Abstract
A common functional variant in paraoxonase 1 (PON1), Q192R, was recently reported to be a major determinant of clopidogrel response. This variant was genotyped in 566 participants of the Amish Pharmacogenomics of Anti-Platelet Intervention (PAPI) study and in 227 percutaneous coronary intervention (PCI) patients. Serum paraoxonase activity was measured in a subset of 79 PAPI participants. PON1 Q192R was not associated with pre- or post-clopidogrel platelet aggregation in the PAPI study (P = 0.16 and P = 0.21, respectively) or the PCI cohort (P = 0.47 and P = 0.91, respectively). The Q192 allele was not associated with cardiovascular events (hazard ratio (HR) 0.46, 95% confidence interval (CI) 0.20–1.06; P = 0.07). No correlation was observed between paraoxonase activity and post-clopidogrel platelet aggregation (r2 < 0.01, P = 0.78). None of 49 additional PON1 variants evaluated was associated with post-clopidogrel platelet aggregation. These findings do not support a role for PON1 as a determinant of clopidogrel response.
Dual antiplatelet therapy with clopidogrel and aspirin inhibits platelet function, thereby preventing recurrent cardiovascular events in patients with coronary artery disease (CAD) who are undergoing percutaneous coronary intervention (PCI).1,2 Clopidogrel is a thienopyridine prodrug that requires enzymatic modification in order to produce its bioactive thiol metabolite (SR 26334), which irreversibly binds to the platelet P2Y12 receptor, thereby inhibiting adenosine diphosphate (ADP)-stimulated platelet aggregation. The wide interindividual variability in clopidogrel response has been linked to specific genetic polymorphisms.3–8 Our group and others have identified common loss-of-function genetic variants in the hepatic cytochrome P450 (CYP) isoenzyme CYP2C19 that affect the formation of the active metabolite and on-clopidogrel ADP-stimulated platelet aggregation that are associated with increased cardiovascular event rates in PCI patients on clopidogrel therapy, including stent thrombosis.9–15
Although common CYP2C19 variants account for significant variation in on-clopidogrel ADP-stimulated platelet aggregation, other genetic factors are likely to exist. Paraoxonase 1 (PON1), which is synthesized in the liver, was originally named for its ability to hydrolyze exogenous organophosphates. It is transported with high-density lipoprotein in the plasma and functions as an antioxidant, decreasing the production of atherogenic oxidized low-density lipoprotein. Indeed, a common polymorphism in PON1 (rs662) resulting in a glutamine (Q)-to-arginine (R) amino acid change at position 192 (Q192R) is associated with altered serum PON1 activity and cardiovascular disease.16–18 Recently, Bouman and co-workers reported the PON1 Q192R variant to be associated with lower concentrations of clopidogrel active metabolite, lower platelet inhibition, and a higher risk for stent thrombosis in patients with CAD.19 The variant was found to account for ~70% of the variation in clopidogrel-mediated inhibition of ADP-stimulated platelet aggregation.
To replicate and better understand the genetic factors that impact clopidogrel efficacy, we evaluated the effect of the Q192R variant and other single-nucleotide polymorphisms (SNPs) in PON1 on paraoxonase activity and ADP-stimulated ex vivo platelet aggregation at baseline and in response to clopidogrel treatment in 566 members of the Old Order Amish of Lancaster, Pennsylvania. The Amish are a relatively homogenous, closed founder population in which confounding factors such as life-style variability and medication usage are minimized. Genetic findings in this group were extended by examining the relationship among genotype, platelet function, and ischemic events in an independent population consisting of 227 patients treated with coronary artery stents.
RESULTS
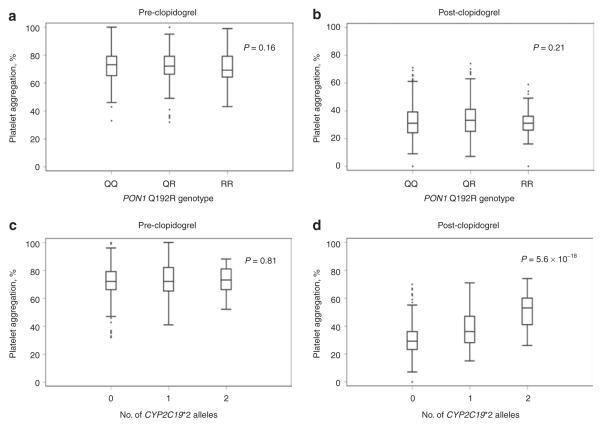
Characteristics of the participants in the Amish Pharmacogenomics of Anti-Platelet Intervention (PAPI) study are shown in Table 1. By design, these individuals were generally healthy and exhibited a low prevalence of hypertension, hyper-cholesterolemia, and diabetes. In this cohort, the minor allele frequency of the PON1 Q192R variant was 0.32, similar to that reported in other Caucasian populations.19–21 In contrast to the report by Bouman et al., the Q192R variant showed no evidence of association with ADP-stimulated platelet aggregation before or after clopidogrel treatment in the PAPI population under an additive model (P = 0.16 and P = 0.21, respectively, Figure 1). This variant was also not associated with high platelet reactivity (HPR) after clopidogrel treatment (P = 0.19). By contrast, CYP2C19*2 genotype (rs4244285) showed strong association with both post-clopidogrel ADP-stimulated platelet aggregation (P = 5.6 × 10−18, Figure 1) and HPR (P = 1.67 × 10−9). After adjustment for CYP2C19*2, there was no association between PON1 Q192R genotype and post-clopidogrel ADP-stimulated platelet aggregation (P = 0.23, additive model).
Table 1.
Characteristics of Amish PAPI study and Sinai Hospital of Baltimore study participants
| Amish PAPIa |
Sinai Hospital of Baltimore |
|||
|---|---|---|---|---|
| Characteristic (units) | Men | Women | Men | Women |
| Number (n) | 276 | 290 | 136 | 91 |
|
| ||||
| Age ± SD (years) | 44.3 ± 12.8 | 46.7 ± 13.6 | 62.5 ± 11.4 | 67.0 ± 11.0 |
|
| ||||
| BMI ± SD (kg/m2) | 25.9 ± 3.7 | 28.0 ± 5.3 | 30.1 ± 6.3 | 30.9 ± 7.1 |
|
| ||||
| Systolic blood pressure ± SD (mm Hg) | 116.1 ± 11.7 | 116.4 ± 13.6 | 138.0 ± 19.9 | 144.6 ± 19.9 |
|
| ||||
| Diastolic blood pressure ± SD (mm Hg) | 70.6 ± 7.3 | 69.4 ± 7.3 | 73.6 ± 13.8 | 70.3 ± 14.2 |
|
| ||||
| Hypertension (%)b | 4.4 | 5.5 | 75.0 | 79.1 |
|
| ||||
| Total cholesterol ± SD (mg/dl) | 207.5 ± 43.5 | 216.4 ± 50.8 | NA | NA |
|
| ||||
| LDL cholesterol ± SD (mg/dl) | 138.7 ± 40.3 | 139.4 ± 47.0 | NA | NA |
|
| ||||
| HDL cholesterol ± SD (mg/dl) | 55.3 ± 14.8 | 62.0 ± 15.3 | NA | NA |
|
| ||||
| Triglycerides ± SD (mg/dl)c | 67.7 ± 38.1 | 74.9 ± 41.5 | NA | NA |
|
| ||||
| Hypercholesterolemia (%)d | 25.0 | 26.2 | 81.6 | 79.1 |
|
| ||||
| Taking aspirin (%) | 2.2 | 1.0 | 100 | 100 |
|
| ||||
| Proton pump inhibitor use (%) | 0.7 | 0.0 | 24.8 | 36.5 |
|
| ||||
| Self-reported diabetes (%) | 1.1 | 0.7 | 28.7 | 48.4 |
|
| ||||
| Hematocrit ± SD (%) | 41.5 ± 2.4 | 37.6 ± 2.3 | 41.8 ± 5.0 | 37.9 ± 4.5 |
|
| ||||
| White blood cell count ± SD (n × 1,000) | 6.1 ± 1.5 | 6.1 ± 1.4 | 6.8 ± 1.3 | 7.5 ± 1.5 |
|
| ||||
| Platelet count ± SD (n × 100,000) | 239.1 ± 43.3 | 246.9 ± 50.5 | 223.6 ± 68.5 | 252.2 ± 71.4 |
|
| ||||
| Current smoker (%)e | 20.1 | 0.0 | 29.4 | 19.8 |
SI conversion factors: to convert HDL cholesterol, LDL cholesterol, and total cholesterol values to mmol/l, multiply by 0.0259; to convert triglycerides to mmol/l, multiply by 0.0113.
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NA, not available; PAPI, Pharmacogenomics of Anti-Platelet Intervention.
For the PAPI study, all participants were withdrawn from prescription and nonprescription medications, vitamins, and supplements 7 days prior to and for the duration of the study. Participants taking anti-hypertensive, lipid-lowering, and diabetes medications accounted for <2% of participants.
Defined as systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg or taking prescription medication for previously diagnosed hypertension.
Logarithm-transformed for analysis and back-transformed for presentation.
Defined as LDL cholesterol >160 mg/dl or taking prescription medication for previously diagnosed hypercholesterolemia.
Self-reported history of cigarette, pipe, or cigar use.
Figure 1.
Association of (a,b) PON1 Q192R variant (rs662) and (c,d) CYP2C19*2 (rs4244285) with adenosine diphosphate–stimulated platelet aggregation before and after clopidogrel treatment in participants of the Amish Pharmacogenomics of Anti-Platelet Intervention study. The horizontal line within each box indicates the median; the top and bottom borders of each box indicate the interquartile range (IQR). The whiskers extending from each box indicate ± 1.5 IQRs; the points beyond the whiskers indicate outliers beyond 1.5 IQRs.
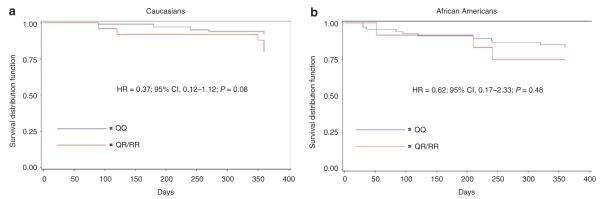
We also evaluated the effect of PON1 Q192R genotype on ADP-stimulated platelet aggregation as well as cardiovascular outcomes in a group of patients from the general population in Baltimore, Maryland, who underwent PCI and had a clinical indication for clopidogrel. Characteristics of these patients are shown in Table 1. Our findings were similar to those of the PAPI study in that we observed no evidence of association with PON1 Q192R genotype and ADP-stimulated platelet aggregation before or after clopidogrel treatment (P = 0.47 and P = 0.91, respectively) or with HPR (P = 0.24). Race-stratified analyses in Caucasians and African Americans also did not reveal an association between this SNP and ADP-stimulated platelet aggregation (P = 0.57 and P = 0.64, respectively) or with HPR (P = 0.22 and P = 0.31, respectively) after clopidogrel treatment. After 1 year of follow-up, the Q192 allele variant previously reported to be associated with stent thrombosis was not associated with cardiovascular event rates in the entire sample (hazard ratio (HR) 0.46, 95% confidence interval (CI) 0.20–1.06; P = 0.07) or when stratified by race (HR 0.37, 95% CI 0.12–1.12; P = 0.08 for Caucasians and HR 0.62, 95% CI 0.17–2.33; P = 0.48 for African Americans, Figure 2). There was no difference in cardiovascular event rates in PON1 192Q carriers vs. noncarriers in the subset of 132 patients not taking clopidogrel (HR 0.55, 95% CI 0.14–2.13; P = 0.39) or in the 95 patients still taking clopidogrel at 1 year of follow-up (HR 0.41, 95% CI 0.14–1.19; P = 0.10). In this same cohort, the CYP2C19*2 genotype was significantly associated with cardiovascular outcomes, as previously reported.9
Figure 2.
Event-free survival over 1 year of follow-up in Sinai Hospital of Baltimore (a) caucasian and (b) African-American patients treated with clopidogrel and aspirin following percutaneous coronary intervention, stratified by PON1 Q192R genotype. Post-discharge ischemic events were defined as myocardial infarction (the occurrence of ischemic symptoms and troponin I value greater than the upper limit of normal), ischemic stroke, stent thrombosis (definite stent thrombosis according to the Academic Research consortium criteria), unplanned target-vessel revascularization (revascularization of vessel treated at the time of study enrollment), hospitalization for coronary ischemia without revascularization (hospitalization for chest pain with evidence of ischemia on electrocardiogram and no evidence of myocardial infarction as assessed by troponin I levels), and death secondary to any cardiovascular cause. Blue and red lines represent rs662 Q/Q and R/– genotypes, respectively. All analyses were adjusted for age and sex.
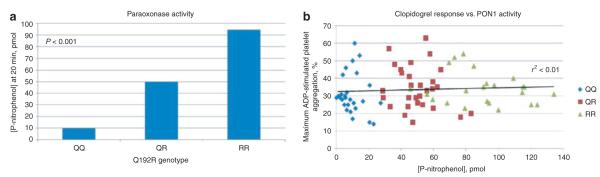
We further evaluated the effect of PON1 Q192R genotype on serum esterase activity using the substrates phenylacetate and paraoxon in 79 members of the PAPI study (27, 29, and 23 with rs662 genotypes QQ, QR, and RR, respectively). Like the authors of previous reports,19,22,23 we found that the Q192R substitution had a significant effect on paraoxonase activity (p-nitrophenol product formation after 20 min = 9.9, 49.7, and 94.6 pmol for QQ, QR, and RR genotypes, respectively, P < 0.001, Figure 3), but it was not a determinant of hydrolysis efficiency of phenylacetate (phenol product formation after 20 min = 254.8, 256.3, and 276.0 nmol for QQ, QR, and RR genotypes, respectively, P = 0.49). No significant correlation was observed between serum paraoxonase activity and ADP-stimulated platelet aggregation at baseline (r2 < 0.01, P = 0.45) or after clopidogrel treatment (r2 < 0.01, P = 0.78) (Figure 3).
Figure 3.
Evaluation of serum paraoxonase activity and adenosine diphosphate–stimulated platelet aggregation by PON1 Q192R genotype. (a) Paraoxonase activity as measured by p-nitrophenol production at 20 min by PON1 Q192R genotype. (b) correlation between paraoxonase activity and adenosine diphosphate–stimulated platelet aggregation after 7 days of clopidogrel administration.
To more comprehensively assess the potential effect of the PON1 locus on ADP-stimulated platelet aggregation and other cardiovascular-related traits, we evaluated 49 PON1 SNPs in 440 PAPI study participants at baseline and after 7 days of clopidogrel treatment using the Illumina 50K HumanCVD BeadChip. The linkage disequilibrium structure of PON1 in this sample is shown in Supplementary Figure S1 online. After correction for multiple comparisons, no SNP in PON1, including the well-studied L55M variant (rs854560), was associated with ADP-stimulated platelet aggregation before or after clopidogrel treatment (Supplementary Table S1).
DISCUSSION
Many studies document wide interindividual variability in clopidogrel response, suggesting a contribution of multiple factors to variation in this trait.24 Defining the factors responsible for variable response may provide an opportunity for physicians to individualize antiplatelet therapy and improve outcomes in patients undergoing PCI. Our previous work indicated that ~70% of the variation in clopidogrel response, as measured by on-clopidogrel ADP-stimulated platelet aggregation, can be attributed to relatedness, suggesting a substantial genetic component.9 Indeed, we and others identified the loss-of-function variant CYP2C19*2, which contributes ~12% of the interindividual variability in clopidogrel response. Thus, there are likely to be other genetic determinants of clopidogrel response.
Recently, Bouman and co-workers reported that PON1 was involved in the metabolic conversion of clopidogrel to its active metabolite in vitro and that the common reduced-function Q-allele of the PON1 Q192R variant was strongly associated with increased on-clopidogrel ADP-stimulated platelet aggregation and an increased risk of stent thrombosis.19 Their data suggested that this variant could account for as much as 70% of the variation in on-clopidogrel ADP-stimulated platelet aggregation and imposes a 3.6-fold increase in risk for stent thrombosis in clopidogrel-treated PCI patients. Curiously, the well-described effect of CYP2C19*2 genotype on clopidogrel response could not be replicated.
The main finding of our study is that the same common functional Q192R variant in PON1 reported by Bouman and co-workers is not associated with baseline or post-clopidogrel ADP-stimulated platelet aggregation in two independent cohorts. Furthermore, this variant is not associated with cardiovascular outcomes in clopidogrel-treated PCI patients. As expected, we detected a strong association between the common CYP2C19*2 loss-of-function variant and post-clopidogrel ADP-stimulated platelet aggregation (and cardiovascular outcomes as previously reported7) in these same cohorts. Interestingly, two recently published independent investigations, by Trenk et al. and Sibbing et al., also failed to observe an association between the PON1 Q192R polymorphism and either ADP-induced platelet aggregation or cardiovascular outcomes.25,26
The reason for the discrepant findings between Bouman and colleagues’ study and our work is unclear. It is possible that differences in populations and/or study design may account for some differences, although both the original report and our studies included predominantly Caucasians of European origin. Allele frequencies of the Q192R variant were also similar. Because Bouman showed that the Q192R variant is the functional variant in vitro, differences in linkage disequilibrium among populations are not likely to explain the differences found between the studies.
Furthermore, we examined 49 SNPs across the PON1 locus and failed to detect significant associations with clopidogrel response. It is unlikely that our failure to replicate the results of Bouman et al. can be attributed to type II error because our sample size provides excellent power to detect effect sizes for post-clopidogrel ADP-stimulated platelet aggregation well below that which was reported, and we were easily able to detect a strong association of CYP2C19*2 with clopidogrel response in the same data set. It is possible, however, that the true effect size is some-what smaller than that initially detected (the so-called winner’s curse). With regard to cardiovascular events, we found a trend toward decreased cardiovascular event rates in carriers of the 192Q allele, which is in the opposite direction of the findings reported by Bouman and co-workers. Although it is possible that differences in cardiovascular event data can be attributed to limited sample size, we feel this is unlikely given our ability to detect differences in event rate by CYP2C19*2 genotype in this same cohort as previously reported.9 Unfortunately, we did not have an adequate sample size to assess the relation of genotype to stent thrombosis.
We did not directly measure levels of clopidogrel active metabolite. However, we were able to detect differences in serum PON1 activity among Q192R genotypes, and the lack of correlation between paraoxonase activity and on-clopidogrel ADP-stimulated platelet aggregation would argue against PON1 playing a major role in clopidogrel activation and/or response.
In summary, our findings do not support a role for genetic variation in PON1 as a major determinant of clopidogrel response. Additional candidate gene and genome-wide approaches in larger sample sizes will be necessary to identify additional genetic variants that influence clopidogrel response.
METHODS
Study populations
Amish PAPI study
Between August 2006 and July 2010, the PAPI study (NCT00799396) recruited 566 healthy Caucasian individuals age ≥20 years. Details of the study have been described previously.9 Briefly, prescription medications, vitamins, and supplements were discontinued 1 week prior to the initial study visit. Participants were extensively phenotyped, and physical examinations, medical and family histories, anthropometric measures, and blood samples after an overnight fast were obtained.
Complete blood count and serum lipid levels (total cholesterol, high-density lipoprotein cholesterol, and triglycerides) were assayed by Quest Diagnostics (Horsham, PA); levels of low-density lipoprotein cholesterol were calculated using the Friedewald equation. Hyperlipidemia was defined as a low-density lipoprotein cholesterol level >160 mg/dl and/or use of prescription cholesterol-lowering medications. Hypertension was designated as systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg and/or use of prescription blood pressure–lowering medications. Diabetes and current smoking status (including cigarette, cigar, or pipe) was obtained by self-report.
Platelet-rich plasma was isolated from blood samples, and platelet counts were adjusted to 200,000 platelets/μl. Baseline platelet function was assessed by optical aggregometry using a PAP8E Aggregometer (Bio/Data, Horsham, PA) after stimulation with ADP (20 μmol/l) and was expressed as the maximal percentage change in light transmittance using platelet-poor plasma as a referent.9 After baseline platelet aggregation measurements, participants were given a 300-mg oral loading dose of clopidogrel followed by 75 mg per day for 6 days. One hour following the last dose of clopidogrel, platelet aggregation measurements were repeated. A second follow-up platelet-aggregation measurement was made later the same day, 1 h after oral ingestion of 324 mg of chewable aspirin.
Sinai Hospital of Baltimore study patients
The Sinai Hospital of Baltimore study (NCT00370045) enrolled 227 patients older than 18 years undergoing nonemergent PCI between January 2004 and May 2007. Detailed recruitment of these patients has been described previously.9 Of the recruited patients, 140 (61.7%) were Caucasian, 83 (36.6%) were African American, and 4 (1.8%) were of other race/ethnicity. Race/ethnicity was established by self-report. Directly preceding the PCI, patients received either a 600-mg (n = 112) or a 300-mg (n = 25) loading dose of clopidogrel; 90 patients were already receiving maintenance therapy with a 75-mg daily dose at the time of PCI and received no loading dose. Patients also received 81–325 mg of aspirin daily for at least 1 week prior to PCI and 325 mg on the day of the procedure. Anticoagulant therapy including bivalirudin or heparin, either with (n = 107) or without (n = 120) eptifibatide, was discontinued at the completion of the procedure in all patients. Platelet function was measured on the day of hospital discharge in patients not treated with eptifibatide or 5 days or more after discharge in patients treated with eptifibatide. There were no differences in baseline characteristic or in the long-term outcomes investigated in stratified analyses of acute clopidogrel dosing or eptifibatide treatment; therefore, these groups were combined for further analyses.
Platelet aggregation was assessed in platelet-rich plasma after stimulation with 20 μmol/l ADP using a Chronolog Lumi-Aggregometer (Model 490-4D; Chronolog, Havertown, PA) and was expressed as the maximum percentage change in light transmittance using platelet-poor plasma as a referent as described previously.27 Aspirin (325 mg/d) and clopidogrel (75 mg/d) were prescribed for all patients at the time of hospital discharge in accordance with the American College of Cardiology and American Heart Association guidelines.28 Medication adherence was assessed by self-report and by review of source documents from hospitalizations for ischemic events.
Enrolled patients were contacted at 1 month and 12 months after PCI to determine the occurrence of post-discharge cardiovascular ischemic events. A physician, blinded to the study results for the patient, adjudicated all end points through review of source documents obtained from medical records. Post-discharge ischemic events were defined as myocardial infarction (the occurrence of ischemic symptoms and a troponin I level greater than the upper limit of normal), ischemic stroke, stent thrombosis (definite stent thrombosis according to the Academic Research Consortium29), unplanned target-vessel revascularization (revascularization of vessel treated at the time of enrollment), hospitalization for coronary ischemia without revascularization (hospitalization for chest pain with evidence of ischemia on electrocardiogram and no evidence of myocardial infarction as assessed by troponin I level, and death secondary to any cardiovascular cause).
All study protocols adhered to the Declaration of Helsinki and were approved by the respective institutional review boards of the University of Maryland and Sinai Hospital of Baltimore. Written informed consent was obtained from all the participants, who were compensated for their participation.
Genotyping
Genotyping of PON1 Q192R and CYP2C19*2 was performed using a TaqMan SNP genotyping assay (Applied Biosystems, Foster City, CA). Genotype concordance rates for both of these polymorphisms in a subset of duplicate samples were 100%. Follow-up genotyping in the PAPI study was performed with the Illumina 50K HumanCVD BeadChip (IBC-array version 2) according to the manufacturer’s instructions (Illumina, San Diego, California). Genotype calls were performed using the Illumina BeadStudio (version 3) Genotyping Module. A total of 49 SNPs spanning the PON1 locus, with minor allele frequencies >1%, were included in our analyses, including the well-described L55M variant. The mean genotype call rate was 99.7%. All SNPs were in Hardy–Weinberg equilibrium (P > 0.05) except rs705381, rs705382, rs854571, and rs854573 (P = 0.03, 0.03, 0.04, and 0.01, respectively).
PON1 enzyme activity
PON1 arylesterase activity was determined in 250-μl samples containing a 1,250-fold dilution of serum, 3.4 mmol/l phenylacetate, 9 mmol/l Tris-HCl (pH 8.0), and 0.9 mmol/l CaCl2. PON1 activity was determined in 100-μl samples containing a 10-fold dilution of serum, 1.5 mmol/l paraoxon, 10 mmol/l Tris-HCl (pH 8.0), 2 mmol/l CaCl2, and 1 mol/l NaCl. Both substrate groups were run in duplicate at 26 °C. The formation of phenol from phenylacetate was measured every 30 s for 20 min at an absorbance wavelength of 280 nm. The formation of p-nitrophenol from paraoxon was measured every 60 s for 20 min at an absorbance wavelength of 405 nm. Reactions were monitored using a PerkinElmer VICTOR X3 Multilabel Plate Reader (PerkinElmer, Waltham, MA).
Statistical analysis
Summary statistics, frequencies, and distributions for the PAPI study and Sinai Hospital of Baltimore cohorts were generated using SAS version 9.1 (SAS Institute, Cary, NC). Measures of Hardy–Weinberg equilibrium were calculated using a χ2-test. All statistical tests were two-sided. For the Illumina HumanCVD BeadChip genotypes, we considered a P value <0.001 (0.05/49 SNPs tested) to be statistically significant. For all other analyses, P values <0.05 were considered statistically significant. Pairwise linkage disequilibrium statistics (D’ and r2) were calculated using Haploview version 4.2.
Amish PAPI study
Association analyses between SNPs and ADP-stimulated platelet aggregation at baseline and following clopidogrel treatment were performed under a variance component model that assesses the effect of genotype as an additive effect on the quantitative trait while adjusting for age, sex, body mass index, diabetes, smoking, renal function, proton pump inhibitor use, pre-clopidogrel platelet aggregation, and relatedness among study participants. Relatedness among participants was accounted for by including a polygenic component as a random effect as previously described.9 The impact of PON1 Q192R and CYP2C19*2 on HPR was evaluated using the consensus cutoff value of HPR previously described (maximal platelet aggregation >0.59, 20 μmol/l ADP).30 Similar analyses were performed comparing PON1 activity among Q192R genotypes. The relationship between PON1 activity and ADP-stimulated platelet aggregation at baseline and following clopidog rel treatment was performed using SAS version 9.1 (SAS Institute, Cary, NC). Power calculations indicated 80% power to detect SNPs with minor allele frequencies of 0.2–0.4 in the PAPI study (n = 566), accounting for 1.3–1.4% of the phenotypic variation at ά and 2.9–3.0% of the phenotypic variation at ά.
Sinai Hospital of Baltimore study
We estimated the effect of PON1 Q192R genotype on pre-clopidogrel and post-clopidogrel ADP-stimulated platelet aggregation under an additive genetic model. The genotype effect was estimated using analysis of variance with adjustment for age, sex, body mass index, diabetes, smoking, proton pump inhibitor use, race, study (Peri-Procedural Myocardial Infarction, Platelet Reactivity, Thrombin Generation, and Clot Strength: Differential Effects of Eptifibatide + Bivalirudin vs. Bivalirudin study, CLEAR PLATELETS-1 (Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets), and CLEAR PLATELETS-2), and treatment (clopidogrel dose and use of eptifibatide). Race-stratified analyses were also performed.
Cardiovascular event–free survival at 1 year of follow-up was evaluated between participants with and without the PON1 rs662 minor allele using a proportional hazards model while simultaneously adjusting for age and sex. Survival analysis stratified by PON1 rs662 genotype was also performed in the subset of 95 patients still taking clopidogrel at the time of event or at 1 year of follow-up and the 132 patients who were not receiving clopidogrel at these times.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by research grants NIH U01 GM074518 and U01 HL105198, the University of Maryland General Clinical Research Center (grant M01 RR 16500), the General Clinical Research Centers Program, the National Center for Research Resources (NCRR), the NIH, the Baltimore Veterans Administration Geriatric Research and Education Clinical Center (GRECC), and Sinai Hospital of Baltimore. We gratefully acknowledge our Amish liaisons and field workers and the extraordinary cooperation and support of the Amish community, without whom these studies would not have been possible.
P.A.G. receives grant support from AstraZeneca, Daiichi-Sankyo, Bayer Healthcare, Eli Lilly, Portola Pharmaceuticals, Haemonetics, Pozen, and Sanofi-Aventis, and honoraria/consulting income from Accumetrics, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Merck, Pozen, Portola/Novartis, and Sanofi-Aventis. A.R.S. receives grant support from the National Institutes of Health to conduct pharmacogenomic studies of antiplatelet agents. He serves as a consultant to Bristol-Myers Squibb and Sanofi-Aventis.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
CONFLICT OF INTEREST The other authors declared no conflict of interest.
References
- 1.Kushner FG, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Catheter. Cardiovasc. Interv. 2009;74:E25–E68. doi: 10.1002/ccd.22351. [DOI] [PubMed] [Google Scholar]
- 2.King SB, 3rd, et al. Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation. 2008;117:261–295. doi: 10.1161/CIRCULATIONAHA.107.188208. [DOI] [PubMed] [Google Scholar]
- 3.Feher G, et al. Clinical importance of aspirin and clopidogrel resistance. World J. Cardiol. 2010;2:171–186. doi: 10.4330/wjc.v2.i7.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo KS, et al. Determination of the prevalence of aspirin and clopidogrel resistances in patients with coronary artery disease by using various platelet-function tests. Korean J. Lab. Med. 2010;30:460–468. doi: 10.3343/kjlm.2010.30.5.460. [DOI] [PubMed] [Google Scholar]
- 5.Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J. Am. Coll. Cardiol. 2005;45:1392–1396. doi: 10.1016/j.jacc.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Hochholzer W, et al. Time dependence of platelet inhibition after a 600-mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary intervention. Circulation. 2005;111:2560–2564. doi: 10.1161/01.CIR.0000160869.75810.98. [DOI] [PubMed] [Google Scholar]
- 7.Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J. Am. Coll. Cardiol. 2005;45:246–251. doi: 10.1016/j.jacc.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 8.Brandt JT, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 2007;5:2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 9.Shuldiner AR, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mega JL, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 11.Hulot JS, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 12.Frere C, et al. Effect of cytochrome p450 polymorphisms on platelet reactivity after treatment with clopidogrel in acute coronary syndrome. Am. J. Cardiol. 2008;101:1088–1093. doi: 10.1016/j.amjcard.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 13.Mega JL, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulot JS, et al. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J. Am. Coll. Cardiol. 2010;56:134–143. doi: 10.1016/j.jacc.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 15.Simon T, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 16.Sentí M, Tomás M, Marrugat J, Elosua R. Paraoxonase1-192 polymorphism modulates the nonfatal myocardial infarction risk associated with decreased HDLs. Arterioscler. Thromb. Vasc. Biol. 2001;21:415–420. doi: 10.1161/01.atv.21.3.415. [DOI] [PubMed] [Google Scholar]
- 17.Turban S, et al. A prospective study of paraoxonase gene Q/R192 polymorphism and severity, progression and regression of coronary atherosclerosis, plasma lipid levels, clinical events and response to fluvastatin. Atherosclerosis. 2001;154:633–640. doi: 10.1016/s0021-9150(00)00495-0. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya T, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouman HJ, et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat. Med. 2011;17:110–116. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee I. Relationship between Paraoxonase 1 (PON1) gene polymorphisms and susceptibility of stroke: a meta-analysis. Eur. J. Epidemiol. 2010;25:449–458. doi: 10.1007/s10654-010-9470-4. [DOI] [PubMed] [Google Scholar]
- 21.Thyagarajan B, et al. Factors associated with paraoxonase genotypes and activity in a diverse, young, healthy population: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Clin. Chem. 2008;54:738–746. doi: 10.1373/clinchem.2007.099044. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B, et al. Paraoxonase (Pon1) Q192R polymorphism and serum Pon1 activity in diabetic patients on maintenance hemodialysis. Clin. Nephrol. 2003;60:257–265. doi: 10.5414/cnp60257. [DOI] [PubMed] [Google Scholar]
- 23.Flekac M, Skrha J, Zídková K, Lacinová Z, Hilgertová J. Paraoxonase 1 gene polymorphisms and enzyme activities in diabetes mellitus. Physiol. Res. 2008;57:717–726. doi: 10.33549/physiolres.931285. [DOI] [PubMed] [Google Scholar]
- 24.Gurbel PA, Antonino MJ, Tantry US. Recent developments in clopidogrel pharmacology and their relation to clinical outcomes. Expert Opin. Drug Metab. Toxicol. 2009;5:989–1004. doi: 10.1517/17425250903107772. [DOI] [PubMed] [Google Scholar]
- 25.Trenk D, et al. Paraoxonase-1 Q192R polymorphism and antiplatelet effects of clopidogrel in patients undergoing elective coronary stent placement. Cardiovasc. Genet. 2011 doi: 10.1161/CIRCGENETICS.111.960112. e-pub ahead of print 11 June 2011. [DOI] [PubMed] [Google Scholar]
- 26.Sibbing D, et al. No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur. Heart J. 2011;32:1605–1613. doi: 10.1093/eurheartj/ehr155. [DOI] [PubMed] [Google Scholar]
- 27.Gurbel PA, Bliden KP, Hiatt BL, O’connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 28.Antman EM, et al. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 29.Applegate RJ, Sacrinty MT, Little WC, Santos RM, Gandhi SK, Kutcher MA. Incidence of coronary stent thrombosis based on academic research consortium definitions. Am. J. Cardiol. 2008;102:683–688. doi: 10.1016/j.amjcard.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 30.Bonello L, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 2010;56:919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.