Abstract
Study Objectives:
Respiratory cycle-related EEG changes (RCREC) quantify statistically significant synchrony between respiratory cycles and EEG spectral power, vary to some extent with work of breathing, and may help to predict sleepiness in patients with obstructive sleep apnea. This study was designed to assess the acute response of RCREC to relief of upper airway obstruction by positive airway pressure (PAP).
Design:
Comparison of RCREC between baseline diagnostic polysomnograms and PAP titration studies.
Setting:
Accredited academic sleep disorders center.
Patients:
Fifty adults referred for suspected sleep disordered breathing.
Interventions:
For each recording, the RCREC in specific physiologic EEG frequency ranges were computed as previously described for the last 3 h of sleep not occupied by apneic events.
Results:
The sample included 27 women; mean age was 47 ± 11 (SD) years; and median respiratory disturbance index at baseline was 24 (inter-quartile range 15-43). Decrements in RCREC, from baseline to PAP titration, reached 43%, 24%, 14%, 22%, and 31% for delta (P = 0.0004), theta (P = 0.01), alpha (P = 0.10), sigma (P = 0.08), and beta (P = 0.01) EEG frequency ranges, respectively. Within each specific sleep stage, these reductions from baseline to PAP studies in synchrony between EEG power and respiratory cycles still reached significance (P < 0.05) for one or more EEG frequency ranges and for all frequency ranges during REM sleep.
Conclusions:
RCREC tends to diminish acutely with alleviation of upper airway obstruction by PAP. These data in combination with previous observations support the hypothesis that RCREC reflect numerous, subtle, brief, but consequential inspiratory microarousals.
Citation:
Chervin RD; Shelgikar AV; Burns JW. Respiratory cycle-related EEG changes: response to CPAP. SLEEP 2012;35(2):203-209.
Keywords: Polysomnography, sleep apnea, obstructive, signal processing, computer-assisted, respiratory cycle-related EEG changes, RCREC, respiration, electroencephalography
INTRODUCTION
Polysomnograms performed to assess for obstructive sleep apnea (OSA) focus on rates of apneas, hypopneas, and respiratory effort-related arousals as the main measures of disease severity.1 However, studies of children and adults repeatedly show that the respiratory disturbance index, or rate of all apneic events per hour of sleep, fails to predict much of the sleepiness, cognitive, or behavioral morbidity thought to reflect key consequences of OSA.2,3 To test the hypothesis that cortical function could be adversely affected by labored breathing outside traditionally scored periods of apnea and hypopnea, a computer algorithm was developed to assess for respiratory cycle-related EEG changes (RCREC), over many breath cycles, that might be invisible to the human eye.4 Application of this algorithm has allowed quantification of RCREC as the extent to which the EEG varies on average in synchrony with the respiratory cycle. Most OSA patients do appear to have highly statistically significant RCREC, meaning that EEG activity in several physiologically relevant frequency bands varies in a predictable way with inspiration and expiration.5
Several observations also suggest that RCREC are clinically relevant. The RCREC appear to be larger in children with sleep apnea than in children without sleep apnea or children treated for sleep apnea by adenotonsillectomy.6 The RCREC in sigma and beta EEG frequency ranges predict subjective sleepiness in children about as well as, and independently of, the respiratory disturbance index.7 In adults evaluated for suspected sleep apnea, and assessed for objective evidence of daytime sleepiness with a multiple sleep latency test, sigma range RCREC adds substantially to the ability of other, more standard polysomnographic variables such as the respiratory disturbance index to predict daytime mean sleep latency.8 Furthermore, during inspiration EEG delta power on average decreases while sigma power increases, both more prominently in sleepy subjects than in non-sleepy subjects.8 This suggests that the RCREC may represent numerous, subtle inspiratory microarousals in response to the labored breathing that is well known to occur for much of the night between standard apneas and hypopneas. Partial correlation of RCREC with results of esophageal pressure monitoring during sleep have added further credence to this hypothesis.5
Nonetheless, much remains unknown about RCREC. In particular, among adults the response of RCREC to continuous or bilevel positive airway pressure (PAP), the most common treatment for OSA, or to any other treatment, has never been assessed. In the current study, we hypothesized that RCREC in patients with OSA should diminish upon application of PAP, and do so in proportion to the baseline severity of the OSA. Affirmation of these hypotheses would suggest that RCREC improve with upper airway patency and decreased work of breathing, and would support the physiological significance of RCREC.
METHODS
Subjects
Subjects for this study were identified retrospectively from among those who underwent PAP titration at the University of Michigan Sleep Disorders Center between March 2, 2009, and March 31, 2009. Under a protocol approved by the University of Michigan medical IRB, a sleep center clinical database was used to identify subjects who would meet the following inclusion criteria: (1) age ≥ 18 years; (2) full-night PAP titration study (continuous PAP, bilevel PAP, or combination) performed for confirmed obstructive sleep apnea did not show central or complex sleep apnea; (3) this titration represented the patient's first experience with PAP (i.e., he or she was not recently on PAP at home); (4) full-night diagnostic polysomnography performed within the previous 6 months; (5) the reason for the diagnostic study had been mainly to assess for OSA; (6) ≥ 4 h of sleep (total sleep time) were recorded on (a) the diagnostic study and (b) the PAP titration; (7) polysomnographic recordings from both the oro-nasal thermocouple and C4-M1 EEG derivations (required for the RCREC analyses) both showed good quality.
Recordings
Diagnostic polysomnograms and PAP titration studies were performed and scored in accordance with standard guidelines.1 Compumedics digital polysomnography equipment and associated Profusion software were used (Compumedics USA Inc, Charlotte, NC). Hypopneas were scored using the alternative rule that allows their identification when followed by either arousals or ≥ 3% oxygen desaturations.1 All studies were performed or supervised by registered polysomnographic technologists in a sleep laboratory accredited by the American Academy of Sleep Medicine. Required quarterly quality assurance assessments helped to maintain reliability, with a targeted 85% concordance between scoring of each technologist and that of the most experienced technologists and faculty in the laboratory. All recordings were converted to European Data Format, with associated XML files that contained scoring information, for RCREC analysis.
Computation of RCREC
To compute RCREC, the EEG and thoracic excursion signals during the last 3 h of recorded sleep were analyzed. Although most previous RCREC analyses have been performed on the first 3 h of recorded sleep, the RCREC can be demonstrated over periods as short as 100 respiratory cycles.4 In this study, we focused on the last 3 h of sleep for both baseline and titration studies to capture time at which OSA was more likely to be effectively controlled, during titration studies, than during the first 3 h. As in our previous studies, to avoid analysis of RCREC during sleep time occupied by apneas, hypopneas, and airflow signal artifacts, on baseline and titration studies, only respiration cycles with airflow amplitudes and durations between the 5 th and 95th percentile were used in the calculations.8
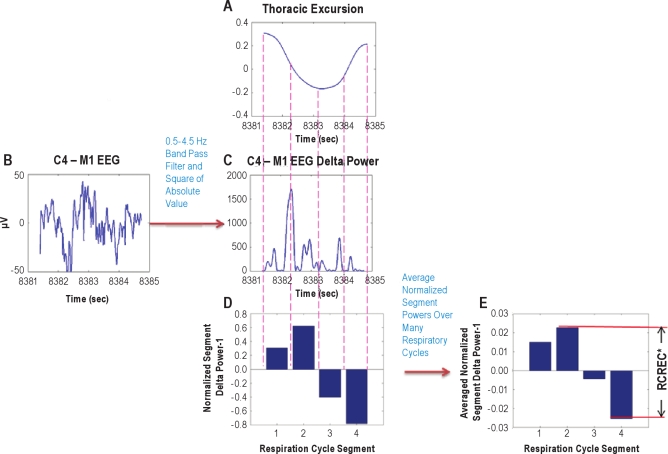
The RCREC were computed as previously described using an algorithm written in MatLab (Mathworks, Natick, MA).8 Figure 1 outlines the procedure. Briefly, the C4-M1 EEG channel was band pass digitally filtered to form 5 time series corresponding to the delta (0.5-4.5 Hz), theta (4.5-8.5 Hz), alpha (8.5-12.5 Hz), sigma (12.5-15.5 Hz), or beta frequencies (15.5-30.5 Hz). Filtering of the C4-M1 EEG data in each case was performed with an appropriate 5th order Butterworth digital filter, implemented using a zero-phase forward and reverse filtering technique (provided by the MatLab filtfilt function) that has zero phase distortion.
Figure 1.
Computation of respiratory cycle-related EEG changes (RCREC) is illustrated. The variation of the EEG signal power is computed for a specifi c frequency band within the time period of each respiratory cycle. The process begins by applying a Savitzky-Golay (polynomial) smoothing fi lter to the recorded thoracic excursion to produce a respiration signal with reduced artifacts (A). The minimums and maximums of this signal, along with temporal midpoints in between, are used to defi ne 4 time segments corresponding to different intervals of the respiratory cycle. A digital bandpass fi lter is applied to the measured EEG signal (B) to produce a time series corresponding to a specifi c frequency band. This signal is squared to produce a time series giving the variation of EEG power with time in the frequency band (C). The mean EEG power in the frequency band (from C) is then computed for each of the 4 respiration cycle time segments defi ned by the fi ltered respiration cycle (A). The mean power for each interval is normalized by the mean frequency-specifi c power over the entire respiration cycle. One is subtracted from each result to get the measures shown for the 4 segments in (D). The segment-specifi c EEG powers are averaged over many respiratory cycles to compute the average measure for each of the 4 respiratory segments (E). The RCREC for this subject, on this sleep study, is computed as the difference between the maximum and minimum mean relative EEG powers for each of the 4 respiratory segments. The RCREC then refl ects the extent to which EEG power varies in synchrony with the average respiratory cycle. *RCREC is maximum difference in normalized segment power after averaging segment power over many respiration cycles.
To define respiratory cycles and component phases, thoracic excursion as recorded by inductance plethysmography was used. Although an oronasal thermocouple has been used in the past,6,8 the thoracic excursion signal was used in the present study to minimize methodological differences between baseline and PAP titration studies, during which machine-generated flow estimates rather than oronasal thermocouples are used to monitor airflow. Previous comparison of RCREC computed from oronasal airflow vs. thoracic excursion suggested that the latter also worked well.4 A Savitzky-Golay (polynomial) smoothing filter was applied to the recorded thoracic excursion signal to produce a respiratory signal with reduced artifacts. Maxima, minima, and intervening temporal mid-points for each thoracic signal-defined respiratory cycle were then used to identify 4 segments that make up each respiratory cycle: early expiration, late expiration, early inspiration, and late inspiration. For each specific EEG frequency band, the mean EEG intensity over each respiratory cycle segment was calculated and divided by the mean intensity over the entire relevant respiratory cycle. In this manner, the EEG frequency-specific, normalized average power was computed for each of the 4 phases of each respiration cycle. The RCREC for any given study was then computed as the maximum difference between the mean EEG powers associated with each of the 4 respiratory cycle segments. The RCREC thus provided quantification of the extent to which EEG power in specified frequency ranges varied in synchrony with the respiratory cycle, on average, over many non-apneic respiratory cycles.
Analyses
The statistical significance of the RCREC for any given study was assessed by a balanced ANOVA in which each respiratory segment power was treated as a group; the ANOVA then tested whether the difference in group means was statistically significant. As respiratory segment powers did not show normal distributions, they were natural log transformed (ln[x + 1]). The RCREC values across subjects also were not normally distributed. Therefore, the hypothesis that RCREC would diminish on PAP, as compared to baseline, was tested using a nonpara-metric Wilcoxon signed rank test. To test the hypothesis that apnea severity at baseline would predict diminution of RCREC on PAP, linear regression models were used. The RCREC and respiratory disturbance index variables were natural log transformed to achieve normal distributions. Minimum oxygen saturation, used as an additional measure of apnea severity, did not require transformation. The secondary hypothesis that RCREC would correlate with apnea severity at baseline was assessed with linear regression. As age and sex showed sporadic weak correlation with RCREC, all regression models were adjusted for these 2 variables to eliminate possible confounding. Finally, as specific sleep stages may influence the likelihood of signifi-cant RCREC,9 and sleep stage distributions are likely to change from baseline to titration studies, RCREC during specific sleep stages captured within the last 3 h of sleep were also explored. The level of significance was set at P < 0.05.
RESULTS
Subjects
The 50 adults whose data were used in this analysis included 23 men and 27 women. The average subject had moderate to severe OSA, with a median respiratory disturbance index of 24 (interquartile range [15-43]) and a median minimum oxygen saturation of 85% [80%-87%]. Demographic and baseline polysomnographic variables are summarized in Table 1.
Table 1.
Demographics and data from baseline polysomnography for N = 50 subjects
| Variable | Mean | Std Dev | Minimum | Maximum |
|---|---|---|---|---|
| Age (years) | 47 | 11 | 28 | 71 |
| Sex (% male) | 46 | |||
| Total Sleep Time (min) | 361 | 44 | 268 | 525 |
| Sleep Efficiency (%) | 81 | 8 | 63 | 95 |
| Stage 1 (%) | 18 | 8 | 3 | 47 |
| Stage 2 (%) | 59 | 12 | 39 | 89 |
| Stage 3 (%) | 7.9 | 8.3 | 0.0 | 26.7 |
| Stage REM (%) | 15 | 7 | 1 | 28 |
| Resp. Disturbance Index | 32 | 25 | 5 | 125 |
| Minimum oxygen sat. (%) | 83 | 6 | 66 | 92 |
Do RCREC diminish or become less statistically significant on PAP?
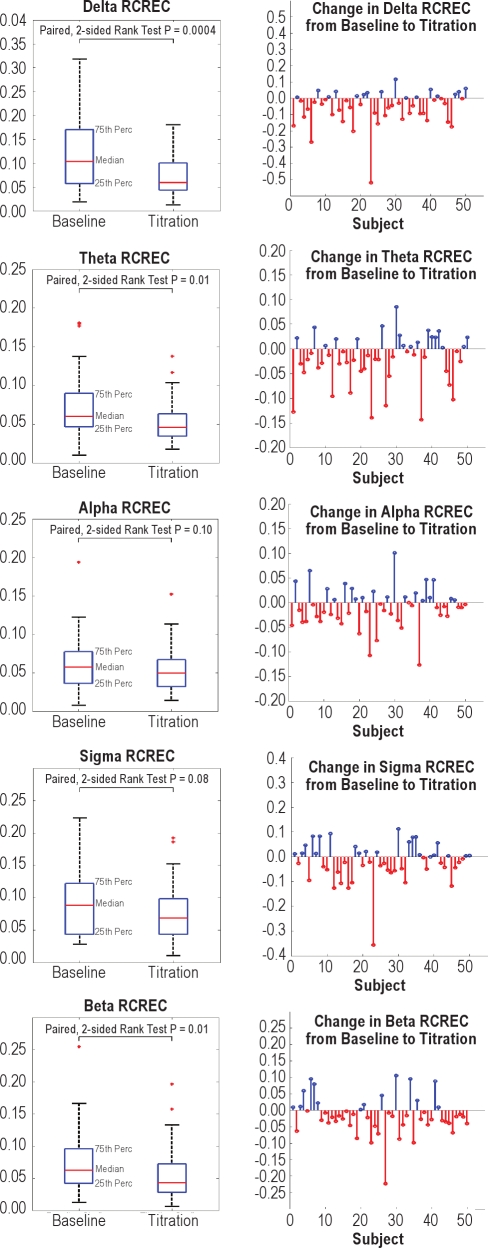
The median decrements in RCREC from baseline to PAP titration studies were 43%, 24%, 14%, 22%, and 31% of their baseline values, for delta (P = 0.0004), theta (P = 0.01), alpha (P = 0.10), sigma (P = 0.08), and beta (P = 0.01) frequency ranges, respectively (Figure 2). These changes and their uniform directions, if not uniform statistical significance, confirm the hypothesis that in general, the tendency for EEG power in specified physiologic frequency ranges to vary in synchrony with non-apneic respiratory cycles diminished on PAP as compared to baseline recordings. Among the 100 polysomno-grams analyzed for this report, only 1 of the 50 PAP studies, and none of the 50 baseline studies, showed no intra-study statistical significance for any RCREC frequency band. For each specific EEG band, from the baseline to PAP titration time points, the frequency of studies showing statistically significant, intra-individual RCREC by ANOVA (P < 0.05) diminished somewhat in absolute terms, though this change reached significance (P < 0.05) or a trend (P < 0.10) only for the delta, theta, alpha, and beta frequency bands (McNemar Test, Table 2).
Figure 2.
Changes from baseline to titration studies in respiratory cycle-related EEG changes (RCREC), for delta, theta, alpha, sigma, and beta EEG frequency ranges, are summarized (left panels) and also shown individually for each of 50 subjects (right panels). Perc, percentile; serifs show 90th and 10th percentiles. These graphs show that in general, the RCREC—as a measure of the extent to which EEG activity is gated by or at least synchronized to the respiratory cycle—diminishes from baseline values when positive airway pressure (PAP) is applied.
Table 2.
Number of subjects (among 50) whose respiratory cycle-related EEG changes (RCREC) achieved statistical significance (ANOVA P < 0.05) during the last 3 h of baseline and positive airway pressure (PAP) titration sleep studies
| RCREC | Baseline | PAP | p-value* |
|---|---|---|---|
| Delta | 42 | 34 | 0.059 |
| Theta | 43 | 35 | 0.074 |
| Alpha | 41 | 32 | 0.050 |
| Sigma | 40 | 35 | 0.251 |
| Beta | 47 | 39 | 0.021 |
McNemar Test.
Interestingly, frequency band-specific RCREC during baseline studies did not correlate strongly with RCREC in the same frequency bands during administration of PAP, though initial beta RCREC did correlate significantly with beta RCREC on PAP (Table 3). Among the 26 subjects with median or greater levels of OSA, sigma and beta RCREC did show more prominent correlation between their respective baseline and PAP values, whereas no such correlations were observed among the remaining 24 subjects with less severe OSA.
Table 3.
Spearman correlations between respiratory cycle-related EEG changes (RCREC) measured during baseline vs. positive airway pressure (PAP) titration studies
| RCREC Frequency Band | All Subjects (n = 50) |
RDI* < 24 (n = 24) |
RDI* ≥ 24 (n = 26) |
|||
|---|---|---|---|---|---|---|
| Rho | P | Rho | P | Rho | P | |
| Delta | 0.17 | 0.230 | 0.14 | 0.519 | 0.25 | 0.216 |
| Theta | 0.17 | 0.228 | −0.03 | 0.888 | 0.35 | 0.077 |
| Alpha | 0.17 | 0.240 | 0.27 | 0.204 | 0.19 | 0.359 |
| Sigma | 0.14 | 0.335 | 0.00 | 0.990 | 0.47 | 0.014 |
| Beta | 0.32 | 0.022 | 0.23 | 0.290 | 0.48 | 0.013 |
RDI, respiratory disturbance index.
Apnea Severity and RCREC
At baseline, RDI (after transformation to normality) showed no significant association or trend toward association with any of the RCREC (natural log transforms), either before or after taking age and sex into account (Table 4). However, baseline RDI, adjusted for age and sex, did explain at least some of the change seen in RCREC after administration of PAP (PAP minus baseline, Table 5). Specifically, baseline RDI predicted change in sigma and beta RCREC, though RDI explained only 9% of the variance in each case.
Table 4.
Regression of baseline respiratory cycle-related EEG changes (RCREC) on baseline respiratory disturbance index*
| RCREC | Overall Model |
Respiratory Disturbance Index |
||||
|---|---|---|---|---|---|---|
| R2 | P | Beta | SE | Partial R2** | P | |
| Delta | 0.021 | 0.80 | 0.127 | 0.149 | 0.015 | 0.40 |
| Theta | 0.046 | 0.53 | 0.079 | 0.130 | 0.008 | 0.54 |
| Alpha | 0.048 | 0.51 | −0.014 | 0.115 | 0.000 | 0.90 |
| Sigma | 0.050 | 0.50 | 0.075 | 0.129 | 0.007 | 0.56 |
| Beta | 0.065 | 0.38 | 0.017 | 0.142 | 0.000 | 0.91 |
RCREC and respiratory disturbance index subjected to natural log transforms; each model adjusted for age and sex.
Squared semi-partial correlation type II.
Table 5.
Regression of differences in respiratory cycle-related EEG changes (RCREC), between PAP and baseline (PAP - baseline), on baseline respiratory disturbance indices*
| RCREC | Overall Model |
Respiratory Disturbance Index |
||||
|---|---|---|---|---|---|---|
| R2 | P | Beta | SE | Partial R2** | P | |
| Delta | 0.023 | 0.77 | −0.140 | 0.167 | 0.015 | 0.41 |
| Theta | 0.035 | 0.65 | −0.160 | 0.149 | 0.025 | 0.29 |
| Alpha | 0.098 | 0.19 | −0.272 | 0.138 | 0.076 | 0.05 |
| Sigma | 0.095 | 0.20 | −0.368 | 0.169 | 0.094 | 0.03 |
| Beta | 0.167 | 0.04 | −0.393 | 0.172 | 0.095 | 0.03 |
RCREC subjected to natural log transforms; each model adjusted for age and sex.
Squared semi-partial correlation type II.
RCREC during Specific Sleep Stages
The RCREC reached within-subject statistical significance in each sleep stage, though the numbers of subjects that showed significant stage-specific RCREC varied (Table 6). Significant RCREC seemed to be more readily demonstrated in stage 2 sleep than in other stages, as previously reported,9 possibly because more stage 2 sleep was recorded. Conversely, the dearth of stage 3 sleep at the end of the night may explain the relative rarity of significant RCREC in this stage, despite the high magnitudes of RCREC during stage 3. Statistically significant reductions in RCREC, from baseline to titration studies, were observed for each specific sleep stage, with the most uniform reductions during REM sleep and the least prominent reductions in stage 1 sleep.
Table 6.
Comparisons of sleep stages and respiratory cycle-related EEG changes (RCREC) within the last 3 hours of sleep, for all sleep stages and each specific sleep stage
| Median baseline time (min) in specified sleep stage | Median titration time (min) in specified sleep stage | Baseline Number of Studies with Significant RCREC* | Titration Number of Studies with Significant RCREC* | Baseline Median RCREC | Titration Median RCREC | Wilcoxon Signed Rank Test p-value | |
|---|---|---|---|---|---|---|---|
| All Stages | 180 | 180 | |||||
| Delta RCREC | 42 | 34 | 0.1039 | 0.0592 | 0.0004 | ||
| Theta RCREC | 43 | 35 | 0.0599 | 0.0455 | 0.0104 | ||
| Alpha RCREC | 41 | 32 | 0.0573 | 0.0493 | 0.1038 | ||
| Sigma RCREC | 40 | 35 | 0.0881 | 0.0684 | 0.0765 | ||
| Beta RCREC | 47 | 39 | 0.0625 | 0.0429 | 0.0122 | ||
| Stage 1 NREM | 31.8 | 18.2 | |||||
| Delta RCREC | 25 | 11 | 0.1493 | 0.1537 | 0.8583 | ||
| Theta RCREC | 20 | 17 | 0.1061 | 0.1265 | 0.0055 | ||
| Alpha RCREC | 19 | 10 | 0.0838 | 0.1180 | 0.0566 | ||
| Sigma RCREC | 20 | 8 | 0.1070 | 0.1032 | 0.8205 | ||
| Beta RCREC | 27 | 17 | 0.0863 | 0.0906 | 0.3417 | ||
| Stage 2 NREM | 105.5 | 106.1 | |||||
| Delta RCREC | 39 | 29 | 0.1289 | 0.0720 | 0.0007 | ||
| Theta RCREC | 39 | 29 | 0.0705 | 0.0532 | 0.0054 | ||
| Alpha RCREC | 32 | 30 | 0.0663 | 0.0613 | 0.0718 | ||
| Sigma RCREC | 40 | 34 | 0.1065 | 0.0905 | 0.0541 | ||
| Beta RCREC | 44 | 38 | 0.0759 | 0.0553 | 0.0224 | ||
| Stage 3 NREM** | 3.9 | 7.3 | |||||
| Delta RCREC | 3 | 1 | 0.1979 | 0.0992 | 0.0100 | ||
| Theta RCREC | 4 | 5 | 0.1057 | 0.0901 | 0.0836 | ||
| Alpha RCREC | 3 | 5 | 0.1435 | 0.0984 | 0.0062 | ||
| Sigma RCREC | 7 | 10 | 0.2186 | 0.1319 | 0.0586 | ||
| Beta RCREC | 10 | 11 | 0.1598 | 0.1118 | 0.0176 | ||
| Stage REM | 38.8 | 48.5 | |||||
| Delta RCREC | 30 | 19 | 0.1332 | 0.0797 | 0.0001 | ||
| Theta RCREC | 29 | 25 | 0.0907 | 0.0705 | 0.0030 | ||
| Alpha RCREC | 22 | 17 | 0.0850 | 0.0602 | 0.0007 | ||
| Sigma RCREC | 20 | 28 | 0.0994 | 0.0797 | 0.0431 | ||
| Beta RCREC | 33 | 27 | 0.0891 | 0.0635 | 0.0052 |
Statistically significant differences between median RCREC on baseline and titration studies are highlighted in red.
Significance assessed by ANOVA (P < 0.05) that compared mean respiratory cycle segment-specific EEG powers, transformed to normality (ln[x + 1]). Sleep studies that attained statistical significance are those for which the observed average degree of synchrony, between EEG activity and respiratory cycle phases, was unlikely to have occurred by chance alone.
Data for Stage 3 NREM based on 19 subjects who showed at least some of this stage during the last 3 hours of sleep on both baseline and titration recordings.
DISCUSSION
This analysis of 100 polysomnograms from 50 adults with OSA is the first to examine the response of respiratory cycle-related EEG changes to positive airway pressure. Results confirm the primary hypothesis that RCREC, at least in the delta, theta, beta, and possibly sigma frequency ranges, diminish significantly from baseline even on the first night that PAP is applied. In parallel to the decrements observed in median RCREC values, from baseline to PAP titration studies, the frequency with which intra-individual RCREC attained statistical significance also decreased significantly for alpha and beta RCREC, and trended downward for delta and theta RCREC. In other words, EEG power varied less closely with the respiratory cycle, on average, when PAP was applied than when PAP was not applied. These findings are consistent with the idea that coupling of brain activity, or its entrainment, to labored respiratory cycles diminishes when upper airway obstruction is alleviated. In combination with previous observations that baseline RCREC during nocturnal sleep predict subjective and objective daytime sleepiness,7,8 that RCREC vary in part with esophageal pressure,5 and that sigma RCREC increase while delta RCREC decrease during inspiration,8 the current results strengthen the hypothesis that OSA may produce sleepiness through mechanisms beyond apneas, hypopneas, and respiratory effort-related arousals that are visible to the human eye. Specifically, OSA may cause sleepiness in substantial part because of numerous, subtle microarousals that appear in tandem with labored inspiration, become visible only by computer analysis over many respiratory cycles, and—as we now report—diminish even on the first night that PAP is applied.
If RCREC do represent coupling of brain activity to labored, obstructed respiratory cycles, an interesting question raised by our data is why reductions in RCREC on PAP, across all sleep stages, did not exceed 14% to 43%. Subjects without OSA generally do still show some degree of RCREC,6 so reduction of RCREC to normal levels may not require their elimination. Furthermore, reduction in RCREC could in theory progress with continued nights of PAP use, beyond the first, just as daytime sleepiness often takes time to resolve. In addition, the current data do not compare RCREC during baseline to RCREC during steady administration of ideal PAP levels, but rather to RCREC during near-optimal, though probably still evolving PAP settings.
A somewhat surprising observation was that sigma-range RCREC diminished much less than some others (such as delta and beta). Two previous studies, one in adults and another in children, found sigma RCREC as compared to RCREC in other frequency ranges to be most sensitive to objectively or subjectively assessed levels of sleepiness.7,8 Sleepy vs. less sleepy adults showed more prominent increases in sigma power during inspiration, suggesting more substantial microarousal with each labored respiratory cycle.8 However, sensitivity of sigma RCREC to sleepiness and failure to improve dramatically on PAP are not necessarily divergent observations. Sleepiness does not always resolve after thorough treatment with PAP, and commonly does not resolve completely.10,11 Standard sleep laboratory titration-determined settings may leave some patients undertreated, with a level of upper airway resistance that is still capable of producing daytime sleepiness.12–14 Treatment of OSA on average reduces the mean sleep latency on a multiple sleep latency test or maintenance of wakefulness by only 0 to 2 minutes in adults15 and children.3 Sleepiness, like hypertension or other consequences of chronic OSA, may not be completely reversible, even when OSA is optimally treated. For these reasons, we wonder whether lack of better improvement in sigma RCREC after OSA is treated may eventually prove informative rather than misleading, in understanding resolution or persistence of sleepiness.
The limited and nonsignificant correlation we found between RCREC and RDI at baseline resembles our previous findings among 38 adults studied for suspected OSA.8 An earlier study of 10 children found a correlation between the apnea-hypopnea index and theta RCREC, but not delta or alpha RCREC (other ranges were not assessed).6 Although a measure of inspiratory microarousals might be expected to correlate more consistently with the RDI, any new measure with improved predictive ability for OSA outcomes should show considerable divergence from an RDI that explains only about 10% of variance in objectively assessed sleepiness among sleep laboratory-referred patients.2 Subjective sleepiness also is not strongly linked to RDI.16 Therefore, if RCREC diverges from the RDI but still predicts OSA outcomes or treatment response, RCREC could still have significant clinical utility. In the current data, larger baseline RDI did predict greater reduction of RCREC, at least in higher EEG frequencies, upon application of PAP.
Exploration of RCREC within each sleep stage, for the last three hours of baseline and titration recordings, confirmed previous findings that statistically significant RCREC could be demonstrated within any sleep stage.4,9 However, the data also suggested that amelioration of RCREC by administration of PAP may be greatest and most reliable (over all EEG frequency ranges) during REM sleep, even if RCREC itself reaches greatest absolute magnitudes during stage 3 NREM sleep (Table 6). A better understanding of RCREC characteristics and implications during specific sleep stages may require analysis of data from full nights of recording (only small amounts of stage 3 NREM were captured in the current end-of-night study) and comparison with sleepiness and other OSA outcomes.
The poor correlations we found between RCREC on baseline and PAP studies raise the possibility that test-retest reliability of RCREC will require further study. One suggestion in the current data however, that lack of association between baseline and PAP RCREC was in fact due to effective treatment—i.e., maximal acute reduction of RCREC to some intrinsic level not related to baseline apnea severity—is that more severe apneics showed stronger correlations between baseline and PAP RCREC, whereas less severe apneics showed no such correlations (Table 3). Baseline and PAP beta RCREC, in particular, did correlate among all subjects and also those with more severe OSA, an interesting observation in view of the previous finding that among all RCREC ranges, beta RCREC correlate most strongly with simultaneous esophageal pressure measurements.5 Subjects with severe apnea at baseline might well be those at risk for less completely effective PAP titrations, less effective control of OSA during the last 3 hours of their titration studies, and therefore stronger correlation between RCREC assessed during baseline and PAP titration studies. However, current results could also suggest that in more severe OSA patients, synchrony between EEG and respiratory cycles, regardless of the magnitude, is more ingrained and more difficult to disassociate when PAP is applied.
Findings from the current study augment previous evidence that RCREC, computed from sleep that occurs outside times at which apneas or hypopneas occur, provide information not captured by a standard RDI. If RCREC do represent numerous, brief, subtle, but consequential inspiratory microarousals, we speculate that routine assessment of RCREC could eventually prove useful clinically in several ways. For example, incorporation of automatic RCREC analyses into real-time monitoring might improve effectiveness of diagnostic polysomnography, laboratory-based PAP titrations, or automatically adjusting PAP machines. In the meantime, many questions remain that merit additional research. De-synchronization of the EEG and labored respiratory cycle might be accomplished by means other than PAP. Algorithms even simpler than RCREC could be devised to assess the same phenomenon. Eventually, with better understanding of exactly what causes sleepiness in obstructive sleep apnea, polysomnography could be further simplified and scoring automated to an extent that might preserve or enhance clinical utility while greatly reducing time and expense.17,18
DISCLOSURE STATEMENT
This was not an industry supported study. The algorithm to compute RCREC is protected by a patent, owned by the University of Michigan and Michigan Technological University. Drs. Chervin and Burns are named as inventors. Dr. Chervin is named as an inventor of intellectual property related to diagnosis and treatment of sleep disorders and owned by the University of Michigan. He has received royalties from Up To Date. He has served on the advisory board for Pavad Medical and as a consultant for Arena Pharmaceuticals. Dr. Shelgikar has indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Morton B. Brown, PhD, for statistical review and advice on the analyses presented. This work was performed at the University of Michigan and Michigan Tech Research Institute, Ann Arbor, Michigan. Support for this study provided by: NIH (R01 HL080941, UL1 RR024986).
Footnotes
A commentary on this article appears in this issue on page 173.
REFERENCES
- 1.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 2.Chervin RD, Aldrich MS. Characteristics of apneas and hypopneas during sleep and relation to excessive daytime sleepiness. Sleep. 1998;21:799–806. [PubMed] [Google Scholar]
- 3.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117:e769–78. doi: 10.1542/peds.2005-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chervin RD, Burns JW, Subotic NS, Roussi C, Thelen B, Ruzicka DL. Method for detection of respiratory cycle-related EEG changes in sleep-disordered breathing. Sleep. 2004;27:110–5. doi: 10.1093/sleep/27.1.110. [DOI] [PubMed] [Google Scholar]
- 5.Chervin RD, Malhotra RK, Burns JW. Respiratory cycle-related eeg changes during sleep reflect esophageal pressures. Sleep. 2008;31:1713–20. doi: 10.1093/sleep/31.12.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chervin RD, Burns JW, Subotic NS, Roussi C, Thelen B, Ruzicka DL. Correlates of respiratory cycle-related EEG changes in children with sleep-disordered breathing. Sleep. 2004;27:116–21. doi: 10.1093/sleep/27.1.116. [DOI] [PubMed] [Google Scholar]
- 7.Chervin RD, Weatherly RA, Ruzicka DL, et al. Subjective sleepiness and polysomnographic correlates in children scheduled for adenotonsillectomy vs. other surgical care. Sleep. 2006;29:495–503. [PMC free article] [PubMed] [Google Scholar]
- 8.Chervin RD, Burns JW, Ruzicka DL. Electroencephalographic changes during respiratory cycles predict sleepiness in sleep apnea. Am J Respir Crit Care Med. 2005;171:652–8. doi: 10.1164/rccm.200408-1056OC. [DOI] [PubMed] [Google Scholar]
- 9.Burns JW, Ruzicka DL, Chervin RD. Respiratory cycle-related EEG changes (RCREC) during specific sleep stages. Sleep. 2005;28:A186. [Google Scholar]
- 10.Sforza E, Krieger J. Daytime sleepiness after long-term continuous positive airway pressure (CPAP) treatment in obstructive sleep apnea syndrome. J Neurol Sci. 1992;110:21–26. doi: 10.1016/0022-510x(92)90004-5. [DOI] [PubMed] [Google Scholar]
- 11.Black J. Pro: modafinil has a role in management of sleep apnea. Am J Respir Crit Care Med. 2003;167:105–6. doi: 10.1164/rccm.2209006. discussion 108. [DOI] [PubMed] [Google Scholar]
- 12.Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness: the upper airway resistance syndrome. Chest. 1993;104:781–7. doi: 10.1378/chest.104.3.781. [DOI] [PubMed] [Google Scholar]
- 13.Guilleminault C, Winkle R, Korobkin R, Simmons B. Children and nocturnal snoring-- evaluation of the effects of sleep related respiratory resistive load and daytime functioning. Eur J Pediatr. 1982;139:165–71. doi: 10.1007/BF01377349. [DOI] [PubMed] [Google Scholar]
- 14.Sforza E, Krieger J, Bacon W, Petiau C, Zamagni M, Boudewijns A. Determinants of effective continuous positive airway pressure in obstructive sleep apnea: role of respiratory effort. Am J Respir Crit Care Med. 1995;151:1852–6. doi: 10.1164/ajrccm.151.6.7767530. [DOI] [PubMed] [Google Scholar]
- 15.Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430–4. doi: 10.1136/thx.2005.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–7. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 17.Bennett LS, Langford BA, Stradling JR, Davies RJO. Sleep fragmentation indices as predictors of daytime sleepiness and nCPAP response in obstructive sleep apnea. Am J Respir Crit Care Med. 1998;158:778–86. doi: 10.1164/ajrccm.158.3.9711033. [DOI] [PubMed] [Google Scholar]
- 18.Chervin RD, Burns JW. Engineering better sleep. Med Biol Eng Comput. 2011;49:623–5. doi: 10.1007/s11517-011-0777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]