Serine phosphorylation of the FcγRI CY domain directs its migration to lipid rafts and its interaction with protein 4.1G, suggesting a tethering role for protein 4.1G.
Keywords: Fc receptors, signaling
Abstract
The high-affinity IgG receptor (CD64, FcγRI) has several special capacities, including the receptor-stimulated cleavage of the cell surface B cell-activating factor of the TNF superfamily (TNFSF13B). With the use of the yeast two-hybrid system, we and others have shown that FcγRI interacts with protein 4.1G (EPB41L2). Our mutational analyses identified two required 4.1G-interacting regions in the FcγRI CY and one FcγRI-interacting site in the C-terminus of protein 4.1G. Herein, we explore mechanism(s) that may regulate the interaction between protein 4.1G and FcγRI CY and influence FcγRI membrane mobility and function. We show that FcγRI CY interacts with protein 4.1G in vitro and that FcγRI coimmunoprecipitates protein 4.1G in freshly isolated human PBMC. With the use of immunostaining, we show that FcγRI colocalizes with protein 4.1G in unstimulated U937 cells, in which the FcγRI CY is constitutively serine-phosphorylated, but significant uncoupling occurs following FcγRI cross-linking, suggesting phosphoserine-regulated interaction. In vitro, protein 4.1G interacted preferentially with CK2-phosphorylated FcγRI CY, and compared with WT FcγRI, a nonphosphorylatable FcγRI mutant receptor was excluded from lipid rafts, suggesting a key role for protein 4.1G in targeting phosphorylated FcγRI to rafts. These data are consistent with a phosphoserine-dependent tethering role for protein 4.1G in maintaining FcγRI in lipid rafts and provide insight into the unique phosphoserine-based regulation of receptor signaling by FcγRI CY.
Introduction
FcγRI, the high-affinity IgG receptor, is expressed as a multiprotein complex on the surface of myeloid cells. The ligand-binding α-chain (CD64) noncovalently associates with the FcεRI γ-chain homodimer, which mediates signaling via its ITAM motif [1–5]. Receptor aggregation initiates signaling with tyrosine phosphorylation of the ITAM in the γ-chain, leading to syk recruitment and activation and subsequent downstream cellular functions, including phagocytosis, gene transcription, and antigen presentation [1, 6–8].
Despite signaling through the common γ-chain subunit shared by other FcRs, FcγRI mediates distinct cellular programs, including cleavage and release of functional, soluble B lymphocyte stimulator/B cell-activating factor of the TNF family from the surface of monocytes and IL-10 production, both of which may promote B cell survival and antibody production [9, 10]. Recent observations also indicate that net FcγRI complex signaling is modulated by the unique α-chain CY [11–13], although the underlying mechanism is not well defined. The FcγRI CY domain, which lacks traditional tyrosine-based signaling motifs, contains serine residues that are constitutively phosphorylated in resting cells and dephosphorylated upon receptor aggregation [13, 14]. Serine/threonine phosphatase inhibitors, which inhibit serine dephosphorylation of FcγRI CY, impair phagocytosis [11, 13]. Therefore, FcγRI-induced cellular functions, although engaging phosphotyrosine-based γ-chain signaling, appear to be modulated by a phosphoserine-based mechanism mediated by the α-chain CY.
FcR signaling has been shown to involve dynamic rearrangement of the actin cytoskelton and redistribution of the receptors to membrane microdomains (lipid rafts) containing signaling proteins [15–20]. However, FcγRI has been shown to reside constitutively in lipid microdomains [5, 21]. As FcγRI is constitutively phosphorylated on serine residues and resides constitutively in lipid rafts, we considered that FcγRI partitioning to rafts might be regulated by the FcγRI CY domain and its interaction with cytoskeletal proteins. We initially sought to identify proteins that are recruited by FcγRI CY with the yeast two-hybrid system and to explore the role of phosphorylation of the CY domain in FcγRI partitioning to lipid rafts and function.
Our results show that FcγRI binds to protein 4.1G, consistent with others [22]. In vitro fusion protein pull-down assays confirm the interaction, and FcγRI coprecipitates with protein 4.1G in freshly isolated human PBMCs. In U937 cells, cross-linking of FcγRI resulted in decreased colocalization of FcγRI and protein 4.1G with time. In vitro studies indicate that FcγRI CY is phosphorylated by CK2, and phosphorylated FcγRI CY preferentially coprecipitated more protein 4.1G. Functional studies show that in transfected cells, a mutant FcγRI, which lacks CY serine residues (and is therefore incapable of being phosphorylated), is excluded from lipid rafts, unlike the WT receptor, indicating a role for FcγRI CY serine phosphorylation in receptor localization to lipid rafts. In addition, upon receptor stimulation, the mutant FcγRI resulted in increased actin polymerization compared with the WT receptor, whereas receptors, in which the CY domain serines had been changed to aspartic acid to mimic serine phosphorylation, resulted in lower levels of actin polymerization. These data indicate that serine phosphorylation of FcγRI CY regulates its partitioning to lipid microdomains and its interaction with protein 4.1G, which may play a role in tethering FcγRI to membrane lipid microdomains.
MATERIALS AND METHODS
Reagents
Yeast growth and maintenance, transformations, plasmid isolations, and LacZ filter assays were performed according to the manufacturers' protocols. The monocyte cDNA library was kindly provided by Dr. Daniel Kastner (Genetics and Genomics Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH, Bethesda, MD, USA). The Matchmaker yeast two-hybrid system, Yeastmaker yeast transformation system, and the human PBL two-hybrid library were from Clontech (Palo Alto, CA, USA). PBMCs were isolated from the blood of normal, healthy donors by centrifugation through lymphocyte separation media (Mediatech, Manassas, VA, USA). All donors gave written, informed consent, and all protocols were preapproved by the University of Alabama at Birmingham Institutional Review Board for Human Use (Birmingham, AL, USA). U937 cells (American Type Culture Collection, Manassas, VA, USA) were maintained as described previously [13] and stimulated with 100 U/ml human IFN-γ for 18 h prior to use. For DNA mutagenesis, the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) was used, and changes were confirmed by DNA sequencing with the ABI Prism BigDye terminator cycle sequencing kit (PerkinElmer Applied Biosystems, Carlsbad, CA, USA).
DNA techniques and transfections
All DNA manipulations were performed using standard techniques. Plasmids were propagated in Escherichia coli DH5α, and recombinants were verified by sequencing inserts and junctions completely. To create the FcγRI CY two-hybrid bait (V-FcγRI CY), a 0.3-kb DNA fragment encoding a six-glycine linker and FcγRI CY was cloned into the EcoRI-Pst1 site of pGBT9 or the Spe1 site of pDBLeu. For mapping-binding sites, mutagenic oligonucleotides were used to create internal deletions and C-terminal truncations in FcγRI CY and protein 4.1G. To create GAL4 activation domain fusions to C-terminal amino acid residues of protein 4.1G, DNA fragments encoding protein 4.1G sequences were PCR-amplified and cloned into the pACT vector. The GST-FcγRI CY, GST-4.1G, ThioHis-FcγRI CY, and ThioHis-protein 4.1G plasmid constructs were created by cloning DNA fragments encoding FcγRI CY and the protein 4.1G C-terminal 321-aa residues into pGEX2T (Pharmacia, Sweden) or Thio-His (Invitrogen, Carlsbad, CA, USA) vectors. Fusion proteins were expressed and purified according to the manufacturers' instructions.
Immunoprecipitation, immunoblotting, and fusion proteins
PBMCs (1×108) were lysed in 2 ml 1% digitonin-0.05% sodium deoxycholate and clarified lysate incubated at 4°C for 2 h with protein-G sepharose beads or beads loaded with 2.5 μg anti-FcγRI (mAb 197) or anti-FcγRIIa (mAb IV.3) antibodies. Beads were then washed three times with lysis buffer and boiled in reducing (2-ME) SDS sample buffer for 5 min. Immunoprecipitates were electrophoresed on 10% SDS-PAGE gels, transferred to nitrocellulose membrane, and probed with rabbit antisera against protein 4.1G (UABN42) and FcγRI (3535) and goat antibody against FcγRIIa (Santa Cruz Biotechnology, Santa Cruz, CA, USA). For fusion protein interactions, glutathione sepharose beads loaded with GST-FcγRI CY were incubated overnight at 4°C with clarified lysate from E. coli cells expressing ThioHis vector protein or ThioHis-protein 4.1G. Beads were washed four times in lysis buffer, boiled in reducing (2-ME) 2× SDS buffer, and electrophoresed on a 12% SDS-PAGE gel. Separated proteins were transferred to nitrocellulose, blocked in 10% nonfat dried milk, and probed with anti-4.1G antibody. Protein bands were visualized using chemiluminescence (Supersignal West Pico, Thermo Scientific, Waltham, MA, USA) and HyBlot CL autoradiography film (Denville Scientific, Metuchen, NJ, USA).
Immunostaining
FcγRI surface expression was confirmed using FACS analysis [13]. For colocalization studies, U937 cells were incubated with 32.2 mAb for 40 min at 4°C, washed, and then incubated at 37°C with goat anti-mouse IgG secondary antibody for various times. Following receptor cross-linking, cells were fixed in 4% formaldehyde for 20 min at 4°C and Fc-binding sites blocked by incubation with 20 μg/ml aggregated human IgG for 1 h at 4°C. Washed cells were permeabilized in HBSS/0.2% Triton X-100. Protein 4.1G was detected using specific rabbit antiserum followed by FITC-conjugated goat anti-rabbit IgG, and FcγRI was detected using PE-conjugated F(ab′)2 fragments of goat anti-mouse IgG. For lipid raft localization, P388D1 murine macrophages stably expressing FcγRI were grown overnight on poly-d-lysine-coated coverslips (BD Biosciences, San Jose, CA, USA), fixed in 3.5% formaldehyde, and stained with 10 ug/ml mAb 32.2-FITC and 8 ug/ml Alexa 555-conjugated cholera toxin subunit B, which binds to GM1 in microdomains (Molecular Probes, Eugene, OR, USA). Slides were analyzed using a Nikon Eclipse TE-2000U inverted high-resolution digital microscope system.
In vitro kinase assay
Glutathione beads loaded with 20 μg GST protein, GST-FcγRI CY fusion protein, or serine-to-alanine mutants of FcγRI CY were incubated with 2 μCi 32P-ATP and 0.5 U CK2 (Promega, Madison, WI, USA), according to the manufacturer′s suggestions. Beads were washed twice in 50 mM Tris-HCl/5 mM EDTA, pH 8.5/0.5 M NaCl, resuspended in 2× SDS buffer, electrophoresed on 15% agarose gels, and then dried and exposed to film. For binding experiments, glutathione beads loaded with 10 μg GST-FcγRI CY fusion or GST-Cntl CY (FcγRI CY) were incubated with CK2, as detailed above, washed three times with buffer, and incubated with 50 μg Thio-His-4.1G fusion protein for 4–6 h at 4°C. Beads were washed extensively with Tris/EDTA/NaCl buffer, electrophoresed on 12% gels, transferred to nitrocellulose membrane, and blotted with rabbit antisera against protein 4.1G and polyclonal anti-GST antibodies.
RESULTS
FcγRI CY coprecipitates with protein 4.1G in human PBMCs
With the use of FcγRI CY as bait in a yeast two-hybrid system screen of human cDNA libraries, we identified the protein 1G (EPB41L2) as a binding partner for FcγRI but not FcγRIIIa [22] (Supplemental Fig. 1). With the use of deletion analysis, we have identified two binding sites in the FcγRI CY, both of which are required for binding to protein 4.1G, and a single binding site in the C-terminal domain of protein 4.1G, which is necessary and sufficient for interaction with FcγRI CY (Supplemental Figs. 2 and 3). To further confirm the interaction between FcγRI CY and protein 4.1G, we used fusion protein pulldowns and coimmunoprecipitation assays. GST-FcγRI CY fusion protein, but not GST alone, was able to pull down a protein 4.1G-ThioHis fusion from bacterial lysate (Fig. 1A, lane 4 vs. lane 2). Neither GST alone nor the GST-FcγRI CY fusion interacted with the ThioHis control protein (Fig. 1A, lanes 1 and 3). Additionally, when immunoprecipitated with receptor-specific antibodies, FcγRI, but not FcγRIIa, coimmunoprecipitated the 110-kDa protein 4.1G from human PBMC lysates (Fig. 1B, lane 2 vs. lane 3). Immunoblots stripped and reprobed with receptor-specific antibodies show the presence of the immunoprecipitated FcγRI and FcγRIIa (Fig. 1B, bottom two panels). Taken together, these data indicate that FcγRI, but not FcγRIIa or FcγRIIIa, interacts with protein 4.1G.
Figure 1. FcγRI CY coprecipitates and colocalizes with protein 4.1G.
(A) Interaction between the GST-FcγRI CY fusion protein and protein 4.1G C-terminal region fused to ThioHis-protein 4.1G. Immobilized GST-FcγRI CY was incubated with lysate from bacteria expressing the ThioHis-protein 4.1G fusion protein. Blots [immunoblots (IB)] were probed with polyclonal antibodies against protein 4.1G. Binding assays were repeated three times with similar results. (B) Coimmunoprecipitation (IP) of protein 4.1G with FcγRI. PBMCs were lysed in 1% digitonin containing 0.05% sodium deoxycholate, and clarified lysate was incubated with anti-FcγRI mAb 32.2 or anti-FcγRIIa mAb IV.3 adsorbed to protein G beads or with protein G beads only. Blots of immunoprecipitates were probed with rabbit anti-protein 4.1G and stripped and reprobed sequentially with rabbit anti-FcγRI and goat anti-FcγRIIa. Data are representative of three experiments. (C) U937 cells were treated with 100 units/ml IFN-γ for 16–24 h before stimulation with FcγRI-specific antibodies for various times. Protein 4.1G and FcγRI were then detected using specific antibodies, as outlined in Materials and Methods. (D) Quantitation of colocalization of FcγRI and protein 4.1G. With the use of Metamorph software, the number of pixels showing overlapping red (FcγRI) and green (protein 4.1G) spectra was determined for seven to 10 randomly chosen cells, and averages were determined from two fields for each time-point. The degree of colocalization was then expressed as the spectral ratio of green and red stains. For unstimulated versus 15-min stimulation, P = 0.0006; for unstimulated versus 30-min stimulation, P = 0.01 (Student′s t test). (E) Lack of colocalization of FcγRIIa with protein 4.1G. U937 cells was treated with IFN-γ, as above, before stimulation with FcγRIIa-specific antibodies for 15 min. Protein 4.1G and FcγRIIa were then detected as described.
Stimulation-dependent decrease in FcγRI interaction with protein 4.1G
FcγRI CY is serine-phosphorylated in resting cells and dephosphorylated upon receptor aggregation, and it is possible that receptor stimulation alters its interaction with protein 4.1G. To assess whether interaction of FcγRI with protein 4.1G is independent of a receptor aggregation state, we used specific antibodies to determine whether FcγRI and protein 4.1G colocalized in untreated and FcγRI-aggregated U937 cells, which endogenously express both proteins. Following treatment of U937 cells with IFN-γ for 16–24 h to enhance FcγRI expression, FcγRI was cross-linked for various times with anti-FcγRI mAb 32.2 F(ab′)2 and goat anti-mouse IgG F(ab′)2, and cells were stained for protein 4.1G and FcγRI using FITC- and PE-conjugated antibodies. FcγRI and protein 4.1G gave similar staining patterns in unstimulated cells (Fig. 1C, first row), and merging of the two images gave the characteristic yellow color, which results from spectral overlap and indicates colocalization of the two proteins (Fig. 1C, third column). Less colocalization was observed at 15 min (Fig. 1C, second and third rows) and at 30 min (Fig. 1C, fourth and fifth rows) after receptor cross-linking, indicating reduced interaction between FcγRI and protein 4.1G. Quantitation of the amount of colocalization using Metamorph analysis indicated that compared with unstimulated cells, FcγRI-stimulated cells showed 40–50% less colocalization at 15 min and 30 min after receptor stimulation (Fig. 1D). These data indicate significant coupling of the receptor and protein 4.1G in resting cells when FcγRI CY is known to be phosphorylated and significant uncoupling when the receptor is cross-linked and dephosphorylated. Consistent with our two-hybrid and biochemical data, FcγRIIa (CD32a), also expressed in U937 cells, did not colocalize with protein 4.1G (Fig. 1E).
Serine-phosphorylated FcγRI CY preferentially binds to protein 4.1G
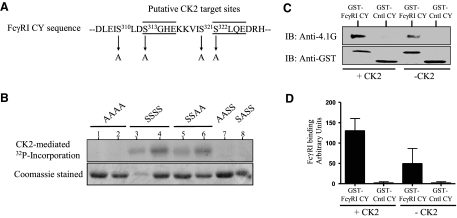
In human and murine myeloid cells, FcγRI CYs are constitutively phosphorylated on serine residues in resting cells, and human FcγRI is dephosphorylated following receptor cross-linking [13, 14]. Our colocalization data indicated stronger association of FcγRI and protein 4.1G in resting cells, in which the FcγRI CY is serine-phosphorylated, suggesting phosphoserine-regulated interaction. Therefore, we wished to determine whether serine phosphorylation of FcγRI CY altered its binding to protein 4.1G in vitro. FcγRI CY contains two protein kinase CK2 phosphorylation sites at serine313 (DS313GHE) and serine322 (SS322LQE) (Fig. 2A). To determine whether CK2 could phosphorylate FcγRI CY, immobilized GST-FcγRI CY fusion proteins were treated with CK2 and 32P-ATP. Fig. 2B shows that CK2 phosphorylates WT FcγRI CY (lanes 3 and 4), but not mutant CY, in which all four serines were changed to alanines (lanes 1 and 2). This indicates that one or more of the serine residues, but not the C-terminal threonine (unchanged in the alanine mutants), are phosphorylated by CK2. In addition, mutational analysis showed that CK2 phosphorylated only GST-FcγRI CY proteins, in which serine313 remained unchanged (Fig. 2B, lanes 3–6 vs. lanes 7–8), indicating that only serine313 is targeted by CK2, which also phosphorylated a ThioHis-FcγRI CY fusion protein (data not shown). Other kinases, including CLK1, GSK-3β, and several isoforms of PKC, were unable to phosphorylate FcγRI CY and point mutants in similar assays, suggesting that CK2 specifically targets FcγRI CY (data not shown).
Figure 2. Protein 4.1G interacts preferentially with CK2-phosphorylated FcγRI CY.
(A) Partial sequence of FcγRI CY (residues 306–328) showing putative CK2 phosphorylation sites. Residues that were mutated to alanines (S>A) are also shown. (B). CK2 specifically phosphorylates serine313 in FcγRI CY. Twenty micrograms of GST-FcγRI CY WT (SSSS) and mutant (AAAA, SSAA, AASS, SASS) fusion proteins were adsorbed to glutathione beads and incubated with 0.5 unit of CK2 and 2 μCi 32P-ATP for a quantitative in vitro kinase assay. Coomassie-stained parallel aliquots of the fusion proteins (lower panel) confirm comparable loading. Lanes 1 and 2 (both FcγRI CY mutant AAAA), lanes 3 and 4 (both WT FcγRI CY SSSS), and lanes 5 and 6 (both FcγRI CY mutant SSAA) represent pairs of independent clones used in the assay. Members of each pair carry identical serine mutations. Data shown represent one of three assays that gave similar results. (C) Phosphorylated FcγRI CY preferentially pulled down protein 4.1G. GST-FcγRI CY or a GST-Cntl CY was incubated with CK2 and ATP, washed, and then incubated with phosphorylated ThioHis-protein 4.1G C-terminal domain for 4–6 h. Bound proteins were immunoblotted with anti-protein 4.1G and stripped and reprobed with GST antibody. CK2 activity was confirmed using 32P-ATP with 30 ng unphosphorylated casein and with 10 μg GST-FcγRI CY in similar reactions (data not shown). Lanes 1 and 3 show equal GST-FcγRI CY loading. Data represent one of three assays giving similar results. (D) Image-J densitometry measure of the protein 4.1G bands represents the average of three assays (for 4.1G binding, +CK2 vs. –CK2; P < 0.001, t test).
To determine whether phosphorylation of FcγRI CY altered its binding to protein 4.1G, immobilized GST-FcγRI CY or GST-Cntl CY was treated with CK2 and ATP before incubation with protein 4.1G. Despite equal protein input, phosphorylated FcγRI CY significantly bound more protein 4.1G (Fig. 2C, lane 1 vs. lane 3), and protein 4.1G failed to bind significantly to the GST-Cntl CY (lanes 2 and 4). These data indicate that protein 4.1G binds preferentially to phosphorylated FcγRI CY and are consistent with increased colocalization of the two proteins in unstimulated U937 cells when FcγRI CY is serine-phosphorylated. These data are also consistent with a role for protein 4.1G in tethering FcγRI to the actin cytoskeleton and to lipid rafts.
FcγRI CY serine-to-alanine mutations alter migration to lipid raft domains and F-actin polymerization
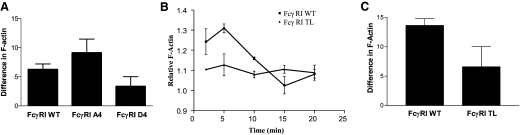
Mutant FcγRIs, carrying serine-to-alanine changes in the CY or a complete deletion of the CY, mediate inefficient signaling [11, 13]. Our current colocalization and biochemical data indicate phosphoserine-regulated interaction between FcγRI CY and protein 4.1G. To further explore the function of this phosphoserine-regulated interaction on the membrane distribution of FcγRI, we used stable transfectants expressing FcγRI, in which the CY serines had been mutated to alanines (A4). Surprisingly, compared with WT receptors, FcγRI, lacking phosphorylatable CY serines, was predominantly excluded from GM1-positive lipid rafts (Fig. 3A, second row). Metamorph quantitation indicated 70–80% reduction in lipid raft localization in cells expressing the FcγRI A4 mutant (Fig. 3B). As FcR-mediated lipid raft reorganization is associated with actin polymerization and cytoskeletal rearrangement [23], we also examined whether FcγRI CY serine mutants could alter the ability of the receptor to initiate F-actin polymerization. Compared with WT receptors, FcγRI A4 mutants, lacking the serine residues, resulted in increased F-actin polymerization (Fig. 4A), whereas receptors, in which the serines were replaced by aspartic acid residues to mimic phosphorylation, resulted in reduced F-actin polymerization (Fig. 4A). Additionally, mutant receptors lacking the CY entirely were less efficient at signaling for F-actin polymerization (Fig. 4B and C). These data indicate that serine phosphorylation of the FcγRI CY is required to tether the receptor to lipid rafts and plays a role in efficient actin polymerization. These data are also consistent with a tethering role for protein 4.1G in maintaining FcγRI in lipid raft domains.
Figure 3. FcγRI serine-to-alanine mutants are excluded from lipid rafts.
(A) Decreased colocalization of mutant FcγRI A4 with GM1-positive lipid rafts. P388DI cells expressing FcγRI WT or a mutant receptor, in which all four serine residues were changed to alanines (A4; see Fig. 2A), were stained for FcγRI (green) and GM1 (red), a marker for microdomains. Nuclei (blue) were stained with Hoescht reagent. (B) Quantitation of colocalization of FcγRI and lipid rafts. With the use of Metamorph software, the number of pixels showing overlapping red (FcγRI) and green (GM1) spectra was determined for seven to 10 randomly chosen cells from two fields for each cell line. The degree of colocalization was then expressed as above. Colocalization experiments were repeated three times with similar results.
Figure 4. FcγRI CY serines are required for regulated FcγRI-induced actin polymerization.
P388D1 cells expressing FcγRI WT, mutants, in which all four serines had been changed to alanines (FcγRI A4) or to aspartic acid (FcγRI D4) or a mutant lacking the CY [FcγRI tail-less (TL)], were stimulated for the indicated times with F(ab′)2 fragments of FcγRI-specific mAb, 22.2 or 32.2, and F(ab′)2 fragments of goat anti-mouse IgG secondary antibodies. Cells were then fixed with formaldehyde and permeabilized and F-actin content detected using AlexaFluor 488-conjugated Phalloidin. (A) Difference in F-actin between serine mutants at the 5-min time-point. Values represent the average of five replicates ± sd (for FcγRI WT vs. FcγRI A4, P=0.03; for FcγRI WT vs. FcγRI D4, P=0.03; for FcγRI A4 vs. FcγRI D4, P=0.01, paired t test). (B) Time-course determination of differences in the relative F-actin content induced by WT and tail-less mutant receptors lacking the CY. (C) Difference in F-actin polymerization between FcγRI WT and FcγRI TL-expressing cells at the 5-min time-point. Values for each time-point represent the average of four replicates ± sd (P<0.05, paired t test).
DISCUSSION
Despite signaling through a canonical ITAM in the associated common FcεRI γ-chain shared by FcγRIIIa, FcεRI, and FcαRI, net signaling of the FcγRI complex includes a critical role for the CY of the FcγRI α-chain, which modulates γ-chain phosphorylation [13]. Previous work has implicated nonmuscle filamin (ABP280) and PPL as α-chain-associated molecules affecting receptor expression and receptor cycling [24–26]. We show that protein 4.1G interacts specifically with FcγRI among the γ-chain-associated FcRs, that this binding is regulated by CK2-mediated phosphorylation of the FcγRI CY, and that this interaction influences receptor stability in lipid rafts. We propose that protein 4.1G influences receptor partitioning into lipid microdomains, thereby influencing src-family kinase phosphorylation of the receptor complex. Furthermore, the CK2-mediated phosphorylation of FcγRI CY, specifically on serine313, which alters the FcγRI CY-4.1G interaction, may modulate FKBP12-4.1G interactions and indirectly, cellular mTOR activity [27, 28]. Thus, the unique α-chain CY domain of FcγRI enables receptor-specific interactions with other intracellular proteins, which have biological implications that are distinct for this high-affinity FcR.
Homology searches of protein databases do not identify any homologues of the unique CY of the FcγRI α-chain. However, motif analysis suggests two putative CK2 phosphorylation sites (Fig. 2A), unlike the CYs of other common FcεRI-γ-chain-associated FcRs. These in silico observations are strongly supported by our direct biochemical studies, which have shown robust phosphorylation with purified CK2 specific to serine313 in the first predicted CK2 motif of the FcγRI α-chain CY. Other serine/threonine kinases, including CLK1, GSK-3β, and several isoforms of PKC, failed to phosphorylate FcγRI CY in WT or point-mutant constructs. Similarly, CK2 was not effective in phosphorylating the CYs of other FcRs associated with the common FcεRI-γ-chain. Interestingly, Hirata and Suzuki [29] observed increased CK2-like kinase activity coimmunoprecipitating with FcγRI following stimulation. Although these observations, together with our data, strongly suggest that CK2 is the likely intracellular kinase in this regulation of FcγRI, unequivocal demonstration of the responsible kinase is currently not established.
Our data, showing preferential interaction between phosphorylated FcγRI CY and protein 4.1G, suggest a phosphoserine-based, regulated interaction that affects surface receptor linkage to the actin cytoskeleton and to lipid microdomains. The effect of FcγRI CY serine mutants on efficient actin polymerization (Fig. 4A) establishes a clear link between FcγRI CY serines and receptor-initiated rearrangement of the actin cytoskeleton, and the exclusion of the serine mutant receptor from lipid rafts establishes a requirement for FcγRI CY serines in receptor stability in lipid microdomains. Rearrangement of the actin cytoskeleton is a requirement for the formation of lipid microdomain assemblies containing receptors and signaling molecules [23, 30–32]. Unlike FcαRI and FcεRI, FcγRI constitutively resides in microdomains in resting and stimulated cells [5, 21], and studies show that following activation of FcγRs, microdomains containing incomplete signaling pathways coalesce into larger lipid domains to create complete signaling platforms [23, 30]. Studies also show that receptor interaction with 4.1 protein, ezrin, radixin, moesin family proteins regulates receptor-associated cytoskeletal and lipid raft rearrangements, which facilitate signaling [31, 33]. Therefore, it is possible that protein 4.1G serves to tether FcγRI CY to lipid rafts in unstimulated cells, and following receptor stimulation, 4.1G disengages from the receptor to allow its migration into larger signaling raft assemblies. This model is consistent with our in vitro data, indicating that protein 4.1G interacts preferentially with phosphorylated FcγRI CY, the predominant form present in resting cells, and with our immunostaining data, which indicate less colocalization at later times following receptor aggregation. Therefore, we propose a model, in which protein 4.1G engages phosphorylated FcγRI CY in resting cells to link the receptor to the actin cytoskeleton and to lipid microdomains.
The C-terminal domain of protein 4.1G is essential for binding to FcγRI CY, which may explain the interaction between protein 4.1B and FcγRI CY (see Supplemental Fig. 1; protein 4.1G and protein 4.1B are 73% homologous in their C-terminal domains). Interestingly, a histidine-proline group, located in the C-terminus of human 4.1G, is required for interaction with FcγRI CY, and a similar histidine-proline group in the C-terminal 94-aa residues of murine 4.1G was previously shown to be critical for binding with FKBP13 [27] (Supplemental Fig. 3). This shared binding requirement suggests that by balancing the relative binding to FcγRI or FKBP12, there may be an important link between the 4.1G-FcγRI interaction and mTOR activity within the cell.
Although signaling through a canonical ITAM in the associated common FcεRI γ-chain is an established mechanism for FcR signal transduction, it is increasingly evident that other mechanisms and pathways play an important role. FcαRI CY has been shown to modulate receptor signaling by a phosphorylated CY-dependent mechanism [34, 35], and we have demonstrated recently that naturally occurring FcαRI CY serine245/glycine245 variants alter receptor signaling, in part, through direct recruitment of Lyn kinase to the CY [36]. Similarly, the CY of the FcγRIIIa α-chain is required for efficient signaling [37], although our data indicate that the mechanism does not involve protein 4.1G or CK2-mediated events characteristic of FcγRI. Given that efficient endocytosis, phagocytosis, antigen presentation, and cytokine gene expression are all modulated by FcγRI CY [11–13, 38], it is likely that FcγRI CY acts, at least in part, as a scaffold for intracellular structural and effector proteins. Consistent with this framework, FcγRI has been shown to interact with PPL, with the ABP280 or nonmuscle filamin, and with protein 4.1G [22, 24, 26]. Our data indicate that the interaction of FcγRI with protein 4.1G, mediated predominantly by the C-terminus of 4.1G, is a regulated event, which links FcγRI to lipid microdomains and possibly to mTOR signaling. These interactions emphasize the functional differences between the different common FcεRI γ-chain-associated receptors and provide a framework for selective therapeutic intervention.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (AR33062 and P30 AR48311). We wish to thank Dr. Xiaoli Yang for assistance with immunostaining.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- ABP
- actin-binding protein
- CK2
- casein kinase 2
- CLK1
- CDC-like kinase 1
- CY
- cytoplasmic domain
- FKBP
- FK506-binding protein
- GST-Cntl CY
- a control protein CY fusion
- mTOR
- mammalian target of rapamycin
- PPL
- periplakin
- ThioHis
- His patch thioredoxin
AUTHORSHIP
A.W.G., J.C.E., and R.P.K. designed the experiments and analyzed the data. A.W.G. and R.P.K. wrote the manuscript. A.W.G., X.L., and J.G.B. performed the experiments. J.W. and C.R. provided critical reagents and advice on experimental protocols.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1. Scholl P. R., Geha R. S. (1993) Physical association between the high-affinity IgG receptor (FcγRI) and the γ subunit of the high-affinity IgE receptor (FcεRI γ). Proc. Natl. Acad. Sci. USA 90, 8847–8850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrison P. T., Bjorkhaug L., Hutchinson M. J., Allen J. M. (1995) The interaction between human FcγRI and the γ-chain is mediated solely via the 21 amino acid transmembrane domain of FcγRI. Mol. Membr. Biol. 12, 309–312 [DOI] [PubMed] [Google Scholar]
- 3. Ernst L. K., Duchemin A. M., Anderson C. L. (1993) Association of the high-affinity receptor for IgG (FcγRI) with the γ subunit of the IgE receptor. Proc. Natl. Acad. Sci. USA 90, 6023–6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller K. L., Duchemin A. M., Anderson C. L. (1996) A novel role for the Fc receptor γ subunit: enhancement of FcγR ligand affinity. J. Exp. Med. 183, 2227–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hutchinson M. J., Harrison P. T., Floto R. A., Allen J. M. (1995) Fcγ receptor-mediated phagocytosis requires tyrosine kinase activity and is ligand independent. Eur. J. Immunol. 25, 481–487 [DOI] [PubMed] [Google Scholar]
- 6. Duchemin A. M., Ernst L. K., Anderson C. L. (1994) Clustering of the high affinity Fc receptor for immunoglobulin G (Fc γ RI) results in phosphorylation of its associated γ-chain. J. Biol. Chem. 269, 12111–12117 [PubMed] [Google Scholar]
- 7. Duchemin A. M., Anderson C. L. (1997) Association of non-receptor protein tyrosine kinases with the FcγRI/γ-chain complex in monocytic cells. J. Immunol. 158, 865–871 [PubMed] [Google Scholar]
- 8. Durden D. L., Rosen H., Cooper J. A. (1994) Serine/threonine phosphorylation of the γ-subunit after activation of the high-affinity Fc receptor for immunoglobulin G. Biochem. J. 299, 569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li X., Su K., Ji C., Szalai A. J., Wu J., Zhang Y., Zhou T., Kimberly R. P., Edberg J. C. (2008) Immune opsonins modulate BLyS/BAFF release in a receptor-specific fashion. J. Immunol. 181, 1012–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sutterwala F. S., Noel G. J., Salgame P., Mosser D. M. (1998) Reversal of proinflammatory responses by ligating the macrophage Fcγ receptor type I. J. Exp. Med. 188, 217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edberg J. C., Yee A. M., Rakshit D. S., Chang D. J., Gokhale J. A., Indik Z. K., Schreiber A. D., Kimberly R. P. (1999) The cytoplasmic domain of human FcγRIa alters the functional properties of the FcγRI.γ-chain receptor complex. J. Biol. Chem. 274, 30328–30333 [DOI] [PubMed] [Google Scholar]
- 12. Van Vugt M. J., Kleijmeer M. J., Keler T., Zeelenberg I., van Dijk M. A., Leusen J. H., Geuze H. J., van de Winkel J. G. (1999) The FcγRIa (CD64) ligand binding chain triggers major histocompatibility complex class II antigen presentation independently of its associated FcR γ-chain. Blood 94, 808–817 [PubMed] [Google Scholar]
- 13. Edberg J. C., Qin H., Gibson A. W., Yee A. M., Redecha P. B., Indik Z. K., Schreiber A. D., Kimberly R. P. (2002) The CY domain of the Fcγ RIa α-chain (CD64) alters γ-chain tyrosine-based signaling and phagocytosis. J. Biol. Chem. 277, 41287–41293 [DOI] [PubMed] [Google Scholar]
- 14. Quilliam A. L., Osman N., McKenzie I. F., Hogarth P. M. (1993) Biochemical characterization of murine FcγRI. Immunology 78, 358–363 [PMC free article] [PubMed] [Google Scholar]
- 15. Barabé F., Rollet-Labelle E., Gilbert C., Fernandes M. J., Naccache S. N., Naccache P. H. (2002) Early events in the activation of Fc γ RIIA in human neutrophils: stimulated insolubilization, translocation to detergent-resistant domains, and degradation of Fc γ RIIA. J. Immunol. 168, 4042–4049 [DOI] [PubMed] [Google Scholar]
- 16. Field K. A., Holowka D., Baird B. (1997) Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J. Biol. Chem. 272, 4276–4280 [DOI] [PubMed] [Google Scholar]
- 17. Katsumata O., Hara-Yokoyama M., Sautes-Fridman C., Nagatsuka Y., Katada T., Hirabayashi Y., Shimizu K., Fujita-Yoshigaki J., Sugiya H., Furuyama S. (2001) Association of FcγRII with low-density detergent-resistant membranes is important for cross-linking-dependent initiation of the tyrosine phosphorylation pathway and superoxide generation. J. Immunol. 167, 5814–5823 [DOI] [PubMed] [Google Scholar]
- 18. Lang M. L., Chen Y. W., Shen L., Gao H., Lang G. A., Wade T. K., Wade W. F. (2002) IgA Fc receptor (FcαR) cross-linking recruits tyrosine kinases, phosphoinositide kinases and serine/threonine kinases to glycolipid rafts. Biochem. J. 364, 517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lang M. L., Shen L., Gao H., Cusack W. F., Lang G. A., Wade W. F. (2001) Fc α receptor cross-linking causes translocation of phosphatidylinositol-dependent protein kinase 1 and protein kinase B α to MHC class II peptide-loading-like compartments. J. Immunol. 166, 5585–5593 [DOI] [PubMed] [Google Scholar]
- 20. Wilson B. S., Steinberg S. L., Liederman K., Pfeiffer J. R., Surviladze Z., Zhang J., Samelson L. E., Yang L. H., Kotula P. G., Oliver J. M. (2004) Markers for detergent-resistant lipid rafts occupy distinct and dynamic domains in native membranes. Mol. Biol. Cell 15, 2580–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beekman J. M., van der Linden J. A., van de Winkel J. G., Leusen J. H. (2008) FcγRI (CD64) resides constitutively in lipid rafts. Immunol. Lett. 116, 149–155 [DOI] [PubMed] [Google Scholar]
- 22. Beekman J. M., Bakema J. E., van der Poel C. E., van der Linden J. A., van de Winkel J. G., Leusen J. H. (2008) Protein 4.1G binds to a unique motif within the Fc γ RI cytoplasmic tail. Mol. Immunol. 45, 2069–2075 [DOI] [PubMed] [Google Scholar]
- 23. Kono H., Suzuki T., Yamamoto K., Okada M., Yamamoto T., Honda Z. (2002) Spatial raft coalescence represents an initial step in Fc γ R signaling. J. Immunol. 169, 193–203 [DOI] [PubMed] [Google Scholar]
- 24. Beekman J. M., Bakema J. E., van de Winkel J. G., Leusen J. H. (2004) Direct interaction between FcγRI (CD64) and periplakin controls receptor endocytosis and ligand binding capacity. Proc. Natl. Acad. Sci. USA 101, 10392–10397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beekman J. M., van der Poel C. E., van der Linden J. A., van den Berg D. L., van den Berghe P. V., van de Winkel J. G., Leusen J. H. (2008) Filamin A stabilizes Fc γ RI surface expression and prevents its lysosomal routing. J. Immunol. 180, 3938–3945 [DOI] [PubMed] [Google Scholar]
- 26. Ohta Y., Stossel T. P., Hartwig J. H. (1991) Ligand-sensitive binding of actin-binding protein to immunoglobulin G Fc receptor I (FcγRI). Cell 67, 275–282 [DOI] [PubMed] [Google Scholar]
- 27. Walensky L. D., Gascard P., Fields M. E., Blackshaw S., Conboy J. G., Mohandas N., Snyder S. H. (1998) The 13-kD FK506 binding protein, FKBP13, interacts with a novel homologue of the erythrocyte membrane cytoskeletal protein 4.1. J. Cell Biol. 141, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Foster K. G., Fingar D. C. (2010) Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J. Biol. Chem. 285, 14071–14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirata Y., Suzuki T. (1987) Protein kinase activity associated with Fc γ 2a receptor of a murine macrophage like cell line, P388D1. Biochemistry 26, 8189–8195 [DOI] [PubMed] [Google Scholar]
- 30. Pike L. J. (2003) Lipid rafts: bringing order to chaos. J. Lipid Res. 44, 655–667 [DOI] [PubMed] [Google Scholar]
- 31. Gupta N., Wollscheid B., Watts J. D., Scheer B., Aebersold R., DeFranco A. L. (2006) Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nat. Immunol. 7, 625–633 [DOI] [PubMed] [Google Scholar]
- 32. Villalba M., Bi K., Rodriguez F., Tanaka Y., Schoenberger S., Altman A. (2001) Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J. Cell Biol. 155, 331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Allenspach E. J., Cullinan P., Tong J., Tang Q., Tesciuba A. G., Cannon J. L., Takahashi S. M., Morgan R., Burkhardt J. K., Sperling A. I. (2001) ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 15, 739–750 [DOI] [PubMed] [Google Scholar]
- 34. Bracke M., Lammers J. W., Coffer P. J., Koenderman L. (2001) Cytokine-induced inside-out activation of FcαR (CD89) is mediated by a single serine residue (S263) in the intracellular domain of the receptor. Blood 97, 3478–3483 [DOI] [PubMed] [Google Scholar]
- 35. Bakema J. E., Bakker A., de Haij S., Honing H., Bracke M., Koenderman L., Vidarsson G., van de Winkel J. G., Leusen J. H. (2008) Inside-out regulation of Fc α RI (CD89) depends on PP2A. J. Immunol. 181, 4080–4088 [DOI] [PubMed] [Google Scholar]
- 36. Wu J., Ji C., Xie F., Langefeld C. D., Qian K., Gibson A. W., Edberg J. C., Kimberly R. P. (2007) FcαRI (CD89) alleles determine the proinflammatory potential of serum IgA. J. Immunol. 178, 3973–3982 [DOI] [PubMed] [Google Scholar]
- 37. Hou X., Dietrich J., Odum N., Geisler C. (1996) The cytoplasmic tail of FcγRIIIAα is involved in signaling by the low affinity receptor for immunoglobulin G. J. Biol. Chem. 271, 22815–22822 [DOI] [PubMed] [Google Scholar]
- 38. Qin H., Edberg J. C., Gibson A. W., Page G. P., Teng L., Kimberly R. P. (2004) Differential gene expression modulated by the cytoplasmic domain of Fc γ RIa (CD64) α-chain. J. Immunol. 173, 6211–6219 [DOI] [PubMed] [Google Scholar]