitoring of adult neuronal stem cell migration in mouse brain using bioluminescence imaging. Stem Cells. 2008;26:2382–2390. doi: 10.1634/stemcells.2007-1062. [DOI] [PubMed] [Google Scholar]
- 129.Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mehler MF, Mabie PC, Zhu G, Gokhan S, Kessler JA. Developmental changes in progenitor cell responsiveness to bone morphogenetic proteins differentially modulate progressive CNS lineage fate. Dev Neurosci. 2000;22:74–85. doi: 10.1159/000017429. [DOI] [PubMed] [Google Scholar]
- 131.Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/S0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- 132.Lim D, Tramontin A, Trevejo J, Herrera D, Garcia-Verdugo J, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/S0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 133.Valenzuela DM, Economides AN, Rojas E, et al. Identification of mammalian noggin and its expression in the adult nervous system. J Neurosci. 1995;15:6077–6084. doi: 10.1523/JNEUROSCI.15-09-06077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith WC, Harland RM. Expression cloning of noggin a new dorsalizing factor localized to the spemann organizer in xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- 135.Mabie PC, Mehler MF, Kessler JA. Multiple roles of bone morphogenetic protein signaling in the regulation of cortical cell number and phenotype. J Neurosci. 1999;19:7077–7088. doi: 10.1523/JNEUROSCI.19-16-07077.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Developmental Biology. 2003;255:164–177. doi: 10.1016/S0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 137.Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. Journal of Neuroscience. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chevigny A, Cooper O, Vinuela A, et al. Fate mapping and lineage analyses demonstrate the production of a large number of striatal neuroblasts after transforming growth factor alpha and noggin striatal infusions into the dopamine-depleted striatum. Stem Cells. 2008;26:2349–2360. doi: 10.1634/stemcells.2008-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Benraiss A, Bruel-Jungerman E, Lu G, Economides EN, Davidson B, Goldman SA. Sustained induction of neuronal addition to the adult rat neostriatum by AAV4-delivered noggin and BDNF. Gene Ther (in press). [DOI] [PMC free article] [PubMed]
- 140.Mangiarini L, Sathasivam K, Seller M, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/S0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 141.Li JY, Popovic N, Brundin P. The use of the R6 transgenic mouse models of Huntington's disease in attempts to develop novel therapeutic strategies. NeuroRx. 2005;2:447–464. doi: 10.1602/neurorx.2.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bedard A, Gravel C, Parent A. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp Brain Res. 2006;170:501–512. doi: 10.1007/s00221-005-0233-5. [DOI] [PubMed] [Google Scholar]
- 143.Lee SW, Trapnell BC, Rade JJ, Virmani R, Dichek DA. In vivo adenoviral vector-mediated gene transfer into balloon-injured rat carotid arteries. Circ Res. 1993;73:797–807. doi: 10.1161/01.res.73.5.797. [DOI] [PubMed] [Google Scholar]
- 144.Kozarsky KF, McKinley DR, Austin LL, Raper SE, Stratford-Perricaudet LD, Wilson JM. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J Biol Chem. 1994;269:13695–13702. [PubMed] [Google Scholar]
- 145.Dai Y, Schwarz EM, Gu D, Zhang WW, Sarvetnick N, Verma IM. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci U S A. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sevin C, Benraiss A, Dam D, et al. Intracerebral adeno-associated virus-mediated gene transfer in rapidly progressive forms of metachromatic leukodystrophy. Hum Mol Genet. 2006;15:53–64. doi: 10.1093/hmg/ddi425. [DOI] [PubMed] [Google Scholar]
- 147.Peel AL, Klein RL. Adeno-associated virus vectors: activity and applications in the CNS. J Neurosci Methods. 2000;98:95–104. doi: 10.1016/S0165-0270(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 148.Bankiewicz KS, Forsayeth J, Eberling JL, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 149.Imayoshi I, Sakamoto M, Ohtsuka T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)