Abstract
Purpose
Vogt-Koyanagi-Harada (VKH) disease is a serious ocular inflammatory autoimmune insult directed against antigens associated with melanocytes. The repertoire of killer cell immunoglobulin-like receptors (KIRs) is known to play a significant role in the pathogenesis of various autoimmune disorders. Accordingly, we sought to determine the incidence of KIR genes and KIR ligand (Human leukocytes antigen [HLA-C]) interaction in a cohort of Saudi VKH patients and to compare the findings to normal controls.
Methods
A total of 30 patients with VKH and 125 control subjects were included. PCR using sequence-specific oligonucleotide primers were employed to determine the genotype of the KIR genes and HLA-C alleles.
Results
The frequency of KIR2DS3 was significantly higher in the VKH patients than in the control group (p=0.048). Two unique genotypes; VKHN*1 and VKHN*2 were observed in the VKH patients and not in normal controls. In addition, the majority of the VKH patients (82%) in this study carry Bx genotypes that encode 2–5 activating KIR receptors. The genotype Bx5 was found to be positively associated with the VKH patients (p=0.053). Significantly higher homozygosity of HLA-C2 was observed in the VKH patients than in controls (p=0.005). Furthermore, HLA-C alleles-Cw*14 and Cw*17 were significantly prevalent in the VKH patients (p=0.037 and p=0.0001, respectively), whereas, Cw*15 significantly increased in the control group (p=0.0205). Among potential KIR-HLA interactions, we observed KIR2DL2/2DL3+HLA-C1 to be higher in the control subjects compared with the VKH patients (p=0.018).
Conclusions
Our findings indicated that KIR2DS3 and HLA-class I alleles (-Cw*14 and -Cw*17) may play a role in the pathogenesis of VKH disease. Additionally, the predominance of KIR2DL2/2DL3+HLA-C1 in the controls may imply that this KIR-ligand interaction could possibly play a role in the prevention of VKH disease, or could decrease its severity. These observations may contribute to our understanding of the pathogenesis of VKH and other autoimmune diseases.
Introduction
Vogt-Koyanagi-Harada (VKH) disease is a multisystem disorder characterized by granulomatous panuvetitis and exudative retinal detachment and is often associated with neurologic and cutaneous manifestations. Ocular inflammation may occur and the onset of VKH disease can be associated with aseptic meningitis, as well as with the subsequent development of vitiligo and hearing changes, as part of the putative cell-mediated autoimmune response affecting melanocytes [1,2]. Several human leukocyte antigens (HLA) have been reported to be associated with VKH disease, including HLA-DR4, HLA-DR53, and HLA-DQ4. Strong association with the HLA-DRB1*0405 subtype has been described in Japanese, Korean, and Saudi populations [3–5], but not in the Mestizo population [6]. We recently reported a significant association of the HLA-DRB1*0405 allele with a Saudi VKH cohort. It is likely that other genetic components of the immune system play a role in conferring risk for VKH disease.
Several studies have shown the influence of killer cell immunoglobulin-like receptors (KIRs), and KIR-ligand pairs, in terms of the susceptibility to and outcome of various autoimmune and infectious diseases, such as AIDS [7,8], hepatitis C virus infection [9], tuberculosis [10], leprosy [11], bird-shot chorioretinopathy [12], idiopathic brochiectasis [13], diabetes mellitus [14,15], Systemic lupus erythromatous [16], Scleroderma [17], Sjogren’s syndrome [18], and ankylosing spondilitis [19].
The polymorphic nature of KIR encodes receptors that inhibit or activate natural killer (NK) cells and certain T-lymphocyte subsets [20–22]. The inhibitory KIR (“iKIRs”) 2DL/3DL recognizes distinct HLA class I molecules and triggers signals to stop NK cell killing. Although the ligands for activating KIRs (“aKIRs”) 2DS/3DS are not well acknowledged, certain aKIRs are predicted to bind to the same HLA-class I ligands in a peptide-dependent manner as their structurally related iKIRs [23]. In addition, the aKIRs interact with the DAP-12 molecules, which modulate granulation and cytokine production in NK cells [24]. Based on the genetic content and the pattern of segregation at the population level, KIR haplotypes are divided into two groups, A and B. Group A haplotypes are defined by the presence of seven genes, KIR2DL1, 2DL3, 2DL4, 3DL1, 3DL2, 3DL3, and 2DS4, and two pseudo genes. However, group B haplotypes show high genetic diversity and are characterized by the presence of more than one aKIRs. KIR2DL4, 3DL2, 3DL3, and 3DP1 are ubiquitously present in all individuals and are termed “framework” genes.
The activity of NK cells is controlled by the balance of contra-regulatory signals derived from a wide variety of inhibitory and activating receptors [25].The balance in KIR signaling is provided by the recognition of HLA molecules on the surface of target cells, and some of these ligands are already known. Dimorphism at residue 80 of the α1- helix in HLA-C alleles defines two groups of KIR ligands, HLA-C1, Cw*01, *03,*07,*08,*12,*13,*14, and *16- and -HLA-C2, -Cw*02, *04, *05, *06,*15,*17, and *18, which are specific for KIR2DL2/2DL3/2DS2 and KIR2DL1/2DS1, respectively [26]. Genes encoding KIR and HLA are located on different chromosomes and vary in both number and type. The independent segregation of KIR and HLA genes results in variable KIR-HLA combinations in individuals, which might ultimately determine the individual’s immunity and susceptibility to disease [27]. Therefore, the objective of the present study was to investigate the association of KIR genes and potential KIR-ligand interactions in patients with VKH disease in a cohort of the Saudi population.
Methods
Patients and samples
Genomic DNA samples were collected from 30 patients with VKH disease, as reported previously [5], were stored at −80 °C, and were analyzed for the purpose of the present study. A total of 125 controls were selected from the earlier published data [28], which had a complete set of data for KIR and HLA.
The VKH patients and controls were Saudi nationals from different parts of the Kingdom of Saudi Arabia. The study was reviewed and approved by the Institutional Review Board at King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia. Written consent forms were duly signed by all subjects included in the study.
Genotyping of KIR and HLA
DNA samples from the VKH patients were typed for 14 KIR genes and two pseudo genes using a single stranded polymorphism typing system by Dynal KIR genotyping kit (Invitrogen Corp, Carlsbad, CA). HLA-C typing was performed using a high-resolution sequence based typing (SBT) kit (Excellerator HLA kit; Qiagen Inc., Valencia, CA), as previously described [28]. Genotyping tests for all novel genotypes were repeated for the purpose of confirmation.
Data analysis and statistical methods
The phenotypic frequency of each KIR was calculated as the percentage of positive numbers among all individuals. The statistical software package SAS, version 9.2 (SAS Institute Inc., Cary, NC) was used for data analysis. Differences in the frequencies of KIR and HLA-C genes between controls and the VKH patients were tested by two-tailed Fisher’s exact probabilities, and p<0.05 was considered statistically significant. Odds Ratios (OD) with 95% Confidence Intervals (CI) were calculated for comparisons showing significant differences between the patients and the control group.
Results
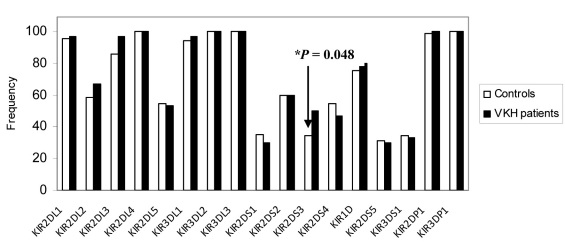
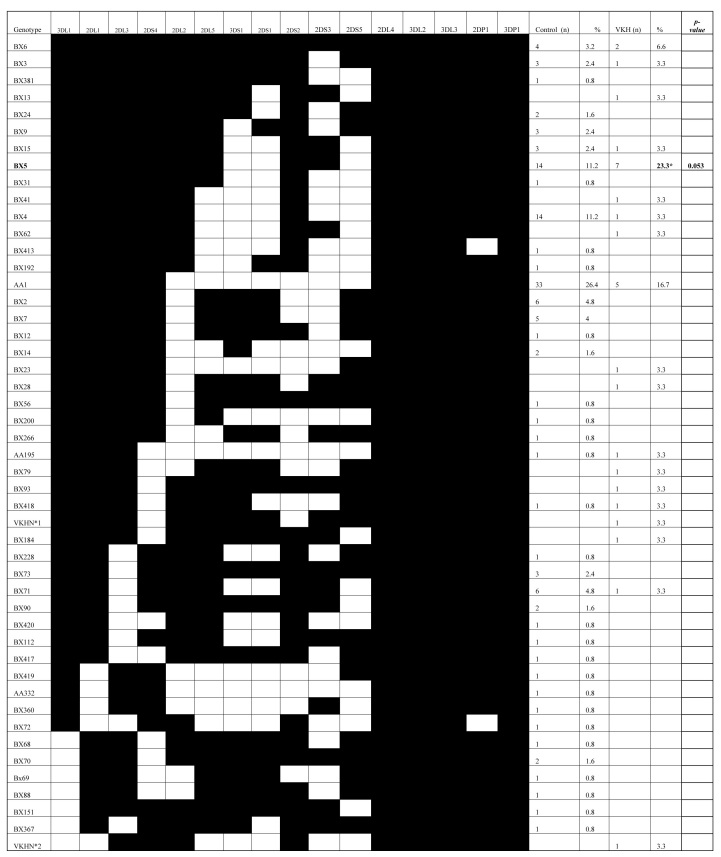
A total of 30 patients with VKH were studied and compared to 125 Saudi controls who had a complete set of KIR and HLA data reported earlier [28].The constituents of the control group were not related to the patients or to each other and were all of the same ethnic origin. All of the 16 KIR genes were identified in our VKH patients and the controls, and the four framework genes (KIR2DL4, 3DL2, 2DP1, and 3DL3) were present in all the subjects tested. The frequencies of iKIR and aKIR genes in the VKH patients and the controls are outlined in Figure 1. The frequencies of individual KIR genes were found to be similar in the controls and the VKH patients, with the exception of KIR2DS3, which was significantly higher in the VKH patients compared to the healthy controls (50% in VKH and 34.4% in controls; p=0.048; OD=1.907; CI=0.85–4.26). Furthermore, 19 [19] KIR genotypes that differed in terms of their gene content were observed in 30 VKH patients. We observed two unique KIR profiles (VKHN*1 and VKHN*2) from two VKH patients that were not found among any of the Saudi control subjects (Figure 2, marked by asterisks). Even though the observed difference did not reach statistical significance, the observation is important as it has not been previously reported elsewhere. Nine [9] genotypes were shared between the VKH patients and the Saudi controls. The frequency of the AA1 genotype was observed to be higher in the controls than in the VKH patients, with a gene frequency of 26.4% and 16.7%, respectively. The ratio of the A:Bx haplotype was found to be 0.33 and 0.20 for the controls and the VKH patients, respectively, (data not shown), indicating the predominance of B haplotype in the VKH patients. Among all the genotypes, only Bx5 reached statistical significance in the VKH patients (p=0.053; OD=2.41; CI=0.73–7.24).
Figure 1.
The distribution of killer cell immunoglobulin-like receptor (KIR) gene frequencies in Saudi patients with Vogt-Koyanagi-Harada (VKH) disease and in control subjects. The frequency of each gene is expressed as a percentage and is defined as the number of individuals possessing the genotype (N+), divided by the total number of individuals studied (n). *KIR2DS3 is significantly higher in the VKH patients compared to the controls. The KIR data for the Saudi controls was obtained from our previous publication [28]. The framework genes (KIR3DL2, 3DL3, 2DL4, and 3DP1) were invariably present in all individuals.
Figure 2.
Killer cell immunoglobulin-like receptor (KIR) genotypes in Saudi patients with Vogt-Koyanagi-Harada (VKH) disease and in control subjects. Data for the Saudi control individuals was previously published [28]. The frequency of each genotype is expressed as a percentage and is defined as the number of individuals possessing the genotype (+N), divided by the number of individuals studied (n) in each group. VKHN*1 and VKHN*2 are two unique genotypes and are marked with an asterisk. **The frequency of the Bx5 genotype was measured as being significantly higher in the VKH patients compared to the normal controls. (p=0.053; OD=2.41; CI=0.73–7.24).
The differences in the frequencies of HLA-C and KIR-HLA-C complex between patients with VKH disease and the control group were statistically significant (Table 1). Significant HLA-C2 homozygosity was measured in the VKH patients (p=0.005; OD=3.23; CI=1.42–7.33). In contrast, the controls were found to have higher HLA-C1 homozygosity than the VKH patients (p=0.029; OD=0.13; CI=0.017–1.009). To explore KIR-HLA class I interactions, the presence of KIR genes (KIR2DL1, 2DL2, 2DL3, 2DS1, and 2DS2) and their specific ligands were screened in both the VKH patients and the controls. The frequency of KIR2DL2/3-HLA-C1 interactions was significantly prevalent in the control subjects (p=0.018; OD=0.36; CI=0.16–0.83) compared with the VKH patients (Table 1). Other potential interactions between iKIRs/aKIRs and HLA ligands were found to be similar between the VKH patients and the controls (Table 1). When the frequencies of HLA-C alleles were compared, HLA-Cw *14 (C1 group) and -Cw*17 (C2 group) were observed to be significantly higher in the VKH patients than in the controls (p=0.037; OD=5.31; CI=4.21–6.69 and p<0.010; OD=21.88; CI=4.59–104.29, respectively) (Table 2). However, HLA-Cw*15 (C2 group) was significantly higher in the controls than in the VKH patients (p=0.020; OD=0.308; CI=0.10–0.89) as shown in Table 2.
Table 1. Comparison of HLA-class I (-Cw) and KIR - HLA-C complex in a cohort of Saudi patients with VKH disease and control subjects.
| Controls (n=125) | VKH patients (n=30) | |||||
|---|---|---|---|---|---|---|
|
HLA-C/KIR-HLA Complex |
(N+) |
%F |
(N+) |
%F |
p-value |
OD 95%(CI) |
|
HLA-Cw* | ||||||
| HLA-C1/C1 |
26 |
20.8 |
1 |
3.3 |
0.0291 |
0.13 (0.017–1.009) |
| HLA-C1/C2 |
63 |
50.4 |
12 |
40 |
|
|
| HLA-C2/C2 |
36 |
28.8 |
17 |
56.7 |
0.0054 |
3.23 (1.42–7.33) |
|
KIR-HLA(KIR ligand) | ||||||
| KIR2DL2/3-HLA-C1 |
88 |
70.4 |
14 |
46.7 |
0.0184 |
0.367 (0.163–0.830) |
| KIR2DL1-HLA-C2 |
97 |
77.6 |
28 |
93.3 |
|
|
| KIR2DS2-HLA-C1 |
52 |
41.6 |
9 |
30 |
|
|
| KIR2DS1-HLA-C2 | 32 | 25.6 | 8 | 26.7 | ||
Table 2. Frequency of HLA-Cw* alleles in Saudi patients with VKH disease and control subjects.
| Controls (n=125) | VKH patients (n=30) | |||||
|---|---|---|---|---|---|---|
|
HLA-C alleles |
Alleles (n) |
% |
Alleles (n) |
% |
p value |
OD 95%(CI) |
| cw*01/C1 |
15 |
6 |
2 |
3.3 |
|
|
| cw*02/C2 |
8 |
3.2 |
2 |
3.3 |
|
|
| cw*03/C1 |
10 |
8 |
1 |
1.6 |
|
|
| cw*04/C2 |
26 |
10.4 |
6 |
10 |
|
|
| cw*05/C1 |
1 |
0.4 |
1 |
1.6 |
|
|
| cw*06/C2 |
52 |
20.8 |
19 |
31.6 |
|
|
| cw*07/C1 |
52 |
20.8 |
9 |
15 |
|
|
| cw*08/C1 |
10 |
4 |
1 |
1.6 |
|
|
| cw*12/C1 |
20 |
8 |
1 |
1.6 |
|
|
| cw*14//C1 |
0 |
0 |
2 |
3.3 |
0.037 |
5.3103(4.211–6.696) |
| cw*15/C2 |
47 |
18.8 |
4 |
6.7 |
0.0205 |
0.3085 (1.06–0.893) |
| cw*1507/C1 |
1 |
0.4 |
0 |
0 |
|
|
| cw*16/C1 |
4 |
1.6 |
0 |
0 |
|
|
| cw*1602/C2 |
2 |
0.8 |
2 |
3.3 |
|
|
| cw*17/C2 |
2 |
0.8 |
9 |
15 |
<0.0001 |
21.88 (4.59–104.29) |
| cw*18/C2 |
0 |
0 |
1 |
1.6 |
|
|
| Total no of alleles (n) | 250 | 60 | ||||
Discussion
KIRs are a relatively recent discovery; while the biologic functions of iKIRs are well described, the function of aKIRs is less clear [29]. Disease association studies have shown that aKIR genotypes and KIR-HLA complexes are, in general, associated with a higher risk of autoimmune diseases [27]. We previously reported a significant association of HLA-DRB1*0405 with the VKH patients [5]. Although the clinical manifestations of VKH are well outlined [2], its exact etiology remains to be elucidated. It is believed that T-lymphocyte-mediated autoimmune processes are directed against an, as yet, unidentified antigen or group of antigens associated with melanocytes [1,4,5]. In this study, we found that the VKH patients have a higher frequency of the activating KIR2DS3 gene compared with healthy controls. Additionally, our results confirm the previous observations made by Levinson et al. [30], who reported a predominance of activating KIR genes in Mestizo VKH patients. Although, the observed difference in their study is not statistically significant, it is in keeping with the trend observed in other autoimmune diseases [12]. Interestingly, the preponderance of aKIRs in the VKH patients compared to normal controls suggests that T cell, T cell subsets, and NK cells bearing KIRs might participate in the pathology of the disease. In support of our findings, other studies have demonstrated the association of aKIR genes with a poor prognosis for patients with the Ebola virus infection [31] and other ocular inflammatory disease, like bird shot chorioretinopathy [12]. Moreover, we observed that group B KIR haplotypes predominate in the VKH patients, compared with the controls. However, only the difference in the frequency of the Bx5 genotype reached statistical significance. This might point to a role played by this genotype in the predisposition or the immunopathology of disease. The predominance of the B haplotype in VKH patients was also found to be consistent with other published studies [30]. Two unique genotypic profiles VKHN*1 and VKHN*2 were only detected in the VKH patients and not in the normal Saudi controls and the difference was not found to be statistically significant, as only one individual was measured in each genotype. It is conceivable to postulate that the presence of these two genotypes, alone or in combination with others, has biologic relevance to the disease. Indeed, more investigations are required to prove the previous hypothesis and to identify their exact role in VKH diseases.
It is recognized that the function of KIR is highly dependent on the HLA molecules expressed on the target cells, and both KIR and HLA show a high degree of polymorphism in the populations. Accordingly, we analyzed the risk conferred by the potential interactions of KIR genes with HLA-C in the VKH patients and the control subjects. Only KIR2DL2/3-HLA-C1 was found to be significantly higher in our control population. This was indicative of the inhibitory or protective role played by NK cells. Data from genetic association studies suggest that the signals transduced by aKIRs, upon binding to their putative HLA class I, may serve to overcome HLA class I-dependent inhibition and trigger NK reactivity, leading to an autoimmune condition, as in VKH disease. Although, the precise physiologic ligands for aKIRs are not well defined, KIR tetramer-binding studies imply that activating and inhibitory receptors recognize the same set of HLA class I molecules, but differ in their binding affinities. This allows fine tuning during cellular activation [23]. There is considerable support for the notion that non-HLA molecules may behave as ligands for aKIRs [25]. It is plausible that KIR2DS3 acts by directly recognizing virally encoded proteins, or by recognizing HLA-loaded viral peptide complexes [25]. It is acknowledged that KIR- positive T-cells expressing the adaptor molecule killer cell-activating receptor associated protein (KARAP)/DAP-12, can be stimulated directly with KIR2DS3 to release cytokines and become cytotoxic, without the need for conventional T-cell receptor (TCR) recognition by antigenic peptides [24]. Both KIR and HLA demonstrate a high degree of polymorphism in a given population [20]. In this study, the HLA-Cw*15 allele was more frequently observed in controls, which might point to the role it plays in offering protection from VKH disease. Alternatively, the strong association of the VKH disease with HLA-Cw*14 and -Cw*17* alleles may suggest that the aKIRs, upon binding to their putative HLA-class I ligands, could overcome HLA class I-dependent inhibition and trigger NK cell reactivity, leading to an autoimmune condition such as VKH disease.
Finally, accumulating evidence indicates that aKIRs, and their corresponding specific HLA-C ligands, might contribute to the pathogenesis of VKH disease by modulating NK cells and T cell functions.
Acknowledgments
This study was approved and funded by Research Advisory Council (RAC), King Faisal Specialist Hospital and Research Centre. We are grateful to the VKH patients and the control volunteers who took part in this study. Also, we thank the Research center administration for their continuous help and support. We are grateful to Dr. Ayodele A. Alaiya and Dr. Andrew Wetzig for critically reviewing the manuscript.
References
- 1.Norose K, Yano A. Melanoma specific Th1 cytotoxic T lymphocyte lines in Vogt- Koyanangi- Harada disease. Br J Ophthalmol. 1996;80:1002–8. doi: 10.1136/bjo.80.11.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugita S, Takase H, Taguchi C, Imai Y, Kamoi K, Kawaguchi T, Sugamoto Y, Futagami Y, Itoh K, Mochizuki M. Ocular infiltrating CD4+ T cells from patients with Vogt- Koyanagi-Harada disease recognize human melanocyte antigens. Invest Ophthalmol Vis Sci. 2006;47:2547–54. doi: 10.1167/iovs.05-1547. [DOI] [PubMed] [Google Scholar]
- 3.Kim MH, Seong MC, Kwak NH, Yoo JS, Huh W, Kim TG, Han H. Association of HLA with Vogt- Koyanagi- Harada syndrome in Koreans. Am J Ophthalmol. 2000;129:173–7. doi: 10.1016/s0002-9394(99)00434-1. [DOI] [PubMed] [Google Scholar]
- 4.Shindo Y, Ohno S, Yamamoto T, Nakamura S, Inoko H. Complete association of the HLA-DRB1*04 and –DQB1*04 alleles with Vogt- Koyanagi- Harada’s disease. Hum Immunol. 1994;39:169–76. doi: 10.1016/0198-8859(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 5.Iqniebi A, Gaafar A, Sheereen A, Al Sulaiman A, Mohammed G, Al Hussein K, Tabbara KF. HLA-DRB1 among patients with Vogt- Koyanangi- Harada disease in Saudi Arabia. Mol Vis. 2009;15:1876–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Levinson RD, See RF, Rajalingam R, Reed EF, Park MS, Rao NA, Holland GN. HLA-DRB1 and –DQB1 alleles in mestizo patients with Vogt- Koyanangi- Harada’s disease in Southern California. Hum Immunol. 2004;65:1477–82. doi: 10.1016/j.humimm.2004.07.236. [DOI] [PubMed] [Google Scholar]
- 7.Gaudieri S, De Santis, Mckinnon E, Moore C, Nolan D, Witt CS, Mallal SA, Christiansen FT. Killer immunoglobulin- like receptors and HLA- act both independently and synergistically to modify HIV disease progression. Genes Immun. 2005;6:683–90. doi: 10.1038/sj.gene.6364256. [DOI] [PubMed] [Google Scholar]
- 8.López-Vázquez A, Miña-Blanco A, Martínez-Borra J, Njobvu PD, Suárez-Alvarez B, Blanco-Gelaz MA, González S, Rodrigo L, López-Larrea C. Interaction between KIR3DL1 and HLA-B*57 supertype alleles influence the progression of HIV-1 infection in a Zambian population. Hum Immunol. 2005;66:285–9. doi: 10.1016/j.humimm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O’Brien SJ, Rosenberg WM, Thomas DL, Carrington M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–4. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 10.Méndez A, Granda H, Meenagh A, Contreras S, Zavaleta R, Mendoza MF, Lzquierdo L, Sarmiento ME, Acosta A, Middleton D. Study of KIR genes in tuberculosis patients. Tissue Antigens. 2006;68:386–9. doi: 10.1111/j.1399-0039.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 11.Franceschi DSA, Mazini PS, Rudnick CCC, Sell AM, Tsuneto LT, De Melo FC, Braga MA, Peixoto PRF, Visentainer JEL. Association between KIR genotypes and Leprosy in Brazil. Tissue Antigens. 2008;72:478–82. doi: 10.1111/j.1399-0039.2008.01127.x. [DOI] [PubMed] [Google Scholar]
- 12.Levinson RD, Du Z, Luo L, Monnet D, Tabbary T, Brezin AP, Zhao L, Gjertson DW, Holland GN, Reed EE, Cohen JHE, Rajalingam R. Combination of KIR and HLA gene variants auguments the risk of developing birdshot chorioretinopathy in HLA-A*29 positive individuals. Genes Immun. 2008;9:249–58. doi: 10.1038/gene.2008.13. [DOI] [PubMed] [Google Scholar]
- 13.Boyton RJ, Smith J, Ward R, Jones M, Ozerovitch L, Wilson R, Rose M, Trowsdale J. HLA-C and killer cell immunoglobulin – like receptor genes in idiopathic bronchiectasis. Am J Respir Crit Care Med. 2006;173:327–33. doi: 10.1164/rccm.200501-124OC. [DOI] [PubMed] [Google Scholar]
- 14.van der Slik AR, Koeleman BP, Verduijn W, Bruining GJ, Roep BO, Giphart MJ. KIR in type 1 diabetes: disparate distribution of activating and inhibitory natural killer cell receptors in patients versus HLA-matched control subjects. Diabetes. 2003;52:2639–42. doi: 10.2337/diabetes.52.10.2639. [DOI] [PubMed] [Google Scholar]
- 15.Middleton D, Halfpenny I, Meenagh A, Williams F, Sivula J. Tuomilehto- Wolf E. Investigation of KIR gene frequencies in type I diabetes mellitus. Hum Immunol. 2006;67:986–90. doi: 10.1016/j.humimm.2006.08.295. [DOI] [PubMed] [Google Scholar]
- 16.Pellett F, Siannis F, Vukin I, Lee P, Urowitz MB, Gladmann DD. KIRs and autoimmune disease: studies in systemic lupus erythematosus and scleroderma. Tissue Antigens. 2007;69:106–8. doi: 10.1111/j.1399-0039.2006.762_6.x. [DOI] [PubMed] [Google Scholar]
- 17.Momot T, Koch S, Hunzelmann N, Kreig T, Ulbricht K, Schmidt RE, Witt T. Association of killer cell immunoglobulin – like receptors with Scleroderma. Arthritis Rheum. 2004;50:1561–5. doi: 10.1002/art.20216. [DOI] [PubMed] [Google Scholar]
- 18.Lowe DP, Cook MA, Simon JB, Briggs DC, Sjogren UK. Association of killer cell immunoglobulin – like receptors with primary Sjogren’s syndrome. Rheumatology. 2009;48:359–62. doi: 10.1093/rheumatology/ken503. [DOI] [PubMed] [Google Scholar]
- 19.Jiao YL, Zhang BC, You L, Li JF, Zhang J, Ma CY, Cui B, Wang LC, Chen ZJ, Zhao YR. Polymorphisms of KIR gene and HLA-C alleles: Possible Association with Susceptibility to HLA-B-27- Positive patients with Ankylosing spondilitis. J Clin Immunol. 2010;30:840–4. doi: 10.1007/s10875-010-9444-z. [DOI] [PubMed] [Google Scholar]
- 20.Lanier LL. NK cell Recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 21.Vilches C, Parham P. KIR: Diverse, Rapidly Evolving Receptors of Innate and Adaptive Immunity. Annu Rev Immunol. 2002;20:217–51. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 22.Rajalingam R. Killer Cell Immunoglobulin- like Receptor Influence the Innate and Adaptive Immune Responses. Iran J Immunol. 2007;4:61–78. [PubMed] [Google Scholar]
- 23.Stewart CA, Laugier AF, Vely F, Saulquin X, Reidmuller J, Tisserant A, Gauthier L, Romagne F, Ferracci G, Arosa FA, Moretta A, Sun PD, Ugolini S, Vivier E. Recognition of peptide- MHC class I complexes by activating Killer immunoglobulin – like receptors. Proc Natl Acad Sci USA. 2005;102:13224–9. doi: 10.1073/pnas.0503594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder MR, Nakajima NT, Leibson PJ, Weyand CM, Goronzy JJ. Stimulatory killer Ig – like receptors modulate T cell activation through DAP-12-dependent and DAP-12- independent mechanisms. J Immunol. 2004;173:3725–31. doi: 10.4049/jimmunol.173.6.3725. [DOI] [PubMed] [Google Scholar]
- 25.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O'Brien SJ, Rosenberg WM, Thomas DL, Carrington M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–4. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 26.Boyton RJ, Attmann DM. Natural killer cells, killer immunoglobulin – like receptors and human leukocyte antigen class I in disease. Clin Exp Immunol. 2007;149:1–8. doi: 10.1111/j.1365-2249.2007.03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Single RM, Martin MP, Meyer D, Gao XJ, Carrington M. Methods for assessing gene diversity of KIR with examples from global set of populations. Immunogenetics. 2008;60:711–25. doi: 10.1007/s00251-008-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaafar A, Sheereen A, Iqneibi A, Mohamed G, Al Sulaiman A, Turpeinen H, Al Hussein K. Killer cell immunoglobulin-like receptor gene diversity in the Saudi population. Mol Biol Rep. 2011;38:2603–10. doi: 10.1007/s11033-010-0401-y. [DOI] [PubMed] [Google Scholar]
- 29.Namekawa T, Synder MR, Yen JH, Goehring BE, Leibson PJ, Weynand CM, Goronzy JJ. Killer cell activating receptors function as costimulatory molecules on CD4+CD28 null T cells clonally expanded in Rheumatoid Arthritis. J Immunol. 2000;165:1138–45. doi: 10.4049/jimmunol.165.2.1138. [DOI] [PubMed] [Google Scholar]
- 30.Levinson RD, Du Z, Luo L, Holland GN, Rao NA, Reed EF, Rajalingam R. KIR and HLA gene combinations in Vogt- Koyanagi-Harada disease. Hum Immunol. 2008;69:349–53. doi: 10.1016/j.humimm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Wauquier N, Padilla C, Becquart P, Leroy E, Vieillard V. Association of KIR2DS1 and KIR2DS3 with fatal outcome in Ebola virus infection. Immunogenetics. 2010;62:767–71. doi: 10.1007/s00251-010-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]