Abstract
Study Objectives:
Patients with obstructive sleep apnea may have difficulty exhaling against positive pressure, hence limiting their acceptance of continuous positive airway pressure (CPAP). C-Flex is designed to improve comfort by reducing pressure in the mask during expiration proportionally to expiratory airflow (3 settings correspond to increasing pressure changes). When patients use CPAP, nasal resistance determines how much higher supraglottic pressure is than mask pressure. We hypothesized that increased nasal resistance results in increased expiratory supraglottic pressure swings that could be mitigated by the effects of C-Flex on mask pressure.
Design:
Cohort study.
Setting:
Sleep center.
Participants:
Seventeen patients with obstructive sleep apnea/hypopnea syndrome and a mechanical model of the upper airway.
Interventions:
In patients on fixed CPAP, CPAP with different C-Flex levels was applied multiple times during the night. In the model, 2 different respiratory patterns and resistances were tested.
Measurements and Results:
Airflow, expiratory mask, and supraglottic pressures were measured on CPAP and on C-Flex. Swings in pressure during expiration were determined. On CPAP, higher nasal resistance produced greater expiratory pressure swings in the supraglottis in the patients and in the model, as expected. C-Flex 3 produced expiratory drops in mask pressure (range −0.03 to −2.49 cm H2O) but mitigated the expira-tory pressure rise in the supraglottis only during a sinusoidal respiratory pattern in the model.
Conclusions:
Expiratory changes in mask pressure induced by C-Flex did not uniformly transmit to the supraglottis in either patients with obstructive sleep apnea on CPAP or in a mechanical model of the upper airway with fixed resistance. Data suggest that the observed lack of expiratory drop in supraglottic pressure swings is related to dynamics of the C-Flex algorithm.
Citation:
Masdeu MJ; Patel AV; Seelall V; Rapoport DM; Ayappa I. The supraglottic effect of a reduction in expiratory mask pressure during continuous positive airway pressure. SLEEP 2012;35(2):263-272.
Keywords: Upper airway resistance, nasal resistance, obstructive sleep apnea, fixed CPAP, flexible CPAP, CPAP compliance
INTRODUCTION
Continuous positive airway pressure (CPAP) is the primary treatment for obstructive sleep apnea/hypopnea syndrome (OSAHS).1,2 CPAP use normalizes breathing, improves sleep architecture,3 enhances daily function,4,5 and reduces the number and severity of cardiovascular events.6–8 Despite the efficacy of CPAP, studies defining adherence as use for at least 4 hours per night have reported that 29% to 83% of patients did not adhere to CPAP therapy.9,10 Although multiple factors may contribute to CPAP intolerance, including mask fit, humidity, excessive mask leak, claustrophobia, and nasal symptoms,11 pressure intolerance is a frequent complaint.9 In 2 studies,12,13 29% and 18% of patients reported “difficulty exhaling” during CPAP treatment. Several small studies have also suggested that initial rejection of CPAP correlates with increased nasal resistance.14–16 Nasal resistance may contribute to CPAP intolerance through several mechanisms, including alterations in the route of breathing,17,18 the need for high CPAP pressure, and increased leak.19 During expiration, nasal resistance causes the pressure experienced by the patient to be higher than the prescribed CPAP. This is because the resistance interacts with expiratory flow to produce a backpressure that adds to the CPAP (mask pressure), and this may contribute to discomfort.
Multiple technologic strategies have been proposed to improve CPAP adherence (including continuous automatic titration and bilevel therapy), but there is little evidence that these strategies significantly improve patient adherence.20–23 A recently introduced approach, C-Flex (Respironics; Murraysville, PA), is designed to improve comfort by modifying pressure in the mask during CPAP only during expiratory flow (in contrast with bilevel therapy, which maintains a low expiratory pressure throughout expiration). Although it is possible that any increased comfort (and consequent effect on compliance) achieved through reducing expiratory pressure may be achieved by reducing the pressure affecting the nose, it seems more likely that improved comfort would arise from reduction of excessive supraglottic pressure swings (i.e., that the drop in mask pressure would offset the expiratory rise in pharyngeal pressure above the prescribed CPAP).
Prospective randomized studies have demonstrated that C-Flex is not inferior to conventional fixed CPAP,24,30 but increased adherence rates have not been uniformly demonstrated. Some studies have shown that C-Flex reduces discomfort26,28 and improves satisfaction25 and compliance,27,31 but larger randomized studies29,30 have shown no difference in compliance between CPAP and C-Flex.
During well-titrated CPAP, collapsibility of the upper airway (UA) is abolished. In this condition, total UA resistance is dictated by nasal resistance, which, at constant CPAP, necessarily produces flow-related effects on supraglottic pressure. During expiration, this supraglottic pressure (pressure from the mask plus any pressure resulting from the expiratory flow) determines the expiratory work of breathing and could contribute to patient symptoms and CPAP intolerance. The purpose of the present study was to examine whether C-Flex decreases supraglottic pressure swings during expiration, providing a mechanism for improved comfort. This study did not examine comfort, treatment adherence, or clinical outcomes per se; our goal was to define the underlying physiology and effects of C-Flex to better address the role it has in the clinical setting (e.g., defining its relevance to patients with high nasal resistance). Specifically, we examined the expiratory pressure profile at the mask and in the upper airway at the supraglottis in asleep patients while they were on CPAP with and without C-Flex. We also examined whether expiratory supraglottic pressure swings could be mitigated with the application of C-Flex. In addition to testing the application of C-Flex in patients with OSAHS, we also used a mechanical model of the UA to control respiratory flow and pattern and to eliminate reflex changes seen in patients
METHODS
Patients with OSAHS
Twenty-two adults presenting for evaluation of OSAHS with complaints of snoring and excessive daytime sleepiness were recruited for this study. Demographic and clinical variables were documented. Patients were excluded if they had a medically unstable condition (i.e., recent myocardial infarction, congestive heart failure) or if they were unable to sleep with CPAP.
All patients underwent full nocturnal polysomnography to confirm the diagnosis of OSAHS, and the polysomnogram was performed as per American Academy of Sleep Medicine recommended clinical guidelines.32,33 If CPAP treatment was clinically indicated, the patients were referred for in-laboratory CPAP-titration polysomnography.34 In addition to the usual measurements of mask flow and mask pressure, supraglottic pressure measurements were also obtained during optimal fixed CPAP and at the same CPAP level with expiratory pressure reduction (C-Flex). C-Flex produces a constant inhalation pressure but reduces airway pressure during exhalation in proportion to the patient's expiratory airflow (thus, a drop in pressure occurs primarily during early expiration). C-Flex allows for 3 setting, C-Flex 1, C-Flex 2, C-Flex 3, which correspond to an increasing proportionality constant between expiratory flow and pressure reduction.
During the CPAP-titration polysomnography, pressure was directly measured at the mask using a pressure transducer (Ultima Dual Airflow Pressure SensorTM, Braebon 0585, Ontario, Canada). Airflow was recorded from the output of a Respironics BiPAP Auto M Series device in CPAP mode. CPAP was titrated manually during the first hour of the study to a level that eliminated all sleep disordered breathing events, including obstructive apneas and hypopneas and runs of inspiratory flow limitation. The optimal pressure was defined as the minimum pressure at which flow limitation disappeared. This pressure was determined by performing step-down measures, i.e., dropping the pressure every 2 minutes by 1 cm H2O until indications of inspiratory flow limitation occurred. The pressure prior to the appearance of flow limitation established the minimum therapeutic pressure.
In addition to standard monitoring, supraglottic pressure was obtained using a pressure transducer-tipped catheter (Millar MPC 500, Millar Instruments, Houston, TX). The patient's nose was anesthetized using atomized lidocaine 5% and lido-caine 2% jelly for the throat. The Millar catheter was introduced transnasally, and the tip of the catheter was placed just below the uvula. The catheter position was confirmed visually through the mouth. The catheter was taped to the nose to secure its position throughout the study. The nasal CPAP mask was then applied, and leak at the exit site of the catheter was minimized. The output of the Millar catheter was amplified and recorded at 64 Hz. To verify that the supraglottic catheter tip was placed just below the collapsible segment of the UA, the tracings from the supraglottic and CPAP inspiratory pressures after the patient fell asleep were inspected during a brief “step-down” of CPAP pressure. Correct positioning of the catheter tip required that the delta pressure between the mask and the supraglottic area increased substantially during inspiration and that evidence of inspiratory flow limitation appeared simultaneously. If this increase in delta pressure was not observed while the CPAP was reduced, the technician assumed that the catheter position was too high and advanced the catheter.
Interventions were performed after 5 minutes of stable stage N2 sleep with the patient on optimal CPAP. The data were discarded if an arousal occurred. Three different levels of C-Flex were applied cyclically multiple times throughout the night. The order of application of C-Flex level was not randomized. Each level was maintained for 1 minute, and fixed CPAP was restored at the end of the sequence, which was repeated at least twice, up to 10 times, across the night. Changes in pressure were accomplished with a single machine while patients were asleep, and, thus, patients were effectively blinded to the intervention.
Subjects signed a consent form approved by the Institutional Review Board of the New York University School of Medicine.
Mechanical Model of the Upper Airway
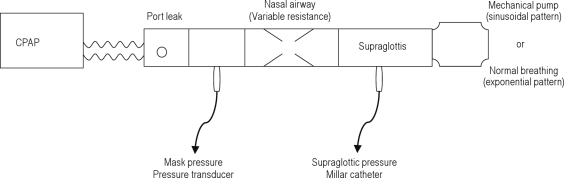
To create a bench test for some of our observations in patients, we designed a mechanical model of the upper airway in patients on CPAP (i.e., without a collapsible airway) (Figure 1). This model consisted of a rigid resistive tube, the resistance of which could be varied by changing the aperture size. A pure sinusoidal respiratory pattern was generated using a mechanical pump (Respiration Pump 607, Harvard Apparatus Co, INC. Dover, MA). In a separate data collection, a healthy volunteer breathing through the system generated a “normal” breathing pattern (exponential expiration with pause). Airflow was measured from the output of a Respironics BiPAP Auto M Series device in CPAP mode. Simulated mask pressure was measured with a pressure transducer (Ultima Dual Airflow Pressure Sensor). Simulated supraglottic pressure was obtained using the Millar catheter. Measurements were obtained using these 2 respiratory patterns at 2 respiratory rates and 2 tidal volumes. All measurements were performed with 2 different resistances on CPAP and on C-Flex settings. Three different levels of C-Flex were applied and maintained for 1 minute each, and fixed CPAP was restored at the end of the sequence, which was repeated.
Figure 1.
Mechanical model of the upper airway. The model consists of a rigid resistive tube with a variable “upstream” upper airway resistance controlled by changing the aperture size to mimic a patient using nasal continuous positive airway pressure (CPAP). A rigid tube was used to model the upper airway because dynamic collapse does not occur in patients on CPAP. The pressure taps are placed within the model to obtain measurements that simulate nasal and supraglottic pressures in a patient. Patterns of breathing were applied by a mechanical pump (sinusoidal) or a healthy volunteer breathing on a mouthpiece (“normal” pattern).
Analysis
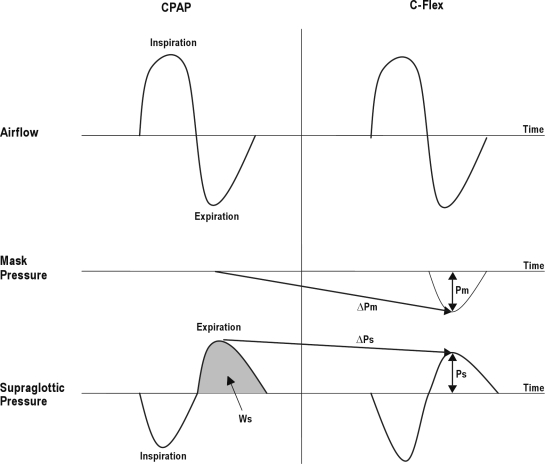
Figure 2 shows a drawing of airflow, mask pressure, and supraglottic pressure signals and the derived variables. Respiratory variables were analyzed only during the expiratory phase. We assessed values of variables on CPAP and on different C-Flex settings. Mask pressure (Pm in Figure 2) is the expiratory pressure swing in the mask (the difference between mask pressure at peak expiratory airflow and mask pressure at the end of expiration, which is the set CPAP). Supraglottic pressure (Ps) is the expiratory pressure swing in the supraglottis (the difference between the supraglottic pressure at peak expiratory airflow and the supraglottic pressure at the end of expiration). Delta Pm (ΔPm) is the change in mask-pressure swings with application of C-Flex (Pm on C-Flex minus Pm on CPAP). Delta Ps (ΔPs) is the change in supraglottic pressure swings with application of C-Flex (Ps on C-Flex minus Ps on CPAP). We calculated the integrated supraglottic pressure during expiration (Ws) as a surrogate for expiratory work. The UA resistance was calculated as the difference between mask pressure and supraglottic pressure at peak airflow divided by peak airflow.
Figure 2.
This drawing shows data collected and variables analyzed for a single breath. The left panel shows continuous positive airway pressure (CPAP) and the right shows C-Flex 3. The top tracing shows airflow (inspiration up). The middle tracing is pressure at the mask, and the bottom tracing is supraglottic pressure (inspiration down). Pm refers to the expiratory pressure swing in the mask; Ps, expiratory pressure swing in the supraglottis; ΔPm, change in expiratory mask pressure swings (C-Flex 3 minus CPAP); ΔPs, change in expiratory pressure swings in the supraglottis (C-Flex 3 minus CPAP); Ws, estimated expiratory work by calculating the integrated supraglottic pressure during expiration (grey area).
In patients (during stage N2 sleep and in the same position) and in the UA model, we identified 2 separate periods suitable for data collection during which stable respiration was present. In each of these periods, data from 3 consecutive breaths were averaged to obtain the value for each variable on C-Flex 1, C-Flex 2, C-Flex 3, and fixed CPAP. The average value from 2 segments is reported as a single value for each variable on C-Flex 1, C-Flex 2, C-Flex 3, and fixed CPAP. Analysis of the supraglottic pressure signal was done without hiding the mask pressure signal, and the investigator was, thus, not blinded as to the presence of C-Flex.
Statistical analysis was performed using SPSS for Windows (version 17; SPSS, Chicago, IL). An independent samples t-test was used for comparisons between low and high resistance. Comparisons of ΔPm and ΔPs were made using paired-samples t-test comparing CPAP with C-Flex 3. Significance was assumed at a P value of less than 0.05. Values are shown as mean ± SD.
RESULTS
Patients with OSAHS
Of the 22 patients with OSAHS who were recruited, 17 (13 men/4 women) completed the study: the remaining potential subjects were excluded due to insufficient sleep (n = 2), excessive mask leak (n = 2), and poor signal quality from the supraglottic catheter (n = 1). The mean age was 49.2 ± 11.1 years, mean body mass index, 35.1 ± 9.8 kg/m2; the mean apnea-hypopnea index, 61.2 ± 35.1 events/h; the mean respiratory index disturbance 64.8 ± 35.1 events/h, mean Epworth Sleepiness Scale score, 12.7 ± 5.4; and the mean CPAP level, 10.11 ± 3.5 cm H2O.
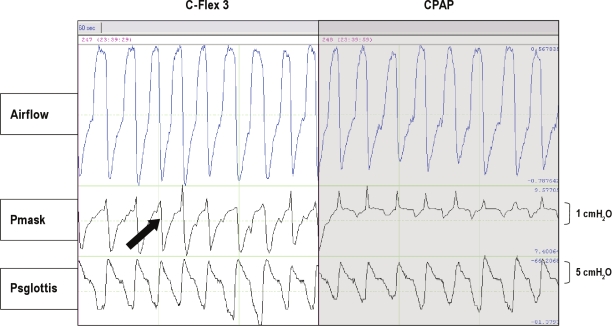
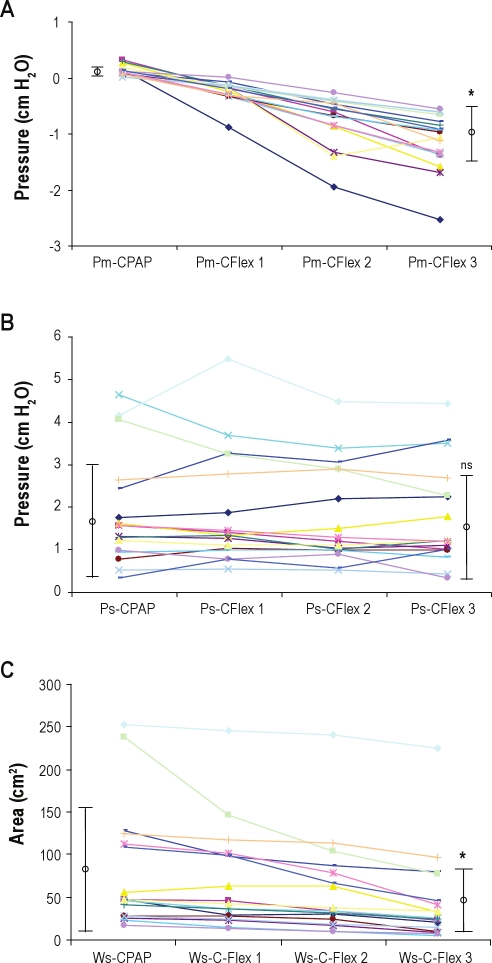
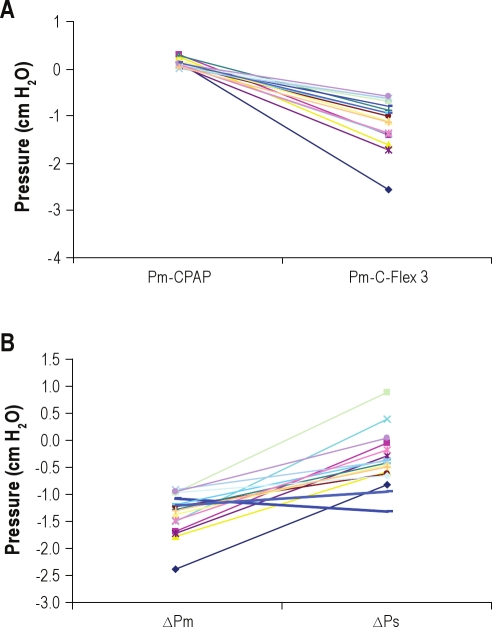
Figure 3 shows raw-tracing data of airflow, mask pressure, and supraglottic pressure from 1 patient with OSAHS. Swings in the expiratory mask pressure in the patients during CPAP were near 0 (Pm = +0.09 ± 0.08 cm H2O) and, as expected, swings in the supraglottic expiratory pressure did occur (Ps = +1.87 ± 1.30 cm H2O). During C-Flex 3, all patients developed expiratory mask pressure dips (Pm = −1.13 ± 0.48 cm H2O), and the drop of Pm was progressive as C-Flex went from setting 1 to setting 3 (Figure 4A). Concurrently, expiratory supraglottic pressure swings (Ps) were +1.75 ± 1.19 cm H2O (Figure 4B). Thus, unexpectedly, there was no significant reduction in supraglottic expiratory pressure swings during C-Flex, compared with the swings present in Ps during CPAP alone (P = 0.46). Figure 5A shows the effect of C-Flex compared with CPAP on Pm in the individual patients. The transmission of the expira-tory mask pressure swings to the supraglottis did not occur in 15 of the 17 patients (e.g., ΔPm was −1.23 ± 0.53 cm H2O and ΔPs was −0.06 ± 0.47 cm H2O, P = 0.000, see Figure 5B). This behavior was in contrast to the expectation that C-Flex would reduce or abolish changes in expiratory supraglottic pressures.
Figure 3.
The raw tracing data of airfl ow, mask pressure (Pmask), and supraglottic pressure (Psglottis) from a patient with obstructive sleep apneahypopnea syndrome on C-Flex 3 and continuous positive airway pressure. The arrow shows the reduction of mask pressure during expiration with the application of C-Flex 3.
Figure 4.
Data from patients with obstructive sleep apnea-hypopnea syndrome—the effect of C-Flex compared with continuous positive airway pressure (CPAP) on mask pressure swings (Pm), supraglottic pressure swings (Ps), and the estimated expiratory work (Ws). Each line represents a patient (n = 17) with lines connecting the magnitude of the expiratory pressure swing within the mask when the patient was using CPAP to the expiratory pressure swing within the mask when the patient was using C-Flex 3. (A) All patients developed expiratory dips, and the Pm showed progressive reduction on C-Flex 1, C-Flex 2, and C-Flex 3 compared with CPAP. The mean ± SD values of the CPAP and C-Flex 3 are shown, and the * indicates a significant difference in the mean (P < 0.0001). (B) Patients did not show a reduction in expiratory supraglottic pressure swings (Ps) with the application of various levels of C-Flex. The mean ± SD value of the CPAP and C-Flex 3 are shown, and ns indicates no significant difference in the means. (C) Patients showed a variable reduction of the integrated expiratory pressure in the supraglottis with C-Flex. This reduction may be most evident when comparing CPAP with the highest level of C-Flex 3. The mean ± SD value of the CPAP and C-Flex 3 are shown, and the * indicates a significant difference in the mean (P < 0.0001).
Figure 5.
(A) Patients with obstructive sleep apnea-hypopnea syndrome— the effect of C-Flex compared with the effect of continuous positive airway pressure (CPAP) on mask pressure swings (Pm). Each line represents a patient (n = 17) with lines connecting the magnitude of expiratory pressure swing within the mask when the patient was using CPAP to the expiratory pressure swing within the mask when the patient was using C-Flex 3. All patients developed expiratory dips, and Pm showed reduction when patients were using C-Flex 3, compared with CPAP. (B) Patients with obstructive sleep apnea-hypopnea syndrome—the change in expiratory pressure swings with C-Flex 3. Each line represents a patient with lines connecting the change of expiratory mask pressure (Pm) between continuous positive airway pressure and C-Flex 3 and the change in expiratory supraglottic pressure (Ps). There was no transmission of the mask pressure swings to the supraglottis in 15 of the 17 patients (e.g., change in Pm [ΔPm] was more negative than change in Ps [ΔPs]). The 2 thick lines represent the 2 patients with a parallel drop in Pm and ΔPs.
When patients were using CPAP during sleep, no differences occurred between the mean inspiratory and mean expiratory instantaneous UA resistance (0.12 ± 0.08 cm H2O·L-1·min-1 vs 0.10 ± 0.09 cm H2O·L-1·min-1, P = 0.11). Table 1 examines the role of expiratory UA (upstream) resistance on our findings by separating our patients whose expiratory UA resistance was less than (n = 12) and greater than (n = 5) 0.1 cm H2O·L-1·min-1, in accord with what has been shown in the literature on nasal resistance.35–37 Patients with low UA resistance during CPAP use (constant mask pressure) showed expiratory pressure swings at the supraglottis (Ps) of +1.15 ± 0.45 cm H2O. As expected, Ps was significantly greater (P = 0.001) in patients with high levels of UA resistance (+3.59 ± 0.99 cm H2O). On C-Flex 3, there were no discernable differences between groups for ΔPm and ΔPs.
Table 1.
Expiratory mask and supraglottic pressure swings and effect of C-Flex compared to CPAP in OSAHS patients
| OSAHS Patients | Low UA resistance (n = 12) (< 0.1 cm H2O/L/min) |
High UA resistance (n = 5) (> 0.1 cm H2O/L/min) |
||
|---|---|---|---|---|
| CPAP | C-Flex 3 | CPAP | C-Flex 3 | |
| Pm (cm H2O) | 0.11 ± 0.09 | −1.21 ± 0.53 | 0.03 ± 0.02 | −0.93 ± 0.31 |
| Ps (cm H2O) | +1.15 ± 0.45 | +1.11 ± 0.52 | +3.59 ± 0.99 | +3.29 ± 0.84 |
| ΔPm (cm H2O) | −1.32 ± 0.55 | −0.96 ± 0.31 | ||
| ΔPs (cm H2O) | −0.04 ± 0.39 | −0.29 ± 1.18 | ||
Values are means ± standard deviation. UA, upper airway; CPAP, continuous positive airway pressure; C-Flex, reduction of expiratory pressure during CPAP; Pm, expiratory pressure swing in the mask; Ps, expiratory pressure swing in the supraglottis; ΔPm, change in mask pressure swings (C-Flex 3 minus CPAP); ΔPs, change in supraglottic pressure swings (C-Flex 3 minus CPAP).
To examine this unexpected lack of change in supraglottic pressure—despite a drop in expiratory pressure at the mask— we used a mechanical model in which the pattern of airflow could be controlled.
Mechanical Model of the Upper Airway
Sinusoidal respiratory pattern
When we implemented our mechanical model of the upper airway with a sinusoidal respiratory pattern and a low simulated UA resistance during CPAP (constant mask pressure), expira-tory pressure swings at the simulated supraglottis (Ps) were +1.94 ± 1.47 cm H2O. As expected, with a highsimulated UA resistance, Ps increased to +4.40 ± 3.03 cm H2O.
During the highest level of C-Flex (C-Flex 3), mask pressure developed expiratory dips and Pm was −1.45 ± 0.74 cm H2O on low simulated UA resistance and −1.57 ± 0.66 cm H2O on high simulated UA resistance. Concurrently, expiratory Ps was +0.51 ± 1.11 cm H2O on low simulated UA resistance and +2.87 ± 2.41 cm H2O on high simulated UA resistance.
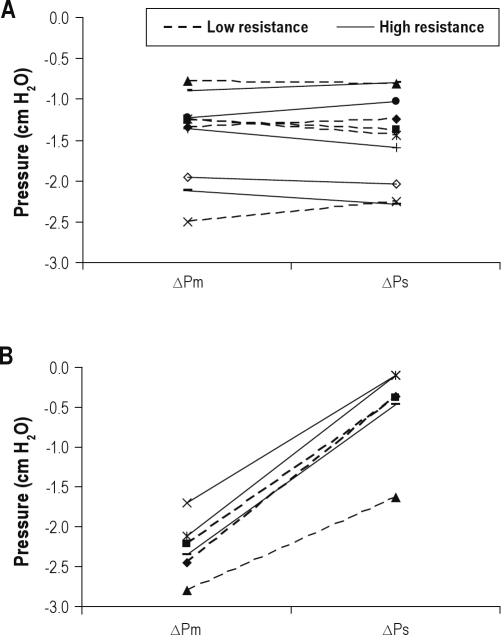
Table 2 shows the effect of C-Flex compared with CPAP on Pm and Ps in our mechanical-model data. The data across a range of imposed tidal volumes and frequencies are grouped according to whether there was a low or high simulated upstream “UA” resistance. The change in Ps (ΔPs) when going from CPAP to C-Flex 3 was similar to the change in Pm (ΔPm) (e.g., there was no statistically significant difference in the magnitude of the swings between Ps and Pm). Furthermore, in the model, expiratory pressure swings were transmitted similarly from mask to supraglottis for all patterns of breathing and for low and high UA resistance.
Table 2.
Effect of C-Flex compared with CPAP on mask and supraglottic expiratory pressure swings in the mechanical model with sinusoidal breathing
| Upper Airway Model | Change (C-Flex 3 minus CPAP) |
|
|---|---|---|
| Sinusoidal respiratory pattern | ΔPm (cm H2O) | ΔPs (cm H2O) |
| Low upper airway resistance (0.028 ± 0.018 cm H2O/L/min) | ||
| RR 10 bpm, TV 450 ml | −0.80 | −0.81 |
| RR 16 bpm, TV 450 ml | −1.34 | −1.25 |
| RR 24 bpm, TV 450 ml | −1.26 | −1.37 |
| RR 12 bpm, TV 800 ml | −1.25 | −1.44 |
| RR 24 bpm, TV 800 ml | −2.5 | −2.25 |
| Total group | −1.43 ± 0.64 | −1.42 ± 0.52 |
| High upper airway resistance (0.059 ± 0.022 cm H2O/L/min) | ||
| RR 12 bpm, TV 450 ml | −0.89 | −0.80 |
| RR 16 bpm, TV 450 ml | −1.22 | −1.02 |
| RR 24 bpm, TV 450 ml | −1.35 | −1.60 |
| RR 12 bpm, TV 800 ml | −1.95 | −2.04 |
| RR 24 bpm, TV 800 ml | −2.13 | −2.29 |
| Total group | −1.51 ± 0.52 | −1.55 ± 0.64 |
Values for total group are means ± standard deviation. RR, respiratory rate; TV, tidal volume; bpm, breath per minute; ΔPm, change in mask pressure swings (C-Flex 3 minus CPAP); ΔPs, change in supraglottic pressure swings (C-Flex 3 minus CPAP).
Exponential respiratory pattern
When a healthy volunteer breathing on the upper airway model produced a nonsinusoidal (normal) respiratory pattern with a rapid peak in expiratory airflow followed by an exponential decay of flow, low simulated UA resistance during CPAP (constant mask pressure) produced expiratory pressure swings at the supraglottis (Ps) of +4.09 ± 2.74 cm H2O. Again, as expected, high simulated UA resistance increased Ps to +6.61 ± 4.86 cm H2O.
During the highest level of C-Flex (C-Flex 3), mask pressure developed expiratory dips and Pm was −2.61 ± 0.62 cm H2O on low simulated UA resistance and −2.35 ± 0.70 cm H2O on high simulated UA resistance. Concurrently, expiratory supraglottic pressure swings (Ps) were +3.30 ± 2.01 cm H2O on low simulated UA resistance and +6.42 ± 4.60 cm H2O on high simulated UA resistance.
Table 3 shows the effect of C-Flex compared with CPAP on mask and supraglottic pressure swings in the model data. The data across a range of imposed tidal volumes and frequencies are grouped according to whether there was a low or high simulated upstream “UA” resistance. In contrast with the findings during sinusoidal breathing, ΔPs was lower than the ΔPm (P = 0.024 for low simulated UA resistance and P = 0.003 for high simulated UA resistance). The lack of transmission of pressure swings from mask to supraglottis was most evident during the simulated high UA resistance.
Table 3.
Effect of C-Flex compared to CPAP on mask and supraglottic expiratory pressure swings in the mechanical model with exponential breathing
| Upper Airway Model | Change (C-Flex 3 minus CPAP) |
|
|---|---|---|
| Exponential respiratory pattern | ΔPm (cm H2O) | ΔPs (cm H2O) |
| Low upper airway resistance (0.040 ± 0.014 cm H2O/L/min) | ||
| RR 14 bpm, TV∼500 ml | −2.45 | −0.37 |
| RR 28 bpm, TV∼500 ml | −2.21 | −0.38 |
| RR 16 bpm, TV∼(2× baseline) ml | −2.80 | −1.63 |
| Total group | −2.48 ± 0.3 | −0.79 ± 0.72 |
| High upper airway resistance (0.075 ± 0.004 cm H2O/L/min) | ||
| RR 16 bpm, TV∼500 ml | −1.69 | 0 |
| RR 26 bpm, TV∼500 ml | −2.11 | −0.09 |
| RR 16 bpm, TV∼(2× baseline) ml | −2.34 | −0.47 |
| Total group | −2.05 ± 0.33 | −0.19 ± 0.25 |
Values for total group are means ± standard deviation. RR, respiratory rate; TV, tidal volume; bpm, breath per minute; ΔPm, change in mask pressure swings (C-Flex 3 minus CPAP); ΔPs, change in supraglottic pressure swings (C-Flex 3 minus CPAP).
Figure 6 combines the data in Tables 2 and 3 to contrast the effect of sinusoidal (Figure 6A) and “normal” nonsinusoidal (Figure 6B) breathing patterns on ΔPm and ΔPs in the model. Whereas there is a consistent transmission of mask pressure swings to the supraglottis in sinusoidal breathing patterns, mask pressure swings were NOT transmitted to the supraglottis during “normal” nonsinusoidal breathing (e.g., ΔPm was significantly more negative than ΔPs [p = 0.024 for low simulated UA resistance and P = 0.003 for high simulated UA resistance]).
Figure 6.
Upper airway model—change in expiratory pressure swings with C-Flex 3. Each line represents a different tidal volume or frequency. Dashed lines are simulations with low upper airway resistance, and the solid lines are simulations with high upper airway resistance connecting the change of expiratory expiratory mask pressure between continuous positive airway pressure and C-Flex 3 and the change of expiratory supraglottic pressure. (A) Sinusoidal respiratory pattern. There is a consistent transmission of expiratory mask pressure swings to the supraglottis (eg ΔPm is similar to ΔPs). (B) “Normal” (exponential expiration) respiratory pattern. Expiratory mask pressure swings were not transmitted to the supraglottis (eg ΔPm was significantly more negative than ΔPs).
Analysis of Expiratory Pressure-Time Curve
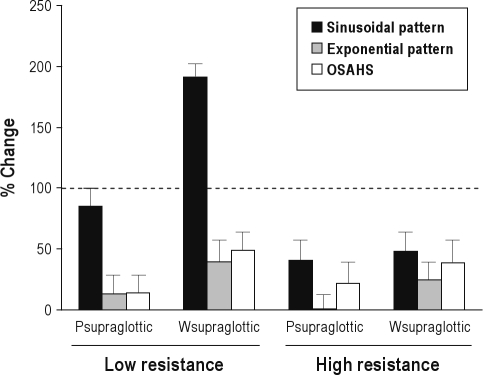
In addition to the analysis of the effect of C-Flex on expira-tory peak pressures at the supraglottis, we also integrated the pressure-time curve as an estimate of expiratory work in patient and model data. This area measurement was used to re-evaluate the effectiveness of application of C-Flex to the UA of patients with OSAHS and our UA model (Figures 4C, 7). In Figure 7, for each condition (low and high resistance, sinusoidal and non-sinusoidal model data and patient data) the percentage change from CPAP to C-Flex 3 is shown for supraglottic expiratory pressure swings and expiratory area. We defined a change of 100% from the CPAP to C-Flex 3 value as complete reversal of the expiratory pressure swing in the supraglottis. In the UA model when UA resistance was low, application of C-Flex 3 produced complete reversal of expiratory Ps with sinusoidal breathing but produced a partial reversal with “normal” breath shape. Patients with OSAHS behaved similarly to the model data with “normal” breath and did not show much reversal of the expiratory pattern for Ps or Ws. When UA resistance was high, application of C-Flex 3 produced incomplete reversal of expiratory Ps and Ws in all cases for the model and patients. Thus, application of C-Flex reduced the integrated expiratory pressure in the supraglottis but not the peak (Figure 4C). This indicates that mask pressure is transmitted to the supraglottis but the transmission is not fast enough to reduce peak Ps. However, it does reduce the integrated pressure and may reduce expiratory work.
Figure 7.
The effectiveness of application of C-Flex to the upper airway model and to patients with obstructive sleep apnea-hypopnea syndrome. The Y axis shows the percentage change from continuous positive airway pressure to C-Flex 3 values for peak expiratory supraglottic pressure and the estimated expiratory work (Ws) for conditions with low and high resistance. The black bars show data from simulations done using a sinusoidal respiratory pattern. The gray bars show data in simulations done using an exponential respiratory pattern. The white bars show data in patients with obstructive sleep apnea-hypopnea syndrome. On the left are shown data collected in situations of low resistance, and, on the right, with high resistance. The dashed line (100% change) represents complete reversal of the expiratory pressure swing in the supraglottis, defined by a change of 100% from the continuous positive airway pressure to C-Flex 3 value and is the desired result of applying C-Flex. See text for discussion.
DISCUSSION
Our data show that, when mask pressure is constant during CPAP use, significant pressure swings occur in the supraglottis during expiration. The essential new finding of this study is that imposed expiratory changes in mask pressure produced by C-Flex did not uniformly transmit to the supraglottis in either patients with OSAHS on CPAP or in a mechanical model of the upper airway with a fixed resistance. Our model data comparing breaths with a sinusoidal shape to breaths with an exponential expiratory decay (“normal”) of airflow suggest to us that the observed lack of expiratory drop in supraglottic pressure swings is related to dynamics of the C-Flex algorithm that controls mask pressure rather than to intrinsic properties of theupper airway.
In our mechanical model of the UA on CPAP, we found that, during expiration, as expected, high nasal resistance (upstream) produced greater pressure swings in the supraglottis than low nasal resistance. Only with a sinusoidal respiratory pattern did the expiratory pressure drop in the mask produced by C-Flex successfully mitigate the expiratory rise in pressure seen in the supraglottis, which is the intended purpose we attribute to C-Flex. In contrast, when tested in our model with breaths having the more physiologically typical exponential respiratory pattern, application of C-Flex caused little reduction of supraglottic pressure swings during expiration, despite a similar drop of mask pressure. Similarly, in the patients with OSAHS, application of C-Flex produced a drop in expiratory mask pressure in all patients; however, most patients did not demonstrate the expected fall in supraglottic pressures swings.
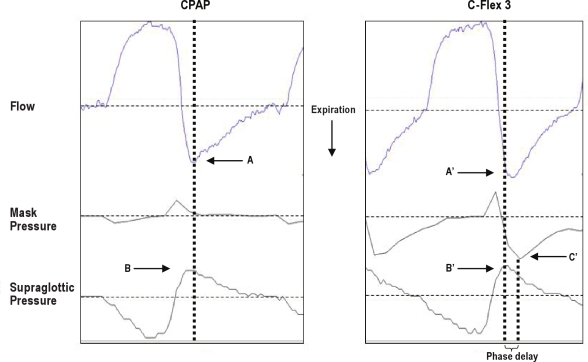
One explanation of our primary finding, i.e., that the C-Flex algorithm may not work as well with nonsinusoidal patterns of breathing as with pure sinusoidal expiration, may be related to the occurrence of rapid changes in flow during early expiration with an exponential pattern. Inspection of the pressure and flow tracings suggests that a phase delay in the pressure response to expiratory flow was present. Figure 8 shows a typical example from one patient. The drop in mask pressure occurs well after the initiation of the rise of supraglottic pressure during early expiration. This phase lag between flow and mask pressure, and the persistence of supraglottic pressure swings on C-Flex, was seen in all of the patients (mean phase lag 0.31 ± 0.06 sec; range, 0.19-0.42 sec) and also during the exponential expira-tory pattern in the model data (mean phase lag 0.28 ± 0.10 sec; range, 0.15-0.41 sec). To further understand this phenomenon, we attempted to find a relationship between the presence of a phase lag between peak expiratory flow and peak mask expira-tory pressure drop and respiratory frequency but were not able to do so within the range of respiratory patterns recorded. Thus, we cannot say with certainty whether the failure of C-Flex to abolish the expiratory supraglottic pressure swings was due to only a rapid change in expiratory flow or to some other aspect of nonsinusoidal breathing.
Figure 8.
Phase delay in pressure response to expiratory airflow. Representative tracing of 1 breath obtained in 1 patient of airflow, mask pressure, and supraglottic pressure. A and A' indicate timing of expiratory peak flow on continuous positive airway pressure, and C-Flex, B, and B' indicate the peak of expiratory supraglotic pressure. C indicates the timing of peak drop in mask pressure on C-Flex. The phase delay is indicated.
An alternate explanation of our findings of a lack of change in expiratory supraglottic pressure despite a drop in mask pressure during C-Flex in the patients with OSAHSis that there was unexplained development of expiratory flow limitation in the upper airway that occurred only in association with C-Flex. We are aware of no neural or mechanical reason for such a behavior of the relatively rigid nasal airway on CPAP. Specifically, the behavior of the UA while the patient is on CPAP should be relatively invariant because the collapsible segment of the UA that is usually responsible for changes in airway resistance during sleep is being “splinted” throughout the respiratory cycle above optimal CPAP.
Although C-Flex did not show much effect on peak expira-tory supraglottic pressure swings, we did record some reduction in the Ws, (our estimate of expiratory work). However, we achieved only a partial reversal of the “expiratory phenomenon” with the maximum available settings of C-Flex (Figure 7). We have no way of assessing whether comfort, or the perception of discomfort by a patient during expiration, is affected more by mitigating the peak pressure or by mitigating work of exhalation; this may need to be tested directly.
One limitation of our study is that we did not recruit patients based on nasal resistance and, thus, did not have a large number of patients with high nasal resistance. We could have attempted to increase the number of patients in this study who had high nasal resistance by recruiting based on awake subjects' complaints of nasal symptoms or on the results of testing obtained during wake (such as with rhinomanometry or acoustic rhinometry) that showed a high nasal resistance. However, we have previously shown that awake noninvasive measures of nasal resistance are not predictive of nasal resistance asleep.38 Furthermore, despite a limited range in nasal resistances in the present data, we did show in the present dataset that, on CPAP, patients with high UA resistance had greater supraglottic pressure swings than did patients with low resistance.
A second possible limitation is that we did not specifically select patients who had reported intolerance to CPAP, and, in this study, we did not assess level of comfort. Thus, we cannot relate increased expiratory supraglottic pressure swings (or work) to perceived comfort on CPAP or an effect of C-Flex on reported comfort. However, this was not the objective of the present study. Furthermore, our patients were being studied during a first exposure to CPAP, during which they had multiple interventions (CPAP titration, trial with different settings of C-Flex), and these circumstances would have made collecting patients' acute impressions of comfort difficult to interpret.
A final caveat exists in interpreting the results of these data: by design, we studied the effect of C-Flex-induced pressure drops at the mask on supraglottic pressure in patients only during sleep; “comfort” may be partially or wholly affected by the conditions during wake. The analysis reported here is based on measurements made during stage N2 sleep; in our current data set, we did not record much data when subjects were breathing in the wake state. In the limited wake periods available for analysis, we saw no trends toward a greater transmission of mask to supraglottic pressure swings while patients were on C-Flex.
In conclusion, we were not able to show that C-Flex reduces expiratory pressure swings in the supraglottis in patients with OSAHS on CPAP during sleep. Although C-Flex did succeed in reducing “supraglottic” pressure swings in our modeling studies using sinusoidal breathing, the magnitude of C-Flex mask-pressure reductions was not sufficient to eliminate expiratory supraglottic pressure swings at anysetting for other patterns. These nonsinusoidal model data are similar to the data in patients. Our observations suggest that maximum potential physiologic impact of C-Flex on supraglottic pressure may not have been achieved by the present algorithm, and this may account for the recent data showing little effect of C-Flex use on overall CPAP compliance.29,30 Because there was surprisingly little physiologic expiratory effect at the supraglottis of C-Flex during sleep with the present implementation of expiratory pressure modification by C-Flex, it is not possible to test the hypothesis that optimal mitigation of supraglottis expiratory pressure swings will improve patient comfort and compliance. However, if C-Flex does improve comfort, it is unlikely to do so by the mechanism of reducing the peak expiratory supraglottic pressure
DISCLOSURE STATEMENT
This was not an industry supported study. Drs. Rapoport and Ayappa have received research support from Fisher – Paykel Healthcare and Ventus Medical, speaking honoraria from Fisher – Paykel Healthcare. Drs. Rapoport and Ayappa have received assistance with the licensing of various patents from Biologics, Fisher and Paykel Healthcare, Advanced Brain Monitoring and Tyco. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge Guo-Ming Chen, Boris Opancha, Reni Pillai, and Rakhil Kanevskaya for their technical support during this research. This research was supported by grants from ALANY, NCRR M01RR00096, Foundation for Research in Sleep Disorders, Instituto de Salud Carlos III, Societat Cata-lana de Pneumologia, Sociedad Española de Neumología y Cirugía Torácica. The work was performed at the Sleep Disorders Center of Pulmonary, Critical Care and Sleep Medicine Department, New York University School of Medicine, New York, NY.
ABBREVIATIONS
- CPAP
continuous positive airway pressure
- OSAHS
obstructive sleep apnea/hypopnea syndrome
- UA
upper airway
- Pm
mask pressure
- Ps
supraglottic pressure
- ΔPm
delta mask pressure
- ΔPm
delta supraglottic pressure
- Ws
integral of pressure × expiratory time (surrogate for expiratory work)
REFERENCES
- 1.McDaid C, Duree KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev. 2009;13:427–36. doi: 10.1016/j.smrv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Sanders MH, Montserrat JM, Farre R, Givelber RJ. Positive pressure therapy: a perspective on evidence-based outcomes and methods of application. Proc Am Thorac Soc. 2008;5:161–72. doi: 10.1513/pats.200709-150MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med. 2001;164:1459–63. doi: 10.1164/ajrccm.164.8.2008146. [DOI] [PubMed] [Google Scholar]
- 4.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–13. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 5.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565–71. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 6.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 7.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 8.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128:624–33. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 9.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 10.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 11.Grunstein RR. Sleep-related breathing disorders. 5. Nasal continuous positive airway pressure treatment for obstructive sleep apnoea. Thorax. 1995;50:1106–13. doi: 10.1136/thx.50.10.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lojander J, Brander PE, Ammala K. Nasopharyngeal symptoms and nasal continuous positive airway pressure therapy in obstructive sleep apnoea syndrome. Acta Otolaryngol. 1999;119:497–502. doi: 10.1080/00016489950181062. [DOI] [PubMed] [Google Scholar]
- 13.Engleman HM, Asgari-Jirhandeh N, McLeod AL, Ramsay CF, Deary IJ, Douglas NJ. Self-reported use of CPAP and benefits of CPAP therapy: a patient survey. Chest. 1996;109:1470–6. doi: 10.1378/chest.109.6.1470. [DOI] [PubMed] [Google Scholar]
- 14.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 15.Sugiura T, Noda A, Nakata S, et al. Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respiration. 2007;74:56–60. doi: 10.1159/000089836. [DOI] [PubMed] [Google Scholar]
- 16.Virkkula P, Maasilta P, Hytonen M, Salmi T, Malmberg H. Nasal obstruction and sleep-disordered breathing: the effect of supine body position on nasal measurements in snorers. Acta Otolaryngol. 2003;123:648–54. doi: 10.1080/00016480310001493. [DOI] [PubMed] [Google Scholar]
- 17.Cole P. Nasal and oral airflow resistors. Site, function, and assessment. Arch Otolaryngol Head Neck Surg. 1992;118:790–3. doi: 10.1001/archotol.1992.01880080012004. [DOI] [PubMed] [Google Scholar]
- 18.Ohki M, Usui N, Kanazawa H, Hara I, Kawano K. Relationship between oral breathing and nasal obstruction in patients with obstructive sleep apnea. Acta Otolaryngol Suppl. 1996;523:228–30. [PubMed] [Google Scholar]
- 19.Bachour A, Maasilta P. Mouth breathing compromises adherence to nasal continuous positive airway pressure therapy. Chest. 2004;126:1248–54. doi: 10.1378/chest.126.4.1248. [DOI] [PubMed] [Google Scholar]
- 20.Teschler H, Berthon-Jones M, Thompson AB, Henkel A, Henry J, Konietzko N. Automated continuous positive airway pressure titration for obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;154:734–40. doi: 10.1164/ajrccm.154.3.8810613. [DOI] [PubMed] [Google Scholar]
- 21.Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep. 2004;27:249–53. doi: 10.1093/sleep/27.2.249. [DOI] [PubMed] [Google Scholar]
- 22.Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest. 1990;98:317–24. doi: 10.1378/chest.98.2.317. [DOI] [PubMed] [Google Scholar]
- 23.Reeves-Hoche MK, Hudgel DW, Meck R, Witteman R, Ross A, Zwillich CW. Continuous versus bilevel positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:443–9. doi: 10.1164/ajrccm.151.2.7842204. [DOI] [PubMed] [Google Scholar]
- 24.Nilius G, Happel A, Domanski U, Ruhle KH. Pressure-relief continuous positive airway pressure vs constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest. 2006;130:1018–24. doi: 10.1378/chest.130.4.1018. [DOI] [PubMed] [Google Scholar]
- 25.Mulgrew AT, Cheema R, Fleetham J, Ryan CF, Ayas NT. Efficacy and patient satisfaction with autoadjusting CPAP with variable expiratory pressure vs standard CPAP: a two-night randomized crossover trial. Sleep Breath. 2007;11:31–7. doi: 10.1007/s11325-006-0078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenzel M, Kerl J, Dellweg D, Barchfeld T, Wenzel G, Kohler D. Expira-tory pressure reduction (C-Flex Method) versus fixed CPAP in the therapy for obstructive sleep apnoea. Pneumologie. 2007;61:692–5. doi: 10.1055/s-2007-980075. [DOI] [PubMed] [Google Scholar]
- 27.Marshall NS, Neill AM, Campbell AJ. Randomised trial of compliance with flexible (C-Flex) and standard continuous positive airway pressure for severe obstructive sleep apnea. Sleep Breath. 2008;12:393–6. doi: 10.1007/s11325-008-0189-3. [DOI] [PubMed] [Google Scholar]
- 28.Dolan DC, Okonkwo R, Gfullner F, Hansbrough JR, Strobel RJ, Rosenthal L. Longitudinal comparison study of pressure relief (C-Flex) vs. CPAP in OSA patients. Sleep Breath. 2009;13:73–7. doi: 10.1007/s11325-008-0199-1. [DOI] [PubMed] [Google Scholar]
- 29.Bakker J, Campbell A, Neill A. Randomized controlled trial comparing flexible and continuous positive airway pressure delivery: effects on compliance, objective and subjective sleepiness and vigilance. Sleep. 2010;33:523–9. doi: 10.1093/sleep/33.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepin JL, Muir JF, Gentina T, et al. Pressure reduction during exhalation in sleep apnea patients treated by continuous positive airway pressure. Chest. 2009;136:490–7. doi: 10.1378/chest.08-2646. [DOI] [PubMed] [Google Scholar]
- 31.Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest. 2005;127:2085–93. doi: 10.1378/chest.127.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iber C. 2007. The AASM manual for the scoring of sleep and associated events: rules terminology and technical specifications. [Google Scholar]
- 33.The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 34.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–71. [PMC free article] [PubMed] [Google Scholar]
- 35.Leiter JC, Knuth SL, Bartlett D, Jr Dependence of pharyngeal resistance on genioglossal EMG activity, nasal resistance, and airflow. J Appl Physiol. 1992;73:584–90. doi: 10.1152/jappl.1992.73.2.584. [DOI] [PubMed] [Google Scholar]
- 36.Fitzpatrick MF, McLean H, Urton AM, Tan A, O'Donnell D, Driver HS. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur Respir J. 2003;22:827–32. doi: 10.1183/09031936.03.00047903. [DOI] [PubMed] [Google Scholar]
- 37.Laine MT, Warren DW. Perceptual and respiratory responses to added nasal airway resistance loads in older adults. Laryngoscope. 1995;105:425–8. doi: 10.1288/00005537-199504000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Masdeu MJ, Seelall V, Patel AV, Ayappa I, Rapoport DM. Awake measures of nasal resistance and upper airway resistance on CPAP during sleep. J Clin Sleep Med. 2011;7:31–40. [PMC free article] [PubMed] [Google Scholar]