Abstract
Objectives:
This study evaluated slow wave activity homeostatic response to a mild sleep challenge in alcohol-dependent adults compared to healthy controls.
Design:
Participants maintained a 23:00-06:00 schedule for 5 days verified by actigraphy and diary, followed by 3 nights in the lab: adaptation, baseline, and a sleep delay night with an 02:00-09:00 schedule.
Setting:
Sleep ' Chronophysiology laboratory.
Participants:
48 alcohol-dependent adults (39 men, 9 women) who were abstinent for at least 3 weeks and 16 healthy control adults (13 men, 3 women), 21-55 years of age participated in study.
Interventions:
N/A.
Measurements and Results:
Slow wave EEG activity (SWA) in consecutive NREM periods was compared between baseline and sleep delay nights and between AD and HC groups, using age and sex as statistical covariates. The AD group showed a blunted SWA response to sleep delay with significantly lower SWA power than the HC group. Exponential regression analyses confirmed lower asymptotic SWA with a slower decay rate over NREM sleep time in the AD group. Results were similar for raw SWA and %SWA on the delay night expressed relative to baseline SWA.
Conclusions:
Alcohol dependence is associated with impaired SWA regulation and a blunted response to a mild homeostatic sleep challenge.
Citation:
Armitage R; Hoffmann R; Conroy DA; Arnedt JT; Brower KJ. Effects of a 3-hour sleep delay on sleep homeostasis in alcohol dependent adults. SLEEP 2012;35(2):273-278.
Keywords: Alcohol dependence, polysomnography, sleep, slow wave activity, sleep homeostasis
INTRODUCTION
Alcohol dependence is associated with substantial insomnia complaints during abstinence that are correlated with an increased risk of relapse.1,2 Sleep laboratory studies have generally confirmed polysomnographic features consistent with insomnia complaints, including prolonged sleep latency and impaired sleep continuity, as well as decreased slow wave sleep (SWS), increased amounts of REM sleep, and a short latency to the first episode of REM sleep (REML).1,3,4
Computer-analyzed sleep electroencephalographic (EEG) findings have identified differences between alcohol-dependent (AD) and healthy control (HC) participants. Those with alcohol dependence show less spectral power in the 1-6 Hz frequency range during sleep.4–6 Some studies have also shown increased fast-frequency beta (> 16 Hz) power in AD5 or among those who relapse to drinking,7 whereas others have failed to differentiate beta activity between AD patients from controls.6 Despite the discrepant findings, both reduced slow-frequency delta and increased beta activity are consistent with a reduced drive for deep sleep in AD. The underlying mechanism could be one of either hyperarousal (reflected by increased beta activity) and/or impairment in homeostatic regulation of delta activity in NREM sleep, also known as slow wave activity (SWA) sleep. However, few studies have investigated homeostatic regulation of sleep in those with AD.5,8
The standard approach to assessing homeostatic regulation is to assess the SWA response to sleep challenge such as sleep restriction or deprivation.5,9,10 SWA is typically increased in response to these sleep challenges, particularly in the first NREM episode in recovery sleep, reflecting an increase in homeostatic sleep drive. How SWA dissipates over NREM sleep time after sleep challenge is presumed to reflect the recovery function of sleep. A preliminary study8 from our group has shown that the time course of SWA following a sleep challenge is abnormal in AD men, with a lower accumulation of SWA and a slower dissipation across successive NREM sleep episodes compared to HC men. These preliminary findings support an earlier study by Irwin and colleagues that showed a blunted SWA response to partial sleep deprivation in AD men.5 However the Irwin study did not evaluate the time course of SWA, and our own study only included 10 AD men.
The present study investigated SWA response to a mild sleep challenge, using a 3-h sleep delay paradigm, in 48 AD men and women compared to healthy controls (HC). We hypothesized that AD individuals would show a blunted accumulation and a slower dissipation of SWA across the night in response to a sleep challenge. This sleep delay procedure has been used effectively to study sleep homeostatic response in depressed patients9 and those with chronic fatigue syndrome.10 Two benefits of this procedure over total sleep deprivation are that available total sleep time is held constant before and after recovery sleep and that recovery sleep is still occurring within the nocturnal sleep window, rather than during the daytime.9
METHODS
Participants
Participants were 54 men and 12 women, 20-60 years of age. All participants were recruited through a combination of advertisements, including flyers posted in the Ann Arbor community and in UM-affiliated clinics. Clinician- or self-referral for patients was also permitted.
Forty-eight participants (39 men, 9 women) who met past-year DSM-IV diagnostic criteria11 for alcohol dependence as determined by the Structured Clinical Interview for DSM-IV12,13 were recruited and studied 3-12 weeks after their last drink, as determined by the timeline follow-back interview14–16 and negative breath testing during the screening period. AD participants were excluded if they met DSM-IV criteria for dependence on any substance other than nicotine; if they met current criteria for any mood disorder, anxiety disorder, or eating disorder; or had a lifetime diagnosis of bipolar disorder or any psychotic disorder. Medical illness or taking medications that could interfere with sleep were also cause for exclusion. On average, their duration of problem drinking was 16.1 ± 11.8 years, with an age of onset at 20.7 ± 9.3 years. The group also averaged 8.2 ± 6.2 on the Obsessive-Compulsive Drinking Scale.17
HC participants included 13 men and 3 women, who met the same exclusion criteria as AD participants, and never met lifetime criteria for alcohol dependence. Fewer HCs were purposely recruited to maximize study resources and because differences between groups were expected to be robust.
The mean age of participants was 35.6 ± 10.7 years in the HC group and 36.5 ± 11.0 years in the AD group (not different between groups). The HC group was 2 years better educated (t62 = 3.8, P < 0.05) and more likely to be employed (t62 = 17.5, P < 0.05). The HC group was exclusively Caucasian, whereas the AD group had 33 Caucasians, 11 African Americans, and 4 Hispanics. The sample was medication-free for 37.1 days prior to sleep study. Only 2/48 subjects had undergone detoxification and had received benzodiazepines 3 months prior to sleep study. Three subjects had taken medication within 2 weeks of the laboratory study—one taking diphenhydramine for allergies 6 days prior to study, one taking tramadol for arthritis 11 days before the sleep study, and another who took one propoxyphene/acetaminophen (Darvocet) pill 12 days before night 1.
As expected, the Short Inventory of Problems score, a measure of adverse consequences from drinking,18 was significantly lower in the HC group compared to those who were AD (t62 = 4.8, P < 0.05). Demographic and clinical data are shown in Table 1.
Table 1.
Demographic and clinical data in healthy controls (HC) and alcohol-dependent (AD) group
| HC | AD | |
|---|---|---|
| N | 16 | 48 |
| Number of women (n) | 3 | 9 |
| Age (yrs) | 35.6 (10.6) | 36.4 (10.9) |
| Education (yrs) | 15.5 (2.0) | 13.2 (2.1) |
| Race (n) | 16 C | 33 C, 11 AA, 4H |
| Employed (%) | 68.7 (4.8) | 43.7 (5.0) |
| Short Inventory of Problems | 0.07 (0.27) | 13.2 (10.9) |
| Age of onset of drinking problem | – | 20.7 (9.3) |
| Duration of problem drinking (yrs) | – | 16.1 (11.8) |
| Obsessive-Compulsive Drinking Scale | – | 8.2 (6.2) |
| Past history of MDD (n), (%) | 6 (12.5%) |
Bold font indicates group difference P < 0.05 by t-test. C, Caucasian; AA, African American; H, Hispanic.
Sleep Procedures
Participants kept a 23:00-06:00 schedule for ≥ 5 days prior to lab study, verified by sleep diary and actigraphy. Napping was proscribed and caffeine was restricted to 1 cup equivalent per day, prior to noon. Participants were not taking alcohol or drugs of abuse as confirmed by urine drug screens and breath testing on ≥ 4 occasions during the screening/baseline period, including each night participants slept in the laboratory.
Each subject spent 3 consecutive nights in the UM Sleep ' Chronophysiology Laboratory. Night 1 served as lab adaptation and as a further screening for intrinsic sleep disorders such as sleep apnea and periodic limb movements during sleep. All records from the screening night were reviewed by a board-certified sleep specialist (DC). The second night served as the baseline for sleep EEG data. The sleep schedule was fixed at 23:00-06:00 on Nights 1 and 2. On the third night, bedtime was delayed for 3 h until 02:00, with rise time at 09:00. This procedure kept available sleep time constant at 7 h each night, but still permitted a challenge to SWA regulation.
EEG was recorded from C3 and C4, referenced to the earlobes and connected to a 10-kΩ resistor to minimize nonhomogeneous current flow. The electrode montage also included left and right electro-oculogram (EOG) leads placed on both the upper and lower canthi; a bipolar, chin-cheek electromyography (EMG); leg leads; chest and abdomen respiration bands; and a nasal-oral thermistor. Impedances were maintained < 2 kΩ. EEG was also monitored throughout the 3-h sleep delay period to ensure that no subject fell asleep before 02:00.
All electrophysiological signals were transduced by Vitaport III digital amplifiers with an equivalent sensitivity of 5 (50 μV, 0.5-s duration calibration) corresponding to a gain of 50,000. Filter settings were set at 0.3 and 70 Hz for EEG and 30 and 100 Hz for EOG. All data were digitized at 256 Hz, and digitized signals were displayed in real-time in analog form on a computer monitor external to the Vitaport.
Polysomnographic (PSG) records were scored in 30-sec epochs using standard criteria19 by research personnel trained to better than 90% agreement on an epoch-by-epoch basis. Sleep continuity variables included the total sleep period (TSP) from lights out to morning awakening; latency to persistent sleep (SOL), defined as the time from lights out to the first 10-min block of sleep with ≥ 8 min of any sleep stage; time and percentage of TSP spent awake and/or moving (Awake ' %Awake, respectively); and sleep efficiency (SE), calculated as TSP over total time in bed × 100%. The percentage of TSP spent in stage 1, stage 2, slow wave sleep (SWS, defined as stage 3 + stage 4), and REM sleep (%REM%) was also calculated. REM latency was defined in minutes from sleep onset to the first epoch of REM. REM density, reflecting the number of eye movements in REM was scored on a 0-5 scale.20
PSG data were coded for group and repeated-measures multivariate analysis of covariance (MANCOVA, with age and sex controlled) evaluated differences between HC and AD groups and the effects of sleep delay on PSG characteristics. Univariate analyses were only conducted if an overall significant effect was evident from MANCOVA, following statistical convention.
Quantifying SWA
During visual scoring, epochs were tagged for artifact rejection. Records were inspected visually a second time by RA or RH to ensure that epochs with any movement or electrical artifact, baseline shift, or electrode problems were excluded from analysis. Fewer than 2% of epochs were excluded.
Power spectral analysis (PSA) was performed on digitized EEG signals at both electrode sites. Although the full EEG spectrumwas quantified, primary statistical analyses for the present paper focused on delta (0.5 to < 4 Hz) power from PSA. The PSA algorithm, based on a fast Fourier transform, was taken from Press et al.,21 processing data in 2-s epochs (512 samples for each 2 s) with a Hanning window taper. The PSA generates power (area under the curve) in the delta band (0.5 - 3.9 Hz), expressed as μV2. Delta power was then averaged in 30-s epochs to provide identical epoch lengths to the stage-score data9,22,23
The delta power data were then sorted by NREM period (determined by stage-score data), again separately for each subject on baseline and sleep delay nights to compare SWA between groups. The definition of NREM periods was chosen to match previous studies.9,22,23 NREM periods were defined as the succession of stages 2, 3, or 4 of ≥ 15-min duration and terminated by stage REM or a period of wakefulness of ≥ 5 minutes. Stage 1 sleep epochs were excluded. No minimum REM duration was required for the first or last REM period. Delta power was summed and then averaged relative to the number of epochs in each NREM period, for each subject, henceforth referred to as slow wave activity (SWA).
SWA measures were computed on the baseline night and on the 3-h sleep delay night. The percentage of SWA (%SWA) was also computed as SWA on delay night relative to baseline, to normalize power and control for individual differences across subjects. Data were coded for group (AD or HC) and repeated-measures multivariate analyses of covariance (MANCOVAs) were computed on SWA and %SWA measures, using NREM period as a 4-level repeated measure. Exponential regressions described the time course of SWA in each group. All SWA statistical analyses were conducted using SAS general linear models and regression routines.
Although there were no significant age differences between groups and due to the small number of women included in the study, age and sex were used as statistical covariates for all SWA analyses.
RESULTS
PSG Measures
Group main effects in PSG measures
The means and standard deviations of key PSG measures are shown in Table 2. As can be seen, there were few differences between HC or AD groups. Only REM latency and the minutes of SWS in the first NREM period showed significant group main effects from repeated-measures MANCOVA (F1,60 = 6.1, P < 0.02; F1,60 = 6.7, P < 0.02; respectively). The minutes of SWS in the first NREM period were significantly higher in the HC group than in the AD. REM latency was shorter in the AD group than in HCs.
Table 2.
Means and standard deviations (in brackets) of PSG measures at baseline and following sleep delay
| Healthy Controls (n = 16) |
Alcohol Dependent (n = 48) |
|||
|---|---|---|---|---|
| Baseline | Delay | Baseline | Delay | |
| Total Sleep Period (min) | 412.9 (5.1) | 419.4 (4.1) | 406.1 (18.1) | 405.4 (22.6) |
| Sleep Latency (min)* | 12.1 (4.1) | 4.3 (5.5) | 9.0 (11.9) | 3.8 (3.3) |
| Sleep Efficiency (%) | 95.9 (1.7) | 93.0 (5.9) | 92.5 (6.0) | 92.2 (6.4) |
| Awake and movement (%) | 2.6 (2.4) | 4.3 (3.3) | 4.8 (4.4) | 5.3 (4.1) |
| % Stage 1 | 3.4 (2.4) | 5.1 (4.2) | 7.0 (4.8) | 6.3 (3.7) |
| % Stage 2* | 56.7 (8.0) | 54.1 (7.5) | 54.7 (9.0) | 50.8 (9.1) |
| % SWS | 11.3 (5.1) | 12.2 (4.9) | 9.9 (5.4) | 10.5 (5.5) |
| %REM | 24.0 (4.9) | 24.2 (6.5) | 23.5 (6.4) | 27.2 (6.0) |
| REM Latency | 73.3 (22.0) | 65.8 (12.7) | 65.5 (24.4) | 43.4 (28.5) |
| REM Density | 3.1 (0.9) | 2.9 (0.8) | 2.7 (1.2) | 2.7 (1.2) |
| Minute SWS in 1st NREM* | 18.6 (9.4) | 22.2 (5.2) | 16.1 (9.3) | 13.3 (10.2) |
Bold font denotes significant group main effect;
denotes significant sleep delay main effect; (min) in minutes; % expressed relative to total sleep period.
Sleep delay main effect
With regard to the effects of sleep delay, a significant repeated-measures effect was obtained for sleep latency (F1,60 = 7.1, P < 0.01), the minutes of SWS in the first NREM period (F1,60 = 4.9, P < 0.04), and % stage 2 sleep (F1,60 = 7.9, P < 0.008). Sleep latency was significantly shorter, and stage 2 sleep decreased after sleep delay. The minutes of SWS increased in the first NREM period after sleep delay, an effect that was driven by the HC group, as SWS decreased after sleep delay in the AD group.
Group by sleep delay interactions on PSG
None of the PSG measures showed significant group by sleep delay interactions. Mean differences, shown in Table 2, suggested that the effect of sleep delay on REM latency was larger in the AD group than in HCs, but the effect failed to reach statistical significance, largely due to the variability in REM latency in the AD group.
SWA Measures
Group main effect
The means and standard deviations for SWA power and the latencies and durations of each of the NREM periods are shown in Table 3 by group. A significant overall group main effect was evident for SWA power by MANCOVA (F1,60 = 5.1, P < 0.03), indicating the SWA power was lower overall in the AD group. Univariate analyses confirmed lower baseline SWA power in the AD group compared to the HC group but only in the first NREM period (F1,60 = 6.9, P < 0.02). A significant overall group effect was also evident after sleep delay, with lower SWA power in the AD group in the first (F1,60 = 15.0, P < 0.003) and second (F1,60 = 4.5, P < 0.04) NREM periods.
Table 3.
Means and standard deviations (in brackets) of SWA power averaged across electrode site and the latencies and durations of each of the NREM periods on the baseline and delay nights, by group
| Healthy Controls |
Alcohol-Dependent |
|||
|---|---|---|---|---|
| Baseline | Delay | Baseline | Delay | |
| NREM 1 | ||||
| Latency (min) | 6.9 (3.2) | 9.7 (6.4) | 13.9 (19.1) | 16.8 (14.0) |
| Duration (min) | 69.5 (22.3) | 58.2 (10.5) | 54.4 (23.1) | 57.3 (15.3) |
| SWA Power (μV2) | 375.9 (98.4) | 409.5 (91.2) | 308.3 (89.8) | 310.8 (86.6) |
| NREM 2 | ||||
| Latency (min) | 93.4 (27.3) | 89.6 (19.8) | 90.4 (31.8) | 99.6 (32.8) |
| Duration (min) | 73.1 (17.2) | 62.3 (15.1) | 70.9 (32.0) | 64.0 (20.6) |
| SWA Power (μV2) | 300.0 (66.9) | 333.5 (70.1) | 284.7 (72.5) | 284.5 (85.1) |
| NREM 3 | ||||
| Latency (min) | 197.6 (49.5) | 186.3 (40.9) | 191.0 (54.6) | 199.1 (45.2) |
| Duration (min) | 72.4 (28.6) | 65.2 (42.2) | 65.5 (19.2) | 54.0 (18.4) |
| SWA Power (μV2) | 259.7 (68.3) | 267.8 (52.0) | 246.3 (61.4) | 236.3 (62.5) |
| NREM 4 | ||||
| Latency (min) | 294.2 (49.6) | 287.6 (59.9) | 282.0 (49.7) | 291.5 (46.5) |
| Duration (min) | 55.5 (19.4) | 45.0 (15.4) | 52.4 (15.6) | 48.8 (13.8) |
| SWA Power (μV2) | 232.4 (55.2) | 248.3 (72.7) | 222.9 (50.9) | 229.6 (67.6) |
Latencies defined as minutes from sleep onset to the beginning of each NREM period. Italic font denotes significant group effect at baseline; bold font denotes significant group effect after delay.
Sleep delay main effect
A sleep delay main effect was obtained for the duration of the individual NREM periods (F1,56 = 7.1, P < 0.01). Overall, the durations of NREM periods were shorter after sleep delay, as seen in Table 3.
The latencies to each NREM period did not show sleep delay main effects or interactions and thus no further analyses were conducted on these measures.
Sleep delay by group interactions
SWA power did show a significant overall repeated-measures sleep delay by group interaction (F1,60 = 4.0, P < 0.05), indicating a smaller change in SWA power in response to challenge in the AD group. A significant repeated-measures NREM period by group interaction (F1,60 = 9.1, P < 0.0002) was also obtained, indicating that the effect of sleep delay on SWA was greater in the first REM period than in subsequent NREM periods and was smaller in the AD group overall (See Table 3).
Exponential regression analysis
To describe this group difference in the time course of SWA, we also computed parameters for exponential regressions (i.e., asymptotes and decay rates). The resultant means and confidence intervals for these regression parameters are shown in Table 4. For comparisons between groups, the SWA parameter estimates in the AD group were compared to the 95% confidence intervals (CI) of the HC group. For comparisons of within-group sleep delay effects, the SWA parameter estimates are compared against the 95% CI at baseline. As seen in Table 4, asymptotic SWA was significantly lower and the rate of decay significantly slower in the AD group than in the HC group, both at baseline and following sleep delay. The SWA parameter estimate for the AD group at baseline was 333.9 with a decay rate of 29.4, outside the 95% CI of the HC group. Following sleep delay, asymptotic SWA was 327.1 in the AD group, with a decay rate of −29.2, also outside the 95% CI for both parameters in the HC group. Moreover, there was a significant increase in asymptotic SWA within the HC group during sleep delay. Asymptotic SWA was 479.8, outside their baseline 95% CI of the baseline SWA parameter. The AD group did not show a higher SWA asymptote after sleep delay, falling within their baseline confidence interval. Neither group showed a significant sleep delay effect on the regression decay parameter. Thus, the decay of SWA across NREM sleep did not differ after the sleep challenge.
Table 4.
Exponential regression parameters and 95% confidence intervals (CI) on baseline and delay nights by group
| Baseline |
Delay |
|||||||
|---|---|---|---|---|---|---|---|---|
| SWA | 95% CI | Decay | 95% CI | SWA | 95% CI | Decay | 95% CI | |
| HC | 416.4 | 361.5 to 458.7 | −47.5 | −65.3 to −29.7 | 479.8 | 408.4 to 511.7 | −57.9 | −76.8 to −39.1 |
| AD | 333.9 | 314.9 to 363.6 | −29.4 | −38.3 to −20.6 | 327.1 | 311.7 to 364.9 | −29.2 | −38.9 to −19.5 |
Italics denote significant group difference; bold font denotes significant delay effect within group.
Relative SWA measures (%SWA)
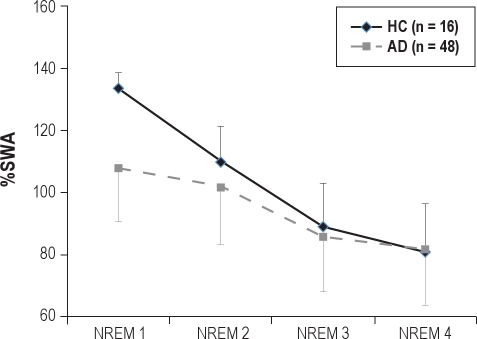
To ensure that individual differences in SWA power did not mask the sleep delay effects or influence between group differences, we also conducted a MANCOVA on %SWA, computed as SWA on the delay night expressed relative to baseline SWA (i.e., normalized within individuals). Figure 1 presents the %SWA data. Confirming what was observed without normalizing SWA, the AD group showed a lower accumulation of SWA on the sleep delay night with a slower dissipation across NREM sleep time. MANCOVA revealed a signifi-cant NREM period effect (F3,168 = 4.5, P < 0.005) and a significant NREM period by group interaction (F3,168 = 4.4 P < 0.005). Univariate analysis confirmed lower accumulation of %SWA in the AD group, restricted to the first NREM period (F1,60 = 12.9, P < 0.001).
Figure 1.
SWA power after sleep delay, expressed relative to baseline SWA by group. Amounts > 100 indicate an enhancement over baseline.
DISCUSSION
The major finding in this study was that homeostatic regulation of SWA was impaired in the AD group. SWA was lower than HCs at baseline and failed to increase in response to sleep delay. Results from the exponential regression analysis or SWA changes across NREM periods were in agreement; the AD group showed both reduced homeostatic drive, reflected in a lower initial accumulation of SWA after sleep onset and reduced homeostatic recovery, reflected in a slower dissipation of SWA over the night, in response to sleep delay. Moreover, the group differences were evident in both the raw SWA power and %SWA, normalized for individual differences, and whether MANCOVA models or exponential regressions were used.
Blunted SWA homeostasis has been associated with both a failure to generate SWA through thalamic cortical circuitry in the brain and a failure to propagate across the scalp.8–10 Both of these mechanisms could reflect a reduction in neural synchronization. It is not clear, however, whether a vulnerability in thalamic cortical circuitry was antecedent to alcohol dependence, or emerged as a consequence of alcohol exposure. Studies assessing sleep regulation in individuals at high risk for alcohol dependence, such as those with a positive parental history, may help identify whether blunted homeostasis is a risk factor or a consequence of alcohol dependence.
Alternatively, the sleep delay procedure may not have been of sufficient strength to elicit a response in the AD group. If this was the case, it would suggest that homeostatic response could be increased with a stronger challenge. However, shorter sleep latency and REM latency were evident in the AD group after sleep delay suggesting that the procedure did have an effect on sleep, but did not provoke a change in SWA. Since the SWA response in the HC group was consistent with that reported elsewhere,9,10,22,23 it does not appear that the blunted SWA response was due to insufficient strength of the sleep challenge. What's more, Irwin and colleagues have shown that a 4-hour partial sleep deprivation protocol also resulted in a lower ratio of SWA power in the first to second NREM period, consistent with our finding of lower initial accumulation of SWA following sleep delay.5 Thus, the findings reported here are consistent with both impaired homeostatic drive and homeostatic recovery in AD.
Impaired sleep homeostatic response to a 3-hour sleep delay has also been reported in major depressive disorders, but only in men.8,9,22,23 Women with MDD do not show evidence of reduced homeostatic drive or recovery in response to sleep challenge.9,22,23 In fact, women with MDD show a larger response to sleep challenge, prompting several groups to suggest that they are under higher homeostatic pressure than healthy women, even in the absence of a sleep challenge.9,22–25
There were more men than women in the present study and as such, our finding of impaired homeostasis may be more of a characteristic of men than women with AD, as reported among those with depression. Although none of the AD subjects currently met criteria for MDD, based on a structured clinical interview, a past history of depression was evident in 6/48 participants in the AD group (all men) and we cannot rule out that past MDD contributed to the blunted homeostatic response. Thus, it seems that impaired sleep homeostasis is not specific to MDD, but is also evident in AD, albeit predominantly among men.
While there has been historical interest in establishing sleep measures as unique markers of psychiatric illness,26 most notably from the perspective of REM sleep regulation, more recent viewpoints recognize the overlap in symptoms and biological markers across diagnostic groups and the role that sleep loss plays in risk for multiple diseases. A recent PubMed search revealed more than 100 papers on sleep disturbances and immune and cardiovascular disease, pain syndromes, chronic fatigue, and diabetes just in the past 12 months. In our view, impaired sleep homeostasis need not be specific to one disorder to be of clinical relevance, and likely reflects the ubiquitous roles that sleep plays in illness and wellness. Similarly, impaired SWA homeostasis may relate to clinical course across a number of clinical conditions with worse outcomes in those with the smallest response to a sleep challenge. This relationship will be addressed in our future studies.
This study did not indicate robust differences in PSG measures between those with AD and HCs in contrast to several review articles27 and to some previous original research.5,6,28 Compared to those reports, however, participants in this study were on average 5-15 years younger, and recruited from the community rather than from inpatient treatment facilities, and had less severe and chronic AD than those reported in Gann et al.28 and Irwin et al.5 Moreover, neither Colrain et al.6 nor Irwin et al.5 found differences between AD and HC participants in PSG-measured sleep continuity variables. Therefore, at least some of our PSG findings are in line with previous work and suggest that our AD population was not unusual. Further, recent studies of other clinical populations such as those with MDD also report fewer PSG differences between patients and controls when groups are matched on age and sex.9 Perhaps study designs are more rigorous or sleep schedules prior to lab studies are better controlled in more recent studies and have reduced the degree of PSG-measured sleep problems in clinical groups. Alternatively, abstinent alcoholics from the community may have less severe PSG disturbances. It is also noteworthy that the present study had fixed and regularized bed and rise times of 23:00-06:00. Subjects who deviated more than 1 hour from this schedule over the week prior to lab study were excluded from study. This strict adherence to a fixed sleep schedule may have contributed to improved sleep in the AD group.
The present study is also limited in that it does not address whether women with AD also specifically show impaired homeostasis. It could not be addressed here because of the small number of women included. The distribution of male and female participants matched the clinic flow and the greater incidence of AD in men. We did use sex (and age) as statistical covariates for all analyses, but we recognize the need to address potential sex differences in this and other clinical disorders, and that sufficient numbers of women will need to be included to assess whether SWA homeostasis is sex-dependent in AD as it is in MDD.
Finally, we have shown that abstinent alcohol-dependent adults show a blunted response to a SWA homeostatic challenge. We have not addressed the clinical relevance of this finding. For example, it is not clear if blunted SWA and an abnormal time course relate to relapse to drinking or whether low SWA precedes alcohol dependence and represents a biological risk factor for onset of drinking problems. However, there is evidence that children of alcoholics have lower NREM delta power than control children,29 suggesting that some aspects of impaired SWA may be antecedent to the onset of AD. Further, Colrain et al.6 reported decreased SWA in alcoholics relative to controls without any effect of sobriety duration, which ranged from 30 to 719 days. With regard to SWS, decreases have been shown to persist in alcoholics for 16 weeks after their last week and not recover until 14 and 27 months.30 In addition, SWA power and homeostasis may not normalize in those with the earliest ethanol exposure, since this has been associated with relatively permanent changes in EEG.31 A longitudinal follow-up of those with AD and their families is necessary to fully address these questions.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Armitage has consulted for Eisai Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by NIH Grants R01 AA016117, K24 AA00304.
REFERENCES
- 1.Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–39. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- 2.Krystal AD, Erman M, Zammit GK, Soubrane C, Roth T, Group ZS. Long-term efficacy and safety of Zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep. 2008;31:79–90. doi: 10.1093/sleep/31.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landolt HP, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs. 2001;15:413–25. doi: 10.2165/00023210-200115050-00006. [DOI] [PubMed] [Google Scholar]
- 4.Irwin M, Miller C, Gillin JC, Demodena A, Ehlers CL. Polysomnographic and spectral sleep EEG in primary alcoholics: an interaction between alcohol dependence and African-American ethnicity. Alcohol Clin Exp Res. 2000;24:1376–84. [PubMed] [Google Scholar]
- 5.Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry. 2002;51:632–41. doi: 10.1016/s0006-3223(01)01304-x. [DOI] [PubMed] [Google Scholar]
- 6.Colrain IM, Turlington S, Baker FC. Impact of alcoholism on sleep architecture and EEG power spectra in men and women. Sleep. 2009;31:1342–52. doi: 10.1093/sleep/32.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feige B, Scaal S, Hornyak M, Gann H, Riemann D. Sleep electroencephalographic spectral power after withdrawal from alcohol in alcohol-dependent patients. Alcohol Clin Exp Res. 2007;31:19–27. doi: 10.1111/j.1530-0277.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 8.Brower KJ, Hoffmann R, Conroy DA, Arnedt JT, Armitage R. Sleep homeostasis in alcohol-dependent, depressed and healthy control men. Eur Arch Psychiatry Clin Neurosci. 2011 Feb 11; doi: 10.1007/s00406-011-0195-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armitage R. Sleep and circadian rhythms in mood disorders. Acta Neurol Scand Suppl. 2007;115:104–15. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 10.Armitage R, Landis C, Hoffmann R, Lentz M, Watson N, Goldberg J, et al. The impact of 4-hour sleep delay on slow-wave activity in twins discordant for chronic fatigue syndrome. Sleep. 2007;30:645–50. doi: 10.1093/sleep/30.5.657. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Washington, DC: American Psychiatric Association; 2000. DSM-IV-TR: Diagnostic and statistical manual of mental disorders, 4th ed., text revision. [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, Williams JBW, editors. User's Guide. Washington, DC: American Psychiatric Press; 1997. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version. [Google Scholar]
- 13.Kranzler HR, Kadden RM, Babor TF, Tennen H, Rounsaville BJ. Validity of the SCID in substance abuse patients. Addiction. 1996;91:859–68. [PubMed] [Google Scholar]
- 14.Maisto SA, Sobell MB, Cooper AM, Sobell LC. Test-retest reliability of retrospective self-reports in three populations of alcohol abusers. J Behav Assess. 1979;1:315–26. [Google Scholar]
- 15.Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behav Res Ther. 1979;17:157–60. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- 16.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: Assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 17.Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–9. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 18.Feinn R, Tennen H, Kranzler HR. Psychometric properties of the short index of problems as a measure of recent alcohol-related problems. Alcohol Clin Exp Res. 2003;27:1436–41. doi: 10.1097/01.ALC.0000087582.44674.AF. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 20.Armitage R, Rush AJ, Trivedi M, Cain J, Roffwarg HP. The effects of nefazodone on sleep architecture in depression. Neuropsychopharmacology. 1994;10:123–7. doi: 10.1038/npp.1994.14. [DOI] [PubMed] [Google Scholar]
- 21.Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical recipes in Pascal. New York: Cambridge University Press; 1989. [Google Scholar]
- 22.Armitage R, Hoffmann R, Trivedi M, Rush AJ. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000;95:201–13. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- 23.Armitage R, Hoffmann R, Fitch T, Trivedi M, Rush AJ. Temporal characteristics of delta activity during NREM sleep in depressed outpatients and healthy adults: group and sex effects. Sleep. 2000;23:607–17. [PubMed] [Google Scholar]
- 24.Armitage R, Hoffmann R. Sleep EEG, depression and gender. Sleep Med Rev. 2001;5:237–46. doi: 10.1053/smrv.2000.0144. [DOI] [PubMed] [Google Scholar]
- 25.Frey S, Birchler Pedross A, Bruner P, Gotz T, Knoblauch V, Cajochen C. Women with major depression live under higher homeostatic sleep pressure. Presented at the European Sleep Research Society Meeting; Sept 14-18,2010; Lisbon, Portugal. [Google Scholar]
- 26.Giles DE, Roffwarg HP, Rush AJ, Guzick DS. Age-adjusted threshold values for reduced REM latency in unipolar depression using ROC analysis. Biol Psychiatry. 1990;27:841–53. doi: 10.1016/0006-3223(90)90465-e. [DOI] [PubMed] [Google Scholar]
- 27.Benca RM, Obermeyer WH, Thisted RA Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 28.Gann H, Feige B, Hohagen F, van Calker D, Geiss D, Dieter R. Sleep and thc cholinergic rapid eye movement sleep induction test in patients with primary alcohol dependence. Biol Psychiatry. 2001;50:383–90. doi: 10.1016/s0006-3223(01)01172-6. [DOI] [PubMed] [Google Scholar]
- 29.Tarkoh L, Carskadon M. Sleep electroencephalogram in children with a parental history of alcohol abuse/dependence. J Sleep Res. 2010;19:165–74. doi: 10.1111/j.1365-2869.2009.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drummond S, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–802. [PubMed] [Google Scholar]
- 31.Ehlers CL, Criado JR. Adolescent ethanol exposure: does it produce long-lasting electrophysiological effects? Alcohol. 2010;44:27–37. doi: 10.1016/j.alcohol.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]