Abstract
Study Objectives:
Sleep quality commonly diminishes with age, and, further, aging men often exhibit a wider range of sleep pathologies than women. We used a freely available, web-based discovery technique (Semantic MEDLINE) supported by semantic relationships to automatically extract information from MEDLINE titles and abstracts.
Design:
We assumed that testosterone is associated with sleep (the A-C relationship in the paradigm) and looked for a mechanism to explain this association (B explanatory link) as a potential or partial mechanism underpinning the etiology of eroded sleep quality in aging men.
Measurements and Results:
Review of full-text papers in critical nodes discovered in this manner resulted in the proposal that testosterone enhances sleep by inhibiting cortisol. Using this discovery method, we posit, and could confirm as a novel hypothesis, cortisol as part of a mechanistic link elucidating the observed correlation between decreased testosterone in aging men and diminished sleep quality.
Conclusions:
This approach is publically available and useful not only in this manner but also to generate from the literature alternative explanatory models for observed experimental results.
Citation:
Miller CM; Rindflesch TC; Fiszman M; Hristovski D; Shin D; Rosemblat G; Zhang H; Strohl KP. A closed literature-based discovery technique finds a mechanistic link between hypogonadism and diminished sleep quality in aging men. SLEEP 2012;35(2):279-285.
Keywords: Information technology, literature-based discovery, testosterone, cortisol, sleep, men
INTRODUCTION
Primary insomnia is particularly common and is often co-morbid with aging-related disorders (e.g., depression, arthritis, cancer).1 Changes in sleep due to the normal physiology of aging in healthy adults include more frequent awakenings, shorter total sleep duration, and decreased REM sleep, among others.2 Clinical lore suggests that hormonal changes are responsible for a shorter sleep duration, but no mechanism is proposed.3
Since later-life onset of hypogonadism is common4 and middle-aged men secrete less testosterone at night than young healthy men,5 the relationships of aging and sleep in men might be informed by examination of the non-sleep literature. To this end, we utilized a database discovery method—never before used in sleep medicine—to comprehensively examine the literature and uncover mechanisms underpinning the etiology of diminished sleep quality in aging men. Such approaches are based on the premise that a literature review by any one person or group cannot survey the entire published literature and, more importantly, is subject to bias, fatigue, and failure to comprehensively determine if equipoise exists for one or more relationships. The information collected or “computable knowledge” obtained by these methods is used to probe for linkages or test hypotheses given that a reference literature and unknown causality exist, as is the case here. We concentrated on references to normal physiologic changes, not pathology, and used literature-based discovery techniques6 enhanced with semantic predications extracted from journal titles and abstracts.7,8 The result was a testable hypothesis that cortisol is a mechanistic link connecting declining testosterone levels to age-related alterations in sleep behavior in men.
BACKGROUND
Testosterone and Sleep
There is some indication that testosterone affects sleep physiology. For example, plasma testosterone concentrations correlate with REM-NREM cycles,9 and reproductive hormones, including testosterone, affect the ability of stress to alter sleep in mice.10 Sleep deprivation has been associated with lowered testosterone levels,11 and sleep fragmentation impairs testosterone production.12 Additionally, decreased sleep duration has been correlated with androgen concentrations in aging men.13
Semantic Predications
The specific approach in this study exploits predications (formal representations of textual content) extracted from MEDLINE titles and abstracts by the SemRep software application.7 For example, SemRep extracts the predication “Sulfones treats Sleep Disorders” from the following title of a citation (PMID:20493697) “Non-basic ligands for aminergic GPCRs: the discovery and development diaryl sulfones as selective, orally bioavailable 5-HT2A receptor antagonists for the treatment of sleep disorders.”
SemRep predications have a subject argument (“Sulfones”), a predicate (“TREATS”), and an object argument (“Sleep Disorders”). Arguments are concepts from the Unified Medical Language System (UMLS) Metathesaurus,14 and the predicate is from the Semantic Network.15 Such predications provide a normalized representation of part of the meaning of the text from which they were extracted and serve as “computable knowledge” supporting further processing. SemRep has been used to extract 24,405,702 semantic predications from 7,131,316 MEDLINE citations (titles and abstracts) ranging in date from January 1, 1999 through June 30, 2010. These are stored in an SQL database and made available for applications.
Semantic MEDLINE16 is a Web-based application that manages the results of PubMed searches by visualizing SemRep predications extracted from MEDLINE citations. Predications are presented to the user as an interactive graph in which the nodes represent arguments and the arcs predicates which are color coded. For example yellow is used for disrupts, green for affects, magenta for interacts_with, and blue for treats. Hyperlinks are maintained between predications and citations.
Literature-Based Discovery
Literature-based discovery6 (LBD) is a methodology for uncovering previously unnoticed relationships in the published research literature, thereby providing insight into some phenomenon. LBD can be implemented according to either of two different paradigms. In open discovery, the paradigm is articulated with concepts A, B, and C, where a relationship between A and B (A-B) is known, as is a relationship between B and C (B-C), but the relationship between A and C (A-C) has gone unnoticed. Based on the known relationships, the unknown A-C is inferred (or “discovered”). Closed discovery17 is used to explicate an observed relationship. Processing is implemented by assuming A-C; relationships A-B and B-C are then sought to provide B as a mechanistic link between A and C. In exploiting the closed discovery paradigm, we assumed a relationship between testosterone and sleep (A-C) and sought a B mechanism to connect them (cortisol).
Swanson implemented relationships as words and phrases cooccurring in documents. Subsequent research developed the implementation of Swanson's ideas17 based on concepts (e.g., Weeber et al.18). Hristovski et al.8 introduced the use of semantic predications in LBD. We exploit semantic predications and the Semantic MEDLINE application to pursue a method that involves user interaction to develop a hypothesis incrementally and iteratively.
METHODS
There are two parts to the methodology: (a) using Semantic MEDLINE to gain insight into testosterone and sleep generally, and, guided by the first part, (b) getting predications on testosterone and cortisol specifically. The first part involved significant user involvement. A series of queries was issued to PubMed in order to identify MEDLINE citations likely to give insight into the relationship between testosterone and sleep. All queries were limited by date (01/01/1999 through 03/31/2009). An initial, general query (“hormones AND sleep”) was followed by one specific for testosterone (“testosterone AND sleep”). These were followed by two focused queries: “steroid hormones AND sleep architecture” and “testosterone AND circadian rhythms.” For each query, predications extracted from the citations retrieved were presented by Semantic MEDLINE as a graph with links to the relevant text. Each graph was then visually inspected for predications involving testosterone and sleep. The full-text articles of the citations from which such predications had been extracted were then examined to determine their contribution to the developing hypothesis. The number of such citations was considerably less than the number retrieved by the original PubMed search.
This first step served as a guide to the published research literature regarding the relationship between testosterone and cortisol: We next extracted all predications from the SemRep predication database involving these two substances. This extraction was not limited to citations involving sleep. We issued a query to the database that maximized the likelihood of finding papers that involved the interaction of these two substances, retrieving all predications in which either argument was either the UMLS concept “Testosterone or “Hydrocortisone” (the UMLS preferred name for cortisol). The predicate was specified as being either inhibits, stimulates, or interacts_with. All citations containing returned predications were examined. This step evaluates the strength of the data outside the field of sleep that might support or refute a hypothesis.
RESULTS
Semantic MEDLINE
Results for each query
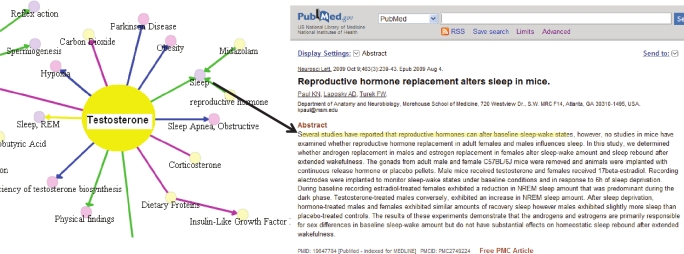
Semantic MEDLINE16 manages the results of PubMed searches by visualizing SemRep predications extracted from MEDLINE citations. Predications are presented to the user as an interactive graph in which the nodes represent arguments and the connecting arcs predicates which are color coded. Figure 1 shows a screen shot from this web-based application for 206 citations retrieved with the PubMed query “testosterone AND sleep.” Online, semantic MEDLINE helps users navigate through core information contained in retrieved citations.
Figure 1.
Semantic MEDLINE representation for testosterone, showing a citation from which the predication “reproductive hormone affects Sleep” was extracted. Online, this is an interactive graph in which the nodes represent arguments, and the arcs, predicates which are color coded. Yellow is used for disrupts, green for affects, magenta for interacts with, and blue for treats. Links are maintained between predications and citations. The black arrow links the predication “reproductive hormone affects sleep” to the sentence (highlighted) in a citation from which it was extracted.
The number of citations (Cits) returned with all queries are given in Table 1, along with the number of predications extracted from those citations (Preds). Predications were displayed in separate graphs for each query by Semantic MEDLINE, and visual inspection of these resulted in seven “interesting” predications extracted from nine citations.
Table 1.
Semantic MEDLINE results
| Query | Cits | Preds | Useful Predications (Citations) |
|---|---|---|---|
| hormones AND sleep | 1000 | 222 | Reproductive Hormones affects Sleep (2) Testosterone affects Sleep (1) |
| testosterone AND sleep | 171 | 54 | Testosterone affects Sleep (2) Corticosterone interacts_with Testosterone (1) |
| sleep architecture AND steroid hormones | 33 | 22 | Testosterone associated_with Sleep Disturbances (1) Steroids affects Sleep (1) |
| testosterone AND circadian rhythms | 280 | 404 | Testosterone coexists_with Corticosterone (1) |
| 4 queries | 1484 | 702 | 7 predications (9 citations) |
The full-text articles for these citations were retrieved and used to develop a hypothesis about the relationship between testosterone and sleep.
Information determined from citations
Two lines of information from the citations found with Semantic MEDLINE graphs were: (a) the relationships between testosterone and sleep and (b) interactions of testosterone and cortisol in general or specifically for sleep in aging men.
Relationship between testosterone and sleep:
One can immediately notice citations providing evidence for a bidirectional relationship between testosterone and sleep. In their review, Andersen and Tufik19 report that testosterone levels are sleep related in that they begin to increase at first REM onset, and peaks correlate with subsequent REM periods. Further, low testosterone levels are associated with decreased sleep efficiency and fewer REM episodes (predication “Testosterone affects Sleep”). Other identified studies emphasize the effect of sleep on testosterone (as opposed to a strictly circadian controller). Axelsson et al.20 report that peak testosterone production occurs during sleep, even in acute phase shift (“Testosterone AFFECTS Sleep”).
Several citations retrieved suggest that hormones (including testosterone) have an effect on sleep. In an early review,21 Empson and Purdie report that testosterone secretion is at the maximum shortly before awakening. They comment in general on the papers they cite: “It is clear that sex steroids are all implicated in sleep and thermoregulation, although we cannot as yet define their precise roles.” (“Steroids AFFECTS Sleep”). Paul et al.22 review studies that also suggest that sex hormones influence sleep and may correlate with differing sleep disorders in males and females; they do not discuss a mechanism (“Reproductive Hormones affects Sleep”). In further evidence of the influence of testosterone on sleep, Barrett-Connor et al.23 found that low testosterone levels are associated with poor sleep efficiency, increased nocturnal waking, and less slow wave sleep (“Testosterone associated_with Sleep Disturbances”). In an animal study,24 Paul, Laposky, and Turek found that testosterone-treated castrated male mice exhibit an increase in NREM sleep amount (“Reproductive Hormones affects Sleep”). Finally, Liu et al.25 report that high dose, exogenous testosterone replacement therapy shortens sleep and worsens sleep apnea (“Testosterone affects Sleep”).
Relationship between testosterone and cortisol:
Two papers discuss the reciprocal relationship between testosterone and cortisol, one in the context of sleep and one not in the context of sleep. Andersen et al.26 report that diminished testosterone is correlated with increased corticosteroids in rats, although they do not posit a direct connection: “We found a correlation between testosterone and corticosterone during SD [sleep deprivation] r = −051; P < 0.01).” They further state that “Testosterone and estrone decreased, whereas progesterone, prolactin, corticosterone, ACTH, DA and NOR increased.” (“Corticosterone interacts_with Testosterone”). Waite et al.27 report on the inhibitory effect of HPA products on reproductive function: “Castrated rats exhibit a feminized pattern of corticosterone secretion with increased pulse number, amplitude and frequency (1), and this effect is reversible by dihydrotestosterone treatment (2).” They do not refer to sleep (“Testosterone coexists_with Corticosterone”). Based on the reciprocal relationship of testosterone and cortisol26 and the inhibitory effect of testosterone on cortisol,27 we examined the predications extracted from the entire SemRep predication database for further information on a mechanistic relationship between testosterone and cortisol in the context of sleep.
Database of Semantic Predications
The follow-up query to the database retrieved 16 predications linking testosterone and cortisol (Table 2). We examined the citations from which each of these predications had been extracted. We also pursued references in these citations. Predication accuracy (as reflecting the meaning of the sentence) did not necessarily correlate with usefulness of the citation for this study.
Table 2.
Predications extracted from the database
| Predication type | Instances | Correct |
|---|---|---|
| Hydrocortisone inhibits Testosterone | 8 | 6 |
| Hydrocortisone stimulates Testosterone | 2 | 0 |
| Testosterone inhibits Hydrocortisone | 4 | 1 |
| Testosterone stimulates Hydrocortisone | 2 | 0 |
Eight citations found with predications from the database were relevant to this study. From them we gathered further general support for the reciprocal relationship between testosterone and cortisol. In addition, we determined specifically that testosterone inhibits cortisol, thus providing a mechanistic link between the two in the context of sleep.
Considerable research indicates that testosterone and cortisol are in inverse proportion, and many papers report decreased testosterone and increased cortisol. Gitau et al.28 state that in humans “Unlike the norm in adults, where testosterone production is often inhibited by cortisol, fetal plasma testosterone shows a positive link with cortisol.” (“Hydrocortisone inhibits Testosterone” [Predication correct]). They cite papers that indicate increased cortisol and decreased testosterone (without necessarily giving a mechanistic connection). For example, Cumming et al.29 [referred to in Gitau et al.28] report that the acute activation of the ACTH-adrenal axis by administration of hydrocortisone results in a decrease in circulating testosterone. Several studies found with predications from the database discuss the relationships between cortisol and testosterone in pathology. Cernak et al.30 report that two days post trauma, testosterone was decreased and cortisol was increased (“Testosterone inhibits Hydrocortisone” [Predication incorrect]). In Couvade syndrome, Storey et al.31 found that men and women had higher concentrations of prolactin and cortisol in the period just before the birth and lower postnatal concentrations of sex steroids (testosterone or estradiol) (“Hydrocortisone inhibits Testosterone” [Predication correct]). Tuberculosis patients show increased cortisol and profoundly decreased testosterone32 (referred to in Botasso et al.33) (“Hydrocortisone inhibits Testosterone” [Predication correct]). In an animal study, Medhamurthy et al.34 found that the stress of short-term fasting leads to an increase in cortisol levels and a corresponding decrease in testosterone secretion in the bonnet monkey (“Testosterone stimulates Hydrocortisone” [Predication incorrect]).
Two papers emphasize concurrent increased testosterone and decreased cortisol levels. Kraemer et al.35 report on an increase in total testosterone in response to exercise stress along with significant decreases in resting cortisol (“Testosterone inhibits Hydrocortisone” [Predication incorrect]). Bosco et al.36 report that whole body vibration has an effect similar to explosive power training: increased testosterone and decreased cortisol (“Hydrocortisone stimulates Testosterone” [Predication incorrect]).
The previous papers do not commit to a mechanistic relationship between testosterone and cortisol. However, Rubinow et al.37 claim that testosterone has a significant suppressive effect on cortisol (“Testosterone inhibits Hydrocortisone” [Predication correct]), but do not discuss this relationship in the context of sleep.
Originality of the hypothesis
In order to determine whether our hypothesis had previously been proposed, we conducted a high-recall (sensitivity) search in MEDLINE generally and in addition searched the sleep literature specifically. The PubMed query was constructed for high recall and conducted on June 30, 2010: testosterone AND sleep AND (cortisone OR cortisol OR hydrocortisone OR corticosterone OR glucocorticoid OR corticoid OR corticosteroid OR glucocorticosteroids OR “corticotropin releasing hormone” OR “adrenocorticotropic hormone”). This query returned 136 citations, and we examined all of them. None stated that testosterone affects sleep by inhibiting cortisol (or related HPA phenomena).
There are ten journals indexed in MEDLINE with “sleep” as part of their title. PubMed queries on testosterone were issued on July 27, 2010, and limited to each of these journals: (< journal title > [source] AND testosterone). Nineteen citations were returned, 13 of which occurred in one journal (Sleep).
Four of these journals have no papers mentioning testosterone: Behavioral Sleep Medicine, Open Sleep Journal, Sleep and Biological Rhythms, and Sleep Medicine Clinics. Four journals have one paper each in which testosterone is mentioned. However, only one of the papers is relevant to this study, and it had already been found with our method.19 Three papers mentioned testosterone but concentrated on other issues: meno-pause (Journal of Clinical Sleep Medicine), sleep apnea (Sleep & Breathing), and erectile dysfunction (Sleep Medicine). One journal, Journal of Sleep Research, has two papers that mention testosterone; one was found with our method26 (and the other is on electromagnetic fields). The journal Sleep has 13 papers that mention testosterone. Nine of these focus on topics other than how testosterone affects sleep in aging men (e.g., sleep apnea, women, and impotence). Two papers report that sleep affects testosterone,38,39 a point we already make. One paper40 is a comment on a review paper on gender differences in insomnia,41 which cites several studies already discovered in the graphs suggesting the influence of sex hormones on sleep, and points out the need for further research in this area. Finally, one paper42 addresses melatonin in hypogonadal men. It cites a paper43 which investigated the effect of testosterone on sleep without reference to cortisol, but is relevant as potential counterevidence (as discussed below).
DISCUSSION
Use of literature-based discovery tools is a process that identifies the major papers on a question and can be constructed to document information to support a causal pathway previously not uncovered or proposed. This approach has not been used in sleep medicine or in many other fields, as it is so new. In this demonstration a novel phenomenon, namely an inhibitory effect of testosterone on cortisol, is offered as an explanation for diminished sleep quality in aging men. A corollary would be that in men, a certain amount of testosterone may be needed to counteract the wakefulness-promoting aspect of cortisol. Thus, as testosterone levels decline in aging men, cortisol levels increase and sleep quality is diminished.
Cortisol as a Mechanistic Link between Testosterone and Sleep
Cortisol secretion has long been associated with sleep.44 Cortisol impairs sleep and promotes wakefulness and has been shown to increase EEG frequency, which is correlated with increased wakefulness and/or shallower sleep stage. Otte et al.45 report that serum cortisol level is inversely related to slow wave sleep. There is an increase in serum cortisol levels soon after awakening, called the cortisol awakening response.46 Chang and Opp47 demonstrate that when the action of corticotrophin-releasing hormone is blocked using antisense oligonucleotides, spontaneous waking is reduced in rats. Buckley and Schatzberg48 review the extensive evidence that HPA activation disrupts sleep and promotes wakefulness.
Further, it is known that cortisol increases with age and that this is related to diminished sleep quality in aging. Cortisol levels are elevated in aging in both men and women.49 Cortisol impairs sleep in aging,50 and middle-aged (37-54 y) men are more sensitive to HPA effects.51 Increased cortisol levels are seen in aging (> 50) men, along with reduced amounts of REM.52
Potential counterevidence
While the hypothesis seems to be an original one based on a forward and backward inquiry of the database, our method also identified two papers which appeared to report potential counterevidence. One43 asserts that testosterone has minimal effect on sleep, while the other53 reports that testosterone does not affect nocturnal cortisol secretion in healthy older men. We suggest mitigating considerations.
Leibenluft et al.43 chemically induced hypogonadism in young men for three months. Subjects were then administered either exogenous testosterone or placebo for an additional month, and sleep was compared in the two groups. The authors state, “However, pharmacologically induced hypogonadism and testosterone replacement did not have significant effects on melatonin or TSH secretion or on the timing or duration of sleep.” (p. 3207) Nonetheless, their results show significant increase in length of NREM 4, which is consistent with results of Wauquier et al.,54 who report that aging men (with presumably diminished testosterone) have less NREM 4. We further note that artificial hypogonadism induced in young men for three months may not share crucial similarities with long-term hypogonadism in aging men.
Muniyappa et al.53 administered exogenous testosterone to healthy older men for 26 weeks and report that nocturnal cortisol secretion was not significantly altered. They did not study sleep characteristics and reported cortisol measurements taken at 8:00 am only. The cortisol diurnal secretion pattern is complicated.55–58 We have not made a commitment to which particular aspect of that pattern interacts with testosterone regarding sleep. Further, there is evidence to show that testosterone inhibits cortisol under some conditions,37 and our hypothesis is neutral as to whether testosterone interacts with cortisol in the context of sleep by inhibiting its secretion or blocking its affect. Thus, this discovery platform can be used to support equipoise in the hypothesis and provide lessons for the design of future experimental approaches.
Implications
This methodology contributes to biomedical research by generating hypotheses to chart the course of future experimentation. Furthermore, extraction of semantic predications will identify mechanisms from several different models, including studies which may not be obvious, and evidence useful in the testing of a hypothesis. In the context of this study, feasible research can be designed to examine testosterone's interaction with cortisol as part of the mechanism regarding sleep in aging men. Such studies might investigate substances known to interact with testosterone or with cortisol for their potential effect on sleep; notable in this regard are the cytokines,59 nitric oxide,60 and melatonin.42
CONCLUSION
The utility of a freely available, web-based discovery method was demonstrated to explicate the fact that aging men often suffer diminished sleep quality, particularly decreased total sleep duration and increased frequency of nocturnal awakenings. We exploited a literature-based paradigm as it is well known that serum testosterone levels decrease with aging. We assumed that testosterone is associated with sleep (the A-C relationship in the paradigm) and looked for a mechanism to explain the association (B explanatory link). In addition to finding additional support for the connection between testosterone and sleep, we found research evidence indicating that testosterone inhibits cortisol. We consider these findings important given the context of known facts about cortisol—that it promotes wakefulness, increases with age, and impairs sleep in the elderly. Based on this novel approach we posit cortisol as part of a mechanistic link elucidating the observed correlation between decreased testosterone in aging men and diminished sleep quality.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported in part by the Intramural Research Program of the National Institutes of Health, National Library of Medicine. The seventh author was supported by an appointment to the National Library of Medicine Research Participation Program administered by the Oak Ridge Institute for Science and Education through an inter-agency agreement between the U.S. Department of Energy and the National Library of Medicine. For access to Semantic MEDLINE, send email to trindflesch@mail.nih.gov. Research carried out at National Institutes of Health, National Library of Medicine, Lister Hill National Center for Biomedical Communications, Bethesda, MD 20894.
REFERENCES
- 1.Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult - a mini-review. Gerontology. 2010;56:181–9. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espiritu JR. Aging-related sleep changes. Clin Geriatr Med. 2008;24:1–14. doi: 10.1016/j.cger.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Trenell MI, Marshall NS, Rogers NL. Sleep and metabolic control: waking to a problem? Clin Exp Pharmacol Physiol. 2007;34:1–9. doi: 10.1111/j.1440-1681.2007.04541.x. [DOI] [PubMed] [Google Scholar]
- 4.Bassil N, Morley JE. Late-life onset hypogonadism: a review Clin Geriatr Med. 2010;26:197–222. doi: 10.1016/j.cger.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Luboshitzky R, Shen-Orr Z, Herer P. Middle-aged men secrete less testosterone at night than young healthy men. J Clin Endocrinol Metab. 2003;88:3160–6. doi: 10.1210/jc.2002-021920. [DOI] [PubMed] [Google Scholar]
- 6.Swanson DR. Fish oil, Raynaud's syndrome, and undiscovered public knowledge. Perspect Biol Med. 1986;30:7–18. doi: 10.1353/pbm.1986.0087. [DOI] [PubMed] [Google Scholar]
- 7.Rindflesch TC, Fiszman M. The interaction of domain knowledge and linguistic structure in natural language processing: Interpreting hypernymic propositions in bio medical text. J Biomed Inform. 2003;36:462–77. doi: 10.1016/j.jbi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Hristovski D, Friedman C, Rindflesch TC, Peterlin B. AMIA Annu Symp Proc. 2006. Exploiting semantic relations for literature-based discovery; pp. 349–53. [PMC free article] [PubMed] [Google Scholar]
- 9.Roffwarg HP, Sachar EJ, Halpern F, Hellman L. Plasma testosterone and sleep: relationship to sleep stage variables. Psychosom Med. 1982;44:73–84. doi: 10.1097/00006842-198203000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Paul KN, Losee-Olson S, Pinckney L, Turek FW. The ability of stress to alter sleep in mice is sensitive to reproductive hormones. Brain Res. 2009;1305:74–85. doi: 10.1016/j.brainres.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortés-Gallegos V, Castañeda G, Alonso R, et al. Sleep deprivation reduces circulating androgens in healthy men. Arch Androl. 1983;10:33–7. doi: 10.3109/01485018308990167. [DOI] [PubMed] [Google Scholar]
- 12.Luboshitzky R, Zabari Z, Shen-Orr Z, Herer P, Lavie P. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J Clin Endocrinol Metab. 2001;86:1134–9. doi: 10.1210/jcem.86.3.7296. [DOI] [PubMed] [Google Scholar]
- 13.Goh VH, Tong TY. Sleep, sex steroid hormones, sexual activities, and aging in Asian men. J Androl. 2010;31:131–7. doi: 10.2164/jandrol.109.007856. [DOI] [PubMed] [Google Scholar]
- 14.Bodenreider O. The Unified Medical Language System (UMLS): integrating biomedical terminology. Nucleic Acids Res. 2004;32:D267–70. doi: 10.1093/nar/gkh061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCray AT. High-Performance Medical Libraries: Advances in Information Management for the Virtual Era. 1993. Representing biomedical knowledge in the UMLS Semantic Network; pp. 45–55. [Google Scholar]
- 16.Kilicoglu H, Fiszman M, Rodriguez A, Shin D, Ripple AM, Rindflesch TC. Semantic MEDLINE: A Web application to manage the results of PubMed searches. Proc Third International Symposium for Semantic Mining in Biomedicine. 2008:69–76. [Google Scholar]
- 17.Swanson DR. Migraine and magnesium: eleven neglected connections. Perspect Biol Med. 1988;31:526–57. doi: 10.1353/pbm.1988.0009. [DOI] [PubMed] [Google Scholar]
- 18.Weeber M, Klein H, Aronson AR, Mork JG, Jong-Van Den Berg L, Vos R. Proc AMIA Symp. 2000. Text-based discovery in biomedicine: the architecture of the DAD-system; pp. 903–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen ML, Tufik S. The effects of testosterone on sleep and sleep-disordered breathing in men: its bidirectional interaction with erectile function. Sleep Med Rev. 2008;12:365–79. doi: 10.1016/j.smrv.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Axelsson J, Ingre M, Akerstedt T, Holmbäck U. Effects of acutely displaced sleep on testosterone. J Clin Endocrinol Metab. 2005;90:4530–5. doi: 10.1210/jc.2005-0520. [DOI] [PubMed] [Google Scholar]
- 21.Empson JA, Purdie DW. Effects of sex steroids on sleep. Ann Med. 1999;31:141–5. doi: 10.3109/07853899708998790. [DOI] [PubMed] [Google Scholar]
- 22.Paul KN, Turek FW, Kryger MH. Influence of sex on sleep regulatory mechanisms. J Womens Health. 2008;17:1201–8. doi: 10.1089/jwh.2008.0841. [DOI] [PubMed] [Google Scholar]
- 23.Barrett-Connor E, Dam TT, Stone K, Harrison SL, Redline S, Orwoll E Osteoporotic Fractures in Men Study Group. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J Clin Endocrinol Metab. 2008;93:2602–9. doi: 10.1210/jc.2007-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul KN, Laposky AD, Turek FW. Reproductive hormone replacement alters sleep in mice. Neurosci Lett. 2009;463:239–43. doi: 10.1016/j.neulet.2009.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu PY, Yee B, Wishart SM, et al. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab. 2003;88:3605–13. doi: 10.1210/jc.2003-030236. [DOI] [PubMed] [Google Scholar]
- 26.Andersen ML, Martins PJ, D'Almeida V, Bignotto M, Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res. 2005;14:83–90. doi: 10.1111/j.1365-2869.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 27.Waite E, Kershaw Y, Spiga F, Lightman SL. A glucocorticoid sensitive biphasic rhythm of testosterone secretion. J Neuroendocrinol. 2009;21:737–41. doi: 10.1111/j.1365-2826.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- 28.Gitau R, Adams D, Fisk NM, Glover V. Fetal plasma testosterone correlates positively with cortisol. Arch Dis Child Fetal Neonatal Ed. 2005;90:F166–9. doi: 10.1136/adc.2004.049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cumming DC, Quigley ME, Yen SS. Acute suppression of circulating testosterone levels by cortisol in men. J Clin Endocrinol Metab. 1983;57:671–3. doi: 10.1210/jcem-57-3-671. [DOI] [PubMed] [Google Scholar]
- 30.Cernak I, Savic VJ, Lazarov A, Joksimovic M, Markovic S. Neuroendocrine responses following graded traumatic brain injury in male adults. Brain Inj. 1999;13:1005–15. doi: 10.1080/026990599121016. [DOI] [PubMed] [Google Scholar]
- 31.Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol Hum Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- 32.del Rey A, Mahuad CV, Bozza V, et al. Endocrine and cytokine responses in humans with pulmonary tuberculosis. Brain Behav Immun. 2007;21:171–9. doi: 10.1016/j.bbi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Bottasso O, Bay ML, Besedovsky H, del Rey A. Immunoendocrine alterations during human tuberculosis as an integrated view of disease pathology. Neuroimmunomodulation. 2009;16:68–77. doi: 10.1159/000180261. [DOI] [PubMed] [Google Scholar]
- 34.Medhamurthy R, Priyanka G, Vinuthan MK, Manjunatha AM. Short-term fasting leads to inhibition of responsiveness to LH-stimulated testosterone secretion in the adult male bonnet monkey. Am J Primatol. 2007;69:791–801. doi: 10.1002/ajp.20406. [DOI] [PubMed] [Google Scholar]
- 35.Kraemer WJ, Häkkinen K, Newton RU, et al. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol. 1999;87:982–92. doi: 10.1152/jappl.1999.87.3.982. [DOI] [PubMed] [Google Scholar]
- 36.Bosco C, Iacovelli M, Tsarpela O, et al. Hormonal responses to whole-body vibration in men. Eur J Appl Physiol. 2000;81:449–54. doi: 10.1007/s004210050067. [DOI] [PubMed] [Google Scholar]
- 37.Rubinow DR, Roca CA, Schmidt PJ, et al. Testosterone suppression of CRH-stimulated cortisol in men. Neuropsychopharmacology. 2005;30:1906–12. doi: 10.1038/sj.npp.1300742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akerstedt T, Palmblad J, de la Torre B, Marana R, Gillberg M. Adrenocortical and gonadal steroids during sleep deprivation. Sleep. 1980;3:23–30. doi: 10.1093/sleep/3.1.23. [DOI] [PubMed] [Google Scholar]
- 39.Penev PD. Association between sleep and morning testosterone levels in older men. Sleep. 2007;30:427–32. doi: 10.1093/sleep/30.4.427. [DOI] [PubMed] [Google Scholar]
- 40.Turek FW. Gender (sex) and sleep: shh…not in front of the children. Sleep. 2006;29:21–2. doi: 10.1093/sleep/29.1.21. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 42.Luboshitzky R, Lavi S, Lavie P. The association between melatonin and sleep stages in normal adults and hypogonadal men. Sleep. 1999;22:867–74. doi: 10.1093/sleep/22.7.867. [DOI] [PubMed] [Google Scholar]
- 43.Leibenluft E, Schmidt PJ, Turner EH, et al. Effects of leuprolide-induced hypogonadism and testosterone replacement on sleep, melatonin, and prolactin secretion in men. J Clin Endocrinol Metab. 1997;82:3203–7. doi: 10.1210/jcem.82.10.4270. [DOI] [PubMed] [Google Scholar]
- 44.Miyatake A, Morimoto Y, Oishi T, et al. Circadian rhythm of serum testosterone and its relation to sleep: comparison with the variation in serum luteinizing hormone, prolactin, and cortisol in normal men. J Clin Endocrinol Metab. 1980;51:1365–71. doi: 10.1210/jcem-51-6-1365. [DOI] [PubMed] [Google Scholar]
- 45.Otte C, Lenoci M, Metzler T, Yehuda R, Marmar CR, Neylan TC. Hypothalamic-pituitary-adrenal axis activity and sleep in posttraumatic stress disorder. Neuropsychopharmacology. 2005;30:1173–80. doi: 10.1038/sj.npp.1300676. [DOI] [PubMed] [Google Scholar]
- 46.Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: More than a measure of HPA axis functions. Neurosci Biobehav Rev. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 47.Chang FC, Opp MR. A corticotropin-releasing hormone antisense oligodeoxynucleotide reduces spontaneous waking in the rat. Regul Pept. 2004;117:43–52. doi: 10.1016/j.regpep.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–14. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- 49.Peeters GM, van Schoor NM, van Rossum EF, Visser M, Lips P. The relationship between cortisol, muscle mass and muscle strength in older persons and the role of genetic variations in the glucocorticoid receptor. Clin Endocrinol. 2008;69:673–82. doi: 10.1111/j.1365-2265.2008.03212.x. [DOI] [PubMed] [Google Scholar]
- 50.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 51.Vgontzas AN, Bixler EO, Wittman AM, et al. Middle-aged men show higher sensitivity of sleep to the arousing effects of corticotropin-releasing hormone than young men: clinical implications. J Clin Endocrinol Metab. 2001;86:1489–95. doi: 10.1210/jcem.86.4.7370. [DOI] [PubMed] [Google Scholar]
- 52.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–8. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 53.Muniyappa R, Veldhuis JD, Harman SM, Sorkin JD, Blackman MR. Effects of testosterone administration on nocturnal cortisol secretion in healthy older men. J Gerontol A Biol Sci Med Sci. 2010;65:1185–92. doi: 10.1093/gerona/glq128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wauquier A, van Sweden B, Lagaay AM, Kemp B, Kamphuisen HA. Ambulatory monitoring of sleep-wakefulness patterns in healthy elderly males and females (greater than 88 years): the “Senieur” protocol. J Am Geriatr Soc. 1992;40:109–14. doi: 10.1111/j.1532-5415.1992.tb01928.x. [DOI] [PubMed] [Google Scholar]
- 55.Krieger DT, Allen W, Rizzo F, Krieger HP. Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab. 1971;32:266–84. doi: 10.1210/jcem-32-2-266. [DOI] [PubMed] [Google Scholar]
- 56.Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- 57.Weitzman ED, Zimmerman JC, Czeisler CA, Ronda J. Cortisol secretion is inhibited during sleep in normal man. J Clin Endocrinol Metab. 1983;56:352–8. doi: 10.1210/jcem-56-2-352. [DOI] [PubMed] [Google Scholar]
- 58.Kumari M, Badrick E, Sacker A, Kirschbaum C, Marmot M, Chandola T. Identifying patterns in cortisol secretion in an older population. Findings from the Whitehall II study. Psychoneuroendocrinology. 2010;35:1091–9. doi: 10.1016/j.psyneuen.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 59.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14:3408–16. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weissman BA, Sottas CM, Zhou P, Iadecola C, Hardy MP. Testosterone production in mice lacking inducible nitric oxide synthase expression is sensitive to restraint stress. Am J Physiol Endocrinol Metab. 2007;292:E615–20. doi: 10.1152/ajpendo.00412.2006. [DOI] [PubMed] [Google Scholar]