Abstract
Simple and efficient technologies for intradermal immunization have recently been developed, making cutaneous vaccination a valid alternative for vaccine delivery. This raises an urgent need for safe and potent adjuvants suitable for cutaneous vaccination. Many traditional adjuvants like aluminum-based adjuvants may not be appropriate for boosting cutaneous immunization because they evoke strong and persistent inflammation in the skin that would potentially breach its integrity with serious consequences. Laser vaccine adjuvant is induced by brief illumination of a small area of the skin with a safe, noninvasive laser prior to intradermal injection of the vaccine into the site of illumination. It does not stimulate overt inflammation or reactogenicity in the skin and boosts immune responses via enhancing the motility of antigen-presenting cells. Laser vaccine adjuvant is convenient, safe and ideal for augmentation of cutaneous immunization and has distinct advantages over conventional adjuvants, in particular when encountering vaccine shortages during an unpredictable event.
Keywords: adjuvant, cell motility, cutaneous vaccination, laser, noninflammation
Vaccination remains one of the most cost-effective strategies to prevent the spread of infections and protect a large population from infection-induced morbidity and mortality. Current vaccines are mainly administered intramuscularly, which, although relatively convenient and readily managed, delivers the antigen directly into the muscular tissue where antigen-presenting cells (APCs) are scarce. On the contrary, the skin is rich in APCs and it is a preferable site for vaccine delivery, but intradermal (id.) injection currently faces some technical difficulties that limit its use in the clinic. With significant progress made in the development of various microinjection systems specifically for id. injection in the past decade [1], id. vaccination has been widely investigated in recent clinical trials for influenza vaccines [2,3]. It is expected to be a valid alternative to deliver other vaccines in the near future, because it is easier to use, more effective and safer than intramuscular (im.) vaccination [4]. However, this more effective and less invasive route of vaccination demands safer and less inflammatory adjuvants that can sufficiently boost immune responses while warranting the integrity of the skin, crucial to the first line of the body’s defense. In this article, we will discuss how a newly developed laser vaccine adjuvant (LVA) can potentially act as a universal adjuvant for cutaneous vaccination [5].
Cutaneous immunization
The skin is an immunologically active tissue with an abundance of resident APCs, in marked contrast to the muscular tissue. Approximately 40% of the body’s APCs are located in the skin including epidermal Langerhans cells and dermal dendritic cells (DCs). There is also a thick network of capillary lymphatic vessels in the skin that direct the passage of antigens and antigen-captured DCs from the skin to the draining lymph nodes. The first vaccine, smallpox, was delivered by skin scarification nearly two centuries ago [6]. A body of evidence has consistently shown that id. vaccination is superior to im. in the clinic. For instance, id. delivery of one fifth of the influenza vaccine dosage could induce the same level of protection as im. vaccination in humans [7,8]. id. administration of unadjuvanted influenza vaccine stimulated hemagglutination inhibition antibody titers at a level comparable to those induced by MF59-adjuvanted influenza vaccine injected intramuscularly in the elderly [9]. In addition to influenza vaccines, Mikszta et al. showed that one dose of id. immunization of naked recombinant protective antigen (rPA) of Bacillus anthracis gave rise to a 60% seroconversion rate, whereas only 20% of mice generated a detectable antibody response after im. vaccination of alhydrogel-aduvanted rPA [10,11]. Likewise, in comparison with im. delivery, id. delivery provided a tenfold dose-sparing benefit for rabies or HBV vaccine in normal patients [12–14]. Furthermore, id. but not im. vaccination induced significant anti-HBV antibodies in nonresponsive hemodialysis patients [15].
Despite being an effective route of vaccination, current id. delivery techniques, such as the Mantoux method and bifurcated needles, are inconvenient and require specially trained personnel. Thus it is not practical for immunization of a large population in a short period of time by this route [4]. This situation may soon be changed, thanks to the development of a novel microinjection system that is now in clinic trials for convenient id. immunization of influenza vaccines [1]. Similar technologies such as jet injectors, microneedle and microprojection array patches have also been developed for convenient cutaneous immunization and to improve patient compliance [16–19].
Adjuvant for cutaneous vaccination
At present, the majority of vaccine adjuvants are developed and evaluated in im. immunizations. Different from the muscular tissue, the skin tends to develop severe local reactogenicity after id. immunization even in the absence of adjuvants, presumably attributable to the presence of a large number of resident immune cells and a high density of blood and lymphatic vessel networks in the skin [1–3,7–9,20]. The local reactions are also more readily visible in the skin than in the muscle. Importantly, the skin is the organ serving as a sensorial physical barrier between our body and the environment, and its integrity is crucial in fulfilling this task. Thus, adjuvants for cutaneous immunization must have a higher level of safety and induce less inflammation. Many adjuvants used in im. vaccinations cannot be used for cutaneous immunization.
The only US FDA-approved, widely used adjuvant over the past 80 years in the clinic has been aluminum salt-based adjuvant, referred to generically as ‘Alum’ [21]. It is currently included in several child vaccines in the USA, such as hepatitis A/B, diphtheria–tetanus–pertussis, and Haemophilus influenza type b. In 2009, an AS04-adjuvanted recombinant human papillomavirus vaccine was approved by the US FDA to prevent cervical cancer in girls and young women [22]. AS04 is a combinatorial adjuvant containing Alum and monophosphoryl lipid A (MPL), a low-toxicity derivative of lipopolysaccharide that activates Toll-like receptor (TLR)-4. MF59-adjuvanted seasonal influenza vaccine has been used for more than a decade in the elderly in Europe, but not in children, because a major component of MF59 adjuvant is a self substance named squalene that has the potential to induce autoantibodies after repeated use [23–25]. A dozen other adjuvants are approved by foreign authorities to be included or tested in human vaccines. These include the oil-in-water emulsion AS03, water-in-oil emulsions montanide ISA 51 and ISA 720, the TLR-7 agonist imiquimod (R837), the TLR-9 agonist unmethylated CpG oligonucleotides (CpG), saponin QS21 mixed with MPL in liposomes (AS01) or in squalene emulsion (AS02), immunostimulatory complexes, and particle formulation adjuvants, such as liposomes, virosomes and microspheres [26]. All of these adjuvants are tested or used in im. immunizations; however, their safety for id. vaccination remains a concern.
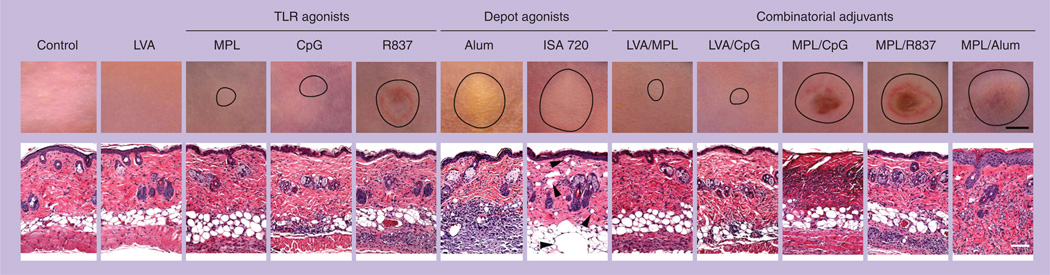
We evaluated the local reaction of some of these adjuvants following id. injection [27–29]. As shown in Figure 1, R837, MPL/CpG, MPL/R837 and MPL/Alum induced the most severe local reactions with a lesion size of 4–6 mm in diameter, concurrent with skin ulceration (upper panels), which was persistent for weeks. Histological examination revealed a heavy infiltration of inflammatory cells into adjuvant-treated skin (lower panel). The strong and persistent reactions in the skin exclude them to be used as cutaneous vaccine adjuvants owing to potential breaching of the skin that would provide the opportunity for local and systemic infections with various microorganisms. Alum and montanide ISA 720 induced a similar lesion as described previously, with infiltration of inflammatory cells evoked by Alum greater than for montanide ISA 720, but both caused little skin ulceration. Deposition of Alum, montanide ISA 720 and MPL/Alum was readily visible at the injection site, appearing as an abscess owing to the white/silver color of the adjuvant and did not disappear for months. Although only two emulsion adjuvants are being tested, it is likely that other emulsion adjuvants (e.g., AS02, MF59 and AS03) may have similar depositions in the skin in light of their similar physiochemical properties. The unpleasant ‘abscess’ and persistent inflammation in the skin question the use of any antigen-depot adjuvants for cutaneous vaccination. In contrast to the adjuvants described earlier, MPL or CpG induced mild local reactions with a lesion size of only about 2 mm in diameter that was completely resolved within 2 weeks. MPL and CpG may thus be safe adjuvants for cutaneous vaccination.
Figure 1. Reactogenicity of various vaccine adjuvants in the skin.
BALB/c mice were illuminated for 2 min with a Q-switched 532-nm Nd:YAG laser of a pulse width 5–7 ns, beam diameter 7 mm, and frequency 10 Hz at 0.3 W and 90 J/cm2 (laser vaccine adjuvant). Or, the mice were intradermally injected with indicated adjuvants with or without laser illumination. The injection volume is 20 µl containing 25 µg monophosphoryl lipid A, 30 µg CpG, 100 µg R837, 50% Alum (v/v), 70% montanide ISA 720 (v/v), or a combination of two indicated adjuvants. After 5 days, photos were taken (upper panel; scale bar: 2 mm), followed by histological examination (lower panel; scale bar: 100 µm). The lesion in the skin is outlined by circle in the upper panel and arrowheads in the lower panel of Montanide ISA 720-injected skin point to void bulbs preoccupied by the water-in-oil adjuvant.
In addition to the persistent inflammation, inclusion of emulsion-based adjuvants to a vaccine faces another challenge due to a small injection volume allowed for id. injection, typically 100 µl per site, in contrast to 0.5–1 ml for im. injection. The sticky solution is also probably difficult to inject through the novel microinjection system. Besides, microneedle array patches and needle-free vaccine patches made of dried vaccines are being developed in order to eliminate cold-chain storages. Addition of emulsion-based adjuvant to the dried vaccine may result in a loss of its adjuvanticity, since a specific physical status of the adjuvant, in particular, antigen-depot adjuvants, is crucial for the immune-enhancing effect of the adjuvant. Accordingly, an ideal adjuvant for cutaneous vaccination should be safe, easily injectable, with little inflammatory response.
Laser vaccine adjuvant
We developed a novel physical type of laser-based vaccine adjuvant capable of enhancing vaccine-induced immune responses without direct contact with the antigen [5]. In brief, a small area (<1 cm2) of mouse skin had hair removed and was exposed to a Q-switched 532-nm Nd:YAG laser (Spectra-Physics Inc., CA, USA) with a pulse width of 5–7 ns, beam diameter of 7 mm and frequency of 10 Hz at 0.3 W for 2 min, a corresponding dose of 90 J/cm2. The illumination did not raise the skin temperature higher than 41°C as measured by an infrared camera [5] or cause alteration in the skin visibly or histologically (Figure 1; LVA). Following the illumination, immunogens were intradermally administered into the site of laser illumination, whereas control mice received the immunogens similarly in the absence of laser illumination. The brief laser illumination was able to enhance ovalbumin (OVA)-specific antibody production by 300–500% over OVA alone (p < 0.001) [5]. Similar immune enhancement was observed with other immunogens like 2009–2010 seasonal influenza vaccine [5], nicotine vaccine and malarial liver stage antigen 1 (data not shown).
Furthermore, when LVA was combined with MPL or CpG, two adjuvants with less reactogenicity either alone or after combination with LVA (Figure 1), a synergistic immune boosting was obtained. Thus, id. administration of a mixture of OVA and MPL into the site of laser illumination augmented OVA-specific antibody production by twofold over nonlaser-treated control, or 22-fold over antigen alone (data not shown). Likewise, when a nicotine vaccine was mixed with MPL at a 1:1 ratio and intradermally injected, nicotine-specific antibody level was increased by 13-fold over nicotine-vaccine alone and incorporation of LVA further increased the antibody level by 33-fold. Importantly, similar synergistic effects were attained with OVA-induced cell-mediated immune responses, as reflected by a significant increase in the number of CD4+ and CD8+ cells secreting IL-4 or IFN-γ in the presence, as compared with the absence, of LVA. LVA also synergistically boosted OVA-induced antibody production and cell-mediated responses when combined with CpG. Taken together, id. vaccination in combination with LVA, LVA/MPL, or LVA/CpG augmented immune responses by approximately 16-, 72- and 40-times, respectively, over im. vaccination in the absence of adjuvants. This physical adjuvant is thus ideal for cutaneous vaccination either alone or in combination with other vaccine adjuvants that are known to activate DCs such as MPL and CpG [30,31].
Mechanisms of LVA function
The mechanism underlying laser-mediated immune enhancement is not well understood at present. It does not appear to be solely caused by photothermal effects, because when the skin was maintained at 42°C by a 10 × 10 × 100 mm steel bar for 2 min followed by immunization at the warm skin with OVA as previously, OVA-specific antibody production was increased by only 20% [5]. Furthermore, immunization conducted 2 or 4 h after laser illumination, with skin temperature having returned to normal, gave rise to a similar boost as immunization did immediately after laser illumination. Russian scientists reported that illumination of the skin with a laser of a higher power and density (0.6 W and 3 W/cm2) enhanced humoral immunity against influenza vaccine id. delivered in mice, by induction of extracellular heat-shock protein 70 production and inflammatory responses [32]. In contrast, laser used in our study did not induce heat-shock protein or a significant inflammatory response in laser-exposed skin [5]. We also observed little alteration in the surface expression of costimulatory molecules CD86 and CD83 or MHC class II molecule on skin DCs. We therefore consider LVA as a noninflammatory vaccine adjuvant.
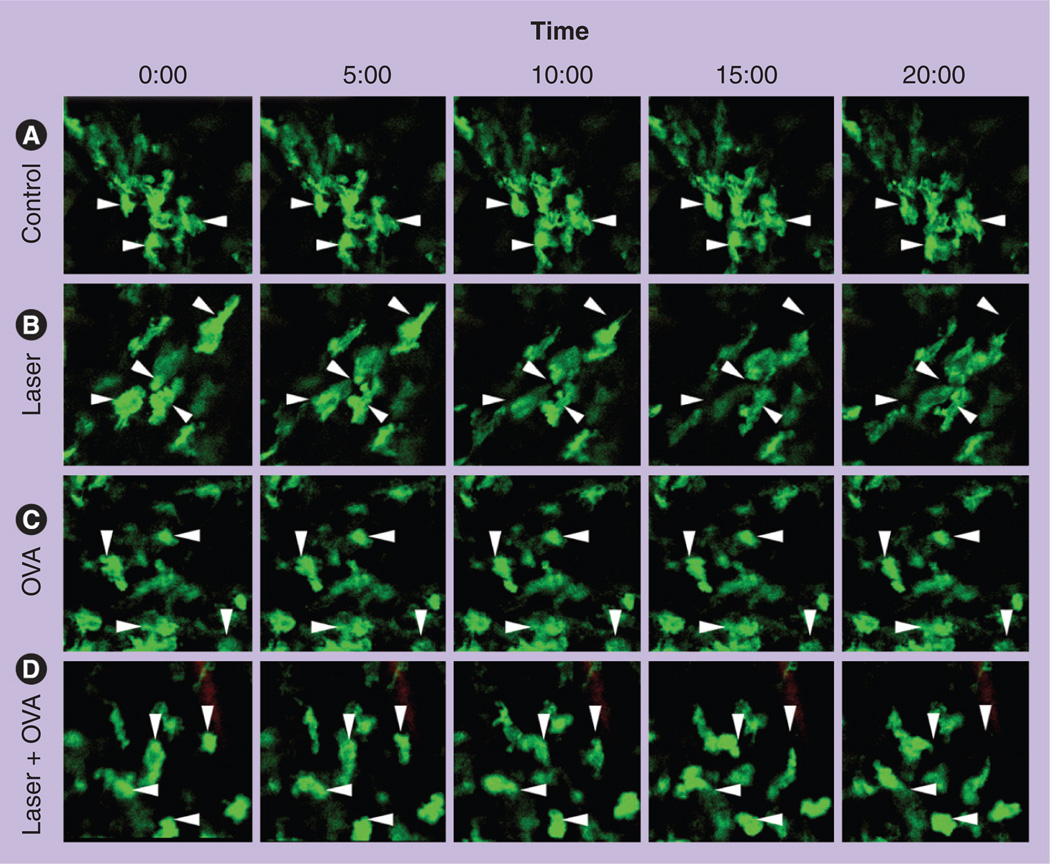
However, injection of the antigen into the site of laser illumination appeared to be crucial since if the antigen was injected into a distal site, for instance, 1 cm away from the laser-illuminated site, the immune-enhancing potential decreased substantially [5]. In accordance with this, we found that the laser illumination greatly accelerated the motility of APCs only at the areas of laser illumination. As shown in Figure 2, dermal GFP+ cells, mostly DCs and macrophages, were constantly shifting, albeit slowly, and extending pseudopods, but most of them remained at original locations during a 20-min period of recording in the control (Figure 2). On the contrary, the cells in the laser-treated mice showed a high migratory ability: cells leaving their original locations and a gap appearing between arrows (original locations) and the individual cells over time. OVA injection also increased migration of APCs, albeit to a lesser extent. Strikingly, a synergistic effect was observed on APC motility when OVA was administrated into the site of laser illumination (Figure 2; laser + OVA). An increase in the motility of APCs is likely to promote them to survey a greater area and facilitate their antigen sampling as recognized using dendrite surveillance extension and retraction cycling habitude (dSEARCH) [33]. The increased motility may also lead to sufficient transportation of antigen-captured DCs to the draining lymph nodes.
Figure 2. Laser significantly enhances dermal antigen-presenting cell motility.
The rear skin of MHC II-EGFP transgenic mice was left untreated (A), exposed to laser illumination at 0.3 W for 2 min (B), intradermally injected with OVA (C), or exposed to laser illumination (0.3 W; 2 min) followed by intradermal injection of OVA (D). 5 h later, dermal layers were subjected to intravital confocal imaging every 30 s for 20 min. Representative time-lapse images were shown. Arrows point to the original location of cells.
OVA: Ovalbumin.
Reproduced from [5].
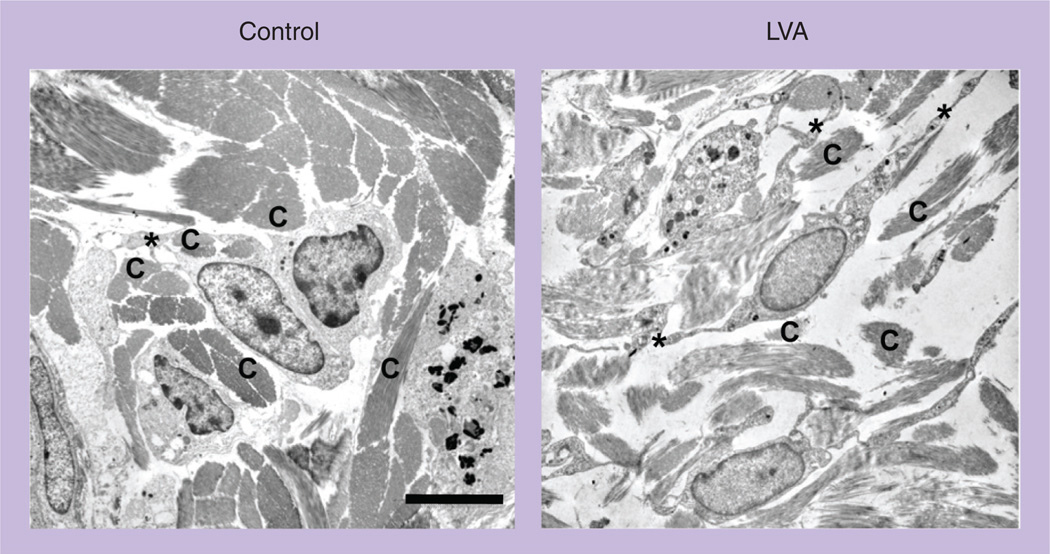
We postulate that brief laser illumination can transiently alter the interstitial microarchitecture, increase the tissue permeability, and permit relatively free movement of APCs in the interstitium. Indeed, upon laser illumination, dermal collagen fibers were dissociated and the interaction between DCs and surrounding tissue scaffolds was disrupted in the site of laser illumination, in sharp contrast to the well-organized microarchitecture in the control dermal connective tissue, as revealed by transmission electron microscopy (Figure 3). Dissociation of APCs with the matrix proteins is expected to free their movement [34]. The laser illumination may also enlarge pre-formed channels in the peri-lymphatic basement membrane to assist entry of APCs into the lymphatic vessel [35]. In support of altered interstitial resistance resulting in enhanced migration of DCs by laser illumination, we found that id. injection of DCs into the site of laser illumination increased the number of DCs migrating into the draining lymph nodes by approximately 300%, when compared with injection of DCs into the control skin, irrespective of DC maturation status. We also found that modification of pulse width, frequency and peak power at 532 nm did not significantly influence laser adjuvant effects, arguing for a physical-based mechanism. Another potential mechanism for laser-mediated immune enhancement may be the ability of laser to transiently permeabilize cellular membranes by a shock wave, which augments antigen uptake by DCs [36]. Laser treatment has been shown to increase uptake of antisense oligonucleotide by three- or 30-fold due to a laser pulse-generated high pressure [37]. Zeira et al. also showed that femtosecond laser sufficiently enhanced DNA delivery into cells and induced immune responses to the encoded antigen [38]. In addition, acceleration of interstitial flow by laser illumination can greatly assist a flow of soluble antigens from the skin to the draining lymph nodes where the antigens are presented to resident DCs. In the skin, the initial lymphatic vessels are blind-end structures with wide lamina and thin walls. These initial lymphatic vessels drain excess fluid and solutes from the interstitial space and pass them to lymph nodes via lymphatic ducts. The draining process is extremely slow under normal physiological conditions but it can be increased as many as ten times by inflammation or fever-range hyperthermia [39,40]. Other mechanisms may be also involved in laser-mediated immune enhancement, including enhanced mitochondrial activity of APCs, chemical releases, altered tissue pressure, and so on. Although most of these mechanisms, such as laser-induced shock wave, accelerated interstitial flow and altered mitochondrial activity, remain largely speculative and more studies are needed to confirm, it is clear that laser augments vaccination by novel and distinct mechanisms over traditional vaccine adjuvants.
Figure 3. Laser illumination disrupts the dense microarchitecture of dermal connective tissue.
The low dorsal skin of BALB/c mice was exposed to laser illumination at 0.3 W for 2 min, excised 30 min later, and subjected to transmission electron microscopy analysis. Nontreated control skin from the same mice was used as a control.
Scale bar: 6 µm.
*: Cell dendrites; C: Collagen fiber; LVA: Laser vaccine adjuvant.
Advantages of LVA over traditional vaccine adjuvants
Vaccine adjuvants have been traditionally defined as molecules, compounds or macromolecular complexes that ideally boost the potency and longevity of specific immune responses to antigens, but cause minimal toxicity or long-lasting immunity on their own [21]. However, LVA is not a chemical or compound, and it thus has the following advantages over conventional vaccine adjuvants. First, it eliminates the complex formulation process of mixing adjuvant and antigens. It also circumvents the problems associated with the maintenance of a stable mixture of the resultant vaccine during the cold storage. Optimal formulation of a safe, stable mixture between vaccine and adjuvant is a challenge for some vaccines such as rPA anthrax vaccine [41]. Alum adjuvant is a noncrystalline gel and antigen must be adsorbed onto highly charged aluminum particles for the adjuvant to be potent. At least two serious issues result from the use of Alum. First, freezing, lyophilization, or cold storage would result in separation of antigen from the aluminum particles and cause a loss of the adjuvant potency [42–44]. Second, the biophysical structure and stability of the resultant product are difficult to assay as an Alum complex. Importance of formulation has also been illustrated by the development of the malaria RTS,S vaccine. When the malaria vaccine was mixed with Alum plus MPL (AS04), it failed to protect immunized subjects against a Plasmodium falciparum challenge, whereas the same antigen mixed with QS21 plus MPL in an oil-in-water emulsion (AS02) or in liposome (AS01) induced protection [45,46]. Second, LVA can be used immediately and repeatedly at any time, which offers a great advantage when facing vaccine shortages in the event of influenza pandemic, an outbreak of a new viral strain or a bioterrorist attack. Conceivably, the vaccine dose-sparing effect of LVA can greatly enhance the bioavailability of a given stockpile vaccine, which can potentially save millions of lives during the early phase of an influenza pandemic. Third, due to the way that the laser stimulates the immune system without direct interaction with vaccine itself, the laser-based vaccine adjuvant platform may work as a type of universal and standalone adjuvant, which is especially significant for the new US National Biodefense Strategy, stressing one more flexible, broad-spectrum approach for protection against multiple diseases. Conceivably, with a valid laser device, it can be conveniently and readily applied to any vaccine whenever it is needed. Fourth, LVA can be readily combined with the newly developed id. or transcutaneous delivery strategies, such as microinjection systems and microneedle array patches. Finally, LVA does not involve administration of any foreign or self substances into the body apart from the immunogen itself and thus would not induce self-destructive immune cross-reactions, also termed ‘molecular mimicry’, which can potentially cause long-term side effects [47,48]. By contrast, other adjuvants, regardless of whether they are foreign or self to the body, have the potential to cause long-term adverse reactions after repeated uses. Recently, the Swedish and Finnish authorities suspended further vaccination with Pandemrix™ (made by GlaxoSmithKline, UK), an AS03-adjuvanted 2009 pandemic H1N1 influenza vaccine, and began to investigate the causative link of Pandemrix vaccination to the rising cases of narcolepsy, a chronic neurological disease with disturbed sleep–wake cycles, in children and adolescents. This concern is raised because narcolepsy did not rise in the USA where a nonadjuvanted H1N1 influenza vaccine was used in the same period of time. In fact, whether or not adjuvanted influenza vaccines are safe is an area of hot debate among scientific communities, health authorities and the general public. Whether adjuvant exposure causes macrophagic myofacititis, Gulf War syndrome, and other rare mental and chronic autoimmune diseases remains an overall public concern.
Expert commentary
Recent development of sufficient and convenient id. and transcutaneous vaccination technologies raises an urgent need for safe and potent vaccine adjuvants for augmentation of cutaneous vaccination. The majority of traditional vaccine adjuvants are not suitable for use in skin immunization because many of them cause unacceptable local reactogenicity owing to high sensitivity of the skin to inflammation. The use of lasers to stimulate skin immune cells is safe, simple, unique and ideal for cutaneous vaccination. It may be possible to adjuvantate both existing and future vaccines. This less inflammatory adjuvant can be potentially used to boost either Th1 or Th2 immune responses dependent on the nature of a given immunogen or the presence of other adjuvants. In this regard, our ongoing study showed that LVA in combination with MPL or CpG greatly boosted both humoral and Th1 immunity, which may be of particular significance for increasing vaccine immunogenicity in the elderly or immunocompromised populations. Furthermore, LVA can be readily incorporated into newly developed id. or transcutaneous vaccine delivery systems, bringing about at least a tenfold antigen-dose-sparing benefit, without any adjuvant injection, as compared with im. vaccination. Conceivably, a hand-held laser device can illuminate the skin for 2 min followed by microinjection or topical application of a vaccine-coated microneedle patch on the site of illumination. These simple vaccine delivery and immune-enhancing strategies will have a great impact on vaccination of a large population in the USA and worldwide, in particular, during an unpredictable vaccine shortage, for instance, during an influenza pandemic, an outbreak of a new viral strain, a bioterrorist attack, or a major natural disaster in fear of cholera outbreaks.
Five-year view
Within the next 5 years, a hand-held, safe prototype laser device will be fabricated and tested first in swine and then in the clinic. The laser vaccine adjuvant is expected to gain US FDA approval and advance to clinical trials because lasers with a much higher energy have been widely used for decades in patients and healthy individuals for a variety of cosmetic and therapeutic purposes. The laser vaccine adjuvant will also be combined with newly developed microinjection or microneedle delivery systems, which will represent saltational improvement in vaccine delivery for both dosage-sparing and a mass vaccination campaign. However, changing the route of delivery and formulation of existing vaccines for vaccination will require more research to determine which vaccines work more sufficiently with this technology and what population the technology should be applied to along with a careful economic assessment.
Key issues.
The skin is a preferable site of vaccination, but safe and effective adjuvants for skin vaccination are critically lacking.
The majority of traditional vaccine adjuvants are not suitable for cutaneous immunization due to high sensitivity of the skin to inflammation.
Laser vaccine adjuvant (LVA), monophosphoryl lipid A or CpG are potent and safe vaccine adjuvants for cutaneous immunization.
LVA is convenient and has distinct advantages over traditional vaccine adjuvants for cutaneous vaccination.
LVA can be readily combined with the novel intradermal or transcutaneous immunization technologies.
LVA may be particularly useful when encountering an unpredictable vaccine shortage.
A combination of LVA with monophosphoryl lipid or CpG can be powerful adjuvants for skin immunization in augmentation of both humoral and Th1-cell immunity.
Acknowledgments
This work is supported in part by the NIH grants AI070785 and RC1 DA028378, Sponsored Research agreement grant #2008A25652 from Boston Biocom LLC., and Grand Challenges Explorations grant #53273 from the Bill & Melinda Gates Foundation (to Mei X Wu).
Footnotes
Financial & competing interest disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Laurent PE, Bonnet S, Alchas P, et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25(52):8833–8842. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Beran J, Ambrozaitis A, Laiskonis A, et al. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009;7:13. doi: 10.1186/1741-7015-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland D, Booy R, De Looze F, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J. Infect. Dis. 2008;198(5):650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 4.Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26(26):3197–3208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 5. Chen X, Kim P, Farinelli B, et al. A novel laser vaccine adjuvant increases the motility of antigen presenting cells. PLoS One. 2010;5(10):e13776. doi: 10.1371/journal.pone.0013776. •• First report demonstrating safety, efficacy and part of potential mechanism for a novel physical-type laser-based vaccine adjuvant.
- 6.Lofquist JM, Weimert NA, Hayney MS. Smallpox: a review of clinical disease and vaccination. Am. J. Health Syst. Pharm. 2003;60(8):749–756. [PubMed] [Google Scholar]
- 7. Belshe RB, Newman FK, Cannon J, et al. Serum antibody responses after intradermal vaccination against influenza. N. Engl. J. Med. 2004;351(22):2286–2294. doi: 10.1056/NEJMoa043555. • Key report for clinical evaluation of intradermal influenza vaccine delivered by hypodermic needles.
- 8. Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 2004;351(22):2295–2301. doi: 10.1056/NEJMoa043540. • Key report for clinical evaluation of intradermal influenza vaccine delivered by hypodermic needles.
- 9. Van Damme P, Arnou R, Kafeja F, et al. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study. BMC Infect. Dis. 2010;10:134. doi: 10.1186/1471-2334-10-134. •• Key study showing comparable immunogenicity between intradermal influenza vaccine and MF59-adjuvanted vaccine in the elderly.
- 10.Mikszta JA, Sullivan VJ, Dean C, et al. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J. Infect. Dis. 2005;191(2):278–288. doi: 10.1086/426865. [DOI] [PubMed] [Google Scholar]
- 11.Mikszta JA, Dekker JP, III, Harvey NG, et al. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infect. Immun. 2006;74(12):6806–6810. doi: 10.1128/IAI.01210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryan JP, Sjogren MH, Perine PL, Legters LJ. Low-dose intradermal and intramuscular vaccination against hepatitis B. Clin. Infect. Dis. 1992;14(3):697–707. doi: 10.1093/clinids/14.3.697. [DOI] [PubMed] [Google Scholar]
- 13.Redfield RR, Innis BL, Scott RM, Cannon HG, Bancroft WH. Clinical evaluation of low-dose intradermally administered hepatitis B virus vaccine. A cost reduction strategy. JAMA. 1985;254(22):3203–3206. [PubMed] [Google Scholar]
- 14.Warrell MJ, Warrell DA, Suntharasamai P, et al. An economical regimen of human diploid cell strain anti-rabies vaccine for post-exposure prophylaxis. Lancet. 1983;2(8345):301–304. doi: 10.1016/s0140-6736(83)90288-x. [DOI] [PubMed] [Google Scholar]
- 15.Barraclough KA, Wiggins KJ, Hawley CM, et al. Intradermal versus intramuscular hepatitis B vaccination in hemodialysis patients: a prospective open-label randomized controlled trial in nonresponders to primary vaccination. Am. J. Kidney Dis. 2009;54(1):95–103. doi: 10.1053/j.ajkd.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Jackson LA, Austin G, Chen RT, et al. Safety and immunogenicity of varying dosages of trivalent inactivated influenza vaccine administered by needle-free jet injectors. Vaccine. 2001;19(32):4703–4709. doi: 10.1016/s0264-410x(01)00225-0. [DOI] [PubMed] [Google Scholar]
- 17.Frech SA, Dupont HL, Bourgeois AL, et al. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a Phase II, randomised, double-blind, placebo-controlled field trial. Lancet. 2008;371(9629):2019–2025. doi: 10.1016/S0140-6736(08)60839-9. [DOI] [PubMed] [Google Scholar]
- 18.Matriano JA, Cormier M, Johnson J, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm. Res. 2002;19(1):63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- 19.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr. Top. Microbiol. Immunol. 2009;333:369–393. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clin. Infect. Dis. 2010;50(10):1331–1338. doi: 10.1086/652144. [DOI] [PubMed] [Google Scholar]
- 21.Wack A, Rappuoli R. Vaccinology at the beginning of the 21st Century. Curr. Opin. Immunol. 2005;17(4):411–418. doi: 10.1016/j.coi.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin K, Doolan K, Hung CF, Wu TC. Perspectives for preventive and therapeutic HPV vaccines. J. Formos. Med. Assoc. 2010;109(1):4–24. doi: 10.1016/s0929-6646(10)60017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asa PB, Wilson RB, Garry RF. Antibodies to squalene in recipients of anthrax vaccine. Exp. Mol. Pathol. 2002;73(1):19–27. doi: 10.1006/exmp.2002.2429. • Describes a possible link of adjuvant with induction of autoimmune disease.
- 24. Asa PB, Cao Y, Garry RF. Antibodies to squalene in Gulf War syndrome. Exp. Mol. Pathol. 2000;68(1):55–64. doi: 10.1006/exmp.1999.2295. • Describes a possible link of adjuvant with induction of autoimmune disease.
- 25. Satoh M, Kuroda Y, Yoshida H, et al. Induction of lupus autoantibodies by adjuvants. J. Autoimmun. 2003;21(1):1–9. doi: 10.1016/s0896-8411(03)00083-0. • Describes a possible link of adjuvant with induction of autoimmune disease.
- 26.Guy B. The perfect mix: recent progress in adjuvant research. Nat. Rev. Microbiol. 2007;5(7):505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 27.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 28.Gu M, Hine PM, James JW, Giri L, Nabors GS. Increased potency of BioThrax anthrax vaccine with the addition of the C-class CpG oligonucleotide adjuvant CPG 10109. Vaccine. 2007;25(3):526–534. doi: 10.1016/j.vaccine.2006.07.056. [DOI] [PubMed] [Google Scholar]
- 29.Fransen F, Boog CJ, van Putten JP, van der LP. Agonists of Toll-like receptors 3, 4, 7, and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup B. Infect. Immun. 2007;75(12):5939–5946. doi: 10.1128/IAI.00846-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin M, Michalek SM, Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect. Immun. 2003;71(5):2498–2507. doi: 10.1128/IAI.71.5.2498-2507.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30(1):23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Onikienko SB, Zemlyanoy AB, Margulis BA, et al. Interactions of bacterial endotoxins and lipophilic xenobiotics with receptors associated with innate immunity. Donosologiya (St Petersburg) 2006;1:32–54. [Google Scholar]
- 33.Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J. Invest. Dermatol. 2006;126(4):787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- 34.Sangaletti S, Gioiosa L, Guiducci C, et al. Accelerated dendritic-cell migration and T-cell priming in SPARC-deficient mice. J. Cell Sci. 2005;118(Pt 16):3685–3694. doi: 10.1242/jcs.02474. [DOI] [PubMed] [Google Scholar]
- 35.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 2009;206(13):2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doukas AG, Kollias N. Transdermal drug delivery with a pressure wave. Adv. Drug Deliv. Rev. 2004;56(5):559–579. doi: 10.1016/j.addr.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 37.Lee WR, Shen SC, Liu CR, Fang CL, Hu CH, Fang JY. Erbium:YAG laser-mediated oligonucleotide and DNA delivery via the skin: an animal study. J. Control Release. 2006;115(3):344–353. doi: 10.1016/j.jconrel.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Zeira E, Manevitch A, Manevitch Z, et al. Femtosecond laser: a new intradermal DNA delivery method for efficient, long-term gene expression and genetic immunization. FASEB J. 2007;21(13):3522–3533. doi: 10.1096/fj.06-7528com. [DOI] [PubMed] [Google Scholar]
- 39.Ng CP, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J. Cell Sci. 2005;118(Pt 20):4731–4739. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 40.Ostberg JR, Kabingu E, Repasky EA. Thermal regulation of dendritic cell activation and migration from skin explants. Int. J. Hyperthermia. 2003;19(5):520–533. doi: 10.1080/02656730310001607986. [DOI] [PubMed] [Google Scholar]
- 41.Klas SD, Petrie CR, Warwood SJ, et al. A single immunization with a dry powder anthrax vaccine protects rabbits against lethal aerosol challenge. Vaccine. 2008;26(43):5494–5502. doi: 10.1016/j.vaccine.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta RK. Vaccine. 16. Vol. 13. Bethesda, Maryland, USA: 1995. Feb 13–15, New advances in vaccine technologies and applications; pp. 1623–1625. [DOI] [PubMed] [Google Scholar]
- 43.Gupta RK, Siber GR. Adjuvants for human vaccines – current status, problems and future prospects. Vaccine. 1995;13(14):1263–1276. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 44.Alving CR, Detrick B, Richards RL, Lewis MG, Shafferman A, Eddy GA. Novel adjuvant strategies for experimental malaria and AIDS vaccines. Ann. NY Acad. Sci. 1993;690:265–275. doi: 10.1111/j.1749-6632.1993.tb44015.x. [DOI] [PubMed] [Google Scholar]
- 45.Bejon P, Lusingu J, Olotu A, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N. Engl. J. Med. 2008;359(24):2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoute JA, Slaoui M, Heppner DG, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N. Engl. J. Med. 1997;336(2):86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 47.Shoenfeld Y, Aron-Maor A. Vaccination and autoimmunity-‘vaccinosis’: a dangerous liaison? J. Autoimmun. 2000;14(1):1–10. doi: 10.1006/jaut.1999.0346. [DOI] [PubMed] [Google Scholar]
- 48.Waisbren BA., Sr Acquired autoimmunity after viral vaccination is caused by molecular mimicry and antigen complimentarity in the presence of an immunologic adjuvant and specific HLA patterns. Med. Hypotheses. 2008;70(2):346–348. doi: 10.1016/j.mehy.2007.04.043. [DOI] [PubMed] [Google Scholar]