Abstract
Study Objectives:
Task-switching is an executive function involving the prefrontal cortex. Switching temporarily attenuates the speed and/or accuracy of performance, phenomena referred to as switch costs. In accordance with the idea that prefrontal function is particularly sensitive to sleep loss, switch-costs increase during prolonged waking in humans. It has been difficult to investigate the underlying neurobiological mechanisms because of the lack of a suitable animal model. Here, we introduce the first switch-task for rats and report the effects of sleep deprivation and inactivation of the medial prefrontal cortex.
Design:
Rats were trained to repeatedly switch between 2 stimulus-response associations, indicated by the presentation of a visual or an auditory stimulus. These stimulus-response associations were offered in blocks, and performance was compared for the first and fifth trials of each block. Performance was tested after exposure to 12 h of total sleep deprivation, sleep fragmentation, and their respective movement control conditions. Finally, it was tested after pharmacological inactivation of the medial prefrontal cortex.
Settings:
Controlled laboratory settings.
Participants:
15 male Wistar rats.
Measurements & Results:
Both accuracy and latency showed switch-costs at baseline. Twelve hours of total sleep deprivation, but not sleep fragmentation, impaired accuracy selectively on the switch-trials. Inactivation of the medial prefrontal cortex by local neuronal inactivation resulted in an overall decrease in accuracy.
Conclusions:
We developed and validated a switch-task that is sensitive to sleep deprivation. This introduces the possibility for in-depth investigations on the neurobiological mechanisms underlying executive impairments after sleep disturbance in a rat model.
Citation:
Leenaars CHC; Joosten RNJMA; Zwart A; Sandberg H; Ruimschotel E; Hanegraaf MAJ; Dematteis M; Feenstra MGP; van Someren EJW. Switch-task performance in rats is disturbed by 12 h of sleep deprivation but not by 12 h of sleep fragmentation. SLEEP 2012;35(2):211-221.
Keywords: Task-switching, prefrontal cortex, sleep deprivation, sleep fragmentation, conditional discrimination
INTRODUCTION
It has been suggested that cognitive functions that depend on the integrity of the prefrontal cortex, often referred to as “executive functions,” are particularly sensitive to sleep.1,2 Prefrontal regions show strong deactivations during sleep and sleep deprivation.2–4 Altered activity of modulatory monoaminergic and cholinergic neurotransmitter systems has been hypothesized to underlie at least some of these findings.1 However, the study of this relationship has been difficult and the use of cognitive tasks leading to reproducible results and allowing for repeated measurements has been advocated.1 As measurements of neurotransmitter systems generally depend on invasive measurements in rodents, there is a need to translate tasks for prefrontal functions affected by sleep deprivation for use in experimental animals.
Executive functions encompass, among others: mental planning, initiation of activity, attentional control, utilization of feed-back, and cognitive flexibility. The latter function can be probed by switch-tasks.5,6 Several groups reported that prolonged wakefulness negatively affects performance on switch-tasks, specifically on the first trials after switching.7–9
Switch-tasks give reproducible results and allow for repeated measurements. While rodent alternatives to human tasks are available for other tasks probing flexibility, such as reversal learning and extradimensional set-shifting,10 rodent models for switch tasks have not yet been described.
The general concept behind most switch-tasks is the combination of two different tasks in blocks of several trials within test sessions. The switch-task therefore contains task-switching trials where subjects must change between tasks, and repetition trials, where subjects continue to perform the same task. Switch-tasks can be as simple as pressing a button matching certain objects,11 or as complex as categorizing words using different rules.12 Irrespective of the precise task used, switch-costs appear on response latency and/or response accuracy; responses are slower on switch-trials compared to repetition-trials, or accuracy is decreased on switch-trials compared to repetition-trials.6
Human switch-tasks are usually performed fairly fast (reaction times < 1 s) and accurate (errors on < 3% of the trials).5 Consequently, small changes in task-performance can be significant and are considered to be biologically relevant.7–9 As rat switch-tasks should ideally be performed with roughly similar speed and accuracy, task design must be kept relatively simple. Based on these considerations, we designed a switch-task for rats in which a simple conditional discrimination task is presented block-wise. Several types of conditional discrimination tasks have been described in rodent literature, but trials of each type were offered randomly instead of in a block-design.13–25 These conditional discrimination tasks usually consist of a simple spatial discrimination where the rewarded lever in a Skinner box is dependent on the presence or absence of certain stimuli.
We here introduce and validate a new switch-task for rats based on a simple conditional discrimination task, where one out of two levers is rewarded when a light stimulus is present, while the other lever is rewarded when an auditory stimulus is present. In contrast to normal conditional discrimination tasks, the switch-task consisted of blocks of 5-10 trials of either stimulus-response association.
We hypothesized that sleep disruption would impair rodent switch-task performance, in line with human findings. Sleep disruption may involve deprivation or fragmentation of sleep by, for example, noise. We therefore first investigated whether 12 h of total sleep deprivation, as a model for one night without sleep, would impair switch-task performance in rats as it does in humans. Cognitive deficits of fragmented sleep have been reported as well,26 but may be less pronounced than is the case after total sleep deprivation. To the best of our knowledge, the effect of sleep fragmentation on task-switching has not yet been investigated in any species. We therefore tested if 12 h of sleep fragmentation, a model for regular sleep disturbance during one night, would also impair switch-task performance. As humans activate their prefrontal cortical (PFC) areas during task-switching,27–29 we would also expect the rat PFC to be involved in switch-task performance. Therefore, we next tested the effect of temporary PFC-inactivation on performance of our switch-task for rats.
We here show that task-switching induces switch-costs on latency and accuracy in conditions of undisturbed sleep, and that a period of 12 h of near total sleep deprivation, but not a similar period of sleep fragmentation, decreases the accuracy specifically on switch-trials. Involvement of the medial prefrontal cortex was indirectly supported by the fact that its inactivation by local infusion of a mixture of muscimol and baclofen results in a decrease in the overall accuracy.
MATERIALS AND METHODS
All behavioral experiments were performed in 15 male Wi-star rats (Harlan, Horst, the Netherlands; weight upon arrival 225-275), housed under reversed light conditions (lights ON at 23:15; lights OFF at 11:15) to enable dark-phase behavioral experiments during regular office hours, in groups of 4 in type-IV Makrolon cages (60 × 38 × 20 cm), in a room with controlled temperature (20°C ± 2°C) and humidity (60% ± 20%). These 15 rats were first trained to perform the switch-task. After training, rats were initially tested under conditions of unrestricted sleep. Secondly, they were tested after sleep deprivation and the sleep deprivation movement control, and thirdly, after sleep fragmentation and the sleep fragmentation movement control. An overview of the experimental protocol is provided in Table 1A. These same rats were cannulated after the sleep experiments to participate in the local infusion experiments. After surgery, rats were housed individually in high type-III Makrolon cages (38 × 21 × 24 cm) to prevent damaging of the cannulae.
Table 1A.
Overview of experimental protocol
| Training | Sleep Deprivation | Sleep Fragmentation | Surgeries Recovery | Local Infusions | ||
|---|---|---|---|---|---|---|
| ± 12 weeks | 3 weeks | 1 week | 3 weeks | 1 week | 1 week | 3 weeks |
Rats performed the switch-task on all workdays (but not in the week of surgery). Experiments were performed over the course of one week (see Table 1B), the two experimental weeks within one experiment were separated by one week of daily testing while otherwise undisturbed in the home cage.
As we use a novel movement control condition in this study, an additional experiment was set-up to quantify sleep in this condition. EEG-experiments were performed in a separate group of 8 rats (Harlan, Horst, the Netherlands; weight upon arrival 225-275), housed under normal light conditions (lights ON at 07:00; lights OFF at 19:00). After surgery, these rats were housed individually in high type-III Makrolon cages.
All rats were left undisturbed for at least one week after arrival for acclimatization, and habituated to daily handling for at least another week prior to starting experiments. Food was restricted for rats in the behavioral experiments. Rats in the EEG experiments were always provided with food ad libitum. Water was unrestricted for all rats.
All experiments were approved by the experimental animal committee of the Royal Netherlands Academy of Arts and Sciences and performed in accordance with Dutch legislation (Wet op de dierproeven, 1996) and European guidelines.
Behavior and Training
Approximately 3 days before the onset of behavioral training, food was restricted. On the days without behavioral testing, rats were fed 15 g/rat/day, on the days with behavioral training this was decreased to 12 g/rat/day, as rats could then supplement their diet with food rewards. All rats increased their body weight during these experiments.
Behavioral experiments were conducted in 8 Skinner boxes (Med-associates, St. Albans, Vermont, USA) controlled by MED-PC software (Med-associates). Skinner boxes (305 × 241 × 210 mm) consisted of Perspex sides and metal modular panels with a grid floor 19 × 4.8 (305 × 241 mm, 19 grids of 4.8 mm∅) suspended over a tray filled with sawdust. The operant panel on one side of the box contained 2 levers (40 × 19 × 2 mm) placed 21 mm above the floor on the left and right side of the food tray (51 × 51 mm, 21 mm above the floor). Two cue-lights (7,9 mm∅) were placed 75 mm above each lever. On the opposite side of the box, a house-light was centrally mounted (200 mm above the floor), next to which the white noise speaker (76 × 83 mm, 175 mm above the floor) was placed.
Each rat was appointed one Skinner box, in which daily (on workdays) training and testing of that specific rat took place at the onset of the active phase (dark-onset).
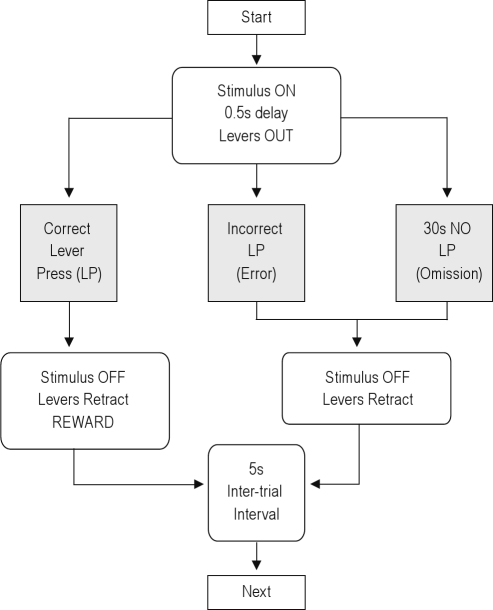
After rats had been trained to associate lever-pressing with food reward (Bio-Serve purified dustless precision pellets, F0021, 45 mg, BioServ, Frenchtown, New Jersey, USA), they were trained on a conditional discrimination task, in which they had to discriminate between 2 distinct stimuli (2 simultaneously lit cue-lights versus acoustic white noise) to decide which response to make to obtain a reward. Stimuli were presented from 0.5 s before lever-presentation until a response was made. Rats were trained in one session per day, and first exposed to 2 sessions consisting of one stimulus-response association only (e.g. after the light stimulus, press the right lever to obtain a reward). On the following days, they were exposed to 2 sessions with the opposite stimulus-response association (e.g. after the sound stimulus, press the left lever to obtain a reward). A flowchart of a trial within the conditional discrimination task is provided in Figure 1. The 2 stimulus-response associations were then gradually integrated within sessions, exposing rats to increasing numbers of switches between both rules within a session. An overview of the general training procedure is provided in Table 2. Although individual differences occurred in the speed of acquisition, all rats performed > 80% accurate at the end of each stage of training.
Figure 1.
Flowchart of one trial of the switch task.
Table 2.
Training procedure
| Session | Number |
|---|---|
| Learn to press the levers | 5 |
| First SRA | 2 |
| Second SRA | 3 |
| 1 switch | 3 |
| 2 switches | 1 |
| 3 switches | 2 |
| 15 to 17 switches | 22 |
SRA, stimulus response association.
Lever pressing was taught with 1-2 sessions per day. After rats had learned to associate a lever press with food reward, they were only exposed to one session per day. At the end of each stage, rats performed the task at hand with an accuracy of > 80%.
Behavior; Switch-Task
Rats were tested in sessions of 119-129 trials, grouped in 15-17 blocks of an unpredictable number of 5-10 trials of one of the 2 stimulus-response associations. This configuration led to 14-16 switches between the 2 rules, the first trial of each block being the switch-trial. An example of a switch-task session is provided in Figure 2. The total number of blocks and the numbers of trials in the blocks were varied across days to prevent sequence predictability. One daily behavioral test session (Monday to Friday) was started within 5 min of the onset of the dark period (the active phase of the rat). All experiments were counterbalanced for both the lever associated with each stimulus and the first rule learned.
Figure 2.
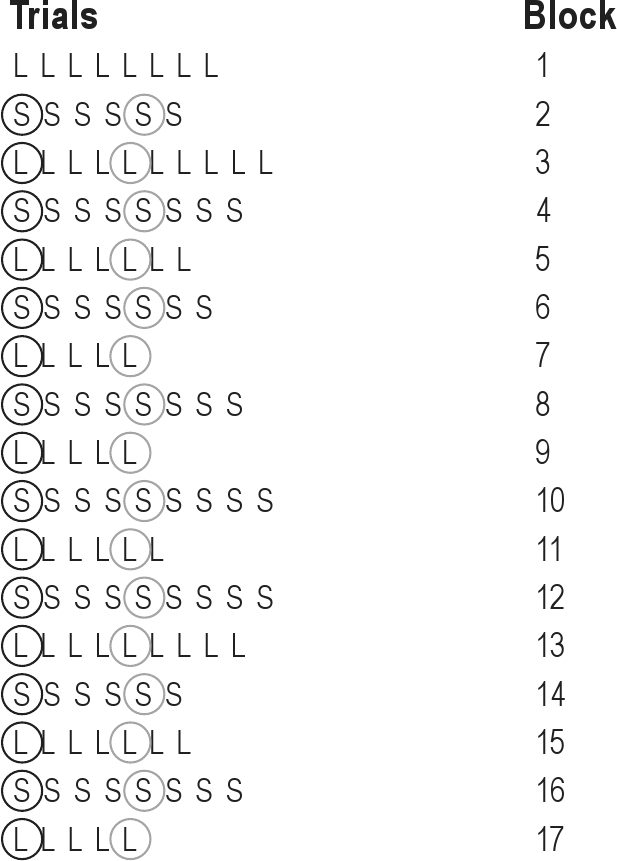
Example of the subsequent trials in a switch-task session. L, Light trial; S, Sound trial; circled trials were used for data-analysis. Black circles indicate switch-trials, gray circles indicate repetition trials.
As each block of the conditional discrimination task consisted of ≥ 5 trials, the fifth trial was chosen as the typical repetition-trial used in later comparisons with switch-trials. For each session and for each rat, average switch-trial and repetition-trial latencies were calculated, as well as the percentages of correctly performed switch-trials and fifth trials. Data from the first block of each session were not included in the analyses, as the first trial of the first block is neither a switch-trial, nor a repetition trial. Data from all other blocks were pooled for each session. Results from blocks with acoustic noise and light stimuli were pooled, comparable to the described analyses of human data.11,28–33 Error trials and omitted trials, as well as trials directly following errors and omissions, were removed from latency analyses. Baseline switch-task performance was analyzed during the last 3 days preceding the first experimental week; during the 19th–22nd training session.
Sleep Deprivation
Sleep deprivation was accomplished with gradually increasing mild forced locomotion, as described previously.34 Briefly, sleep deprivation devices consisted of a rotating drum (∅ 39 cm, height 37 cm), divided into 2 semicircular compartments by a stationary central wall. The bottom moves bidirectionally and at varying speed, and both speed and the number of directional alternations are gradually increased in hourly intervals to compensate for increasing sleep pressure. A movement control condition was created with the intention of allowing rats a relatively normal amount of sleep, while exposing them to the same increases in locomotor activity as produced by the sleep deprivation protocol. To this end, the movement control protocol started 12 h earlier than the sleep deprivation condition and consisted of alternating hours of undisrupted sleep opportunity and hours during which the device rotated as in the sleep deprivation protocol, yet interspersed with hourly rest intervals. As this movement control condition has not been implemented before, an additional experiment was set-up to quantify sleep in this condition (described below).
Rats were housed in the sleep deprivation boxes during a full test week (depicted in Table 1B), from Monday (day 1) - after their daily testing - onwards. Monday and Tuesday were habituation days, Wednesday (day 1) was used for baseline measurements. Sleep deprivation always started at 23:15 (day 1), and rats were tested immediately after deprivation ended at 11:15 (day 2). After testing, rats were placed back in the deprivation devices for one more day (recovery, day 3). Activity levels were measured in the sleep deprivation boxes as described previously,34 for 24 h before sleep deprivation or 12 h before the movement control condition, during these protocols and for 12 h of subsequent recovery.
Table 1B.
Overview of an experimental sleep deprivation week
| Monday | Tuesday | Wednesday | Thursday | Friday |
|---|---|---|---|---|
| Habituation to Sleep | Continued | Baseline | Experiment | Recovery |
| Deprivation Device | habituation | (day 1) | (day 2) | (day 3) |
Rats performed the switch-task on all workdays at dark-onset.
Rats were exposed to both the movement control and the sleep deprivation condition in a counterbalanced order, separated by at least 1 week of testing under unrestricted sleep conditions from the normal home cage.
Sleep Fragmentation
Sleep fragmentation can be accomplished by exposing rats to forced locomotion for brief periods while allowing undisturbed sleep in between.10 We exposed our rats to a protocol in which 30 s of sleep deprivation device movement was followed by 90 s without movement to induce fragmented sleep. In the movement control condition for sleep fragmentation, rats were exposed to 10 min of sleep deprivation device movement, followed by 30 min without movement, resulting in an equivalent total time period of locomotion without major effects on sleep.10 The speed of the sleep deprivation boxes was set at 2 RPM. At this speed, when rats would walk halfway the radius of our circular device, they would walk approximately the same distance as in the treadmill study where the speed was set at 0.02 m/s.10 The direction of the box was reversed for each 30-s movement episode.
Rats were housed in the sleep deprivation boxes during a full test week, from Monday (day −1) - after their daily testing - onwards. Monday and Tuesday were habituation days, Wednesday (day 1) was used for baseline measurements. Sleep fragmentation always started at 23:15 (day 1), and rats were tested immediately after fragmentation ended at 11:15 (day 2). After testing, rats were placed back in the deprivation devices for one more day (recovery, day 3). Activity levels were measured in the sleep deprivation boxes as described previously,34 for 24 h before sleep fragmentation/movement control condition, during these protocols and for 12 h of subsequent recovery.
Rats were exposed to both the movement control and the sleep fragmentation condition in a counterbalanced order, separated by at least 1 week of testing under unrestricted sleep conditions from the normal home cage.
Brain Cannulations
Cannulae for local infusions were placed into mPFC in 13 rats. Surgical procedures were comparable to procedures used in microdialysis probe implantation as described previously.34 Briefly, anesthesia was induced with intramuscular Hypnorm (0.22 mg/kg fentanyl citrate with 7.0 mg/kg fluanisone in 0.7 mL/kg body weight, Janssen Pharmaceuticals, Belgium) and subcutaneous Dormicum (0.75 mg/kg midazolam in 0.3 mL/kg, Roche, Switzerland), the latter also providing muscle relaxation.
Cannulae (C313G, PlasticsOne, Roanoke, Virginia, USA) were stereotactically implanted bilaterally into the mPFC at an angle of 12° to vertical (AP+3.0, L ± 1.8 relative to bregma; V−3.0 mm relative to dura) and secured to the skull with 3 skull screws and dental cement (Kemdent Simplex Rapid, Associated Dental Products Ltd, Wiltshire, UK). Dummies (plastics one) were inserted into the cannulae to prevent blocking. Temgesic (0.10 mg/kg buprenorphine, Schering-Plough, Houten, the Netherlands) was subcutaneously given for postoperative pain relief upon awakening. During post-surgical recovery, no behavioral tests were performed for one week and food was given ad libitum for 5 days.
Local Infusions
After 1.5 weeks of retraining, rats were injected bilaterally with either, a mixture of the GABAA-agonist muscimol (0.01 mM) and the GABAB-agonist baclofen (0.1 mM), dissolved in saline, or with the vehicle (saline). Dummies were removed and microinjection-needles (extending 1 mm below the end of the guide cannulae) were inserted into the guides. A volume of 0.3 μL per hemisphere was injected over a period of 2 min (CMA100 microinjection pump, CMA, Solna, Sweden). Microinjection-needles were replaced by dummies 1 min after the infusion ended. Another 5 min later the rat was placed in the Skinner box, and the behavioral session started. Protocols for local injections were based on previous studies.35,36
Rats received both the saline and the muscimol-baclofen infusion in a counterbalanced order with 2 weeks between repeated infusions allowing for full recovery. Due to technical problems during infusion experiments, 2 rats could only be tested in one condition. These data were not used for analyses.
Histology
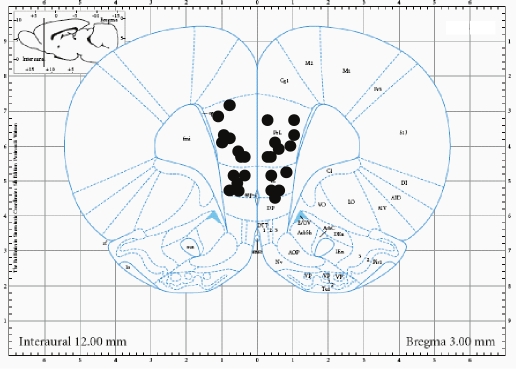
Histology was performed on all cannulated brains. Animals were anesthetized with an overdose of Nembutal (50 mg in 1 mL IP) and transcardially perfused with saline followed by paraformaldehyde (4% in phosphate-buffer 0,1M). Brains were removed and kept in paraformaldehyde for at least one week, and then transferred to sucrose (30%, in Tris-buffered saline with 0.05% sodium azide). After at least one more week, sections were cut on a cryostat, stained with cresyl violet (1%) and tract positions were compared to the Paxinos & Watson atlas. Positioning of the microinjection needle-tips is depicted in Figure 3.
Figure 3.
Location of the tips of the injection needles.
EEG
EEG surgeries, experiments and analysis were performed as described previously.34 Briefly, 5 homemade EEG electrodes were placed on the dura (AP+2.0; L ± 2.0 relative to bregma; AP+2.0; L ± 2.0 relative to lambda; and AP −2.0 mm on midline relative to lambda), 2 EMG electrodes were inserted into the neck muscle and all gold pins were inserted into a connector (Albedo Projects, Belgium). EEG experiments were performed after approximately 2 weeks of postsurgical recovery. Two animals were always recorded simultaneously in one sleep deprivation device. EEG and EMG were sampled at 200 Hz, filtered between 0.5 and 90 Hz, and stored with Somnologica 3.3.1 (Medcare, Reykyavik).
Animals were allowed approximately 10 h of habituation to the experimental environment while EEG was recorded, followed by 24 h of baseline measurements. Afterwards, rats were exposed to the movement control protocol for sleep deprivation, described above. Recording continued throughout the 12 h of subsequent recovery.
EEG-EMG traces were manually scored offline in 10-s epochs for waking, slow wave sleep (SWS) and rapid eye movement sleep (REMS) by one of the experimenters. The duration of SWS and REMS was quantified in hourly intervals.
Statistical Analysis
For baseline switch-task performance during the last baseline week preceding sleep deprivation interventions, latencies and accuracy (%correct) were analyzed separately with ANOVAs including experimental day (day 1–3, Wednesday – Friday) and trial (first versus fifth) as within-subject factors.
For performance after sleep interventions, latencies and accuracy (%correct) were analyzed separately with ANOVA's including experimental day (day 1–3, always Wednesday – Friday), experimental condition (sleep intervention or movement control) and trial (first versus fifth) as within-subject factors.
For local infusion experiments, performance after infusion of the muscimol-baclofen mixture was directly compared with performance after the saline control infusion using an ANOVA including trial (first versus fifth) and condition (saline versus mixture) as within-subject factors.
Besides, performance after saline was compared with baseline performance in the 2 experimental conditions and the baseline week, using an ANOVA with trial and experiment as within-subject factors.
Activity data during the movement protocols were collected in 2-min bins. For sleep deprivation experiments, values were summed over the 24-h interval preceding the behavioral test. For sleep fragmentation experiments, values were summed over the 12-h interval preceding the behavioral test. These totals were compared between conditions with a paired T-test. Data from 10:00-11:00 were excluded from all analyses, as rats were being tested in the Skinner boxes in that interval.
For EEG data, total durations of SWS and REMS during baseline and during the subsequent sleep deprivation movement-control protocol were compared within subjects using paired t-tests. Baseline data can be used as a within-subject reference, as continued EEG recording shows very consistent sleep stage durations over subsequent days.34
For all ANOVAs, a Greenhouse-Geisser correction was applied when the assumption of sphericity was violated. All data are presented as average values ± standard error of the mean (SEM). Statistical analyses were performed with SPSS (Chicago, Illinois, USA). Differences were considered significant at P = 0.05.
RESULTS
Baseline Performance
After 22 sessions of training on the switch-task, rats performed this task at a relatively stable baseline level. Omissions generally occurred in less than 1% of the trials and will therefore be disregarded. Baseline switch versus repetition trial performance comparisons were made for data obtained during training sessions 19–22 of the last baseline week preceding sleep interventions.
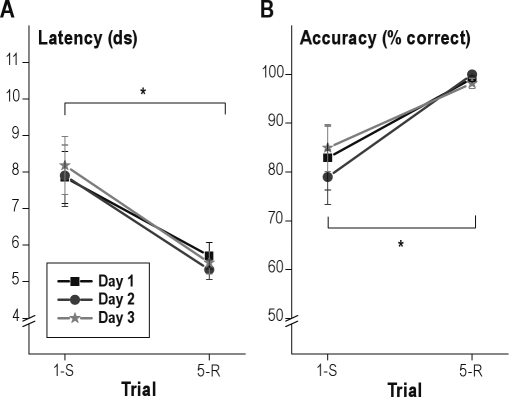
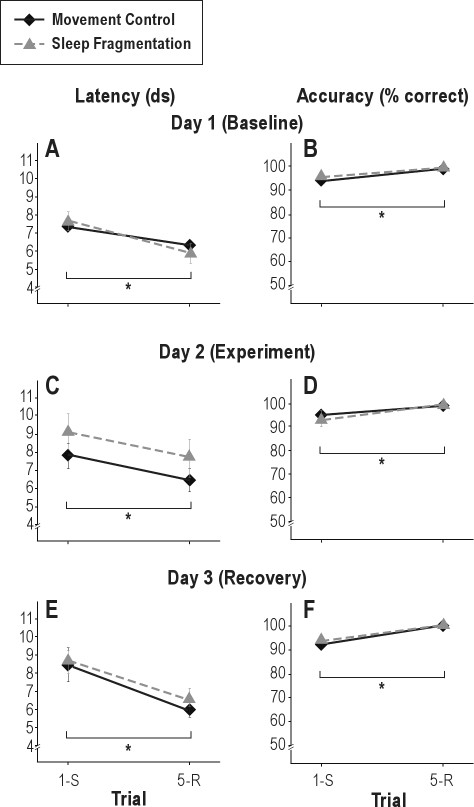
Analysis of response latencies showed a significant effect of trial (1st vs. 5th); repetition-trials were performed faster than switch-trials (F1.0,14.0 = 11.5; P = 0.004) indicating latency switch-costs (slower performance on switch compared to repetition trials, Figure 4). No significant day-effect occurred (P = 0.7) and the day*trial interaction was not significant either (P = 0.6).
Figure 4.
Latencies (A) and accuracy (B) on first (switch, 1-S) and fifth (repetition, 5-R) trials in the switch-task under baseline conditions on 3 subsequent testing days in the last training week. Mean ± standard error of the mean (SEM). *P < 0.05. ds, deciseconds.
Analysis of the response accuracy showed a significant effect of trial (1st vs. 5th); as compared to repetition-trials, a lower percentage of switch-trials was performed correctly (F1.0,14.0 = 11.4; P = 0.005), indicating accuracy switch-costs (more errors in switch compared to repetition trials, Figure 4). No significant day-effect occurred (P = 0.5) and the interaction of day with trial was also not significant (P = 0.2).
Effects of Sleep Deprivation
Testing of switch task performance continued while rats were housed in the deprivation devices for a full week. The effect of 12 h of total sleep deprivation during the inactive phase was always tested immediately after the deprivation period, at dark onset (day 2). Behavioral results on the day before (baseline, day 1) and after (recovery, day 3) were included in the analyses.
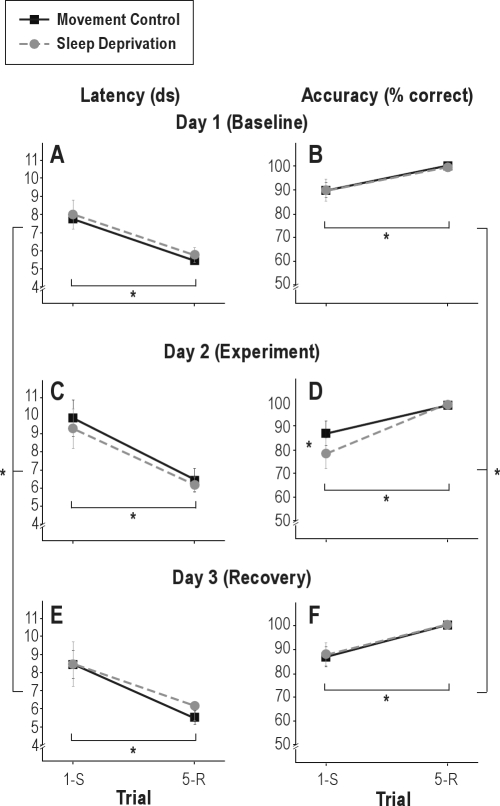
Analysis of the response latencies showed a significant effect of trial (1st vs. 5th); switch-trials were performed slower than repetition-trials (F1,14 = 13.9; P = 0.002), confirming the latency switch-costs observed at baseline. A significant main effect of day occurred (F2,28 = 6.1; P = 0.006). After both sleep deprivation and the respective movement control (day 2), latencies were increased on both first and fifth trials. Post hoc ANOVAs for both conditions separately indicated that the increased latency was only significant for the movement control condition (F1.3,18.8 = 7.7; P = 0.008; P = 0.3 for the sleep deprivation condition). The main effect of condition (sleep deprivation or movement control) was not significant (P = 0.9). No significant interactions between any of the factors occurred either, indicating the absence of further differences between sleep deprivation and movement control. Latencies on the different trials and days are depicted in Figure 5.
Figure 5.
Latencies (A, C, E) and accuracy (B, D, F) on first (switch, 1-S) and fifth (repetition, 5-R) trials in the switch-task under baseline conditions (Day 1; A, B), after sleep deprivation or movement control (Day 2; C, D) and after 24 h of recovery sleep (Day 3; E, F). Mean ± standard error of the mean (SEM). *P < 0.05. ds, deciseconds.
Analysis of the response accuracy showed a significant effect of trial (1st vs. 5th) a lower percentage of switch-trials than repetition-trials were performed correctly (F1,14 = 8.4; P = 0.012) confirming the accuracy switch-costs already observed at baseline. Again, a significant day-effect was found (F2,28 = 6.6; P = 0.004); and planned contrasts showed that the percentage of correctly performed trials was lower on day 2. The main effect of condition (sleep deprivation or control) was not significant (P = 0.3). A significant interaction between day and trial reflected that the decrease in correctly performed trials was restricted to the switch-trials (F2,28 = 5.0; P = 0.014). Furthermore, the 3-way interaction between condition, day and trial was significant, reflecting that this decrease was more pronounced in the sleep deprivation than the movement control condition (F2,28 = 3.8; P = 0.033). The condition-by-day and the condition-by-trial interactions were not significant (P = 0.09 and P = 0.2, respectively). Percentages correct on switch- and repetition trials on these 3 days are depicted in Figure 5.
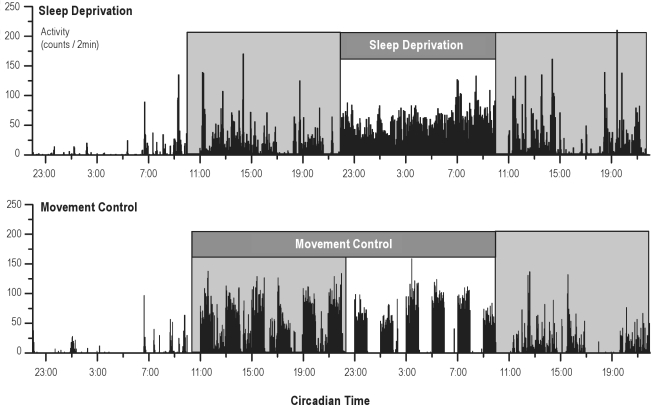
To provide an indication of potential confounding by movement, locomotor activity was measured during the sleep deprivation and control condition. Total activity levels in the 24h preceding behavioral testing did not differ between both protocols (25200 ± 2270 counts in 24 h for sleep deprivation and 26703 ± 1437 for movement control; P = 0.4), although activity did differ from hour to hour within the protocols, as can be observed in 2 representative actigrams of the 2 conditions (Figure 6)
Figure 6.
Representative actigrams of one rat (rat 9) in the sleep deprivation condition and in the matching movement control. Data from 10:00–11:00 are deleted, as rats were then performing the switch-task.
Summarized, 12 h of total sleep deprivation do increase the accuracy-switch-costs, but not the latency-switch-costs compared to the movement control condition.
Effects of Sleep Fragmentation
After at least one week with uninterrupted sleep in the home cage, with daily (weekdays) testing of switch task performance, rats were again housed in the deprivation devices for a full test week. The effect of 12 h of sleep fragmentation during the inactive phase was always tested immediately upon completion of the fragmentation (day 2). Behavioral results on the day before (day 1) and after (recovery, day 3) were included in the analyses.
In the sleep fragmentation experiment, again, a signifi-cant effect of trial (1st vs. 5th) on accuracy was observed for both latency (F1,14 = 8.1; P = 0.013) and accuracy (F1,14 = 7.7; P = 0.015), confirming the robustness of latency- and accuracy switch-costs in our new task. None of the other factors tested were significant (P ≥ 0.2), indicating that 12 h of sleep fragmentation do not affect switch-task performance (Figure 7).
Figure 7.
Latencies (A, C, E) and accuracy (B, D, F) on first (switch, 1-S) and fifth (repetition, 5-R) trials in the switch-task under baseline conditions (Day 1; A, B), after sleep fragmentation or movement control (Day 2; C, D) and after 24 h of recovery sleep (Day 3; E, F). Mean ± standard error of the mean (SEM). *P < 0.05. ds, deciseconds.
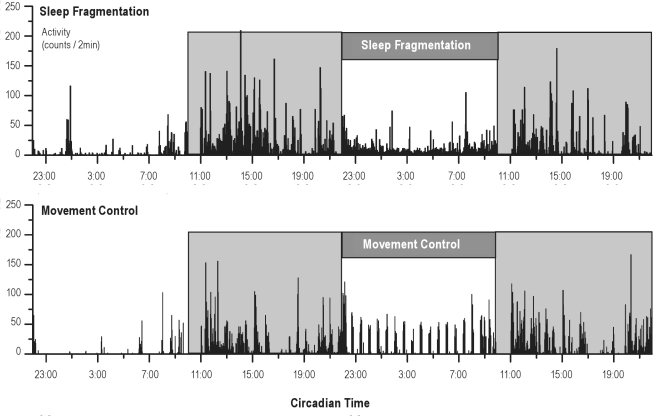
To provide an indication of potential confounding by movement, locomotor activity was measured during the sleep fragmentation and control condition. Activity values in the 12 h preceding behavioral testing did not differ between both protocols (4694 ± 392 counts in 24 h for sleep fragmentation and 4243 ± 321 for movement control, P = 0.2), although activity did differ on specific time points within the protocols, as can be observed in 2 representative actigrams of the 2 conditions (Figure 8).
Figure 8.
Representative actigams of one rat (rat 9) on the sleep fragmentation protocol and on the matching movement control. Data from 10:00–11:00 are deleted, as rats were then performing the switch-task.
Summarized, 12 h of sleep fragmentation do not affect switch-costs.
PFC Dependency
Following the sleep deprivation and sleep fragmentation experiments, rats were equipped with local injection cannulae, and retrained on the switch-task until baseline performance was restored. Rats were tested in a counterbalanced order after muscimol-baclofen injections and control saline injections. Performance immediately following mixture infusion was compared with performance following saline infusion. Besides, performance after saline was compared with the various baseline conditions from the preceding experiments. No effect of experiment on baseline performance was observed (for the latencies: P = 0.4 for the main effect and P = 0.9 for the experiment-by-trial interaction; for the accuracy: P = 0.1 for the main effect and P = 0.2 for the interaction), indicating that baseline performance was not impaired by saline infusion.
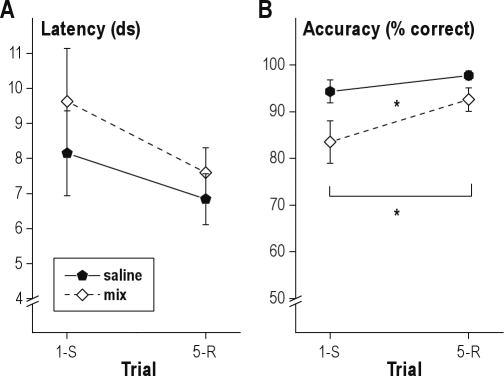
In these infusion experiments, the effect of trial (1st vs. 5th) on latency was not significant (P = 0.1), indicating the absence of noticeable latency switch-costs after local infusions. This was possibly due to increase between-rat variation; during the 3 baseline days, the standard error of the mean for switch-trial latency was 0.08 s, while in infusion conditions, it had nearly doubled to 0.14 s. Treatment (saline versus the muscimol-baclofen mixture) did not affect response latencies (P = 0.4 for the treatment and P = 0.7 for the treatment-by-trial interaction).
The effect of trial (1st vs. 5th) on accuracy was significant (F1,10 = 6.3; P = 0.031). Transient inactivation of the mPFC by local infusion of a mixture of muscimol and baclofen decreased accuracy (F1,10 = 6.0; P = 0.034, Figure 9). The interaction be tween trial and infusion-condition was not significant (P = 0.3), indicating that accuracy decreases were similar for switch and repetition trials.
Figure 9.
Latencies (A) and accuracy (B) on first (switch, 1-S) and fifth (repetition, 5-R) trials after PFC inactivation by local micro-infusion of muscimol-baclofen (Mix) or saline control. Mean ± standard error of the mean (SEM). *P < 0.05. ds, deciseconds. Latency switch-costs are not significant. Accuracy switch costs are significant, and PFC inactivation decreases overall accuracy on the switch-task.
Sleep during the Movement Control Condition for 12-h Sleep Deprivation
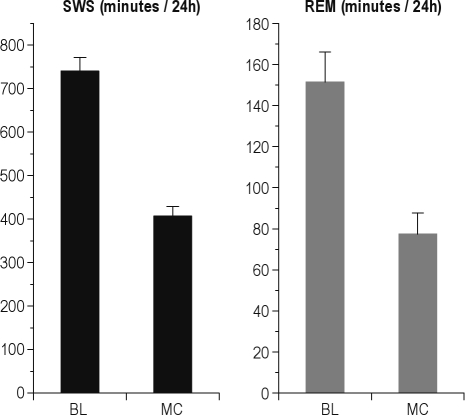
In the movement control condition for 12h of total sleep deprivation, rats had less SWS (407 ± 22 min during the protocol versus 741 ± 32 during baseline) and REMS (77 ± 10 min during the protocol versus 151 ± 15 during baseline) than during undisturbed baseline conditions (t5 = 13.9; P < 0.001 for SWS and t5 = 11.5; P < 0.001 for REMS, Figure 10); movement control rats could not fully compensate for the hours of sleep deprivation in the hours of interspersed rest, but they were also not fully sleep deprived, while being exposed to an equivalent amount of movement as during the total sleep deprivation protocol. During the 14-h period subsequent to the movement control protocol (after task performance), no significant recovery sleep could be observed (for SWS: 323 ± 22 min during 14 h of recovery sleep versus 383 ± 24 during corresponding baseline, P = 0.20; for REMS 65 ± 8 min during recovery versus 74 ± 7 min during baseline, P = 0.46).
Figure 10.
Total amounts of SWS and REMS during baseline and movement control protocol for 12h of sleep deprivation. Mean ± standard error of the mean (SEM). P < 0.001 for both SWS and REMS.
DISCUSSION
Previous studies showed that sleep deprivation impairs human task-switching performance,7–9 supporting the hypothesis that PFC-dependent cognitive functions are particularly sensitive to sleep loss.1,2 We aimed to confirm these findings in rats, so that we would be able to study the underlying neurobiological mechanisms in future experiments. We successfully designed and validated the first switch-task for rats and repeatedly demonstrated switch-cost effects, supporting the robustness of the task. When rats had to switch from one task to another (between blocks) we observed increases in both latency and number of errors, compared to when they performed repetitions of the same task (within a block). Accuracy switch-costs (impaired performance on switch compared to repetition trials) were increased when they were kept awake for 12 h using our novel, highly efficient automated method for sleep deprivation.34 After recovery sleep, their performance returned to baseline levels again. Importantly, we observed impairments in task-switching accuracy only when rats were totally deprived of sleep, but not when they were exposed to increased locomotor activity without substantial sleep deprivation. This confirms the absence of confounding effects of the procedure on corticosterone levels and locomotor activity, as reported before.34
The switch-task that we present can be used to study the neuro-biological mechanisms by which total sleep deprivation impairs executive functions such as task switching. Our task has two important advantages compared to most of the available alternatives for probing executive functions (e.g., attentional set-shifting10). The first is the automation of the test (resulting in decreased handling of animals and decreased work-load for the experimenters), while being closely comparable to commonly used human tasks. The second is that it probes flexibility without requiring new learning, resulting in a clean task for flexibility, independent of mechanisms involved in the learning of new information.
The Switch-Task
We modelled the switch-task for rats after human switch tasks, where a simple conditional discrimination37 is presented in blocks of variable numbers of trials.27 The level of performance in this task for rats resembles that in the human tasks, where performance is usually both accurate (errors on < 3% of the trials) and fast (reaction times < 1s).5–7,9,12,27,38,39 We are not the first to use a conditional discrimination task in rats, but previous authors did not use a specific block design. Instead, in previously used tasks, the trials were presented in a (semi)random fashion, and many of them used more complex stimuli.16,19–22,25 The more complex tasks are difficult to learn for rats and, consequently, compared to our task, the training periods are longer and the performance is worse, making them less suitable as a model for human switch-tasks.
Increasing the task difficulty may however be necessary for certain experimental purposes. The current task could be made more challenging by for example removing the currently used 1-s delay between stimulus onset and lever presentation. If the levers are presented simultaneously with the cue, rats may increasingly perseverate on the pre-switch lever. When not working with sleep deprived animals, another alternative could be to add more repetition trials within blocks. As this will increase the length of the task, the motivation of sleep deprived rats may then become insufficient to finish the task. Our experience during the pilot phase shows that sleep deprived rats do not finish a longer version of the task, in which 30-s delays between trials were used.
Sleep and Task Switching
One night of total sleep deprivation increased accuracy switch-costs in our paradigm. In humans, one night of total sleep deprivation generally increased latency-switch costs.7–9
We did not replicate this observation in our paradigm. This may be due to two experimental factors. First, the speed of lever-pressing by the rats is registered with an accuracy of 0.1 s, the time resolution of the Skinner boxes. This resolution may be insufficient to detect subtle modifications of the switch-effect itself, which is usually in the order of 0.1 s-0.3 s (Figures 4, 5, 7, and 9). Second, cue presentation always preceded lever presentation by 0.5 s, thereby giving the rats some preparation time on each trial. As the size of the switch-effect generally decreases with increases in the preparation time,33 this 0.5-s interval may have rendered latencies less sensitive to disturbance.
The lack of an effect of sleep fragmentation on switch-costs could be explained in several ways. The most likely explanation is that task-switching may not be very sensitive to a mild sleep disturbance, as rats will still have had a substantial amount of sleep during sleep fragmentation. Unfortunately, no data are available on human switch-task performance after comparable experimentally fragmented sleep to confirm this explanation.
As the sleep fragmentation experiment was performed after finishing the sleep deprivation experiment, rats did have previous experience with performing the task in a sleep deprived state. An alternative explanation may therefore be that sleep fragmented rats increase their attentional effort to compensate for tiredness, a phenomenon known as reactive reinforcement, which has been used to explain an absence of increasing switch-costs at certain circadian times after sleep deprivation in humans.7
Movement Control Protocol
In this study, we implemented a novel movement control condition to prevent confounding of the cognitive behavioral results by increases in locomotor activity. A proper movement control condition should induce equal amounts of movement, while leaving sleep relatively unaffected. Indeed, the amount of behavioral activity observed during the protocols for total sleep deprivation and for movement control was similar. However, rats were still partially sleep deprived; 45.0% of SWS and 55.2% of REM sleep were missed, compared to almost total loss of sleep during the sleep deprivation (98.6% of SWS and 100% of REM34). Furthermore, in the movement control condition, no recovery sleep could be observed on the subsequent day, while significant recovery sleep can be observed up to 6 h after 12 h of total sleep deprivation.34 Therefore, although the amount of sleep missed is sufficient to negatively affect latencies, this movement control condition can be a useful procedure to prevent confounding by increases in behavioral activity.
PFC and Task-Switching
Performance of the human switch-task is accompanied by activation of several regions within the PFC in healthy subjects,27–29,31,33 indicating a role of this brain region in normal performance on either discrimination or task-switching. Switch-task performance after PFC-damage in humans is impaired,30,32 as after temporary PFC inactivation in rats in our paradigm: PFC inactivation by co-administration of the GABA-agonists muscimol and baclofen decreased accuracy. However, both rats and humans still manage to perform correctly on most of the trials after PFC damage or inactivation. The lack of an effect of PFC inactivation on latency switch-costs, and the absence of significant latency switch-costs in this condition, can be explained by the increase in latency variability after both control and muscimol-baclofen local injections.
The effect of pharmacological PFC inactivation on switch costs has not been described before, but a few studies describe that acquisition and performance of a conditional discrimination are unaffected by lesions of the prelimbic-infralimbic part of the mPFC in rats,13 while more generalized lesioning of the PFC can affect acquisition, but not performance of a simple conditional discrimination task.24 We now show that when a block design is used with a simple conditional discrimination task, accuracy is impaired after immediate temporary inactivation of the medial PFC. It is important to note that more brain regions than just prefrontal cortex have been implicated in task switching and conditional discrimination. Apart from other cortical areas (e.g., parietal) the basal ganglia and thalamus are involved,40,41 as is dopamine acting on the caudate nucleus.39 Thus, the failure to prove a selective effect of mPFC inactivation on switch trials might also be explained by involvement of other areas.
Although sleep deprivation is thought to affect PFC-dependent processes specifically,1,2,26 the relationship between sleep deprivation and PFC functioning is far from simple. After sleep deprivation, activation of the PFC during task performance can be either increased or decreased42 compared to normal performance, and other brain regions may compensate for decreased PFC functioning. Further research is required to unravel the mechanisms underlying altered PFC functioning after sleep deprivation.
CONCLUSION
In this paper, we describe the first switch-task for rats and demonstrate its robustness. We moreover showed that performance can be disturbed by sleep deprivation and PFC inactivation. In humans, the performance on human switch-tasks is impaired in multiple conditions, including prolonged wakefulness and insomnia, but also, for example, in Parkinson disease.39 The presented switch-task for rats appears to be highly valuable for investigations into the brain mechanisms underlying these cognitive impairments.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the Netherlands Organization for Scientific Research, The Hague, The Netherlands (projects Cognition - Integrated Research Projects 051-04-010; and VICI 453-07-001). This work was performed at the Netherlands Institute for Neuroscience. The authors thank Rebecca Astill for useful advice in developing the switch task and accompanying data analysis, and for proofreading an earlier draft of this manuscript.
REFERENCES
- 1.Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends Cogn Sci. 2002;6:475–81. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- 2.Horne JA. Human sleep, sleep loss and behaviour: Implications for the pre-frontal cortex and psychiatric disorder. Br J Psychiatry. 1993;162:413–9. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- 3.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3:679–93. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 4.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 5.Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–40. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 6.Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. J Exp Psychology. 1995;124:207–31. [Google Scholar]
- 7.Bratzke D, Rolke B, Steinborn MB, Ulrich R. The effect of 40 h constant wakefulness on task-switching efficiency. J Sleep Res. 2009;18:167–72. doi: 10.1111/j.1365-2869.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- 8.Jennings JR, Monk TH, van der Molen MW. Sleep deprivation influences some but not all processes of supervisory attention. Psychol Sci. 2003;14:473–9. doi: 10.1111/1467-9280.02456. [DOI] [PubMed] [Google Scholar]
- 9.Heuer H, Kleinsorge T, Klein W, Kohlisch O. Total sleep deprivation increases the costs of shifting between simple cognitive tasks. Acta Psychol (Amst) 2004;117:29–64. doi: 10.1016/j.actpsy.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 10.McCoy JG, Tartar JL, Bebis AC, et al. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep. 2007;30:52–60. doi: 10.1093/sleep/30.1.52. [DOI] [PubMed] [Google Scholar]
- 11.Dibbets P, Jolles J. The Switch Task for Children: Measuring mental flexibility in young children. Cogn Development. 2006;21:60–71. [Google Scholar]
- 12.Dreisbach G, Goschke T, Haider H. Implicit task sets in task switching? J Exp Psychol Learn Mem Cogn. 2006;32:1221–33. doi: 10.1037/0278-7393.32.6.1221. [DOI] [PubMed] [Google Scholar]
- 13.Delatour B, Gisquet-Verrier P. Lesions of the prelimbic-infralimbic cortices in rats do not disrupt response selection processes but induce delay-dependent deficits: evidence for a role in working memory? Behav Neurosci. 1999;113:941–55. doi: 10.1037//0735-7044.113.5.941. [DOI] [PubMed] [Google Scholar]
- 14.Dunn MJ, Killcross S. Differential attenuation of d-amphetamine-induced disruption of conditional discrimination performance by dopamine and serotonin antagonists. Psychopharmacology (Berl) 2006;188:183–92. doi: 10.1007/s00213-006-0488-y. [DOI] [PubMed] [Google Scholar]
- 15.Everitt BJ, Robbins TW, Evenden JL, Marston HM, Jones GH, Sirkia TE. The effects of excitotoxic lesions of the substantia innominata, ventral and dorsal globus pallidus on the acquisition and retention of a conditional visual discrimination: implications for cholinergic hypotheses of learning and memory. Neuroscience. 1987;22:441–69. doi: 10.1016/0306-4522(87)90346-0. [DOI] [PubMed] [Google Scholar]
- 16.Everitt BJ, Robbins TW, Gaskin M, Fray PJ. The effects of lesions to ascending noradrenergic neurons on discrimination learning and performance in the rat. Neuroscience. 1983;10:397–410. doi: 10.1016/0306-4522(83)90142-2. [DOI] [PubMed] [Google Scholar]
- 17.Featherstone RE, McDonald RJ. Dorsal striatum and stimulus-response learning: lesions of the dorsolateral, but not dorsomedial, striatum impair acquisition of a stimulus-response-based instrumental discrimination task, while sparing conditioned place preference learning. Neuroscience. 2004;124:23–31. doi: 10.1016/j.neuroscience.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 18.Herremans AH, Hijzen TH, Slangen JL. Validity of a delayed conditional discrimination task as a model for working memory in the rat. Physiol Behav. 1994;56:869–75. doi: 10.1016/0031-9384(94)90317-4. [DOI] [PubMed] [Google Scholar]
- 19.Lashley KS. Conditional reactions in the rat. J Psychol. 1938;6:311–24. [Google Scholar]
- 20.McGaughy J, Turchi J, Sarter M. Crossmodal divided attention in rats: effects of chlordiazepoxide and scopolamine. Psychopharmacology (Berl) 1994;115:213–20. doi: 10.1007/BF02244774. [DOI] [PubMed] [Google Scholar]
- 21.Robbins TW, Giardini V, Jones GH, Reading P, Sahakian BJ. Effects of dopamine depletion from the caudate-putamen and nucleus accumbens septi on the acquisition and performance of a conditional discrimination task. Behav Brain Res. 1990;38:243–61. doi: 10.1016/0166-4328(90)90179-i. [DOI] [PubMed] [Google Scholar]
- 22.Turchi J, Sarter M. Cortical acetylcholine and processing capacity: effects of cortical cholinergic deafferentation on crossmodal divided attention in rats. Brain Res Cogn Brain Res. 1997;6:147–58. doi: 10.1016/s0926-6410(97)00027-x. [DOI] [PubMed] [Google Scholar]
- 23.Ward BO, Wilkinson LS, Robbins TW, Everitt BJ. Forebrain serotonin depletion facilitates the acquisition and performance of a conditional visual discrimination task in rats. Behav Brain Res. 1999;100:51–65. doi: 10.1016/s0166-4328(98)00112-0. [DOI] [PubMed] [Google Scholar]
- 24.Winocur G, Eskes G. Prefrontal cortex and caudate nucleus in conditional associative learning: dissociated effects of selective brain lesions in rats. Behav Neurosci. 1998;112:89–101. doi: 10.1037//0735-7044.112.1.89. [DOI] [PubMed] [Google Scholar]
- 25.Pang K, Merkel F, Egeth H, Olton DS. Expectancy and stimulus frequency: a comparative analysis in rats and humans. Percept Psychophys. 1992;51:607–15. doi: 10.3758/bf03211657. [DOI] [PubMed] [Google Scholar]
- 26.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5:463–75. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- 27.Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res. 2000;9:103–9. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- 28.Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. Inaugural article: the role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci U S A. 2000;97:13448–53. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wylie GR, Javitt DC, Foxe JJ. Task switching: a high-density electrical mapping study. Neuroimage. 2003;20:2322–42. doi: 10.1016/j.neuroimage.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–73. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- 31.Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–26. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- 32.Rogers RD, Sahakian BJ, Hodges JR, Polkey CE, Kennard C, Robbins TW. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson's disease. Brain. 1998;121:815–42. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- 33.Shallice T, Stuss DT, Picton TW, Alexander MP, Gillingham S. Mapping task switching in frontal cortex through neuropsychological group studies. Front Neurosci. 2008;2:79–85. doi: 10.3389/neuro.01.013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leenaars CH, Dematteis M, Joosten RN, et al. A new automated method for rat sleep deprivation with minimal confounding effects on corticosterone and locomotor activity. J Neurosci Methods. 2011;196:107–17. doi: 10.1016/j.jneumeth.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Torregrossa MM, Tang XC, Kalivas PW. The glutamatergic projection from the prefrontal cortex to the nucleus accumbens core is required for cocaine-induced decreases in ventral pallidal GABA. Neurosci Lett. 2008;438:142–5. doi: 10.1016/j.neulet.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ovari J, Leri F. Inactivation of the ventromedial prefrontal cortex mimics re-emergence of heroin seeking caused by heroin reconditioning. Neurosci Lett. 2008;444:52–5. doi: 10.1016/j.neulet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Dibbets P, Evers EA, Hurks PP, Bakker K, Jolles J. Differential brain activation patterns in adult attention-deficit hyperactivity disorder (ADHD) associated with task switching. Neuropsychology. 24:413–23. doi: 10.1037/a0018997. [DOI] [PubMed] [Google Scholar]
- 38.Wylie GR, Javitt DC, Foxe JJ. The role of response requirements in task switching: dissolving the residue. Neuroreport. 2004;15:1079–87. doi: 10.1097/00001756-200404290-00030. [DOI] [PubMed] [Google Scholar]
- 39.Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinson's disease. Brain. 2001;124:2503–12. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- 40.Hadj-Bouziane F, Meunier M, Boussaoud D. Conditional visuo-motor learning in primates: a key role for the basal ganglia. J Physiol Paris. 2003;97:567–79. doi: 10.1016/j.jphysparis.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Kimberg DY, Aguirre GK, D'Esposito M. Modulation of task-related neural activity in task-switching: an fMRI study. Brain Res Cogn Brain Res. 2000;10:189–96. doi: 10.1016/s0926-6410(00)00016-1. [DOI] [PubMed] [Google Scholar]
- 42.Drummond SP, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology. 2001;25:S68–73. doi: 10.1016/S0893-133X(01)00325-6. [DOI] [PubMed] [Google Scholar]