Abstract
Study Objectives:
Obesity is a common feature of narcolepsy. In addition, an increased occurrence of non-insulin dependent diabetes has been reported. So far, it is not known whether glucose metabolism in narcolepsy is disturbed due to, or independently of obesity.
Design:
Case-control study.
Setting:
Sleep medicine clinic at a research institute.
Patients:
We studied 17 patients with narcolepsy/cataplexy compared to 17 healthy controls matched for age, sex, and body mass index (BMI).
Interventions:
A 75-g oral glucose tolerance test was performed.
Measurements:
Glucose tolerance was determined by computing plasma glucose curve following oral glucose challenge for 240 minutes; insulin sensitivity and insulin secretion by homeostasis model assessment and minimal model analysis.
Results:
Standard outcome measures and indices of the oral glucose tolerance test did not differ between the patient group and the group of control subjects.
Conclusions:
In this study, no clinically relevant pathologic findings in the glucose metabolism of narcoleptic patients compared to weight matched controls were found. Thus, narcolepsy is unlikely to be a risk factor per se for impaired glucose tolerance or diabetes.
Citation:
Beitinger PA; Fulda S; Dalal MA; Wehrle R; Keckeis M; Wetter TC; Han F; Pollmächer T; Schuld A. Glucose tolerance in patients with narcolepsy. SLEEP 2012;35(2):231-236.
Keywords: Narcolepsy, hypersomnia, metabolism, orexin, hypocretin
INTRODUCTION
Narcolepsy is a disorder of excessive daytime sleepiness, which, in the vast majority of patients, is closely linked to an acquired orexin deficiency.1,2 Biochemical and neuroanatomical studies suggest an almost complete cessation of production of these peptides prior to or around disease onset due to yet unknown causes; autoimmune mechanisms may be involved.3,4 Orexin A and B, also called hypocretin 1 and 2, are derived from a common precursor peptide and thought to be involved in the regulation of arousal, appetite, and various endocrine and metabolic networks including glycemic control.5 Genetically engineered mice losing orexin neurons during early postnatal development display obesity during adulthood without major changes in food intake.6
Increased body weight has also been shown to be a common feature of human narcolepsy.7 Patients with narcolepsy tend to have a lower basal metabolic rate than controls.8 Moreover, three studies suggest impaired glucose metabolism in narcolepsy: One Japanese study suggests that narcolepsy is associated with an increased incidence of type 2 diabetes mellitus9; and during the 1960s, Roberts conducted a large case series, published in two separate reports, where he found a high rate of impaired glucose tolerance and diabetes due to diabetogenic (“functional”) hyperinsulinism in 200 narcoleptic patients.10,11 Recently, differences of the glucose/insulin ratio in narcoleptic patients compared to patients with idiopathic hypersomnia have been reported. The results were statistically controlled for body mass index (BMI) differences between groups.12
So far, there have been no studies comparing glucose metabolism of narcoleptic patients to BMI-matched healthy controls. Thus, it remains unclear at present whether these particular disturbances are secondary to the observed obesity or are independent from obesity in narcolepsy. To address this issue, we performed oral glucose tolerance tests (OGTT) and assessed endocrine and metabolic parameters in narcoleptic patients and BMI-matched healthy controls.
METHODS
Study Sample
Seventeen Caucasian patients with narcolepsy and 17 age-, sex-, and BMI-matched healthy Caucasian controls participated in the study. All narcoleptic subjects were outpatients of the sleep medicine clinic at the Max Planck Institute of Psychiatry. Healthy volunteers were recruited to match patients for age, sex, and BMI. The protocol was approved by the ethics committee of the Bavarian Medical Council and all participants gave written informed consent prior to the study.
The diagnosis of narcolepsy was based on the presence of excessive daytime sleepiness, cataplexy, and on ≥ 2 sleep onset rapid eye movement periods (SOREMs) in the multiple sleep latency test (MSLT). All patients fulfilled original and revised criteria for narcolepsy.13,14
In one patient, mild SRBD with an apnea/hypopnea index of 9.8/h was observed during polysomnography. Subjects with current or recent infections, known history of insulin dependent or independent diabetes mellitus and corresponding treatment, untreated hypothyroidism, and alcohol or substance abuse were excluded.
Experimental Procedure
Body height and weight as well as waist and hip circumferences were measured using calibrated balance and measurement instruments. All subjects arrived at the laboratory in the evening before the oral glucose tolerance test and spent ≥ 8 h in bed to ensure an overnight fast of ' 12 hours. A venipuncture was performed at 07:00 and a venous catheter was placed. A baseline blood sample was drawn one hour later. At 08:00, the subjects took a 75 g/300 mL glucose tea. At 08:30, 09:00, 10:00, 11:00, and 12:00, further blood samples for glucose and insulin measurements were drawn. Plasma glucose was measured by the glucose oxidase method, and serum insulin was measured using solid phase-Enzyme Amplified Sensitive Immunoassay (INSEASIA KAP1251; BioSource Europe S.A., Belgium). Levels of thyroid-stimulating hormone (TSH), glycated hemoglobin (HbA1c), cortisol, and plasma cholesterol were determined according to routine clinical standard procedures.
Statistical Methods
The incremental area under the curve (AUCi) was calculated from 0 to 120 minutes to quantify the overall glucose response to the oral glucose load.15 The homeostasis model assessment 2 (HOMA2) was calculated with the software implementation of the HOMA2 model from Diabetes Trials Unit (University of Oxford) for insulin sensitivity (HOMA2-S) and β-cell index (HOMA-β).16 The insulinogenic index for 30 minutes ([insulin 30 min-insulin 0 min] / [glucose 30 min- glucose 0 min]), and the insulin sensitivity index (ISI) composite (10000 / [glucose 0 min × insulin 0 min × mean glucose 0-120 min × mean insulin 0-120 min]0.5) were computed for each participant.17
To study differences in glucose metabolism and endocrine parameters, the measurements between patients and healthy controls matched on a group level for age, sex, and BMI were statistically compared using univariate analyses of variance. For descriptive purposes only, effect sizes were calculated from t-statistics as Hedges d as described by Morris and DeShon.18 In this exploratory study, uncorrected P values ≤ 0.05 were considered significant. All analyses were performed using the statistical software package R.19
RESULTS
Sample Characteristics
The patients with narcolepsy (6 women,11 men, mean age 33.9 ± 9.0 years, mean BMI 26.7 ± 3.0 kg/m2) did not significantly differ in sex, age, BMI, or waist and hip measures from the healthy controls (6 women,11 men, mean age 36.5 ± 9.2 years, BMI 26.2 ± 2.8 kg/m2). The mean duration of disease was 12.3 ± 10.4 years (minimum 1 year; maximum 38 years, one unclear onset; Table 1). Family history for diabetes mellitus type 2 was positive in 2 narcoleptic patients (father, grandmother) and none of the controls. Twelve of the 13 patients in whom lumbar puncture was performed showed undetectable cerebrospinal fluid orexin A levels. One patient had an orexin CSF concentration of 190 ng/mL. All twelve patients in whom HLA typing was done were HLA DQB1*0602 positive. Eight patients were treated pharmacologically: 3 took an antidepressant medication against cataplexy, one used modafinil, one levothyroxine and liothyronine, one antiepileptics and one bezafibrate (for details see Table 1).
Table 1.
Characteristics of patients with narcolepsy
| Patient No. | Sex | Age (years) | Duration of Narcolepsy (years) | Cataplexy | SOREMs | HLA | Orexin | Medication | Family history for DM | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | m | 19 | 4 | + | 5 | + | - | - | - | 25.2 |
| 2 | f | 30 | 24 | + | 5 | ? | - | - | - | 27.9 |
| 3 | m | 42 | ? | + | 4 | + | - | +(a) | - | 30.9 |
| 4 | f | 23 | 1 | + | 3 | + | - | - | - | 24 |
| 5 | f | 28 | 14 | + | 3 | + | - | +(b) | - | 19.6 |
| 6 | m | 28 | 11 | + | 3 | ? | - | - | - | 29.6 |
| 7 | f | 44 | 1 | + | 2 | + | - | +(c) | - | 27.3 |
| 8 | m | 40 | 25 | + | 5 | + | - | - | - | 25.6 |
| 9 | m | 37 | 15 | + | 3 | ? | ? | +(b) | - | 23.2 |
| 10 | m | 37 | 9 | + | 5 | + | - | - | - | 26.6 |
| 11 | m | 33 | 5 | + | 5 | ? | - | +(d) | ? | 27.4 |
| 12 | f | 58 | 38 | + | 4 | ? | 190 | +(e) | - | 25.5 |
| 13 | m | 36 | 1 | + | 2 | + | - | - | father | 27.7 |
| 14 | m | 29 | 14 | + | 5 | + | ? | +(f) | - | 26.6 |
| 15 | m | 28 | 13 | + | 5 | + | ? | +(g) | grandmother | 29.2 |
| 16 | f | 32 | 19 | + | 4 | + | ? | - | - | 31.5 |
| 17 | m | 33 | 3 | + | 3 | + | - | - | - | 27.6 |
SOREMs, number of sleep onset REM phases in a multiple sleep latency test; HLA: positive in HLA DQB1*0602 typing; Orexin: cerebrospinal fluid orexin levels (- undetectable, otherwise pg/mL). Orexin levels were measured before in 2007 guidelines for the appropriate use of CSF measurements to diagnose narcolepsy have been established, Commercially available RIA form Phoenix were used, mean values of non-narcoleptic subjects were 739.0 +/- 211.0 pg/mL (175-847 pg/mL)2; medication: - none, (a) fluoxetine, (b) iodide, (c) venlafaxine, (d) valproate and lamotrigine, (e) levothyroxine and liothyronine, (f) clomipramine and modafinil, (g) pantoprazole and bezafibrate; BMI: body mass index as body weight in kilograms divided by height in meters square.
Metabolic Parameters
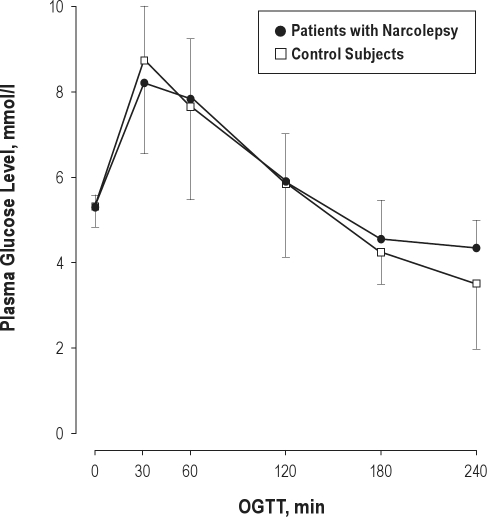
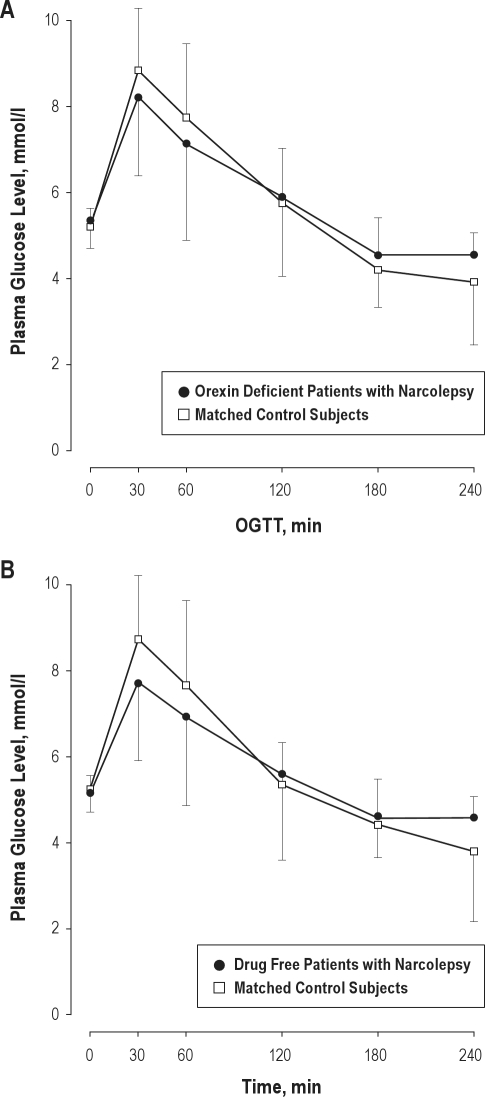
Baseline glucose values ranged between 83 and 105 mg/dL in narcoleptic patients and between 81 and 108 mg/dL in controls. Patients and controls did not significantly differ regarding TSH plasma levels (patients: 1.5 ± 0.8 μU/mL; controls: 1.5 ± 2.9 μU/mL), cholesterol plasma levels (patients: 190.1 ± 32.7 mg/dL; controls: 150.4 ± 66.1 mg/dL), or the percentage of glycated hemoglobin (HbA1c, in patients: 5.6% ± 0.4%; in controls: 5.2% ± 0.3%). Also, no significant differences between groups were observed with respect to glucose response (glucose basal and 120-min level, maximal glucose and maximal insulin levels, AUC glucose incremental, insulinogenic index, HOMA2) or other standard metabolic measurements (see Table 2). Four of the 17 narcoleptic patients and one of the controls had a 120-min blood glucose level > 140 mg/dL, indicating a pathological glucose tolerance according to WHO-criteria (χ2[1] = 0.41; P = 0.52).20 No participant could be classified as suffering from diabetes mellitus with a 120-min glucose response in the 200 mg/dL range. Mean glucose values over time are shown in Figure 1. Effect sizes for the differences between groups were small for most measures and moderate for HOMA2-S, HOMA2-B, and the insulinogenic index. To control for the possible effects of orexin deficiency and medication, the analysis was repeated in the subgroup of 12 patients who were definitely orexin deficient and compared to a matched subsample of the control group. There were no significant differences to the results in total group of patients (see Figure 2A and Table 3A). Also, medication did not significantly affect the results in the present study: If only drug-free patients were compared to matched controls, similar results were obtained (Figure 2B, Table 3B).
Table 2.
Demographics and statistics of glucose and endocrine measures of 17 patients with narcolepsy compared to a group of 17 healthy controls matched for gender and age
| Narcolepsy (6 f, 11 m) Mean (SD) | Control (6 f, 11 m) Mean (SD) | P-Value | Effect Size | |
|---|---|---|---|---|
| Age (years) | 33.9 (9.0) | 36.5 (9.2) | 0.42 | −0.2 |
| BMI (kg/m2) | 26.7 (2.9) | 26.2 (2.8) | 0.58 | 0.14 |
| Glucose 0 Minutes (mmol/L) | 5.2 (0.4) | 5.3 (0.5) | 0.66 | −0.16 |
| Glucose 120 Minutes (mmol/L) | 5.9 (1.8) | 5.9 (1.1) | 0.96 | 0.01 |
| AUCi (mg × min/dL) | 4265 (2851) | 4221 (1792) | 0.96 | 0.01 |
| Insulin max. (μU/mL) | 105.0 (40.8) | 108.6 (51.8) | 0.82 | −0.08 |
| Glucose max. (mmol/L) | 8.8 (1.9) | 9.1 (1.8) | 0.62 | −0.17 |
| HOMA2-S | 59.1 (13.1) | 68.8 (21.5) | 0.13 | −0.54 |
| HOMA2-B | 129.6 (21.5) | 118.9 (34.6) | 0.29 | 0.37 |
| ISI | 4.9. (2.4)a | 4.8 (2.1)a | 0.94 | 0.02 |
| Insgen | 1.5 (1) | 1.1 (0.6) | 0.13 | 0.54 |
AUCi, incremental area under the curve of glucose from 0 to 120 minutes; HOMA2, Homeostasis Model Assessment 2 to estimate model derived steady beta cell function (-B) and insulin sensitivity (-S); ISI, Insulin Sensitivity Index from Matsuda et al. (1000 / ([fasting glucose × fasting insulin] × [mean glucose x mean insulin])0.5; Insgen, Insulinogenic Index (insulin 30 min – insulin 0 min) / (glucose 30 min – glucose 0 min); af, female; m, male.
Figure 1.
Mean plasma glucose levels in patients with narcolepsy and control participants during the oral glucose tolerance test (OGTT). Error bars represent SD. There were no significant differences between groups in the sample. To convert glucose to mg/dl, multiply by 18.0182.
Figure 2.
Mean plasma glucose levels in patients with narcolepsy and matched control participants during the oral glucose tolerance test (OGTT). (A) Orexin deficient patients (4 f, 8 m). (B) Drug free patients (3 f, 6 m). Error bars represent SD. There were no significant differences between groups in the samples. To convert glucose to mg/dl, multiply by 18.0182.
Table 3A.
Demographics and statistics of glucose and endocrine measures of the subgroup of patients with narcolepsy definitely orexin-negative compared to a respective control group, matched for gender and age
| Narcolepsy (4 f, 8 m) Mean (SD) | Control (4 f, 8 m) Mean (SD) | P-Value | Effect Size | |
|---|---|---|---|---|
| Age (years) | 32.75 (7.8) | 34.6 (8.2) | 0.58 | −0.23 |
| BMI (kg/m2) | 26.60 (2.9) | 26.42 (3.0) | 0.88 | 0.06 |
| Glucose 0 Minutes (mmol/L) | 95.00 (6.8) | 93.75 (9.0) | 0.71 | 0.16 |
| Glucose 120 Minutes (mmol/L) | 105.83 (32.8) | 103.75 (22.9) | 0.86 | 0.07 |
| AUCi (mg × min/dL) | 4119 (3358) | 4718 (2037) | 0.60 | −0.22 |
| Insulin max. (μU/mL) | 103.11 (32.9) | 112.78 (36.9) | 0.50 | −0.28 |
| Glucose max. (mmol/L) | 152.0 (34.5) | 165.25 (26.8) | 0.30 | −0.43 |
| HOMA2-S | 58.16 (12.8) | 70.88 (20.4) | 0.08 | −0.75 |
| HOMA2-B | 128.34 (22.3) | 119.33 (34.4) | 0.46 | 0.31 |
| ISI | 4.73 (2.4) | 4.96 (2.2) | 0.81 | −0.10 |
| Insgen | 1.56 (0.8) | 1.09 (0.7) | 0.13 | 0.64 |
AUCi, incremental area under the curve of glucose from 0 to 120 minutes; HOMA2, Homeostasis Model Assessment 2 to estimate model derived steady beta cell function (-B) and insulin sensitivity (-S); ISI, Insulin Sensitivity Index from Matsuda et al. (1000 / ([fasting glucose × fasting insulin] × [mean glucose x mean insulin])0.5; Insgen, Insulinogenic Index (insulin 30 min – insulin 0 min) / (glucose 30 min – glucose 0 min); af, female; m, male.
Table 3B.
Demographics and statistics of glucose and endocrine measures of the subgroup of patients with narcolepsy free of medication compared to a respective control group, matched for gender and age
| Narcolepsy (3 f, 6 m) Mean (SD) | Control (3 f, 6 m) Mean (SD) | P-Value | Effect Size | |
|---|---|---|---|---|
| Age (years) | 30.89 (6.8) | 34.67 (8.1) | 0.30 | −0.51 |
| BMI (kg/m2) | 27.28 (2.3) | 26.66 (2.11) | 0.56 | 0.28 |
| Glucose 0 Minutes (mmol/L) | 93.78 (7.0) | 94.56 (9.6) | 0.85 | −0.09 |
| Glucose 120 Minutes (mmol/L) | 100.78 (36.0) | 96.44 (17.5) | 0.75 | 0.15 |
| AUCi (mg × min/dL) | 3728 (3016) | 4229 (2243) | 0.70 | −0.19 |
| Insulin max. (μU/mL) | 109.05 (36.9) | 111.84 (42.4) | 0.88 | −0.07 |
| Glucose max. (mmol/L) | 146.0 (34.1) | 165.11 (28.1) | 0.21 | −0.61 |
| HOMA2-S | 59.1 (13.3) | 59.9 (11.3) | 0.89 | −0.05 |
| HOMA2-B | 130.48 (22.9) | 128.42 (31.4) | 0.88 | 0.07 |
| ISI | 5.35 (2.74) | 4.14 (0.89) | 0.26 | 0.57 |
| Insgen | 1.94 (1.04) | 1.18 (0.7) | 0.09 | 0.87 |
AUCi, incremental area under the curve of glucose from 0 to 120 minutes; HOMA2, Homeostasis Model Assessment 2 to estimate model derived steady beta cell function (-B) and insulin sensitivity (-S); ISI, Insulin Sensitivity Index from Matsuda et al. (1000 / ([fasting glucose × fasting insulin] × [mean glucose x mean insulin])0.5; Insgen, Insulinogenic Index (insulin 30 min – insulin 0 min) / (glucose 30 min – glucose 0 min); af, female; m, male.
DISCUSSION
In the present study, patients suffering from narcolepsy were compared to healthy controls with respect to glucose metabolism. To disentangle an increased risk for diabetes mellitus type 2 secondary to elevated body weight from a narcolepsy-specific risk, this study compared patients and controls, who were carefully matched for age, gender, and BMI. There were no significant differences between patients and controls according to standard measurements of the OGTT. However, the sample size may have been too small to detect more subtle changes. Therefore, we calculated effect sizes to obtain a standardized estimate of the magnitude of the differences between patients and controls, which suggested β-cell function and insulin sensitivity as possible parameters of disparity. Taken together, however, we found no indication for clinically relevant pathologies of glucose metabolism in narcolepsy that is independent of obesity.
As already mentioned in the introduction, narcolepsy is closely linked to an acquired orexin deficiency.1,2 The presumption of a narcolepsy-specific risk for metabolic changes is based on neurophysiological evidence showing that orexin neurons are very sensitive to changes in extracellular glucose levels.21–24 In addition, orexin knockout mice exhibit deficiencies in glucose tolerance.25
In humans, the literature regarding glucose tolerance in narcolepsy is still conflicting: There have been various anecdotal reports and case series exploring abnormalities of the glucose metabolism in patients with narcolepsy. In 1942, Delay reported the case of a female patient with sleep attacks and cataplexy who showed hypoglycemia after two attacks.26 Twenty years later, Lederer reported on a 9-year-old boy with narcolepsy who showed pronounced sleepiness associated with induced hypoglycemia.27 However, no cataplexy was described and diagnostic certainty is unclear. During the 1960s, Roberts conducted a large case series, published in several separate reports, where he found a high rate of impaired glucose tolerance and diabetes due to diabetogenic (“functional”) hyperinsulinism in 200 narcoleptic patients.10,11 Again, diagnosis of narcolepsy may have been questionable due to a lack of operationalized criteria. The first systematic approach was undertaken by Honda and coworkers in 1986.9 They performed a 50-g glucose OGTT in 48 carefully diagnosed narcoleptic patients (22 women, 26 men, mean age 45.5 years). Four of the patients reported a history of diabetes mellitus, and in a total of six patients (12.5%; 4 male, 2 female, mean age 54.5 years) 120-minute glucose levels were above the Japanese threshold for definite diabetes mellitus. Since in this study no difference was found in body weight (Broca index) between the diabetic and the non-diabetic patients, Honda and coworkers concluded that the findings could not be attributed to differences in obesity. Although both, the Broca index and the average age are comparable between Honda's and our samples, we did not observe a single case with an elevated 120-minute glucose value. In our study, we carefully excluded narcoleptic patients with a known history of diabetes mellitus, which may account for the different findings. It cannot be excluded that the different findings might be due to racial differences between Caucasian and Japanese patients. The subgroup of 12 definitively orexin-deficient patients did not differ from the whole group of patients; thus in the present sample, orexin deficiency had no influence on glucose metabolism in patients with narcolepsy/cataplexy. Nevertheless, this should be studied separately in a larger sample.
In a recent study, Poli et al. showed metabolic alterations in narcoleptic patients compared to patients with idiopathic hyper-somnia.12 The case-control study with 14 subjects in each group showed differences after statistical adjustment for a significantly higher BMI in the narcoleptic group. After adjustment for BMI, waist circumference, HDL cholesterol, and the fasting glucose/insulin ratio were slightly but significantly altered, with a disadvantage for the narcoleptic patients. The area under the curve for glucose of the OGTT did not differ significantly between the two groups, even before adjustment for BMI. In the accurately BMI-matched samples reported herein, narcoleptic patients did not differ from healthy overweight subjects. It nevertheless cannot be excluded whether or not hypersomnia may interfere in/with the glucose metabolism. Unfortunately, we did not measure lipid profiles in the present study; thus the results of the studies cannot be compared with regard to these parameters. It also cannot be excluded that medication had an influence on glucose response, although the reevaluation of those patients who were drug free showed comparable results. To date, no studies are available on the effects of medication on glucose tolerance in human narcolepsy. Only data on BMI changes in those patients are available, which may be an indirect hint. In these studies, patients on medication did not differ in BMI from those who were drug naive.7 Nevertheless this question should be addressed in detail in a larger sample.
As mentioned before, both studies, the study from Poli et al. as well as the study presented herein, may have struggled with the low sample size in view of the high inter- and intraindividual variability of metabolic measurements. The lack of differences might also have been partly due to the medication taken by the patients. Thyroid and psychiatric medication (the latter are quite common in the treatment of narcoleptic symptoms) are known to have an impact on metabolism.28–30 As our patient sample was not completely drug free, this could have affected endocrine measures and the response to glucose challenge.
Tsuneki and colleagues found that aged orexin knockout mice developed an impaired glucose tolerance and insulin resistance that was more pronounced than in control animals.25 In the present study, all patients were younger than 60 years, and the sample was too small to control for the effects of age. Therefore, larger samples including more patients of advanced age would be desirable in future studies
Another limitation may have been the use of an oral glucose tolerance test with comparatively large intervals between measurements. More refined methods such as the frequent sampling intravenous glucose tolerance test might have been more discriminative and should be considered for further research.
In summary, there is a well-established association between obesity and narcolepsy, and clinical and preclinical research links orexin to glucose metabolism on a neurophysiological level. In the present study, we did not find gross abnormalities of glucose metabolism in patients with narcolepsy independent of obesity. Further studies may benefit from a homogeneous age range, more detailed phenotyping including sleep fragmentation, and the utilization of more sensitive tests of glucose metabolism.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The study was, in part, supported by a grant from the Sino-German Science foundation (GZ538).
ABBREVIATIONS
- AUCi
incremental area under the curve
- BMI
body mass index
- HbA1C
glycated hemoglobin
- HLA
human leukocyte antigen
- HOMA1
homeostasis model assessment 1
- HOMA2
homeostasis model assessment 2
- HOMA2-S
homeostasis model assessment insulin sensitivity
- HOMAβ
homeostasis model assessment β-cell index
- ISI
insulin sensitivity index
- MSLT
multiple sleep latency test
- OGTT
oral glucose tolerance test
- SOREM
sleep onset rapid eye movement sleep
- SRBD
sleep related breathing disorders
- TSH
thyroid-stimulating hormone
REFERENCES
- 1.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 2.Dalal MA, Schuld A, Haack M, et al. Normal plasma levels of orexin A (hypocretin-1) in narcoleptic patients. Neurology. 2001;56:1749–51. doi: 10.1212/wnl.56.12.1749. [DOI] [PubMed] [Google Scholar]
- 3.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypo-cretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin L, Hungs M, Mignot E. Narcolepsy and the HLA region. J Neuroimmunol. 2001;117:9–20. doi: 10.1016/s0165-5728(01)00333-2. [DOI] [PubMed] [Google Scholar]
- 5.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–76. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 6.Hara J, Beuckmann CT, Nambu T, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 7.Schuld A, Hebebrand J, Geller F, Pollmächer T. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355:1274–5. doi: 10.1016/S0140-6736(05)74704-8. [DOI] [PubMed] [Google Scholar]
- 8.Chabas D, Foulon C, Gonzalez J, et al. Eating disorder and metabolism in narcoleptic patients. Sleep. 2007;30:1267–73. doi: 10.1093/sleep/30.10.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honda Y, Doi Y, Ninomiya R, Ninomiya C. Increased frequency of non-insulin-dependent diabetes mellitus among narcoleptic patients. Sleep. 1986;9:254–9. doi: 10.1093/sleep/9.1.254. [DOI] [PubMed] [Google Scholar]
- 10.Roberts HJ. The syndrome of narcolepsy and diabetogenic (“functional”) hyperinsulinism, with special reference to obesity, diabetes, idiopathic edema, cerebral dysrhythmias and multiple sclerosis (200 patients) J Am Geriatr Soc. 1964;12:926–76. doi: 10.1111/j.1532-5415.1964.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 11.Roberts HJ. The syndrome of narcolepsy and diabetogenic hyperinsulinism in the American Negro: important clinical, social and public health aspects. J Am Geriatr Soc. 1965;13:852–85. doi: 10.1111/j.1532-5415.1965.tb02068.x. [DOI] [PubMed] [Google Scholar]
- 12.Poli F, Plazzi G, Di Dalmazi, et al. Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep. 2009;29:1491–7. doi: 10.1093/sleep/32.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Sleep Disorders Association. Revised version. Lawrence, KS: Allen Press; 1997. ICSD International classification of sleep disorders: diagnostic and coding manual. [Google Scholar]
- 14.American Academy of Sleep Medicine. diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. The international classification of sleep disorders. [Google Scholar]
- 15.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr. 1986;43:167–72. doi: 10.1093/ajcn/43.1.167. [DOI] [PubMed] [Google Scholar]
- 16.Diabetes Trials Unit, University of Oxford. HOMA2 Calculator v2.2. A software implementation of the HOMA2 model. Available from: http://www.dtu.ox.ac.uk/homa.
- 17.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 18.Morris SB, DeShon RP. Combining effect size estimates in metaanalysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–25. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 19.R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2008. R: A Language and Environment for Statistical Computing. ISBN 3-900051-07-0. URL http://www.R-project.org. [Google Scholar]
- 20.World Health Organisation. report of a WHO/IDF consultation. Geneva: WHO Press; 2006. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. [Google Scholar]
- 21.Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci. 2005;360:2227–35. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamanaka A, Beuckmann CT, Willie JT, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–13. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 23.Burdakov D. K+ channels stimulated by glucose: a new energy-sensing pathway. Pflugers Arch. 2007;454:19–27. doi: 10.1007/s00424-006-0189-8. [DOI] [PubMed] [Google Scholar]
- 24.Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. PNAS. 2008;105:11975–80. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuneki H, Murata S, Anzawa Y, et al. Age-related insulin resistance in hypothalamus and peripheral tissues of orexin knockout mice. Diabetologia. 2008;51:657–67. doi: 10.1007/s00125-008-0929-8. [DOI] [PubMed] [Google Scholar]
- 26.Delay J. Narcolepsie et hypoglycemie. Ann Med Psychol (Paris) 1942;100:375–9. [Google Scholar]
- 27.Lederer J. Narcolepsie par une hypoglycémie due à la leucine. Diabete. 1962;10:254–7. [PubMed] [Google Scholar]
- 28.Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol. 2003;53:154–66. doi: 10.1002/ana.10444. [DOI] [PubMed] [Google Scholar]
- 29.Fava M. Weight gain and antidepressants. J Clin Psychiatry. 2000;61(Suppl 11):37–41. [PubMed] [Google Scholar]
- 30.Ferguson JM. SSRI Antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry. 2001;3:22–7. doi: 10.4088/pcc.v03n0105. [DOI] [PMC free article] [PubMed] [Google Scholar]