Review on the role of leukocyte immunoglobulin-like receptors (LILR) in HIV-1 disease pathogenesis.
Keywords: regulatory cell surface molecules, innate cell-mediated immunity, dendritic cell, immunodeficiency, retroviral/AIDS
Abstract
LILRs represent a group of immunomodulatory molecules that regulate the functional properties of professional APCs and influence immune activation in a variety of disease contexts. Many members of the LILR family recognize peptide/MHC class I complexes as their physiological ligands, and increasing evidence suggests that such interactions are prominently influenced by polymorphisms in HLA class I alleles or sequence variations in the presented antigenic peptides. Emerging data show that LILRs are involved in multiple, different aspects of HIV-1 disease pathogenesis and may critically influence spontaneous HIV-1 disease progression. Here, we review recent progress in understanding the role of LILR during HIV-1 infection by focusing on the dynamic interplay between LILR and HLA class I molecules in determining HIV-1 disease progression, the effects of HIV-1 mutational escape on LILR-mediated immune recognition, the contribution of LILR to HIV-1-associated immune dysfunction, and the unique expression patterns of LILR on circulating myeloid DCs from elite controllers, a small subset of HIV-1-infected patients with natural control of HIV-1 replication. Obtaining a more complete understanding of LILR-mediated immune regulation during HIV-1 infection may ultimately allow for improved strategies to treat or prevent HIV-1-associated disease manifestations.
Introduction
In the vast majority of individuals, infection with HIV-1 leads to progressive immune deficiency, which when left untreated, is almost always fatal. However, how fast the disease progresses and how rapidly this final stage is reached depend on a fine-tuned interplay between various immunomodulatory mechanisms and on specific immunogenetic characteristics [1, 2]. LILRs represent a relatively recently discovered group of immunoregulatory molecules, for which increasing evidence suggests an important function in determining HIV-1 disease outcomes. LILRs are encoded on chromosome 19 within the extended leukocyte receptor complex [3, 4] and are expressed predominantly on cells of the myeloid lineage, although some of these receptors are also observed on T cells, NK cells, and plasmacytoid DCs [5]. A total of 13 different LILRs has been characterized so far and is summarized in Table 1. Depending on associations with ITIM- or ITAM-dependent adaptor molecules, LILRs have been classified into five LILRBs (LILRB1–LILRB5) and six LILRAs (LILRA1–LILRA6); however, functional properties of LILR strongly depend on the cellular context and cannot be predicted based on ITIM or ITAM adaptor molecule use [15]. Most prominently, LILRs are critically involved in the regulation of professional APCs, such as monocytes or DCs, which play important roles for priming and fine-tuning antigen-specific immune responses and for regulating immune activation through cytokine secretion [16–19]. A subgroup of LILRs, in particular, LILRB1, LILRB2, LILRA1, LILRA2, and LILRA3, serves as receptors for HLA class I molecules [9, 16, 18, 20, 21], and emerging data suggest a previously unrecognized, complex LILR-HLA interplay that may be of functional significance for regulating immune activity and host defense against microbial pathogens in a variety of disease contexts. Here, we report recent progress in understanding the functional role of LILR and LILR-HLA interactions in HIV-1 disease pathogenesis.
Table 1. Summary of Currently Identified Members of the LILR Family.
| Group | Type | Receptor | Aliases (according to ref. [6]) | Ligand |
|---|---|---|---|---|
| Group 1 (>70% similarity to LILRB1/B2; conserved HLA class I binding sites) | ITIM adaptor molecules | LILRB1 | LIR-1, ILT2, MIR-7, CD85, LIR1, CD85j | HLA Class I, UL18 [7–11] |
| LILRB2 | LIR-2, ILT4, MIR-10, LIR2, CD85d, MIR10 | HLA Class I, UL18, CD1d [8, 9, 11, 12] | ||
| ITAM adaptor molecules | LILRA1 | LIR-6, CD85i, LIR6a | HLA-B27, HLA-C [9, 13] | |
| LILRA2 | LIR-7, ILT1, CD85h, LIR7 | Unknown | ||
| Soluble | LILRA3 | LIR-4, HM43, ILT6, LIR4, CD85e | HLA-C [9] | |
| Group 2 (<60% similarity to LILRB1/B2; unlikely to bind HLA class I) | ITIM adaptor molecules | LILRB3 | LIR-3, HL9, ILT5, LIR3, CD85a | Unknown |
| LILRB4 | LIR-5, ILT3, HM18, LIR5, CD85k | Unknown | ||
| LILRB5 | LIR-8, LIR8, CD85c | Unknown | ||
| ITAM adaptor molecules | LILRA4 | ILT7, CD85g | BST-2 [14] | |
| LILRA5 | ILT11, LIR9, CD85, CD85f | Unknown | ||
| LILRA6 | ILT8, CD85b | Unknown | ||
| pseudogenes | LILRP1 | ILT9, CD85l, LILRA6P | n/a | |
| LILRP2 | ILT10, CD85m | n/a |
INTERACTIONS BETWEEN HLA CLASS I ISOTYPES AND LILRs
A fundamental observation in HIV-1 infection is the close association between specific HLA class I alleles and HIV-1 disease outcomes; this has been documented in large, prospectively followed immunogenetic cohort investigations [22–25], as well as in genome-wide association studies [1, 2]. An influence of HLA class I alleles on HIV-1 disease outcomes has been most closely demonstrated for HLA-B*57 [26] and HLA-B*27 [27], which represent some of the best predictors of delayed HIV-1 disease progression and are highly enriched in elite controllers, a small group of HIV-1 patients with natural control of HIV-1 replication below detection levels of commercial PCR assays. In contrast, specific HLA-B*35 subtypes are associated with accelerated HIV-1 disease progression [28]. These effects on HIV-1 disease outcomes have, in part, been attributed to the antiviral effects of HIV-1-specific CD8 T cells, which are restricted by HLA class I molecules and may target more- or less-vulnerable segments of HIV-1, depending on the binding motif and epitope preferences of the presenting HLA class I isotype. Moreover, the influence of HLA class I alleles on HIV-1 disease outcomes may in part be related to interactions between HLA isotypes and killer cell Ig-like receptors, a group of immunomodulatory HLA class I receptors expressed predominantly on NK and T cells [29]. LILRs, in particular, LILRB1, LILRB2, LILRA1, LILRA2, and LILRA3, serve as an alternative group of HLA receptors and may influence functional properties of professional APCs following engagement by HLA class I molecules. Is it possible that allele-specific interactions between HLA class I molecules and LILR contribute to the effects of HLA class I on HIV-1 disease outcomes?
Increasing evidence from a number of recent studies suggests that this is indeed the case. Previous investigations have shown that LILRB1 and LILRB2 can recognize all HLA class I molecules, and from a structural perspective, the ability to interact with such a broad range of HLA class I molecules seems to be a result of the location of the LILR binding sites within HLA molecules. LILRB1 and LILRB2 seem to interact with the α3 domain of the HLA complex, which has relatively few allele-specific polymorphisms [7, 30]; additionally, LILRB1 has a binding site in the nonpolymorphic β2m domain [7]. These structural data may suggest that interactions between HLA class I isotypes and LILRs are static and independent of HLA sequence polymorphisms. However, emerging data from various studies indicate that this is not true and instead, suggest that the binding strengths between LILRB1 or LILRB2 and HLA class I molecules are influenced profoundly by the sequence of the HLA class I molecule. Most clearly, such an effect of HLA class I allele polymorphisms on recognition by LILR has been demonstrated in the setting of HLA-B*35 alleles. Prior studies have shown that based on amino acid preferences at position 9 in presented antigenic peptides, HLA-B*35 isotypes can be grouped into Px and PY alleles, and these different subclasses of HLA-B*35 molecules have opposing influences on HIV-1 disease outcomes: Although HLA-B*35 Px alleles represent one of the strongest predictors of accelerated HIV-1 disease progression, HLA-B*35 PY alleles have no detectable influence on spontaneous HIV-1 disease courses [28, 31]. This is true, despite the fact that HLA-B*35 Px and PY alleles differ in as few as 1 amino acid and can present identical viral T cell epitopes, which makes it unlikely that altered, HIV-1-specific CD8 T cell responses are responsible for the divergent effects of HLA-B*35 subgroups on HIV-1 disease outcomes [32]. Instead, a recent study has shown that in contrast to HLA-B*35 PY isotypes, HLA-B*35 Px molecules bind more strongly to the LILRB2 expressed on DCs [33]. This was confirmed by using flow cytometric detection of recombinant HLA-B*35 tetramer binding to DCs, as well as by cell- and label-free surface plasmon resonance technology for direct assessments of protein–protein interactions between recombinant LILRB2 and HLA-B*35 molecules. Interestingly, the preferential recognition of HLA-B*35 Px isotypes by LILRB2 translated into impaired antigen-presenting and cytokine-secretion characteristics of DCs following in vitro exposure to HLA-B*35 Px molecules and was associated with reduced ex vivo activity of DCs in HIV-1-infected carriers of HLA-B*35 Px alleles. The structural correlates of the alternative recognition of HLA-B*35 Px/PY isotypes by LILRB2 still remain unclear, but it is noteworthy that prior structural investigations of the LILRB1/2 interactions with HLA class I molecules were limited to the two most distal extracellular LILR domains [7]. This raises the possibility that the altered recognition of different HLA-B*35 Px/PY subtypes by LILRB2 may be related to as-of-yet undefined HLA binding sites within the two proximal extracellular domains of LILR (Fig. 1). Overall, these data suggest that HLA-B*35 Px subtypes accelerate HIV-1 disease progression by increasing the LILRB2-dependent, functional inhibition of DCs.
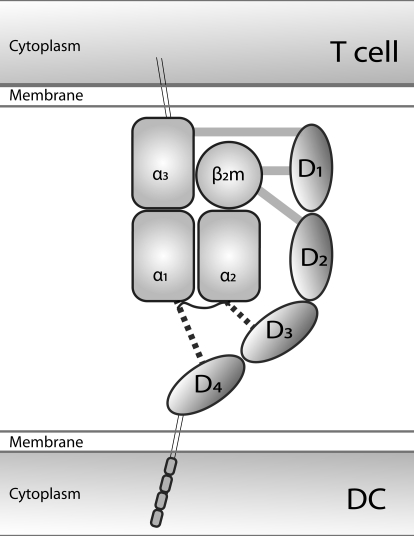
Figure 1. Schematic representation of the structural interactions between extracellular domains of LILRB1/2 (D1–D4) and HLA class I molecules.
LILR binding sites within the α3/β2m domains of the HLA complex interact with D1/D2 regions of LILR. In addition, as-of-yet unidentified binding sites may exist in the α1/α2 domains of the HLA class I complexes that recognize the D3/D4 regions of LILR. Such possible interactions between D3/D4 and α1/α2 domains may explain the peptide/allele-specific recognition of HLA class I molecules by LILR.
Specific interactions with LILRB2 might also be responsible for the beneficial effects of HLA-B*27 and HLA-B*57 on HIV-1 disease progression. Using a cell-free assay to assess binding intensities between a panel of HLA class I beads on the one side and recombinant LILRB1, LILRB2, LILRA1, and LILRA3 molecules on the opposite side, recent investigations showed a wide variability in binding strengths among HLA class I isotypes and LILRB2 [9]. Interestingly, among all HLA class I molecules, weakest binding to LILRB2 was observed for HLA-B*57 and HLA-B*27, the two HLA alleles that have consistently been associated with improved HIV-1 disease progression in a variety of prior investigations. Although functional assays investigating the significance of this finding are currently absent, these observations raise the possibility that beneficial effects of these alleles on HIV-1 disease outcomes may, in part, be related to reduced, LILRB2-dependent inhibition of DCs. Notably, reduced binding of HLA-B*57/B*27 to LILRB2 and ensuing reduction of inhibitory immunoregulatory impulses in DCs are also in line with the observation that HLA-B*57/B*27 alleles are frequently associated with allergic and/or autoimmune diseases [34].
In contrast to LILRB2, recent data suggest that LILRB1 binding to HLA class I molecules appears to be more homogenous, although some variability was observed between LILRB1 and HLA-A isotypes. In particular, amino acids at positions 193 and 194 in the HLA complex, which correspond to previously defined LILR binding sites, seemed to influence binding strength between LILRB1 and HLA-A isotypes [9]. Given the important role of LILRB1 for immunomodulation of DCs and other immune cells [35–37], it is possible that amino acid residues at these positions within HLA-A also influence HIV-1 disease outcomes, although this remains to be investigated further. In addition to amino acid residues at positions 193 and 194, binding of HLA class I isotypes to LILRB1 is influenced by intracytoplasmic cysteine residues, which are present in some but not all HLA class I allele sequences [38]. Using site-directed mutagenesis, previous work has shown that elimination of these cysteine residues abrogates LILRB1-mediated recognition of cell surface-bound HLA class I molecules, suggesting that these cysteine molecules have critical functions for maintaining a structural conformation of the HLA class I complex that is amendable to LILRB1 binding. More detailed studies will be necessary to determine whether the presence of cysteine residues in HLA class I isotypes can influence HIV-1 disease outcomes by facilitating interactions with LILRB1.
A recently identified genetic polymorphism that is strongly associated with delayed progression of HIV-1 infection is known as –35C and encodes for a cysteine residue within the HLA-C promoter region that leads to increased surface expression of HLA-C molecules on T cells [39], likely as a result of micro-RNA-dependent regulation of gene expression [40]. This higher expression of HLA-C molecules may translate into improved, HLA-C-restricted T cell responses against HIV-1; however, recent studies suggested that this genetic variation may also influence interactions between HLA-C and members of the LILR class [9]. In particular, it was shown that FHC complexes of HLA-C, encoded by –35C-linked alleles, had increased binding properties to LILRB2, LILRA1, and LILRA3, as opposed to alternative HLA-C alleles; this may possibly be a result of the dimerization of HLA-C molecules, which is facilitated by –35C-encoded cysteine residues. The identification of how –35C-encoded HLA-C isotypes may mediate immunomodulatory impulses through interactions with these LILRs remains to be determined in future investigations.
MUTATIONAL ESCAPE IN HIV-1 AFFECTS LILR-DEPENDENT IMMUNE RECOGNITION
The ability of HIV-1 to successfully escape from human immune defense mechanisms is to a large part related to its extraordinary genetic plasticity [41–45]. As a result of the error-prone reverse transcriptase, a large number of different viral quasispecies with specific sequence variations are generated each day. This can lead to the rapid selection of viral variants with amino acid substitutions that abrogate T cell-dependent immune recognition by inhibiting peptide binding to the restricting HLA class I molecules [46], blocking antigenic peptide processing [47], or directly altering interactions between peptide/MHC class I complexes and TCR contact residues [48]. As HLA class I receptors from the LILR family may not interact directly with the antigen-presenting domains of the MHC class I complex but instead, recognize binding sites within the α3 domain, it was assumed that interactions between LILR and HLA molecules are antigenic peptide-independent and not affected by viral escape mutations.
Increasing evidence, however, suggests that this is not the case. An important example demonstrating the effects of HIV-1 sequence alternations on immune recognition by HLA class I receptors from the LILR family was provided in the context of typical mutations occurring in the immunodominant, HLA-B*2705-restricted HIV-1 cytotoxic T cell epitope KK10 (gag) [49]. CD8 T cell-mediated immune pressure against this epitope leads to a typical mutation at position 6 (M instead of L), which abrogates T cell-mediated immune recognition and can be associated with higher viral loads and faster HIV-1 disease progression. Using recombinant HLA-B*27 tetramers refolded with the wild type epitopic peptide or the variant peptide, it was shown that the escape variant of this epitope substantially enhanced binding interactions to LILRB2 expressed on DCs and monocytes. Notably, a stronger binding affinity between HLA-B*2705 tetramers presenting the variant peptide and LILRB2 was also observed when assays were performed, using label-free surface plasmon resonance technology in a cell-free system; this suggested that the sequence of the antigenic peptide may indeed influence the binding intensity of HLA class I complexes to LILRB2. Interestingly, these data also showed that the altered recognition of the variant peptide was functionally relevant for inhibiting the antigen-presenting properties of DCs. Subsequent studies demonstrated that peptide- and peptide variant-specific interactions between LILRB2 and HLA molecules also occur in the context of a variety of different HLA molecules and at least three additional, CTL-associated mutations in epitopes presented by HLA-A*11, HLA-B*08, or HLA-B*07 were identified, which led to enhanced binding of the presenting HLA class I complexes to LILRB2 [50]. Overall, these data strongly suggest that HIV-1 sequence variations can critically influence interactions between peptide/MHC class I complexes and LILRB2 and that CTL-driven mutational escape in HIV-1 can contribute to dysfunction of DCs by increasing LILRB2-dependent impulses.
The molecular and structural mechanisms that underlie a peptide-specific recognition of HLA class I molecules by LILR remain unclear at this point. One possible explanation is that amino acid substitutions in the presenting epitope may indirectly alter the conformational structure of LILRB2 binding sites located with the α3 domain and/or the β2m molecule. Such an indirect conformational change has indeed been postulated to explain prior findings showing that polymorphisms in the peptide-presenting domains of the CMV-encoded HLA homologue UL18 can significantly influence binding interactions with LILRB1 [51]. Alternatively, antigenic peptide-specific interactions between HLA class I molecules and LILR may involve additional, as-of-yet undefined LILR binding sites within the antigenic peptide-presenting α1/α2 helices of HLA class I molecules (Fig. 1). In support of such a view, a recent study found that HC10, a HLA class I-specific mAb, which exclusively recognizes binding sites within the antigen-peptide presenting α1 domain, can block interactions between LILRB2 and FHC HLA class I molecules [9]. Interestingly, evidence for a peptide-specific interaction of LILR with its ligands was also found in a recent study investigating the binding of LILRB2 to CD1d, a nonpolymorphic HLA class I-like molecule, which is expressed on monocytes and DCs and presents lipid antigens to NKT cells. These studies demonstrated that native CD1d tetramers (which may present endogenous lipids) strongly bound to LILRB2 on human peripheral blood monocytes, whereas CD1d tetramers, loaded with synthetic α-galactosylceramide lipids, did not [12]. Mechanistic studies demonstrated that the LILRB2 binding sites within CD1d cluster in its antigen-presenting α1/α2 domains and in this way, differ from currently recognized LILRB2 binding sites in classical HLA class I molecules.
IMMUNOREGULATORY EFFECTS OF LILR DURING HIV-1 INFECTION
The most obvious sign of HIV-1-associated immune deficiency is the numerical decline of CD4 T cells; however, infection with HIV-1 also leads to dysfunction of a vast array of other immunological cell groups, including B cells [52], NK cells [53], and NKT cells [54]. Possibly most importantly, HIV-1 causes a profound inhibition of the functional properties of myeloid and plasmcytoid DCs [55, 56]. DCs represent the most effective professional APCs and have critical roles for priming and fine-tuning antigen-specific effector cell responses; moreover, these cells exert immunoregulatory effects through the secretion of cytokines and chemokines. During progressive HIV-1 infection, these functional properties of DCs are progressively disturbed [57]; this DC dysfunction seems to start early during the disease process [58] and represents a sharp contrast to the superior antigen-presenting properties of myeloid DCs, which have been described in elite controllers, a subgroup of HIV-1-infected persons with spontaneous control of HIV-1 replication [59]. This suggests that functional characteristics of DCs are closely correlated to clinical outcomes of HIV-1 infection and may importantly influence the efficacy of host immune defense mechanisms against HIV-1. Molecular pathways that govern the functional activity of DCs in HIV-1 infection therefore represent an important area of investigation.
Increasing evidence suggests that LILRs play an important role in regulating DC activity during HIV-1 infection. For instance, the defective functional profile of DCs in persons with progressive HIV-1 infection is correlated with up-regulation of LILRB2 [60] and simultaneous down-regulation of LILRA1, a stimulatory receptor [59]. This specific LILR surface expression pattern on DCs from progressors closely resembles the tolerogenic profile of DCs, previously observed in the setting of organ transplantation [17], and seems to be, at least in part, related to increased serum concentrations of IL-10 and related immunosuppressive cytokines. Interestingly, the elevated expression of LILRB2 during HIV-1 infection is associated with increased levels of soluble HLA-G in the plasma [61]. HLA-G, a nonclassical HLA class Ib molecule, serves as one of the highest affinity ligands for LILRB2 [8, 62] and can profoundly reduce functional properties of DCs when triggering LILRB2 signaling [61]. The combination of elevated LILRB2 expression on DCs and increased soluble HLA-G in the plasma seems to result into a highly effective inhibition of DCs and contributes substantially to the dysfunctional antigen-presenting properties of DCs during progressive HIV-1 infection.
In contrast to HIV-1 patients with progressive disease, HIV-1 patients with spontaneous control of HIV-1 infection (termed “elite controllers”) were found to have a unique up-regulation of LILRB1 and LILRB3, which exceeded corresponding expression levels in HIV-1 progressors or HIV-1-uninfected control subjects [59]. Importantly, highly specific small interfering RNA-mediated down-regulation of these two receptors resulted in decreased antigen-presenting properties of DCs; moreover, inhibition of freshly isolated, circulating DCs with mAb against LILRB1 and LILRB3 also led to reduced stimulatory activities of these cells. Together, these findings suggest that the selective up-regulation of LILRB1 and LILRB3 mediates extraordinary antigen-presenting properties of myeloid DCs in elite controllers and in this way, may contribute to the effective HIV-1 immune control. Surprisingly, the stimulatory effects of LILRB1 and LILRB3 in DCs were observed, despite the fact that both of these receptors signal via ITIM adaptor proteins, which are typically associated with transmission of inhibitory signals. This suggests that in the specific context of myeloid DCs in elite controllers, these receptors can mediate activating signals and contribute to the increasing recognition that functional properties of LILRs depend on the cellular context and not necessarily on association with ITIM or ITAM adaptor molecules [63, 64]. The reason for the elevated expression of LILRB1 and LILRB3 in elite controllers remains unclear at present, but it is noteworthy that genes encoding for LILRB3 [65] and to a much lesser degree, LILRB1 [66], are highly polymorphic, and the genetic diversity in these alleles may impact the surface expression of LILR molecules, as well as affect the functional properties of DCs. The investigation of the influence of LILR polymorphisms on HIV-1 disease outcomes represents an important goal in future studies.
CONCLUSION
Over the recent years, significant progress has been made in understanding the complex pattern of host-pathogen interactions that regulate immune defense against HIV-1 and determine spontaneous HIV-1 disease progression. Most of these studies have focused on classical T and B cell-mediated immune activity against HIV-1; however, it is now increasingly clear that functional characteristics of myeloid DCs are closely correlated to HIV-1 disease progression and may significantly influence the clinical outcomes of HIV-1 infection. LILRs have critical functions for governing the functional characteristics of DCs, and a growing number of studies suggest that the surface expression pattern of LILRs on DCs and specific interactions between LILRs and HLA class I subtypes have a profound impact on immune activity against HIV-1. Future explorations of novel ligands for LILR, of the genetic diversity of the LILR-HLA interplay, and of the signal transduction mechanisms used by LILRs to influence DC function may allow delineating a more comprehensive understanding of how LILRs and DCs influence immune defense against HIV-1.
ACKNOWLEDGMENTS
X.G.Y. and M.L. are recipients of the Clinical Scientist Development Award from the Doris Duke Charitable Foundation. X.G.Y. is supported by NIH grants AI078799 and AI089339, and M.L. is supported by NIH grant AI093203 and DDCF grant 2009034. The authors thank Dr. Arman Bashirova (NCI Frederick, Maryland, USA) and Mr. Jerome Rogich (Ragon Institute, Boston, MA, USA) for helping with the preparation of the included table and figure.
Footnotes
- FHC
- free heavy-chain
- LILR
- leukocyte Ig-like receptor
- LILRA
- leukocyte Ig-like receptor activating
- LILRB
- leukocyte Ig-like receptor inhibitory
REFERENCES
- 1. Pereyra F., Jia X., McLaren P. J., Telenti A., de Bakker P. I., Walker B. D., Ripke S., Brumme C. J., Pulit S. L., Carrington M., et al. (2010) The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330, 1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fellay J., Shianna K. V., Ge D., Colombo S., Ledergerber B., Weale M., Zhang K., Gumbs C., Castagna A., Cossarizza A., et al. (2007) A whole-genome association study of major determinants for host control of HIV-1. Science 317, 944–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Volz A., Wende H., Laun K., Ziegler A. (2001) Genesis of the ILT/LIR/MIR clusters within the human leukocyte receptor complex. Immunol. Rev. 181, 39–51 [DOI] [PubMed] [Google Scholar]
- 4. Barrow A. D., Trowsdale J. (2008) The extended human leukocyte receptor complex: diverse ways of modulating immune responses. Immunol. Rev. 224, 98–123 [DOI] [PubMed] [Google Scholar]
- 5. Brown D., Trowsdale J., Allen R. (2004) The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens 64, 215–225 [DOI] [PubMed] [Google Scholar]
- 6. Martin A. M., Kulski J. K., Witt C., Pontarotti P., Christiansen F. T. (2002) Leukocyte Ig-like receptor complex (LRC) in mice and men. Trends Immunol. 23, 81–88 [DOI] [PubMed] [Google Scholar]
- 7. Willcox B. E., Thomas L. M., Bjorkman P. J. (2003) Crystal structure of HLA-A2 bound to LIR-1, a host and viral major histocompatibility complex receptor. Nat. Immunol. 4, 913–919 [DOI] [PubMed] [Google Scholar]
- 8. Shiroishi M., Tsumoto K., Amano K., Shirakihara Y., Colonna M., Braud V. M., Allan D. S., Makadzange A., Rowland-Jones S., Willcox B., Jones E. Y., van der Merwe P. A., Kumagai I., Maenaka K. (2003) Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc. Natl. Acad. Sci. USA 100, 8856–8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones D. C., Kosmoliaptsis V., Apps R., Lapaque N., Smith I., Kono A., Chang C., Boyle L. H., Taylor C. J., Trowsdale J., Allen R. L. (2011) HLA class I allelic sequence and conformation regulate leukocyte Ig-like receptor binding. J. Immunol. 186, 2990–2997 [DOI] [PubMed] [Google Scholar]
- 10. Cosman D., Fanger N., Borges L., Kubin M., Chin W., Peterson L., Hsu M. L. (1997) A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity 7, 273–282 [DOI] [PubMed] [Google Scholar]
- 11. Chapman T. L., Heikema A. P., West A. P., Jr., Bjorkman P. J. (2000) Crystal structure and ligand binding properties of the D1D2 region of the inhibitory receptor LIR-1 (ILT2). Immunity 13, 727–736 [DOI] [PubMed] [Google Scholar]
- 12. Li D., Wang L., Yu L., Freundt E. C., Jin B., Screaton G. R., Xu X. N. (2009) Ig-like transcript 4 inhibits lipid antigen presentation through direct CD1d interaction. J. Immunol. 182, 1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allen R. L., Raine T., Haude A., Trowsdale J., Wilson M. J. (2001) Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J. Immunol. 167, 5543–5547 [DOI] [PubMed] [Google Scholar]
- 14. Cao W., Bover L., Cho M., Wen X., Hanabuchi S., Bao M., Rosen D. B., Wang Y. H., Shaw J. L., Du Q., Li C., Arai N., Yao Z., Lanier L. L., Liu Y. J. (2009) Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J. Exp. Med. 206, 1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson K. J., Allen R. L. (2009) Regulation of T-cell immunity by leucocyte immunoglobulin-like receptors: innate immune receptors for self on antigen-presenting cells. Immunology 127, 8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colonna M., Navarro F., Bellon T., Llano M., Garcia P., Samaridis J., Angman L., Cella M., Lopez-Botet M. (1997) A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 186, 1809–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang C. C., Ciubotariu R., Manavalan J. S., Yuan J., Colovai A. I., Piazza F., Lederman S., Colonna M., Cortesini R., Dalla-Favera R., Suciu-Foca N. (2002) Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat. Immunol. 3, 237–243 [DOI] [PubMed] [Google Scholar]
- 18. Colonna M., Samaridis J., Cella M., Angman L., Allen R. L., O′Callaghan C. A., Dunbar R., Ogg G. S., Cerundolo V., Rolink A. (1998) Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J. Immunol. 160, 3096–3100 [PubMed] [Google Scholar]
- 19. Nakajima H., Samaridis J., Angman L., Colonna M. (1999) Human myeloid cells express an activating ILT receptor (ILT1) that associates with Fc receptor γ-chain. J. Immunol. 162, 5–8 [PubMed] [Google Scholar]
- 20. Ryu M., Chen Y., Qi J., Liu J., Fan Z., Nam G., Shi Y., Cheng H., Gao G. F. (2011) LILRA3 binds both classical and non-classical HLA class I molecules but with reduced affinities compared to LILRB1/LILRB2: structural evidence. PLoS ONE 6, e19245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Colonna M., Nakajima H., Navarro F., Lopez-Botet M. (1999) A novel family of Ig-like receptors for HLA class I molecules that modulate function of lymphoid and myeloid cells. J. Leukoc. Biol. 66, 375–381 [DOI] [PubMed] [Google Scholar]
- 22. Carrington M., O′Brien S. J. (2003) The influence of HLA genotype on AIDS. Annu. Rev. Med. 54, 535–551 [DOI] [PubMed] [Google Scholar]
- 23. Kuniholm M. H., Gao X., Xue X., Kovacs A., Anastos K., Marti D., Greenblatt R. M., Cohen M. H., Minkoff H., Gange S. J., Fazzari M., Young M. A., Strickler H. D., Carrington M. (2011) Human leukocyte antigen genotype and risk of HIV disease progression before and after initiation of antiretroviral therapy. J. Virol. 85, 10826–10833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O′Brien S. J., Gao X., Carrington M. (2001) HLA and AIDS: a cautionary tale. Trends Mol. Med. 7, 379–381 [DOI] [PubMed] [Google Scholar]
- 25. Kaslow R. A., Carrington M., Apple R., Park L., Munoz A., Saah A. J., Goedert J. J., Winkler C., O′Brien S. J., Rinaldo C., Detels R., Blattner W., Phair J., Erlich H., Mann D. L. (1996) Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2, 405–411 [DOI] [PubMed] [Google Scholar]
- 26. Migueles S. A., Sabbaghian M. S., Shupert W. L., Bettinotti M. P., Marincola F. M., Martino L., Hallahan C. W., Selig S. M., Schwartz D., Sullivan J., Connors M. (2000) HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 97, 2709–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. den Uyl D., van der Horst-Bruinsma I. E., van Agtmael M. (2004) Progression of HIV to AIDS: a protective role for HLA-B27? AIDS Rev. 6, 89–96 [PubMed] [Google Scholar]
- 28. Gao X., Nelson G. W., Karacki P., Martin M. P., Phair J., Kaslow R., Goedert J. J., Buchbinder S., Hoots K., Vlahov D., O′Brien S. J., Carrington M. (2001) Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N. Engl. J. Med. 344, 1668–1675 [DOI] [PubMed] [Google Scholar]
- 29. Bashirova A. A., Thomas R., Carrington M. (2011) HLA/KIR restraint of HIV: surviving the fittest. Annu. Rev. Immunol. 29, 295–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shiroishi M., Kuroki K., Rasubala L., Tsumoto K., Kumagai I., Kurimoto E., Kato K., Kohda D., Maenaka K. (2006) Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d). Proc. Natl. Acad. Sci. USA 103, 16412–16417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao X., Bashirova A., Iversen A. K., Phair J., Goedert J. J., Buchbinder S., Hoots K., Vlahov D., Altfeld M., O′Brien S. J., Carrington M. (2005) AIDS restriction HLA allotypes target distinct intervals of HIV-1 pathogenesis. Nat. Med. 11, 1290–1292 [DOI] [PubMed] [Google Scholar]
- 32. Jin X., Gao X., Ramanathan M., Jr., Deschenes G. R., Nelson G. W., O′Brien S. J., Goedert J. J., Ho D. D., O′Brien T. R., Carrington M. (2002) Human immunodeficiency virus type 1 (HIV-1)-specific CD8+-T-cell responses for groups of HIV-1-infected individuals with different HLA-B*35 genotypes. J. Virol. 76, 12603–12610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang J., Goedert J. J., Sundberg E. J., Cung T. D., Burke P. S., Martin M. P., Preiss L., Lifson J., Lichterfeld M., Carrington M., Yu X. G. (2009) HLA-B*35-Px-mediated acceleration of HIV-1 infection by increased inhibitory immunoregulatory impulses. J. Exp. Med. 206, 2959–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thorsby E., Lie B. A. (2005) HLA associated genetic predisposition to autoimmune diseases: genes involved and possible mechanisms. Transpl. Immunol. 14, 175–182 [DOI] [PubMed] [Google Scholar]
- 35. Young N. T., Waller E. C., Patel R., Roghanian A., Austyn J. M., Trowsdale J. (2008) The inhibitory receptor LILRB1 modulates the differentiation and regulatory potential of human dendritic cells. Blood 111, 3090–3096 [DOI] [PubMed] [Google Scholar]
- 36. Saverino D., Fabbi M., Ghiotto F., Merlo A., Bruno S., Zarcone D., Tenca C., Tiso M., Santoro G., Anastasi G., Cosman D., Grossi C. E., Ciccone E. (2000) The CD85/LIR-1/ILT2 inhibitory receptor is expressed by all human T lymphocytes and down-regulates their functions. J. Immunol. 165, 3742–3755 [DOI] [PubMed] [Google Scholar]
- 37. Tenca C., Merlo A., Merck E., Bates E. E., Saverino D., Simone R., Zarcone D., Trinchieri G., Grossi C. E., Ciccone E. (2005) CD85j (leukocyte Ig-like receptor-1/Ig-like transcript 2) inhibits human osteoclast-associated receptor-mediated activation of human dendritic cells. J. Immunol. 174, 6757–6763 [DOI] [PubMed] [Google Scholar]
- 38. Gruda R., Achdout H., Stern-Ginossar N., Gazit R., Betser-Cohen G., Manaster I., Katz G., Gonen-Gross T., Tirosh B., Mandelboim O. (2007) Intracellular cysteine residues in the tail of MHC class I proteins are crucial for extracellular recognition by leukocyte Ig-like receptor 1. J. Immunol. 179, 3655–3661 [DOI] [PubMed] [Google Scholar]
- 39. Thomas R., Apps R., Qi Y., Gao X., Male V., O′HUigin C., O′Connor G., Ge D., Fellay J., Martin J. N., Margolick J., Goedert J. J., Buchbinder S., Kirk G. D., Martin M. P., Telenti A., Deeks S. G., Walker B. D., Goldstein D., McVicar D. W., Moffett A., Carrington M. (2009) HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nat. Genet. 41, 1290–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kulkarni S., Savan R., Qi Y., Gao X., Yuki Y., Bass S. E., Martin M. P., Hunt P., Deeks S. G., Telenti A., Pereyra F., Goldstein D., Wolinsky S., Walker B., Young H. A, Carrington M. (2011) Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature 472, 495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ganusov V. V., Goonetilleke N., Liu M. K., Ferrari G., Shaw G. M., McMichael A. J., Borrow P., Korber B. T., Perelson A. (2011) Fitness costs and diversity of CTL response determine the rate of CTL escape during the acute and chronic phases of HIV infection. J. Virol. 85, 10518–10528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goulder P. J., Walker B. D. (1999) The great escape—AIDS viruses and immune control. Nat. Med. 5, 1233–1235 [DOI] [PubMed] [Google Scholar]
- 43. Goulder P. J., Watkins D. I. (2004) HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4, 630–640 [DOI] [PubMed] [Google Scholar]
- 44. Ferrari G., Korber B., Goonetilleke N., Liu M. K., Turnbull E. L., Salazar-Gonzalez J. F., Hawkins N., Self S., Watson S., Betts M. R., et al. (2011) Relationship between functional profile of HIV-1 specific CD8 T cells and epitope variability with the selection of escape mutants in acute HIV-1 infection. PLoS Pathog. 7, e1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fischer W., Ganusov V. V., Giorgi E. E., Hraber P. T., Keele B. F., Leitner T., Han C. S., Gleasner C. D., Green L., Lo C. C., et al. (2010) Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS ONE 5, e12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barouch D. H., Kunstman J., Kuroda M. J., Schmitz J. E., Santra S., Peyerl F. W., Krivulka G. R., Beaudry K., Lifton M. A., Gorgone D. A., Montefiori D.C., Lewis M. G., Wolinsky S. M., Letvin N. L. (2002) Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415, 335–339 [DOI] [PubMed] [Google Scholar]
- 47. Draenert R., Le Gall S., Pfafferott K. J., Leslie A. J., Chetty P., Brander C., Holmes E. C., Chang S. C., Feeney M. E., Addo M. M., et al. (2004) Immune selection for altered antigen processing leads to cytotoxic T lymphocyte escape in chronic HIV-1 infection. J. Exp. Med. 199, 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones N. A., Wei X., Flower D. R., Wong M., Michor F., Saag M. S., Hahn B. H., Nowak M. A., Shaw G. M., Borrow P. (2004) Determinants of human immunodeficiency virus type 1 escape from the primary CD8+ cytotoxic T lymphocyte response. J. Exp. Med. 200, 1243–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lichterfeld M., Kavanagh D. G., Williams K. L., Moza B., Mui S. K., Miura T., Sivamurthy R., Allgaier R., Pereyra F., Trocha A., et al. (2007) A viral CTL escape mutation leading to immunoglobulin-like transcript 4-mediated functional inhibition of myelomonocytic cells. J. Exp. Med. 204, 2813–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang Y., Huang J., Toth I., Lichterfeld M., Yu X. G. (2010) Mutational escape in HIV-1 CTL epitopes leads to increased binding to inhibitory myelomonocytic MHC class I receptors. PLoS ONE 5, e15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cerboni C., Achour A., Warnmark A., Mousavi-Jazi M., Sandalova T., Hsu M. L., Cosman D., Karre K., Carbone E. (2006) Spontaneous mutations in the human CMV HLA class I homologue UL18 affect its binding to the inhibitory receptor LIR-1/ILT2/CD85j. Eur. J. Immunol. 36, 732–741 [DOI] [PubMed] [Google Scholar]
- 52. Moir S., Fauci A. S. (2008) Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J. Allergy Clin. Immunol. 122, 12–19, quiz 20–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hong H. S., Eberhard J. M., Keudel P., Bollmann B. A., Ballmaier M., Bhatnagar N., Zielinska-Skowronek M., Schmidt R. E., Meyer-Olson D. (2010) HIV infection is associated with a preferential decline in less-differentiated CD56dim CD16+ NK cells. J. Virol. 84, 1183–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sandberg J. K., Fast N. M., Palacios E. H., Fennelly G., Dobroszycki J., Palumbo P., Wiznia A., Grant R. M., Bhardwaj N., Rosenberg M. G., Nixon D. E. (2002) Selective loss of innate CD4(+) V α 24 natural killer T cells in human immunodeficiency virus infection. J. Virol. 76, 7528–7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Donaghy H., Gazzard B., Gotch F., Patterson S. (2003) Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 101, 4505–4511 [DOI] [PubMed] [Google Scholar]
- 56. Donaghy H., Pozniak A., Gazzard B., Qazi N., Gilmour J., Gotch F., Patterson S. (2001) Loss of blood CD11c(+) myeloid and CD11c(–) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 98, 2574–2576 [DOI] [PubMed] [Google Scholar]
- 57. Almeida M., Cordero M., Almeida J., Orfao A. (2005) Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS 19, 261–271 [PubMed] [Google Scholar]
- 58. Huang J., Yang Y., Al-Mozaini M., Burke P., Beamon J., Carrington M. F., Seiss K., Rychert J., Rosenberg E. S., Lichterfeld M., Yu X. G. (2011) Dendritic cell dysfunction during primary HIV-1 infection. J. Infect. Dis., Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang J., Burke P. S., Cung T. D., Pereyra F., Toth I., Walker B. D., Borges L., Lichterfeld M., Yu X. G. (2010) Leukocyte immunoglobulin-like receptors maintain unique antigen-presenting properties of circulating myeloid dendritic cells in HIV-1-infected elite controllers. J. Virol. 84, 9463–9471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vlad G., Piazza F., Colovai A., Cortesini R., Della Pietra F., Suciu-Foca N., Manavalan J. S. (2003) Interleukin-10 induces the upregulation of the inhibitory receptor ILT4 in monocytes from HIV positive individuals. Hum. Immunol. 64, 483–489 [DOI] [PubMed] [Google Scholar]
- 61. Huang J., Burke P., Yang Y., Seiss K., Beamon J., Cung T., Toth I., Pereyra F., Lichterfeld M., Yu X. G. (2010) Soluble HLA-G inhibits myeloid dendritic cell function in HIV-1 infection by interacting with leukocyte immunoglobulin-like receptor B2. J. Virol. 84, 10784–10791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Allan D. S., Colonna M., Lanier L. L., Churakova T. D., Abrams J. S., Ellis S. A., McMichael A. J., Braud V. M. (1999) Tetrameric complexes of human histocompatibility leukocyte antigen (HLA)-G bind to peripheral blood myelomonocytic cells. J. Exp. Med. 189, 1149–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pinheiro da Silva F., Aloulou M., Benhamou M., Monteiro R. C. (2008) Inhibitory ITAMs: a matter of life and death. Trends Immunol. 29, 366–373 [DOI] [PubMed] [Google Scholar]
- 64. Barrow A. D., Trowsdale J. (2006) You say ITAM and I say ITIM, let′s call the whole thing off: the ambiguity of immunoreceptor signalling. Eur. J. Immunol. 36, 1646–1653 [DOI] [PubMed] [Google Scholar]
- 65. Pfistershammer K., Lawitschka A., Klauser C., Leitner J., Weigl R., Heemskerk M. H., Pickl W. F., Majdic O., Bohmig G. A., Fischer G. F., Greinix H. T., Steinberger P. (2009) Allogeneic disparities in immunoglobulin-like transcript 5 induce potent antibody responses in hematopoietic stem cell transplant recipients. Blood 114, 2323–2332 [DOI] [PubMed] [Google Scholar]
- 66. Kuroki K., Tsuchiya N., Shiroishi M., Rasubala L., Yamashita Y., Matsuta K., Fukazawa T., Kusaoi M., Murakami Y., Takiguchi M., Juji T., Hashimoto H., Kohda D., Maenaka K., Tokunaga K. (2005) Extensive polymorphisms of LILRB1 (ILT2, LIR1) and their association with HLA-DRB1 shared epitope negative rheumatoid arthritis. Hum. Mol. Genet. 14, 2469–2480 [DOI] [PubMed] [Google Scholar]