Abstract
Purpose
Circulating free DNA (cfDNA) in plasma is promising to be a surrogate for tumor tissue DNA. However, not all epidermal growth factor receptor (EGFR) mutations in tumor tissue DNA has been detected in matched cfDNA, at least partly due to inefficient cfDNA extraction method. The purpose of this study was to establish an efficient plasma cfDNA extraction protocol.
Materials and Methods
The yield of plasma cfDNA extracted by our modified phenol-chloroform (MPC) method from non-small-cell lung cancer (NSCLC) patients was compared with that by QIAamp MinElute Virus Spin kit (Qiagen kit) as control, using the Wilcoxon rank-sum test. TaqMan quantitative polymerase chain reaction (qPCR) assays were used to quantify the plasma cfDNA extracted. Both Mutant-enriched PCR (ME-PCR) coupled sequencing and DxS EGFR mutation test kit were used to evaluate the impact of extraction method on EGFR mutation analysis.
Results
MPC method extracted more plasma cfDNA than Qiagen kit method (p=0.011). The proportion of longer fragment (≥202 bp) in cfDNA extracted by MPC method was significantly higher than by Qiagen kit method (p=0.002). In the sequencing maps of ME-PCR products, a higher mutant peak was observed on plasma cfDNA extracted by MPC method than by Qiagen kit method. In DxS EGFR mutation test kit results, plasma cfDNA extracted by MPC method contained more tumor-origin DNA than by Qiagen kit method.
Conclusion
An improved plasma cfDNA extraction method of MPC is provided, which will be beneficial for EGFR mutation analysis for patients with NSCLC.
Keywords: Circulating free DNA, DNA extraction, methodology, EGFR mutation, non-small-cell lung cancer
INTRODUCTION
Non-small-cell lung cancer (NSCLC) accounts for about 80% of all patients with lung cancer,1 and most of NSCLC patients are first diagnosed at advanced stage. In the treatment of advanced NSCLC, chemotherapy is the mainstay but the response rate is only 17-22%.2 Tyrosine kinase inhibitors (TKI) targeting epidermal growth factor receptor (EGFR) have been used for the treatment of advanced NSCLC, and EGFR activating mutation, which occurs in 30-50% of NSCLC patients in Asian and in 10% in Caucasian, has been proved to be the critical factor for TKI efficacy.3-5 The response rate is around 75% for NSCLC patients with tumor EGFR activating mutation versus less than 10% for those with wild-type EGFR.6 Therefore, EGFR mutation analysis is important for decision of TKI therapy for NSCLC patients.
Tumor tissue is the most optimal sample type for genomic DNA extraction for EGFR mutation analysis. However, tumor tissue samples are not always available from advanced NSCLC patients in clinical practice. Circulating free DNA (cfDNA) in plasma has thus been used as a surrogate for tumor tissue DNA for EGFR mutation analysis, and results from preliminary studies seemed promising.7-9 However, not all EGFR mutations in tumor tissue DNA has been detected in matched cfDNA,7-9 at least partly due to inefficient cfDNA extraction method. In the present study, we provided an improved plasma cfDNA extraction method, i.e. modified phenol-chloroform (MPC) method. Since QIAamp MinElute Virus Spin kit, in comparison with several other commercially available kits, has been reported to yield the highest amount of cfDNA,10 we compared our MPC method to Qiagen kit method on cfDNA extraction yield and the impact of different extraction method on EGFR mutation analysis.
MATERIALS AND METHODS
Subjects and samples
A total of 25 patients with advanced lung adenocarcinoma were recruited from No.3 People's Hospital, School of Medicine, Shanghai Jiaotong University, China between March and September 2009. Written informed consent was obtained from each subject. This study was approved by the ethics review committee of the Institutional Review Board of the hospital.
Five mL of peripheral whole blood were collected from each subject, which was put into an EDTA-Vacutainer tube (BD, Plymouth, UK) to separate plasma. All samples were processed at room temperature within 2 h from the time of blood drawing. Plasma was separated from the cellular fraction by centrifugation at 2,500 g for 10 min at 4℃. After centrifugation, the supernatant was transferred to eppendorf tube and stored immediately at -80℃ until cfDNA extraction.
Extraction of cfDNA
The stored plasma sample was thawed at room temperature and centrifuged at 15,700 g for 15 min at 4℃ to remove residual precipitated cellular components. Three methods were applied for the extraction of cfDNA, i.e. MPC method, traditional phenol-chloroform (PC) method, and commercial QIAamp MinElute Virus Spin kit (Qiagen kit).
For PC/MPC method, 1 mL of plasma was mixed with 50 µL of 25% sodium lauryl sulfate (SDS) and 30 µL of 20 mg/mL proteinase K (Qiagen, Hilden, Germany) and incubated at 55℃ for 16 h. After digestion, equal volume of water-saturated phenol was added into the sample and mixed. Then, the mixture was centrifuged at 16,000 g at room temperature for 15 min (PC method), or transferred into a Phase Lock Gel tube (MaXtract Low Density tube, Qiagen, Hilden, Germany) followed by centrifugation at 16,000 g at room temperature for 5 min (MPC method). The supernatant was transferred into a new 2 mL eppendorf tube and mixed with equal volume of chloroform/isoamyl alcohol (24 : 1) mixture. The mixture was centrifuged at 16,000 g for 15 min (PC method), or transferred into a Phase Lock Gel tube (MaXtract High Density tube, Qiagen, Hilden, Germany) followed by centrifugation at 16,000 g at room temperature for 5 min (MPC method). Sodium acetate (3 mM) with 1 : 10 ratio of the supernatant and equal volume of isopropanol were added for DNA precipitation overnight at -20℃. DNA was dissolved in 50 µL of DNA hydration solution (Qiagen, Hilden, Germany) and stored at -20℃ until use.
For Qiagen kit (Qiagen, Hilden, Germany) method, experiment was performed according to manufacturer's protocol, with the exception of increasing sample volume to 1 mL and accordingly adding the reagent amount to 5-fold. Briefly, 1 mL of plasma was mixed with 62.5 µL of protease and 500 µL of buffer AL. After incubation at 56℃ for 15 min, 250 µL ethanol was added. The mixture was filtered through the column and rinsed by AW1 and AW2 in order. DNA was dissolved in 50 µL of buffer AVE.
Quantification and purity measurement of plasma cfDNA
TaqMan quantitative polymerase chain reaction (qPCR) assays targeting at DNA template of a single copy gene SERPINA1, whose copy number is confirmed to be relatively stable in solid tumors and has been used as internal reference gene indicating copy number of genomic DNA, were used to quantify the extracted cfDNA on ABI7900 real time bePCR machine (Applied Biosystems, Foster City, CA, USA). Forward primers for 77 bp, 123 bp and 202 bp PCR products were 5'-TAC TCA AGG GAA AAT TGT GGA TTT-3', 5'-ACA CCG AAG AGG CCA AGA A-3' and 5'-GGC CTG AAG CTA GTG GAT AAG TT-3', respectively. Common reverse primer was 5'-AGA AGA TGT AAT TCA CCA GAG CAA A-3'. The probe sequence was 5'-FAM-TGT GTC TCT GTC AAG CTC CTT GAC-3'BHQ1. The 15 µL reaction mixture contained 7.5 µL platinum qPCR superMix-UDG (Invitrogen, Paisley, UK), 4.3 µL of pure water, 0.6 µL of 0.1 µM/L primer, 0.6 µL of 0.2 µM/L probe, and 2 µL of cfDNA. PCR was performed at 95℃ for 10 min, followed by 40 cycles of 94℃ for 30 s, 60℃ for 60 s and 72℃ for 15 s. The results were analyzed with software SDS 2.3. Each sample was tested in three replicates and their average was used in the statistical analysis. Quantity of cfDNA was expressed in copies/mL.
Purity of the plasma cfDNA extracted by PC, MPC and Qiagen kit method was spectrophotometrically evaluated using OD (260 nm/280 nm) value determined with the NanoDrop spectrometer ND-1000 (NanoDrop, Rockland, Delaware, USA).
EGFR mutation analysis
For all the 25 patients recruited in the present study, tumor tissue DNA has previously been examined for mutation in exon 19 of the EGFR gene by PCR-based sequencing, and only one small fragmental deletion, 2235-2249delGGAATTAAGAGAAGC, was identified in one patient (unpublished data). Thus, EGFR mutation analysis was performed on plasma cfDNA from this patient, by Mutant-enriched PCR (ME-PCR) coupled sequencing and DxS EGFR mutation test kit. Plasma cfDNA extracted by MPC and Qiagen kit method was used for EGFR mutation analysis.
The principle of the ME-PCR coupled sequencing method was that a wild-type specific enzyme digestion step was inserted before the second PCR of the nest-PCR process, which enriched mutant-type PCR products for subsequent sequencing analysis.8 For the 1st step PCR, forward and reverse primers were 5'-ATC CCA GAA GGT GAG AAA GAT AAA ATT C-3' and 5'-CCT GAG GTT CAG AGC CAT GGA-3', respectively. PCR reaction mixture contained 25 µL of AmpliTaq Gold PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 1 µL of 2.5 M primer (Invitrogen, Paisley, UK), 1.5 µL of 20 mg/mL BSA, 1.5 µL of pure water and 20 µL of cfDNA. PCR was performed at 95℃ for 10 min followed by 35 cycles of 94℃ for 30 s, 56℃ for 60 s, and 72℃ for 60 s. A 5 µL aliquot of 1st PCR product was digested for 4 h at 37℃ in a 20 µL reaction volume containing 5 units of Mse I enzyme (New England Biolabs, Beverly, MA, USA), to digest the TTAA sequence within the deletion target region of wild-type DNA. For the 2nd PCR, forward and reverse primers were 5'-ACT GTA AAA CGA CGG CCA GTA TCC CAG AAG GTG AGA AAG ATA AAA TTC-3' and 5'-ACC AGG AAA CAG CTA TGA CCA CAC AGC AAA GCA GAA ACT CAC ATC GAG-3', respectively. The 2nd PCR was performed with 25 µL reaction volume containing 5 µL of the digested product above and the same concentrations of primers and AmpliTaq Gold PCR Master Mix as the 1st PCR reaction, with the cycling condition of 95℃ for 10 min followed by 40 cycles of 94℃ for 30 s, 60℃ for 30 s, 72℃ for 40 s, ended with 72℃ for 10 min. The 2nd PCR product was applied for Sanger sequencing according to the standard protocol in the manual of ABI prism 3730XL DNA analyzer (Applied Biosystems, Foster City, CA, USA) using common M13R primer (sequence: ACC AGG AAA CAG CTA TGA CC). Sequencing results were analyzed using the SeqScape software v2.5 (Applied Biosystems, Foster City, CA, USA).
A DxS EGFR mutation test kit (DxS Ltd, Manchester, UK), which combines amplification refractory mutation system and Scorpion technologies, was also used to detect known EGFR mutations in real-time PCR as described previously.11,12 All reactions were performed in 25 uL volumes including 5 uLof template DNA, 16 uL of reaction buffer mix, 0.6 uL of Taq polymerase and 3.4 uL water. Real-time PCR was carried out by using MX3005P real-time PCR machine (Stratagene, La Jolla, CA, USA) under the following conditions: initial denaturation at 95℃ for 10 min, 45 cycles of 95℃ 30 s, 61℃ 60 s with fluorescence FAM reading at the end of each cycle. Data analysis was performed with MxPro v4.10 (Stratagene, La Jolla, CA, USA). The Cycle threshold (Ct) represents the threshold at which the signal is detected above background fluorescence. Sample ΔCt values are calculated as the difference between the mutation Ct and control Ct. If the sample's ΔCt is lower than the cut-off ΔCt value, it is judged as positive for a mutation detected by this assay. The cut-off ΔCt value is 12 for deletion in exon 19 of EGFR. The bigger the ΔCt is, the less mutation the sample contains.
Statistical analysis
The nonparametric comparison of median cfDNA yield between groups were evaluated using the Wilcoxon rank-sum test. Exact chi-square test was used to evaluate the difference in fragment distribution of cfDNA between MPC method and Qiagen kit method. All tests were two-sided and a p value of less than 0.05 was considered significant. Statistical analyses were conducted using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA).
RESULTS
Comparison of yield and purity of the plasma cfDNA extracted by MPC, PC and Qiagen kit method
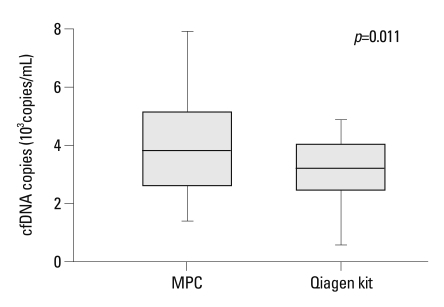
Plasma cfDNA was extracted from all 25 patients by MPC, PC and Qiagen kit methods. As expected, MPC method extracted more cfDNA than PC method (p=0.005). When compared with the Qiagen kit method, significantly elevated cfDNA yield was also observed by MPC method (p=0.011) (Fig. 1).
Fig. 1.
Comparison of cfDNA yield extracted by modified phenol-chloroform (MPC) method and QIAamp MinElute Virus Spin kit (Qiagen kit).
In contrast to the moderate impurities of plasma cfDNA extracted by PC method, the MPC and Qiagen kit methods extracted similarly better purity of cfDNA, as determined by spectrophotometric measurements (data not shown).
Comparison of fragment distribution between cfDNA extracted by MPC and Qiagen kit method
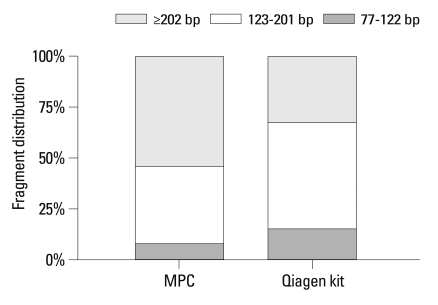
The percentage of cfDNA size of 77-122 bp, 123-201 bp and ≥202 bp extracted by MPC method were 8.2%, 37.6% and 54.2%, respectively, whereas 15.4%, 52.4% and 32.2%, respectively, extracted by Qiagen kit (Fig. 2). The proportion of longer fragment (≥202 bp) in cfDNA extracted by MPC method (54.2%) was significantly higher than by Qiagen kit method (32.2%, p=0.002) (Fig. 2).
Fig. 2.
Comparison of fragment distribution of plasma cfDNA extracted by modified phenol-chloroform (MPC) method and QIAamp MinElute Virus Spin kit (Qiagen kit).
Impact of extraction method on EGFR mutation analysis
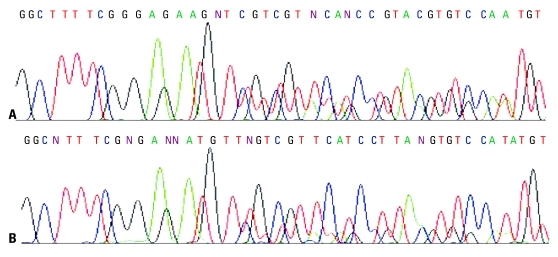
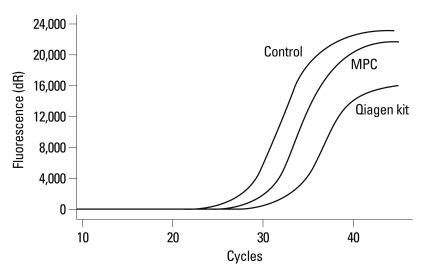
ME-PCR coupled sequencing method and the DxS EGFR mutation test kit were used for EGFR mutation analysis of plasma cfDNA from one patient with a known EGFR fragmental deletion in exon 19, to evaluate the impact of extraction method on EGFR mutation analysis. In the sequencing maps of ME-PCR products, the mutant peak, based on plasma cfDNA extracted by MPC method (Fig. 3A), was higher than that on plasma cfDNA extracted by Qiagen kit (Fig. 3B). In DxS EGFR mutation test kit results, ΔCt of amplification of cfDNA extracted by MPC method and Qiagen kit was 2.24 and 5.45, respectively (Fig. 4).
Fig. 3.
Sequencing maps of known EGFR deletion in exon 19 of plasma cfDNA. cfDNA was extracted by modified phenol-chloroform (MPC) method (A) and QIAamp MinElute Virus Spin kit (Qiagen kit) (B). In (A), the height of mutant peak is similar to that of wild-type peak, while the height of mutant peak in (B) is much lower than that of wild-type peak. EGFR, epidermal growth factor receptor.
Fig. 4.
Amplification plot of cfDNA extracted by modified phenol-chloroform (MPC) method and QIAamp MinElute Virus Spin kit (Qiagen kit) in DxS EGFR mutation test kit. EGFR, epidermal growth factor receptor.
DISCUSSION
Increasing evidence has shown that gene mutation in tumor tissue DNA can be detected in blood using cfDNA.7,8,12 But, three key characteristics of cfDNA in blood from cancer patients hindered the establishment of a reliable and standardized cfDNA extraction method, including that 1) cfDNA concentration is very low with only 10-1,200 ng/mL in plasma;13 2) cfDNA fragment is relatively short with peak fragment of around 180 bp;14 and 3) the percentage of tumor- origin DNA in cfDNA can be very low, especially in serum cfDNA versus plasma cfDNA,10,15,16 since cfDNA is derived from both tumor cells and non-tumor cells.17,18 In the present study, we described an improved cfDNA extraction method, i.e. MPC method, by incorporating Qiagen MaXtract Low and High Density tubes into the traditional PC method, which markedly elevated cfDNA yield in plasma. In addition, this modification offered a better separation between supernatant layer and organic solvents layer, thus minimizing the contamination of PCR inhibition in the extracted cfDNA, as well as making manipulation process much easier and safer. Purity comparision showed that, in contrast to the moderate impurities of plasma cfDNA extracted by PC method which is consistent with previous data by Müller, et al.,19 the purity of cfDNA extracted by MPC method was better, similar to that by Qiagen kit method.
By comparing several commercially available cfDNA extraction kits, Board, et al.10 reported that QIAampVirus Spin kit was the one yielding the highest amount of plasma cfDNA from patients with small cell lung cancer (SCLC).Therefore, we chose QIAamp Virus Spin kit as a reference method to test the performance of the MPC method for cfDNA extraction yield in plasma. Our data showed that MPC method extracted more plasma cfDNA than Qiagen kit method, and that cfDNA extracted by MPC method contained higher percentage of longer fragment (≥202 bp). cfDNA extracted by MPC method was more intact than by Qiagen kit method. Furthermore, we compared the impact of different extraction method of MPC or Qiagen kit on EGFR mutation analysis. Results from both sequencing maps of ME-PCR products and DxS EGFR mutation test suggested that MPC method is superior to Qiagen kit on plasma cfDNA extraction for analysis of mutant EGFR. It has been suggested that tumor-specific cfDNA is enriched in the DNA portion containing shorter fragments, while serum cfDNA isolated by the Qiagen kit method has been demonstrated to be enriched in high-molecular-weight DNA.19,20 Thus, the enhanced sensitivity of detection of mutant EGFR by MPC method versus Qiagen kit method may partly be attributable to more tumor-specific cfDNA extracted by MPC method. Considering that small DNA molecules are partially lost by the Qiagen kit extraction method,19,20 a new kit from Qiagen for the extraction of cfDNA, QIAmp Circulating Nucleic Acid Kit, has most recently been developed, which improves the extraction of small DNA fragments. Further studies are needed to compare our MPC method to the new Qiagen kit method in cfDNA extraction yield, as well as fragment distribution and the impact on EGFR mutation analysis.
In summary, we described an improved cfDNA extraction method of MPC which will be beneficial for EGFR mutation analysis for patients with NSCLC. Additional studies with sufficient number of patients are needed to validate the efficiency and reliability of the MPC method for cfDNA extraction.
ACKNOWLEDGEMENTS
This work was supported by Shanghai Education Committee Foundation grant (No. 08YZ47) and Shanghai Science and Technology Committee Foundation grant (No. 10JC 1409200). We thank technician Yun Sun from Tumor Genetics Capability, Innovation Center China, Astrazeneca Global R&D, Shanghai, China, for her kindly technical assistance.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123:21S–49S. doi: 10.1378/chest.123.1_suppl.21s. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Jänne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J Clin Oncol. 2005;23:3227–3234. doi: 10.1200/JCO.2005.09.985. [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 6.Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 7.Bai H, Mao L, Wang HS, Zhao J, Yang L, An TT, et al. Epidermal growth factor receptor mutations in plasma DNA samples predict tumor response in Chinese patients with stages IIIB to IV non-small-cell lung cancer. J Clin Oncol. 2009;27:2653–2659. doi: 10.1200/JCO.2008.17.3930. [DOI] [PubMed] [Google Scholar]
- 8.He C, Liu M, Zhou C, Zhang J, Ouyang M, Zhong N, et al. Detection of epidermal growth factor receptor mutations in plasma by mutant-enriched PCR assay for prediction of the response to gefitinib in patients with non-small-cell lung cancer. Int J Cancer. 2009;125:2393–2399. doi: 10.1002/ijc.24653. [DOI] [PubMed] [Google Scholar]
- 9.Kuang Y, Rogers A, Yeap BY, Wang L, Makrigiorgos M, Vetrand K, et al. Noninvasive detection of EGFR T790M in gefitinib or erlotinib resistant non-small cell lung cancer. Clin Cancer Res. 2009;15:2630–2636. doi: 10.1158/1078-0432.CCR-08-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Board RE, Williams VS, Knight L, Shaw J, Greystoke A, Ranson M, et al. Isolation and extraction of circulating tumor DNA from patients with small cell lung cancer. Ann N Y Acad Sci. 2008;1137:98–107. doi: 10.1196/annals.1448.020. [DOI] [PubMed] [Google Scholar]
- 11.Kimura H, Kasahara K, Kawaishi M, Kunitoh H, Tamura T, Holloway B, et al. Detection of epidermal growth factor receptor mutations in serum as a predictor of the response to gefitinib in patients with non-small-cell lung cancer. Clin Cancer Res. 2006;12:3915–3921. doi: 10.1158/1078-0432.CCR-05-2324. [DOI] [PubMed] [Google Scholar]
- 12.Kimura H, Suminoe M, Kasahara K, Sone T, Araya T, Tamori S, et al. Evaluation of epidermal growth factor receptor mutation status in serum DNA as a predictor of response to gefitinib (IRESSA) Br J Cancer. 2007;97:778–784. doi: 10.1038/sj.bjc.6603949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 14.Suzuki N, Kamataki A, Yamaki J, Homma Y. Characterization of circulating DNA in healthy human plasma. Clin Chim Acta. 2008;387:55–58. doi: 10.1016/j.cca.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Lee TH, Montalvo L, Chrebtow V, Busch MP. Quantitation of genomic DNA in plasma and serum samples: higher concentrations of genomic DNA found in serum than in plasma. Transfusion. 2001;41:276–282. doi: 10.1046/j.1537-2995.2001.41020276.x. [DOI] [PubMed] [Google Scholar]
- 16.Taback B, O'Day SJ, Hoon DS. Quantification of circulating DNA in the plasma and serum of cancer patients. Ann N Y Acad Sci. 2004;1022:17–24. doi: 10.1196/annals.1318.004. [DOI] [PubMed] [Google Scholar]
- 17.van der Drift MA, Hol BE, Klaassen CH, Prinsen CF, van Aarssen YA, Donders R, et al. Circulating DNA is a non-invasive prognostic factor for survival in non-small cell lung cancer. Lung Cancer. 2010;68:283–287. doi: 10.1016/j.lungcan.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Yoon KA, Park S, Lee SH, Kim JH, Lee JS. Comparison of circulating plasma DNA levels between lung cancer patients and healthy controls. J Mol Diagn. 2009;11:182–185. doi: 10.2353/jmoldx.2009.080098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller I, Beeger C, Alix-Panabières C, Rebillard X, Pantel K, Schwarzenbach H. Identification of loss of heterozygosity on circulating free DNA in peripheral blood of prostate cancer patients: potential and technical improvements. Clin Chem. 2008;54:688–696. doi: 10.1373/clinchem.2007.099333. [DOI] [PubMed] [Google Scholar]
- 20.Wang M, Block TM, Steel L, Brenner DE, Su YH. Preferential isolation of fragmented DNA enhances the detection of circulating mutated k-ras DNA. Clin Chem. 2004;50:211–213. doi: 10.1373/clinchem.2003.026914. [DOI] [PubMed] [Google Scholar]