Abstract
Context
The need for improved therapeutic agents that more quickly and effectively treat depression is critical. In a pilot study we evaluated the role of the cholinergic system in cognitive symptoms of depression and unexpectedly observed rapid reductions in depression severity following the administration of the antimuscarinic drug scopolamine hydrobromide (4 μg/kg intravenously) compared with placebo (P=.002). Subsequently a clinical trial was designed to assess more specifically the antidepressant efficacy of scopolamine.
Objective
To evaluate scopolamine as a potential antidepressant agent.
Design
Two studies were conducted: a double-blind, placebo-controlled, dose-finding study followed by a double-blind, placebo-controlled, crossover clinical trial.
Setting
The National Institute of Mental Health.
Patients
Currently depressed outpatients aged 18 to 50 years meeting DSM-IV criteria for recurrent major depressive disorder or bipolar disorder. Of 39 eligible patients, 19 were randomized and 18 completed the trial.
Interventions
Multiple sessions including intravenous infusions of placebo or scopolamine hydrobromide (4 μg/kg). Individuals were randomized to a placebo/ scopolamine or scopolamine/placebo sequence (series of 3 placebo sessions and series of 3 scopolamine sessions). Sessions occurred 3 to 5 days apart.
Main Outcome Measures
Psychiatric evaluations using the Montgomery-Asberg Depression Rating Scale and the Hamilton Anxiety Rating Scale were performed to assess antidepressant and antianxiety responses to scopolamine.
Results
The placebo/scopolamine group showed no significant change during placebo infusion vs baseline; reductions in depression and anxiety rating scale scores (P<.001 for both) were observed after the administration of scopolamine compared with placebo. The scopolamine/placebo group also showed reductions in depression and anxiety rating scale scores (P<.001 for both) after the administration of scopolamine, relative to baseline, and these effects persisted as they received placebo. In both groups, improvement was significant at the first evaluation after scopolamine administration (P≤.002).
Conclusion
Rapid, robust antidepressant responses to the antimuscarinic scopolamine occurred in currently depressed patients who predominantly had poor prognoses.
Major depression is the leading cause of years lived with disability.1 A range of antidepressant agents is available, but 30% to 40% of patients with major depressive disorder (MDD) do not respond to initial treatment,2 and nonresponse rates are even higher in depressed patients with bipolar disorder (BD).3 Moreover, in patients who experience symptomatic relief after conventional antidepressant drug treatment, improvement generally is not evident for 3 to 4 weeks. The need for improved therapeutic agents that more quickly and effectively treat depression remains critical.
The cholinergic system is one of the neurotransmitter systems implicated in the pathophysiologic mechanism of mood disorders. Increasing cholinergic activity using physostigmine (an anticholinesterase inhibitor) provides a challenge uniquely capable of exacerbating depressive symptoms in currently depressed patients with MDD and inducing depressive symptoms in currently manic patients with BD.4–9 The cholinergic system also is implicated in depression by evidence showing that polysomnographic responses to muscarinic receptor agonists10–12 and neuroendocrine and pupillary responses to cholinomimetics13–16 are exaggerated in depressed patients and that some muscarinic receptor gene polymorphisms are associated with an elevated incidence of depression.17,18 Elevated cholinergic function thus was hypothesized to participate in the pathogenesis of mood disorders.15
Putative animal models of depression also have implicated the muscarinic system. The Porsolt “behavioral despair” model of depression19 that uses the forced swim test is used broadly to evaluate the effect of pharmacologic agents on depressive behaviors. In the context of this model, antimuscarinic agents produced antidepressant-like effects, although these findings were considered “false-positives” based on the assumption that these agents did not exert antidepressant effects in humans.20–22 Moreover, rats bred selectively for increased sensitivity of muscarinic receptors showed putative behavioral analogues of depression, such as lethargy, reductions in self-stimulation, and increased behavioral despair, in the forced swim test in response to cholinomimetic drugs.23,24
Some tricyclic antidepressant (TCA) agents exerted potent antimuscarinic actions,25–27 but these effects were thought primarily to produce adverse effects without contributing to therapeutic efficacy.28–30 As a result, during the past 2 decades, efforts to develop new antidepressant drug treatments emphasized the development of compounds that specifically lack antimuscarinic effects.31
Herein we report the results of 2 studies that each demonstrate rapid, potent antidepressant responses to the antimuscarinic agent scopolamine hydrobromide in depressed patients with MDD or BD. In a pilot study designed to evaluate the role of the cholinergic neurotransmitter system in the cognitive symptoms associated with depression, we unexpectedly observed an antidepressant response to scopolamine in depressed patients. We then designed a second study to more specifically establish the antidepressant effects of scopolamine, and we confirmed the findings of the first experiment by demonstrating antidepressant responses to scopolamine.
STUDY 1
METHODS
Patients
Volunteers aged 18 to 45 years evaluated at the National Institute of Mental Health outpatient clinic were assessed for eligibility if they were nonsmokers and met the DSM-IV32 criteria for recurrent MDD or BD based on an unstructured interview conducted by a psychiatrist and the Structured Clinical Interview for DSM-IV. Exclusion criteria included exposure to psychotropic or other medications likely to affect central nervous system or cholinergic function within 3 weeks (8 weeks for fluoxetine), suicidal ideation, psychosis, lifetime history of substance dependence, substance abuse within 1 year, medical or neurologic disorders, abnormal electrocardiographic findings, narrow-angle glaucoma, hypersensitivity to anticholinergic agents, hepatic dysfunction, electrolyte disturbance, human immunodeficiency virus or hepatitis viral infection, or weight greater than 125 kg. Pregnant or nursing women also were excluded.
Eight currently depressed patients, 5 with MDD (mean±SD age, 28±7.8 years; 4 women and 1 man) and 3 with BD (mean±SD age, 35±9.0 years; 2 women and 1 man), participated. Patients provided written informed consent as approved by the National Institute of Mental Health institutional review board.
Study Design
Four testing sessions were performed in random order under double-blind conditions during which participants received a 15-minute intravenous infusion33 of a saline placebo and 3 doses of scopolamine hydrobromide (2.0, 3.0, and 4.0 μg/kg). Dose sequences were randomized and assigned by patient number at the time of consent. The randomization sequences were restricted only by the rule that no participant would receive 4.0 μg/kg as their first dose. Nonpregnancy was established before each session. Sessions were scheduled 3 to 5 days apart. Follow-up psychiatric evaluations were obtained.
Assessment and Scopolamine Assay
Before each infusion, psychiatric assessments were performed using the Montgomery-Asberg Depression Rating Scale (MADRS)34 at baseline and across days between sessions. Blood samples were obtained at baseline and at 30, 45, 60, 90, 120, and 150 minutes relative to the infusion start time.
For the scopolamine assay, blood samples were centrifuged at 4°C for 10 minutes, and the plasma was transferred to polypropylene tubes, frozen, and stored at –70°C until analysis. Scopolamine plasma levels were determined by CANTEST BioPharma Services (Burnaby, British Columbia).
Data Analysis
Repeated-measures analysis of variance (ANOVA) was used to examine MADRS scores across sessions in series irrespective of dose, as inherently across time individuals received all doses of scopolamine, and paired t tests were used to compare means in the presence of overall significant ANOVA results. The effects of each dose were evaluated using t tests by comparing baseline MADRS scores with scores obtained in the session after administration of each specific dose and by comparing MADRS scores obtained immediately before with those obtained in the session after each dose. Postadministration evaluations for session 4 were provided by follow-up assessments when available (n=5), and last observation carried forward was used for missing follow-up assessments (n=3). The area under the curve concentration from 30 to 150 minutes was estimated for each session, and repeated-measures ANOVA was used to evaluate session differences.
RESULTS
Mean±SD MADRS scores differed across assessments (F=4.8; P=.005), were lower after session 4 (17.6±12.7; P=.008) compared with baseline (29.0±6.1), and trended toward significance after session 3 (20.0±11.0; P=.08).
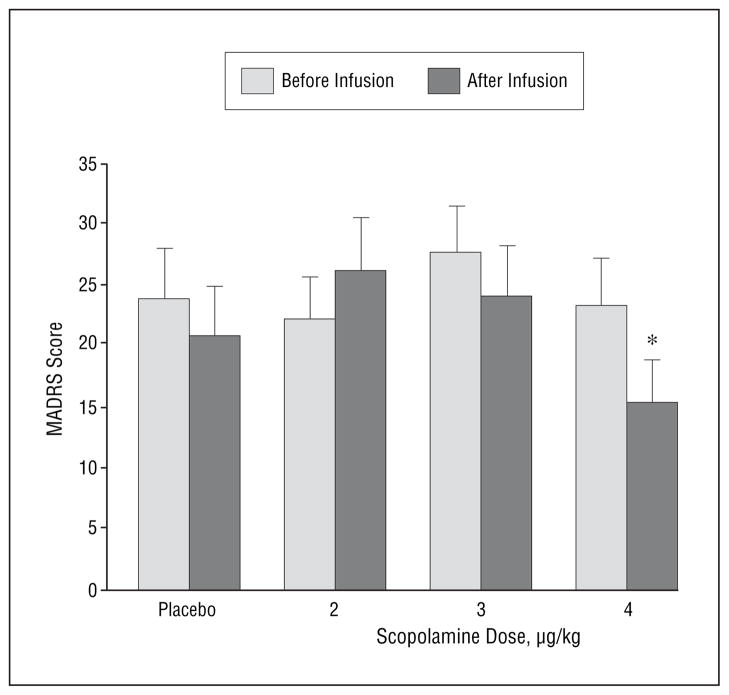
The MADRS scores obtained after administration of scopolamine hydrobromide, 4.0 μg/kg, were lower than baseline (P=.002) and preadministration (P=.02) measures (Figure 1). Moreover, there was a larger reduction in MADRS scores before vs after scopolamine hydrobromide, 4.0 μg/kg, infusion than before vs after placebo infusion (P=.01). No other difference was significant. The mean±SD change in MADRS scores between pretreatment and the evaluation after session 4 was –13.8±7.7 (P<.002). Five patients demonstrated a 50% or greater reduction in MADRS scores, and 3 remitted to the nondepressed range (MADRS score ≤10). Mean±SD area under the curve estimates increased as the dose increased (82.8±43.6, 122.1±60.9, and 176.7±71.3 for the 3 scopolamine doses, respectively; P<.001).
Figure 1.
Mean Montgomery-Asberg Depression Rating Scale (MADRS) scores from study 1 as assessed immediately before and in the first evaluation after infusions of placebo and each of 3 doses of scopolamine hydrobromide. Error bars represent SE. *Significantly different from baseline (P=.002) and from preadministration of 4.0 μg/kg of scopolamine (P=.02).
COMMENT
Depression severity decreased markedly during the study. The improvements seen, particularly after administration of the 4.0-μg/kg dose vs placebo, suggest robust antidepressant responses to scopolamine. The effects occurred rapidly as depressive symptoms were improved during the 3 to 5 days between infusions. Nonetheless, these results were unexpected, and the study was not designed to evaluate an antidepressant response to scopolamine. A second study was designed to establish the antidepressant efficacy of scopolamine.
STUDY 2
METHODS
Patients
Patient recruitment and eligibility criteria were as defined in study 1. A power analysis indicated that we needed a sample of 20 patients to detect an effect size half of that observed in study 1 after administration of scopolamine hydrobromide, 4.0 μg/kg. Participants provided written informed consent as approved by the National Institute of Mental Health institutional review board.
Study Design
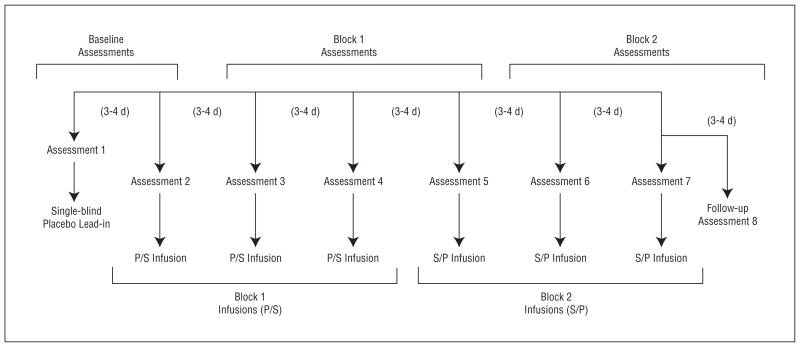
During each of 7 sessions, patients received a 15-minute intravenous infusion of either a placebo saline solution or scopolamine hydrobromide, 4.0 μg/kg. A single-blind lead-in session was used in which all the patients received a placebo infusion. Because psychiatric assessments were obtained before session infusions, the lead-in placebo in session 1 allowed for a second baseline assessment to be obtained in session 2, before the session 2 infusion. Subsequently, individuals were randomized to receive either placebo (3 sessions) followed by scopolamine (3 sessions) or scopolamine (3 sessions) followed by placebo (3 sessions) in a double-blind, placebo-controlled, crossover design (Figure 2). Follow-up evaluations were performed to provide the assessment for session 7. Randomization sequences were determined by the National Institutes of Health outpatient pharmacy and were assigned by patient number at the time of consent. Sessions were scheduled 3 to 4 days apart when possible.
Figure 2.
The study 2 blocked experimental design reflecting infusion series and assessment sessions for the 2 randomized patient groups. P/S indicates placebo followed by scopolamine hydrobromide; S/P, scopolamine followed by placebo.
Assessment and Scopolamine Assay
Before each infusion, psychiatric evaluations were completed using the MADRS, the Hamilton Anxiety Rating Scale (HARS),35 the Young Mania Rating Scale,36 and the Clinical Global Impressions–Improvement scale.34 Visual analog scales (VAS) (components included happy, sad, drowsy, irritated, alert, anxious, and restless) and the Profile of Mood State (POMS)37 were administered at baseline and 20, 60, 120, and 180 minutes after infusion. Blood samples were obtained as described in study 1.
Sixty minutes after the infusion, patients performed a computerized selective attention task. During the task, 2 stimuli composed of superimposed images of faces and houses were presented side by side. Patients were instructed by a cue to attend to either the face or the house component of the stimulus and to decide whether the 2 exemplars from the attended category were of the same person or house. Patients were cued to shift their attention from one stimulus component to the other every 4 to 7 trials. Performance accuracy and reaction time were obtained. The scopolamine assay is described in study 1.
Outcome Measures
The antidepressant and antianxiety responses to scopolamine were evaluated by assessing changes in MADRS and HARS scores, respectively. The Clinical Global Impressions–Improvement scale assessed overall clinical improvement. Secondary outcome measures included the VAS and POMS to assess acute changes in mood within each session. The Young Mania Rating Scale score was obtained to assess the possible development of manic symptoms.
Patients were characterized as achieving (1) a full response (≥50% reduction in MADRS scores from baseline), (2) a partial response (<50% but ≥25% reduction), or (3) no response (<25% reduction).38 Patients achieving remission (posttreatment MADRS score ≤10) also were identified.
Data Analysis
A group (placebo/scopolamine vs scopolamine/ placebo) ×repeated-measures (assessments) ANOVA was performed to evaluate overall group differences in MADRS, HARS, Clinical Global Impressions–Improvement scale, VAS, and POMS scores. To provide a balanced design allowing for group × study block × repeated-assessment analyses, MADRS and HARS data were separated into a baseline block (assessments 1 and 2), the first and last measures of block 1 (assessments 3 and 5), and block 2 (assessments 6 and 8). Between- and within-group t tests were used in planned comparisons to identify where significant effects occurred in the presence of significant overall ANOVAs. Area under the curve was evaluated in a repeated-measures analysis to determine whether scopolamine levels differed across administrations.
RESULTS
Patient Characteristics
Outpatients were recruited from May 28, 2004, through June 7, 2005, at the National Institute of Mental Health. Nineteen patients met the entrance criteria and were randomized to treatment. Another 20 patients were assessed for eligibility but were excluded for not meeting the entrance criteria (n=7) or for refusing to participate (n=13). Ten patients were randomized into the placebo/ scopolamine group and 9 into the scopolamine/placebo group. One patient in the scopolamine/placebo group withdrew after the first infusion (single-blind placebo). The remaining 18 patients (9 with MDD and 9 with BD) received the intended treatment, completed the protocol, and were included in all the analyses, except that the first patient entered was not assessed using the HARS. When follow-up evaluations could not be performed for the assessment after session 7 (n=3), analyses were performed using the last observation (from session 7) carried forward.
In the placebo/scopolamine group (n=10; 7 women and 3 men; 4 were African American, 4 were white, 1 was Hispanic, and 1 was Asian; mean±SD age, 35.1±8.5 years), 6 patients were chronically ill (>2 years), 3 had a co-morbid anxiety disorder, and 1 was unresponsive to previous treatment. In the scopolamine/placebo group (n=8; 7 women and 1 man; 3 were African American, 4 were white, and 1 was Hispanic; mean±SD age, 30.9±9.2 years), 6 patients were chronically ill, 5 had a comorbid anxiety disorder, and 4 were unresponsive to previous treatment. Thus, 13 of 18 patients had a poor prognosis for response to treatment, including 6 in the placebo/ scopolamine group and 7 in the scopolamine/placebo group, based on their history of response to conventional treatment, chronicity, and comorbid anxiety disorders.2,3,39,40
Outcome Indices
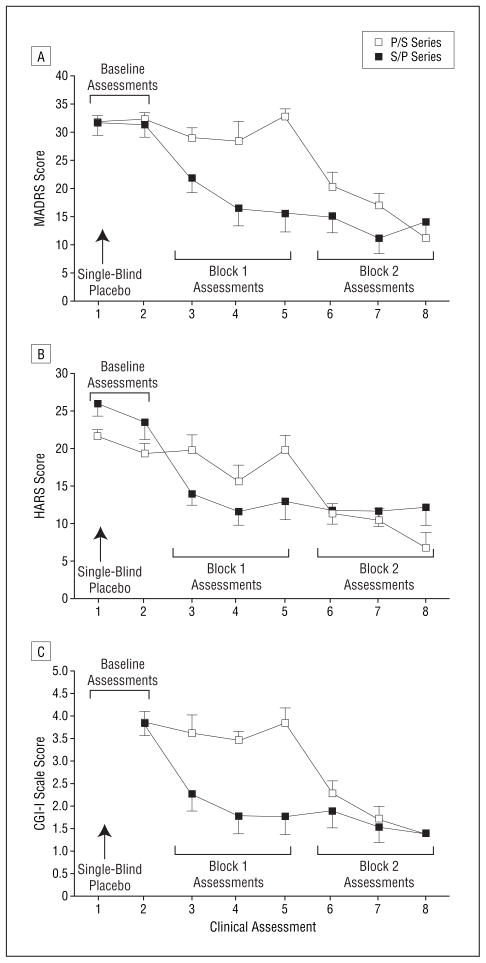
Outcome indices are summarized in Table 1. Mean±SE MADRS scores for the 2 groups across all 8 evaluations are given in Figure 3A. Repeated-measures ANOVA showed a significant group × assessment interaction (F=5.8; P<.001). The 3-way ANOVA (group × study block × assessment) also was significant (F=6.3; P=.005). The groups did not differ in MADRS scores at baseline (F=0.09; P>.20). The groups differed in study block 1 (F=26.7; P<.001; Cohen d=2.7; 95% confidence interval [CI], 1.5–3.9), with the scopolamine/placebo group having lower MADRS scores than the placebo/ scopolamine group, and this difference was significant by the first evaluation in study block 1 (t=2.5; P=.02). The 2 groups did not differ significantly in study block 2 (F = 0.16; P>.20), after both groups had received scopolamine.
Table 1.
Outcome Indices for Patients Treated With Scopolamine Hydrobromide*
| Baseline Block | Block 1 | Block 2 | |
|---|---|---|---|
| P/S group (n = 10) | |||
| CGI-I scale score (vs baseline)† | |||
| 1–2 | 0 | 0 | 9 |
| 3 | 3 | 2 | 1 |
| 4 | 5 | 6 | 0 |
| 5–6 | 2 | 2 | 0 |
| Full response (>50%) | 0 | 0 | 7 |
| Partial response (24%–49%) | 0 | 0 | 3 |
| Remission (MADRS score ≤10) | 0 | 0 | 6 |
| S/P group (n = 8) | |||
| CGI-I scale score (vs baseline)† | |||
| 1–2 | 0 | 6 | 7 |
| 3 | 2 | 2 | 1 |
| 4 | 4 | 0 | 0 |
| 5–6 | 2 | 0 | 0 |
| Full response (>50%) | 0 | 4 | 4 |
| Partial response (25%–49%) | 0 | 2 | 4 |
| Remission (MADRS score ≤10) | 0 | 3 | 4 |
Abbreviations: CGI-I, Clinical Global Impressions–Improvement; MADRS, Montgomery-Asberg Depression Rating Scale; P/S, placebo/scopolamine; S/P, scopolamine/placebo.
Data are given as number of patients.
In the CGI-I scale, 1 indicates very much improved; 2, much improved; 3, minimally improved; 4, no change; 5, minimally worse; and 6, much worse.
Figure 3.
Mean Montgomery-Asberg Depression Rating Scale (MADRS) (A), Hamilton Anxiety Rating Scale (HARS) (B), and Clinical Global Impressions–Improvement (CGI-I) scale (C) scores for the placebo/scopolamine hydrobromide (P/S) group and the scopolamine/ placebo (S/P) group across 8 assessments. Two baseline, 3 block 1, and 3 block 2 assessments are identified in each panel. Because the CGI-I scale reflects change from the first evaluation, no score is associated with assessment 1 (ie, baseline). Error bars represent SE. For each scale, there are significant group × assessment interactions (P<.001 for all).
Within-group analyses in the scopolamine/placebo group also showed that MADRS scores decreased in study block 1 (F=34.8; P<.001; Cohen d=2.2; 95% CI, 0.98–3.4) and in study block 2 (F=61.6; P<.001; Cohen d=2.6; 95% CI, 1.3–3.9) relative to baseline, and this effect was significant with the first assessment in study block 1 (t=4.7; P=.002). Within-group analyses in the placebo/ scopolamine group showed significantly lower MADRS scores in study block 2 compared with the baseline block (F=109.6; P<.001; Cohen d=3.2; 95% CI, 2.0–4.4) and study block 1 (F=94.1; P<.001; Cohen d=3.4; 95% CI, 2.2–4.6), and this effect was significant by the first assessment after scopolamine treatment (t=4.3; P=.002). The MADRS scores in the scopolamine/placebo group in block 2 trended toward a further reduction relative to block 1 (P=.07), indicating that the antidepressant effect persisted as this group received placebo in block 2. Within the scopolamine sessions, the reduction in MADRS scores in the second assessment relative to the first was significant in the placebo/scopolamine group (P=.04) and in the scopolamine/placebo group (P=.002), showing further reduction in symptom severity after repeated scopolamine administration. When considering each item of the MADRS independently as a means to determine which items contribute most to the observed antidepressant response, 9 of the 10 items were reduced significantly at the first assessment after the first infusion of scopolamine compared with the item score in the last session before receiving the drug (P<.01). The item regarding suicidal thoughts (item 10) approached a trend level of reduction (P=.11), although this is not surprising because we excluded patients who had suicidal ideation, and scores on this item at baseline were low. By study end, all the patients had at least a partial response, 11 of 18 had a full response, and 10 of 18 experienced remission.
Mean ± SE HARS scores for the 2 groups across evaluations are given in Figure 3B. Repeated-measures ANOVA identified a group × assessment interaction (F=5.34, P<.001). The 3-way ANOVA (group × study block × repeated assessment) identified a group × block interaction (F=13.4; P<.001). The groups differed in baseline HARS score (t=2.5; P=.03), so the effect of scopolamine on anxiety was evaluated in each group separately.
In the placebo/scopolamine group, a block × assessment analysis indicated differences among study blocks (F=40.1; P<.001) and a trend toward a difference between assessments (F=4.7; P=.06). Anxiety scores in the placebo/scopolamine group were lower in study block 2 compared with baseline (F=63.6) and block 1 (F=56.5) (P<.001 for both); this effect was significant with the first assessment in block 2 (t=8.6; P<.001). In study block 2, the second evaluation trended toward being lower than the first (P=.06), suggesting possible continued improvement in anxiety symptoms during repeated scopolamine infusion. In the scopolamine/placebo group, the study block × assessment analysis also identified differences among study blocks (F=32.23; P<.001). The HARS scores evaluated in block 1 were lower than those at baseline (F=48.76; P<.001). The HARS scores in the scopolamine/placebo group in block 2 were lower than at baseline (F=53.8; P<.001) and did not differ from the scores obtained in block 1 (F=0.69; P=.42), indicating that the antianxiety effect persisted as this group received placebo in study block 2.
To evaluate placebo responses, the 2 baseline measures (assessments 1 and 2) were compared. No difference was observed in MADRS scores (F=0.02; P=.90), but HARS scores were significantly lower during the second baseline assessment (F=6.6; P=.02). Within-group comparisons in the placebo/scopolamine group during block 1 placebo infusions indicate that there is no difference in MADRS (F=0.59; P=.45) or HARS (F=0.21; P=.65) scores obtained during baseline compared with scores in block 1, indicating that evidence of a placebo response was absent by the end of study block 1.
In the diagnostic subgroups, patients with BD (F=53.58) and MDD (F=44.85) (P<.001 for both) separately showed significant reductions in MADRS scores comparing study end with baseline. Consistently, we found no difference in the magnitude of change in MADRS (F=0.004; P=.98) or HARS (F=0.89; P=.36) scores based on diagnosis, suggesting that patients with MDD and BD did not differ in their responses to scopolamine, although the power to address differential effects across subtypes was low. Patients with a good prognosis for response showed larger reductions in MADRS scores than those with a poor prognosis (F=12.01; P=.003). No difference in anxiety response was found based on prognosis (F=2.7; P=.12).
The Clinical Global Impressions–Improvement scale (from session 2 through follow-up) showed a group × assessment interaction (F=6.0; P<.001) (Figure 3C). Individual t tests indicated that the scopolamine/placebo group had lower ratings than the placebo/scopolamine group for each evaluation obtained during block 1 (P<.002), indicating greater clinical improvement in the scopolamine/placebo group. No group difference was observed in ratings from study block 2 evaluations (P>.20), suggesting that the magnitude of clinical improvement did not differ after both groups received scopolamine.
The VAS and POMS ratings indicated that no acute, within-session change occurred during scopolamine use relative to placebo in ratings of depression (P=.30), irritability (P=.59), anxiety (P=.64), or tension (P=.62). Trends were observed toward increased sadness (P=.07) and decreased happiness (P=.08) during scopolamine use vs placebo, whereas confusion (P=.049) and sleepiness (P=.001) increased. No increase in mean ± SD Young Mania Rating Scale scores occurred in patients with BD during scopolamine treatment (P>.20) comparing baseline (4.3±2.2) with study end (2.4±1.2).
No overall change in reaction time on the selective attention task was seen during scopolamine (1839 milliseconds) vs placebo (1823 milliseconds) administration (F=0.15; P=.70). A significant drug× attention condition interaction was observed (F=4.45; P=.05), showing a selective increase in reaction time during the attention to houses condition. A small but significant reduction in performance accuracy was observed during scopolamine (82% correct) relative to placebo (88% correct) treatment (F=6.5; P=.02), although patients were performing well above chance levels during both conditions. Mean ± SD area under the curve estimates did not differ significantly across the 3 scopolamine infusions (135.4±74.3, 132.7±77.5, and 106.8±53.8, respectively).
Adverse Effects
Scopolamine was well-tolerated, and no medically serious adverse events were encountered. Adverse effects reported under the scopolamine and placebo conditions are summarized in Table 2.
Table 2.
Summary of Reported Adverse Effects After Placebo and Scopolamine Hydrobromide Infusions
| Adverse Effect | No. Reported |
|
|---|---|---|
| Placebo (n = 19) | Scopolamine (n = 18) | |
| Drowsiness | 18 | 18 |
| Dry mouth | 12 | 18 |
| Blurred vision | 5 | 17 |
| Lightheadedness | 8 | 17 |
| Dizziness | 0 | 6 |
| Hypotension (no intervention) | 0 | 4 |
| Nausea | 1 | 3 |
| Headache | 1 | 2 |
| Nervousness | 0 | 2 |
| Diplopia | 0 | 2 |
| Palpitations | 0 | 1 |
| Derealization | 1 | 1 |
| Mental clouding | 1 | 0 |
| Irritability | 0 | 1 |
| Restlessness | 0 | 1 |
| Euphoria | 1 | 1 |
COMMENT
Scopolamine produced rapid and robust antidepressant and antianxiety effects in patients with unipolar and bipolar depression. Because this study used a crossover design, the improvement observed independently in the 2 patient groups provided a within-study replication of the antidepressant effect. Thus, together with study 1, we demonstrated potent antidepressant responses to scopolamine in 3 independent samples. This finding is consistent with evidence that the cholinergic system is hypersensitive in depression.
Significant clinical responses were observed in the evaluation after the first scopolamine administration, 3 to 4 days after the first treatment. The assessments evaluated symptoms experienced since the previous visit, and, therefore, this finding indicated that the antidepressant and antianxiety effects were extremely rapid (before 3 days), particularly compared with the 3 to 4 weeks typically required for conventional treatments to become effective. Notably, patients reported experiencing marked improvement in clinical symptoms by the evening of or the morning after scopolamine administration. In addition, patients continued to improve across the 3 drug infusions, suggesting that repeated administrations provided more benefit than a single administration. In the individuals receiving scopolamine first, the improvement that occurred during drug administration persisted as these patients received placebo during block 2, indicating that the clinical effects persisted beyond the treatment period. The persistence of scopolamine’s antidepressant effect suggests a mechanism beyond the direct pharmacologic actions on muscarinic receptors.
The literature reports euphoria associated with the short-term administration of anticholinergics, and thus some physicians may question whether the effects reported herein are associated with anticholinergic euphoria. The antidepressant effects that developed after receiving scopolamine in the scopolamine/placebo group persisted across the placebo sessions, a finding that strongly argues against the idea that we are observing anticholinergic euphoria. Moreover, the within-session assessments using the VAS and the POMS identified no acute effects of scopolamine on mood state (ie, euphoria), and although 1 patient reported euphoria as an adverse effect after receiving scopolamine, 1 patient also reported euphoria as an adverse effect after receiving placebo.
The results of the POMS analysis indicated that patients acutely experienced elevations in confusion during scopolamine. Nonetheless, patients successfully performed the selective attention task with only modest evidence of impaired performance that remained well above chance levels, suggesting that the effect of scopolamine on cognitive functioning was fairly small.
The magnitude of the effect sizes of 2.2 to 3.4 in this study exceeded those typically observed in treatment studies for depression, which range from 0.5 to 1.1 in moderately and severely depressed cases, respectively.41 The large effect size we observed reflects the small and transient placebo response shown by these samples (as expected given the severity and chronicity of illness)42 and the robustness of the antidepressant effect. The latter is highlighted by the proportion of the sample achieving remission with scopolamine vs placebo (56%), which compares with 10% to 20% with antidepressant agents that lack anticholinergic effects compared with placebo.43 This robust response to scopolamine occurred in a patient sample that primarily had poor prognoses with respect to the likelihood of treatment response,2,3,39,40 suggesting that scopolamine may prove beneficial even for patients who are clinically difficult to treat.
A promising aspect of the present findings is the rapid onset of symptom relief observed with scopolamine treatment. One shortcoming of conventional antidepressant treatments is that the several-week delay needed to achieve clinically meaningful improvement prolongs patients’ vulnerability to suicide and disability. Treatments that produce antidepressant responses within 1 week—electroconvulsive therapy, high-dose TCA drug administration, total sleep deprivation, and ketamine use44–48—have not proved amenable to widespread clinical application because of their adverse effects or the transient nature of their therapeutic benefits. In contrast, the absence of serious adverse effects encountered in this study suggests that scopolamine may provide a relatively safe and well-tolerated intervention for achieving rapid antidepressant responses. Notably, electroconvulsive therapy and high-dose TCA drug administration are associated with potent antimuscarinic effects. Electroconvulsive therapy is routinely preceded by administration of the antimuscarinic agent atropine to reduce salivation and stabilize autonomic responses to generalized seizures.49 Whether atropine contributes to the antidepressant efficacy of electroconvulsive therapy has not been investigated, to our knowledge.
The extent to which antimuscarinic effects play a role in the antidepressant efficacy of TCAs also remains unclear. Several TCAs have sufficient muscarinic receptor affinity to produce peripheral anticholinergic adverse effects at parasympathetic neuroeffector junctions, which have much higher sensitivity to antimuscarinic drugs than central muscarinic receptors.50 Amitriptyline hydrochloride is one of the only TCAs with an affinity for muscarinic receptors that is nearly as great as that for relevant monoamine transporters,51–54 indicating that at therapeutic doses of amitriptyline where most serotonin transporter sites are occupied,55,56 a large proportion of muscarinic sites also are occupied. The difference in muscarinic receptor affinity across TCAs may explain why amitriptyline was the only antidepressant drug that proved more effective than more selective agents (eg, selective serotonin reuptake inhibitors) in inpatients with MDD.46,54,57 Nevertheless, in clinical practice, the amitriptyline dose is gradually titrated upward, so potentially rapid responses to full therapeutic amitriptyline doses would not have been detected.
The dose dependency of scopolamine’s antidepressant effect may indicate that a specific muscarinic receptor subtype confers the relevant mechanism of action. The only previous controlled study58 assessing antidepressant effects of an antimuscarinic agent found no significant difference between biperiden and glycopyrrolate (a peripheral antimuscarinic agent). However, biperiden is relatively selective for M1-type muscarinic receptors. In contrast, at M3 receptors, scopolamine is 10-fold more potent than bi-periden and 30-fold more potent than amitriptyline.
Other early studies exploring possible antidepressant effects of antimuscarinic agents reported modest and inconsistent antidepressant responses,59 although these were primarily uncontrolled studies. The powerful effects we report with scopolamine may have been missed because previous studies using this agent in depressed patients used lower effective doses15,60 or assessed clinical effects in the short term only (120 minutes).59 For example, small but significant antidepressant effects were observed the day after administration of scopolamine hydrobromide, 0.4 mg intramuscularly,60 which would approximate 2 μg/kg intravenously.33
Although the specific mechanism through which antimuscarinic effects may impact the pathogenesis of depression is unknown, an effect that scopolamine shares with other somatic antidepressant drug treatments involves modulation of N-methyl-D-aspartate receptor (NMDAR) function. The delay before the onset of the antidepressant response after scopolamine administration seems consistent with an effect on “late-response” gene transcription rather than a direct action on muscarinic receptors.61 The NMDAR gene expression is regulated by muscarinic receptor stimulation in some brain regions, and scopolamine reduces messenger RNA concentrations for NMDAR types 1A and 2A in the rat brain.62 Elevated glutamatergic transmission has been implicated in the pathogenesis of depression, and long-term administration of antidepressant drugs and repeated electro-convulsive shock reduce cortical NMDAR function.63–65 In addition, of treatments with relatively rapid onset, ketamine hydrochloride exerts direct NMDAR antagonist effects,47,63 and sleep deprivation induces internalization of NMDAR, reducing NMDAR function in hippocampal neurons.66,67
CONCLUSIONS
The findings reported herein are consistent with the hypothesis generated by Janowsky et al68 proposing that hypersensitivity of the cholinergic system plays a central role in the pathogenesis of mood disorders. The demonstration that an antimuscarinic agent produces potent antidepressant effects provides a strong link between muscarinic receptor function and mood disorders. Moreover, our results complement the previously reported findings that cholinergic enhancement using physostigmine induces symptoms of depression in currently manic individuals with BD and worsens symptoms in patients with unipolar depression.4,6
Determination of the optimal schedule of administration and the potential long-term use of scopolamine as an antidepressant agent requires further study, particularly because potential adverse effects include confusion and delirium. Future studies also may examine the antidepressant efficacy of scopolamine when using routes of administration that are more clinically practical in outpatient settings. Our results nevertheless hold promise that treatment with scopolamine may offer rapid relief of symptoms to individuals with depression.
Acknowledgments
Funding/Support: This research was supported by the Intramural Program of the National Institute of Mental Health, National Institutes of Health.
We thank Jane Lange and Alice Liu for technical support; Michelle Drevets for patient recruitment; David Luckenbaugh for statistical advice; and Earle Bain, Paul Carlson, and the 5SW Day Hospital nursing staff for medical support.
Footnotes
Additional Information: A use-patent application for the use of scopolamine as an antidepressant agent has been filed.
References
- 1.World Health Organization. The World Health Report 2001: Mental Health: New Understanding, New Hope. [Accessed November 9, 2005]; http://www.who.int/whr/2001/en/
- 2.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10–17. [PubMed] [Google Scholar]
- 3.Kupfer DJ, Spiker DG. Refractory depression: prediction of non-response by clinical indicators. J Clin Psychiatry. 1981;42:307–312. [PubMed] [Google Scholar]
- 4.Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Janowsky EC, Risch C, Janowsky DS. Effects of anesthesia on patients taking psychotropic drugs. J Clin Psychopharmacol. 1981;1:14–20. doi: 10.1097/00004714-198101000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Janowsky DS, el-Yousef MK, Davis JM, Hubbard B, Sekerke HJ. Cholinergic reversal of manic symptoms. Lancet. 1972;1:1236–1237. doi: 10.1016/s0140-6736(72)90956-7. [DOI] [PubMed] [Google Scholar]
- 7.Davis KL, Berger PA, Hollister LE, Defraites E. Physostigmine in mania. Arch Gen Psychiatry. 1978;35:119–122. doi: 10.1001/archpsyc.1978.01770250121012. [DOI] [PubMed] [Google Scholar]
- 8.Risch SC, Kalin NH, Janowsky DS. Cholinergic challenges in affective illness: behavioral and neuroendocrine correlates. J Clin Psychopharmacol. 1981;1:186–192. doi: 10.1097/00004714-198107000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Nurnberger JI, Jr, Jimerson DC, Simmons-Alling S, Tamminga C, Nadi NS, Lawrence D, Sitaram N, Gillin JC, Gershon ES. Behavioral, physiological, and neuroendocrine responses to arecoline in normal twins and “well state” bipolar patients. Psychiatry Res. 1983;9:191–200. doi: 10.1016/0165-1781(83)90043-4. [DOI] [PubMed] [Google Scholar]
- 10.Gillin JC, Sitaram N, Duncan WC. Muscarinic supersensitivity: a possible model for the sleep disturbance of primary depression? Psychiatry Res. 1979;1:17–22. doi: 10.1016/0165-1781(79)90023-4. [DOI] [PubMed] [Google Scholar]
- 11.Berger M, Riemann D, Hochli D, Spiegel R. The cholinergic rapid eye movement sleep induction test with RS-86: state or trait marker of depression? Arch Gen Psychiatry. 1989;46:421–428. doi: 10.1001/archpsyc.1989.01810050035006. [DOI] [PubMed] [Google Scholar]
- 12.Riemann D, Hohagen F, Krieger S, Gann H, Muller WE, Olbrich R, Wark HJ, Bohus M, Low H, Berger M. Cholinergic REM induction test: muscarinic supersensitivity underlies polysomnographic findings in both depression and schizophrenia. J Psychiatr Res. 1994;28:195–210. doi: 10.1016/0022-3956(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 13.Dilsaver SC. Pathophysiology of “cholinoceptor supersensitivity” in affective disorders. Biol Psychiatry. 1986;21:813–829. doi: 10.1016/0006-3223(86)90246-5. [DOI] [PubMed] [Google Scholar]
- 14.Janowsky DS, Risch SC, Huey LY, Kennedy B, Ziegler M. Effects of physostigmine on pulse, blood pressure, and serum epinephrine levels. Am J Psychiatry. 1985;142:738–740. doi: 10.1176/ajp.142.6.738. [DOI] [PubMed] [Google Scholar]
- 15.Janowsky DS, Overstreet DH. The Role of Acetylcholine Mechanisms in Mood Disorders. New York, NY: Raven Press; 1995. [Google Scholar]
- 16.Rubin RT, O’Toole SM, Rhodes ME, Sekula LK, Czambel RK. Hypothalamo-pituitary-adrenal cortical responses to low-dose physostigmine and arginine vasopressin administration: sex differences between major depressives and matched control subjects. Psychiatry Res. 1999;89:1–20. doi: 10.1016/s0165-1781(99)00085-2. [DOI] [PubMed] [Google Scholar]
- 17.Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, Kwon JM, Wu W, Dick DM, Rice J, Jones K, Nurnberger JI, Jr, Tischfield J, Porjesz B, Edenberg HJ, Hesselbrock V, Crowe R, Schuckit M, Begleiter H, Reich T, Goate AM, Bierut LJ. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13:1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- 18.Comings DE, Wu S, Rostamkhani M, McGue M, Iacono WG, MacMurray JP. Association of the muscarinic cholinergic 2 receptor (CHRM2) gene with major depression in women. Am J Med Genet. 2002;114:527–529. doi: 10.1002/ajmg.10406. [DOI] [PubMed] [Google Scholar]
- 19.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 20.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 21.Betin C, DeFeudis FV, Blavet N, Clostre F. Further characterization of the behavioral despair test in mice: positive effects of convulsants. Physiol Behav. 1982;28:307–311. doi: 10.1016/0031-9384(82)90080-4. [DOI] [PubMed] [Google Scholar]
- 22.Browne RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58:331–334. doi: 10.1016/0014-2999(79)90483-7. [DOI] [PubMed] [Google Scholar]
- 23.Overstreet DH, Russell RW, Hay DA, Crocker AD. Selective breeding for increased cholinergic function: biometrical genetic analysis of muscarinic responses. Neuropsychopharmacology. 1992;7:197–204. [PubMed] [Google Scholar]
- 24.Overstreet DH. The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev. 1993;17:51–68. doi: 10.1016/s0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- 25.Raisman R, Briley M, Langer SZ. Specific tricyclic antidepressant binding sites in rat brain. Nature. 1979;281:148–150. doi: 10.1038/281148a0. [DOI] [PubMed] [Google Scholar]
- 26.Richelson E. Antimuscarinic and other receptor-blocking properties of antidepressants. Mayo Clin Proc. 1983;58:40–46. [PubMed] [Google Scholar]
- 27.Stanton T, Bolden-Watson C, Cusack B, Richelson E. Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics. Biochem Pharmacol. 1993;45:2352–2354. doi: 10.1016/0006-2952(93)90211-e. [DOI] [PubMed] [Google Scholar]
- 28.Schatzberg AF. Employing pharmacologic treatment of bipolar disorder to greatest effect. J Clin Psychiatry. 2004;65(suppl 15):15–20. [PubMed] [Google Scholar]
- 29.Pacher P, Kecskemeti V. Trends in the development of new antidepressants: is there a light at the end of the tunnel? Curr Med Chem. 2004;11:925–943. doi: 10.2174/0929867043455594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado PL. How antidepressants help depression: mechanisms of action and clinical response. J Clin Psychiatry. 2004;65(suppl 4):25–30. [PubMed] [Google Scholar]
- 31.Preskorn SH. Rational drug discovery and SSRI’s. In: Preskorn SH, editor. Clinical Pharmacology of Selective Serotonin Reuptake Inhibitors. Caddo, Okla: Professional Communications Inc; 1996. [Google Scholar]
- 32.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 33.Ebert U, Grossmann M, Oertel R, Gramatte T, Kirch W. Pharmacokinetic-pharmacodynamic modeling of the electroencephalogram effects of scopolamine in healthy volunteers. J Clin Pharmacol. 2001;41:51–60. doi: 10.1177/00912700122009836. [DOI] [PubMed] [Google Scholar]
- 34.Khan A, Khan SR, Shankles EB, Polissar NL. Relative sensitivity of the Montgomery-Asberg Depression Rating Scale, the Hamilton Depression rating scale and the Clinical Global Impressions rating scale in antidepressant clinical trials. Int Clin Psychopharmacol. 2002;17:281–285. doi: 10.1097/00004850-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 36.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 37.McNair DM, Lorr M, Droppleman LF. EITS Manual for the Profile of Mood States. San Diego, Calif: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 38.Nierenberg AA, DeCecco LM. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):5–9. [PubMed] [Google Scholar]
- 39.Bagby RM, Ryder AG, Cristi C. Psychosocial and clinical predictors of response to pharmacotherapy for depression. J Psychiatry Neurosci. 2002;27:250–257. [PMC free article] [PubMed] [Google Scholar]
- 40.Klein DN, Schatzberg AF, McCullough JP, Dowling F, Goodman D, Howland RH, Markowitz JC, Smith C, Thase ME, Rush AJ, LaVange L, Harrison WM, Keller MB. Age of onset in chronic major depression: relation to demographic and clinical variables, family history, and treatment response. J Affect Disord. 1999;55:149–157. doi: 10.1016/s0165-0327(99)00020-8. [DOI] [PubMed] [Google Scholar]
- 41.Khan A, Brodhead AE, Kolts RL, Brown WA. Severity of depressive symptoms and response to antidepressants and placebo in antidepressant trials. J Psychiatr Res. 2005;39:145–150. doi: 10.1016/j.jpsychires.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Khan A, Dager SR, Cohen S, Avery DH, Scherzo B, Dunner DL. Chronicity of depressive episode in relation to antidepressant-placebo response. Neuropsychopharmacology. 1991;4:125–130. [PubMed] [Google Scholar]
- 43.Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry. 2001;178:234–241. doi: 10.1192/bjp.178.3.234. [DOI] [PubMed] [Google Scholar]
- 44.Segman RH, Shapira B, Gorfine M, Lerer B. Onset and time course of antidepressant action: psychopharmacological implications of a controlled trial of electroconvulsive therapy. Psychopharmacology (Berl) 1995;119:440–448. doi: 10.1007/BF02245860. [DOI] [PubMed] [Google Scholar]
- 45.Pollock BG, Perel JM, Nathan RS, Kupfer DJ. Acute antidepressant effect following pulse loading with intravenous and oral clomipramine. Arch Gen Psychiatry. 1989;46:29–35. doi: 10.1001/archpsyc.1989.01810010031005. [DOI] [PubMed] [Google Scholar]
- 46.Deisenhammer EA, Whitworth AB, Geretsegger C, Kurzthaler I, Gritsch S, Miller CH, Fleischhacker WW, Stuppack CH. Intravenous versus oral administration of amitriptyline in patients with major depression. J Clin Psychopharmacol. 2000;20:417–422. doi: 10.1097/00004714-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 48.Gerner RH, Post RM, Gillin JC, Bunney WE., Jr Biological and behavioral effects of one night’s sleep deprivation in depressed patients and normals. J Psychiatr Res. 1979;15:21–40. doi: 10.1016/0022-3956(79)90004-9. [DOI] [PubMed] [Google Scholar]
- 49.Kramer BA. Anticholinergics and ECT. Convuls Ther. 1993;9:293–300. [PubMed] [Google Scholar]
- 50.Baldessarini RJ. Drug therapy of depression and anxiety disorders. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman’s: The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill Medical Publishing Division; 2006. pp. 429–459. [Google Scholar]
- 51.Bolden-Watson C, Richelson E. Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci. 1993;52:1023–1029. doi: 10.1016/0024-3205(93)90194-8. [DOI] [PubMed] [Google Scholar]
- 52.Cusack B, Nelson A, Richelson E. Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl) 1994;114:559–565. doi: 10.1007/BF02244985. [DOI] [PubMed] [Google Scholar]
- 53.Hyttel J, Larsen JJ, Christensen AV, Arnt J. Receptor-binding profiles of neuroleptics. Psychopharmacology Suppl. 1985;2:9–18. doi: 10.1007/978-3-642-70140-5_2. [DOI] [PubMed] [Google Scholar]
- 54.Richelson E. Are receptor studies useful for clinical practice? J Clin Psychiatry. 1983;44:4–9. [PubMed] [Google Scholar]
- 55.Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, Houle S. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry. 2001;158:1843–1849. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- 56.Preskorn SH. Clinical Pharmacology of Selective Serotonin Reuptake Inhibitors. Caddo, Okla: Professional Communications Inc; 1996. [Google Scholar]
- 57.Anderson IM. SSRIs versus tricyclic antidepressants in depressed inpatients: a meta-analysis of efficacy and tolerability. Depress Anxiety. 1998;7(suppl 1):11–17. [PubMed] [Google Scholar]
- 58.Richelson E. Cholinergic transduction. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Raven Press; 1995. pp. 125–134. [Google Scholar]
- 59.Newhouse PA, Sunderland T, Tariot PN, Weingartner H, Thompson K, Mellow AM, Cohen RM, Murphy DL. The effects of acute scopolamine in geriatric depression. Arch Gen Psychiatry. 1988;45:906–912. doi: 10.1001/archpsyc.1988.01800340028004. [DOI] [PubMed] [Google Scholar]
- 60.Gillin JC, Sutton L, Ruiz C, Darko D, Golshan S, Risch SC, Janowsky D. The effects of scopolamine on sleep and mood in depressed patients with a history of alcoholism and a normal comparison group. Biol Psychiatry. 1991;30:157–169. doi: 10.1016/0006-3223(91)90170-q. [DOI] [PubMed] [Google Scholar]
- 61.Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia, Pa: Lippincott Williams & Wilkins; 2002. Regulation of gene expression; pp. 217–228. [Google Scholar]
- 62.Liu HF, Zhou WH, Xie XH, Cao JL, Gu J, Yang GD. Muscarinic receptors modulate the mRNA expression of NMDA receptors in brainstem and the release of glutamate in periaqueductal grey during morphine withdrawal in rats [in Chinese] Sheng Li Xue Bao. 2004;56:95–100. [PubMed] [Google Scholar]
- 63.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7(suppl 1):S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 64.Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann N Y Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- 65.Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- 66.Chen C, Hardy M, Zhang J, Lahoste GJ, Bazan NG. Altered NMDA receptor trafficking contributes to sleep deprivation-induced hippocampal synaptic and cognitive impairments. Biochem Biophys Res Commun. 2006;340:435–440. doi: 10.1016/j.bbrc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 67.McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol. 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]