Abstract
Background
Major depressive disorder (MDD) has been associated with adverse medical consequences, including cardiovascular disease and osteoporosis. Patients with MDD may be classified as having melancholic, atypical, or undifferentiated features. The goal of the present study was to assess whether these clinical subtypes of depression have different endocrine and metabolic features and consequently, varying medical outcomes.
Methods
Premenopausal women, ages 21 to 45 years, with MDD (N = 89) and healthy controls (N = 44) were recruited for a prospective study of bone turnover. Women with MDD were classified as having melancholic (N = 51), atypical (N = 16), or undifferentiated (N = 22) features. Outcome measures included: metabolic parameters, body composition, bone mineral density (BMD), and 24 hourly sampling of plasma adrenocorticotropin (ACTH), cortisol, and leptin.
Results
Compared with control subjects, women with undifferentiated and atypical features of MDD exhibited greater BMI, waist/hip ratio, and whole body and abdominal fat mass. Women with undifferentiated MDD characteristics also had higher lipid and fasting glucose levels in addition to a greater prevalence of low BMD at the femoral neck compared to controls. Elevated ACTH levels were demonstrated in women with atypical features of depression, whereas higher mean 24-hour leptin levels were observed in the melancholic subgroup.
Conclusions
Pre-menopausal women with various features of MDD exhibit metabolic, endocrine, and BMD features that may be associated with different health consequences.
Trial Registration
ClinicalTrials.gov NCT00006180
Introduction
Major Depressive Disorder (MDD) is a common condition with a lifetime prevalence of 20.8% [1]. According to the Diagnostic and Statistical Manual of Mental Disorders, (DSM-IV), an episode of MDD can be classified clinically as depression with melancholic features and depression with atypical features. Melancholic depression is characterized by anhedonia, lack of reactivity to pleasurable stimuli and three or more of the following: loss of appetite or weight, insomnia, psychomotor retardation or agitation, sense of guilt, early awakening, depression that is worse in the morning, and a distinct quality of depressed mood. In contrast, atypical depression is defined by mood reactivity (mood brightens in response to positive events), appetite and weight increase, hypersomnia, leaden paralysis, and pathological sensitivity to perceived interpersonal rejection resulting in social or occupational impairment. To further define phenotypes of depressive episodes, distinctive features of clinical subtypes have been studied, including behavioral manifestations, response to antidepressants, regional differences in cerebral blood flow, perceptual asymmetries, electroencephalographic sleep recordings, and endocrine dysregulations [2]–[8]. More recently, cognitive impairment has been reported in subjects with melancholic depression [9]. MDD is characterized by a high level of clinical heterogeneity [10]; for this reason its construct as a nosological entity has been criticized [11]. The classification of episodes into clinical subtypes has been developed in an attempt to limit the clinical heterogeneity of MDD and is currently being re-evaluated in preparation of the DSM-V.
The epidemiology of clinical subtypes of depression has been characterized in the National Comorbidity Survey: 36% of individuals with MDD had atypical features of hypersomnia and hyperphagia, and 34% had melancholic features [12]. Atypical depression is more common in women [13], is associated with an earlier age of onset [2], [14], frequently includes comorbid anxiety [12], [14], or avoidant personality disorder [15], [16], and is associated with drug dependence, suicidal thoughts and attempts, utilization of health services, physical and sexual abuse or neglect, and functional disability [12]. In drug trials, the prevalence of atypical depression ranged from 16% to 42% [16]. Further, patients with atypical features respond better to monoamine oxidase inhibitors than to tricyclic antidepressants [4] and may have a smaller noradrenergic activation than patients with melancholic features [17].
It is becoming increasingly clear that MDD has a substantial impact on physical health [18]. In addition to osteoporosis [19], [20], cardiovascular disease [18], and immune alterations [21], MDD often co-exists with insulin resistance [18], diabetes [22] and obesity [23]. We conducted the POWER (Premenopausal, Osteoporosis, Women, Alendronate, Depression) Study, a longitudinal study of bone turnover in women with MDD, to determine whether major depression was associated with low bone mineral density. We have recently summarized the most important findings of this study [24]: MDD was associated with osteopenia, an increased risk for insulin resistance and cardiovascular disease, a chronic state of subclinical inflammation, increased diathesis to coagulopathy states, major endocrine, immune and neuropeptide alterations, and increased propensity to chronic pain.
The goal of this ancillary study was to determine whether different clinical subtypes of depression were associated with specific clinical or laboratory abnormalities. Thus, we characterized metabolic features, bone mineral density, and endocrine circadian profiles in the clinical subtypes of MDD using validated clinical markers. Our exploratory hypothesis was that individual clinical subtypes would be associated with different endocrine and metabolic features.
Methods
Participants
Study participants were premenopausal women, ages 21 to 45 years who enrolled in the POWER (Premenopausal, Osteoporosis, Women, Alendronate, Depression) Study, a longitudinal study of bone turnover in women with MDD [20]. Recruitment was conducted from July 2001 to February 2003 in the Washington, DC metropolitan area by advertising in newspapers, radio, Internet, and flyers. Written informed consent was obtained from all study participants. This study was approved by the Institutional Review Board of the National Institute of Mental Health.
Psychiatric assessment
Participants were evaluated by clinicians trained in the use of the Structured Clinical Interview for DSM-IV for Axis I disorders, Patient Edition (SCID-IV I/P), version 2.0 [25], to diagnose unipolar MDD, according to criteria from the DSM-IV (American Psychiatric Association 1994) or to rule out any DSM-IV Axis I diagnosis (healthy controls). To minimize recall bias, an additional inclusion criterion for women with depression was having had an episode of MDD within the past three years. Melancholic and atypical subtypes were classified according to the most recent depressive episode. Patients that did not meet criteria for melancholic or atypical depression were classified as unspecified. Lifetime history of depression was estimated, including the number of depressive episodes and their length. Symptoms of depression and anxiety over the past week were assessed with the Hamilton Scale for Depression (HAM-D, 24 items) and the Hamilton Scale for Anxiety (HAM-A, 14 items).
Patients with bipolar disorder, current alcohol abuse or dependence, recent drug abuse or dependence, history of psychosis, anorexia or bulimia, or those at suicidal risk were excluded. Past or current comorbid anxiety and past binge eating disorder were not exclusionary criteria. As previously reported, 120 women with self-reported depression were screened, of whom 89 entered the study [20]. Women with MDD were classified according to the DSM-IV criteria as melancholic (n = 51) or atypical (n = 16). Depressed women whose last MDD episode had features of both melancholic and atypical depression, but did not meet criteria for either subgroup and those who met criteria for neither subgroup, were classified as having undifferentiated depression (n = 22). None of the patients with MDD had symptoms of psychosis, catatonia, or postpartum depressive features. Most patients were taking selective serotonin reuptake inhibitors (SSRIs), and no patient was taking tricyclic or monoamine oxidase inhibitors (MAOI). Sixty-three healthy controls were screened and of those, 44 women with no DSM-IV diagnoses other than past (not within the past five years) alcohol abuse were admitted into the study. Control subjects were excluded if they had any DSM-IV axis I diagnosis. Controls were matched to subjects with MDD based on age (±3 years) and BMI (±2 units), and blinded to the clinical subtype of subjects with depression. Except for two pairs, participants were matched by race as well.
Medical assessment
All participants were in good physical health, as assessed by history and medical examination at screening. Menopause, defined as the absence of spontaneous menses during the preceding 6 months, was an exclusion criterion. Estrogen/progestin contraception was allowed, if unmodified for at least 6 months prior to enrollment.
Anthropometric measurements
Body weight was measured to the nearest 0.1 kg using a platform digital scale; height was measured to the nearest 0.1 cm using a stadiometer. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2) [26]. Waist circumference (WC) was measured with a non-stretch tape to the nearest 0.1 cm at the level of the uppermost lateral border of the right iliac crest. The mean of three measurements was used for analysis.
Frequent blood sampling
Hourly blood collections were performed during a 24-hour period on 87 women who were available to complete an inpatient hospital stay: 32 with melancholic, 15 with undifferentiated, and 10 with atypical MDD features; and 30 healthy controls. Women were admitted to the inpatient unit the day before blood sampling began. Participants were placed on an ad libitum diet. Lights went off at 11 PM. Fasting blood was collected at 8 AM and then samples were taken at 1-hour intervals until 8 AM the next day for a total of 25 samples. Approximately one hour prior to sample collection, a catheter was inserted into a vein in the forearm, and the IV line was connected to the Venous/Arterial Blood Management Protection System (VAMP®, Edwards Lifescience Irvine, CA), a device that permits undiluted blood samples to be obtained from an in-line sampling site. The arm with the sampling catheter was maintained in a heating blanket to ensure arterialization of the venous sample. Blood samples were then placed in pre-chilled tubes using ethylenediaminetetraacetic acid as an anticoagulant. Approximately 0.3 liters of blood were drawn in total.
Bone mineral density and body composition
Bone mineral density (BMD) and whole body composition were assessed by dual energy X-ray absorptiometry (DXA) scan, using the Hologic DXA QDR 4500 (Bedford, MA). The coefficient of variation for BMD was <0.4%. A radiologist (JCR) reviewed the DXA films and reported the percentage of fat for the whole body and the abdominal area extending from the T12/L1 interface to the L4/L5 interface, which includes both visceral and subcutaneous fat.
Cooper test (12-minute walk/run test)
The Cooper test, which measures the distance covered during 12-minutes by running or walking as fast as possible, was used as an indirect index of physical fitness [26].
Blood tests
Plasma ACTH and serum cortisol concentrations were measured using competitive chemiluminescent immunoassays (Nichols Advantage analyzer, San Juan Capistrano, CA, for ACTH; DPC Immulite-2000 analyzer, Los Angeles, CA, for cortisol) at the NIH Clinical Center Department of Laboratory Medicine. Inter-/intra-assay coefficients of variation (CV's) were all <10%. Plasma leptin was measured by ELISA (Linco Research Inc., St. Charles, MI) at Covance Laboratories (Vienna, VA), with inter-/intra-assay CV<10%. Fasting glucose and insulin levels were determined from the 8 AM sample, and insulin resistance was calculated using the homeostasis model for insulin resistance (HOMA-IR), a useful indicator of insulin resistance in patients with diabetes mellitus type 2 and an independent predictor of cardiovascular disease [27].
Statistical analyses
Differences of means among groups were compared by analysis of variance (ANOVA), with pre-planned comparisons of each of the three MDD clinical subtypes with controls, using SAS PROC GLM (SAS Institute, Cary, NC). In case of a significant main group effect (control and the three MDD subtypes), post hoc comparisons between MDD subtypes were conducted using Tukey's least significant difference method. Categorical outcome measures were compared by Fisher's exact test.
The proportion of women with low BMD (t-score<−1.0) at the AP spine, femoral neck, or total femur was compared using logistic regression. To test for differences in the proportion of low BMD at any one or more of the three skeletal sites between women in each subtype and controls, a generalized estimating equation (GEE) model, in which the three bone sites are treated as repeated measures within each subject, was applied [28]. Analyses of BMD measures were adjusted for BMI.
Leptin and ACTH values were log transformed because of positive skewedness and were normalized, as verified by the Shapiro-Wilkes test. ACTH, cortisol, and leptin were each compared in repeated measures with mixed model analyses, using SAS PROC MIXED, where adjacent time-points were averaged for each subject, for a total of 12 values. In these analyses, the main effect of clinical subtype and its interaction with time of day were tested. Each depressive subtype was compared with controls in pre-planned contrasts. In case of a significant interaction, individual time points were compared and reported as significantly different if p<0.01. All data was reported as mean ± standard deviation (SD). Two-sided level of significance was set at a nominal 0.05 value, unless otherwise specified.
Results
Demographic characteristics and clinical features of depression
Table 1 reports the demographic and psychosocial characteristics. Women with undifferentiated MDD were slightly older and had greater BMI than controls. Women with atypical features were heavier, had an earlier age of menarche, were less often married, and less conditioned than controls. Women with melancholic features had demographic features similar to controls. Use of oral contraceptives, history of smoking, and alcohol use did not differ amongst the groups.
Table 1. Demographic characteristics of depressive subtypes of women with unipolar MDD.
| Depressive Subtype | ||||
| Characteristics | Healthy Controls (n = 44) | Undifferentiated (n = 22) | Atypical (n = 16) | Melancholic (n = 51) |
| Age (yrs) | 34.7±6.8 | 38.2±5.4b | 34.3±7.8 | 34.3±7.0 |
| Body mass index (kg/cm2) | 24.2±3.7 | 27.2±6.2b | 28.9±7.1a | 25.8±6.0 |
| Weight (kg) | 68.0±10.6 | 74.2±18.8 | 80.5±21.2a | 70.4±17.3 |
| White race | 86.4% | 90.9% | 75.0% | 90.2% |
| Years of education | 16.3±2.1 | 16.9±2.0 | 15.1±1.9a | 16.7±1.9 |
| Married (%) | 50.0% | 54.6% | 18.8%a | 31.4% |
| Ever Smoked | 31.8% | 27.3% | 50.0% | 28.1% (9/32) |
| Age at Menarche | 13.0±1.6 (n = 43) | 12.7±1.6(n = 20) | 11.9±1.6a(n = 15) | 12.5±1.6 (n = 47) |
| Previous pregnancies | 1.23±1.36 | 1.73±1.70 | 1.56±2.13 | 0.88±1.31 |
| Contraceptive Pill Users | 36.4% | 18.2% | 31.3% | 35.3% |
| Estimated Total Calcium Intake (mg/day) | 1408±755(n = 41) | 1431±844(n = 19) | 1571±699(n = 14) | 1315±561(n = 45) |
| Alcohol Consumption (g/week) | 6.9±11.6(n = 41) | 5.0±4.8 | 3.9±6.1 | 4.3±6.2 |
| Distance covered in the Cooper test (m) | 1438±280(n = 23) | 1252±253(n = 11) | 1129±192a(n = 7) | 1409±370(n = 20) |
Values are reported as mean ± SD or percent.
Sample size in parenthesis, unless otherwise indicated.
Significant comparisons, assessed as p≤.05 nominal value.
Overall: overall test.
Atypical differs from control.
Undifferentiated differs from control.
The clinical characteristics are described in Table 2. Patients were, on average, mildly depressed or in clinical remission, as indicated by Hamilton depression and anxiety scores. However, about 17% of depressed women had experienced an episode of major depression within the past month (data not shown), and most had an average of four episodes and five years of cumulative time in depression. Age of onset of depression for the three subgroups was in the late teens. The most common comorbid Axis I diagnoses were anxiety disorders, present in 44% of depressed women and in similar proportions among the depressive subtypes. More than 80% of depressed patients were taking antidepressants, and approximately 15% used anxiolytics. Specifically, women with undifferentiated features had spent longer time in depressive episodes than women with atypical features.
Table 2. Clinical characteristics of depressive subtypes of women with unipolar MDD.
| Clinical Characteristic | Undifferentiated (n = 22) | Atypical (n = 16) | Melancholic (n = 51) |
| Age of Onset | 17.5±8.6 (n = 17) | 21.6±9.6 (n = 14) | 18.9±9.2 (n = 34) |
| Number of Depressive Episodes | 4.5±2.3 (n = 17) | 4.1±2.5 (n = 14) | 4.4±2.8 (n = 34) |
| Months with Symptoms of Depression (Lifetime) | 113.0±121.4 (n = 17) | 35.7±33.8b (n = 14) | 59.8±54.4 (n = 34) |
| Global Assessment of Function (GAF) | 65±9.1 | 61±8.2 | 62±10.0 (n = 49) |
| Hamilton Scale for Depression | 9.7±8.0 (n = 19) | 11.6±8.1 | 8.1±5.3 (n = 46) |
| Hamilton Scale for Anxiety | 7.8±6.2 (n = 19) | 7.7±4.8 | 6.5±4.2 (n = 46) |
| Other Axis I Diagnoses | |||
| Post-Traumatic Stress Disorders | 22.7% | 12.5% | 8.2% (n = 49) |
| Obsessive compulsive disorders | 9.1% | 6.3% | 2.0% (n = 49) |
| Any anxiety disorder | 50.0% | 56.3% | 36.7% (n = 49) |
| History of binge eating | 4.6% | 0% | 2.0% |
| History of drug abuse | 4.6% | 37.5% | 10.2% (n = 49) |
| History of alcohol abuse | 31.3% | 37.5% | 16.3% |
| Medications | |||
| Antidepressants | 90.9% | 81.3% | 84.3% |
| Anxiolytic | 13.6% | 19.8% | 11.8% |
Values are reported as mean ± SD or percent.
Sample size in parenthesis, unless otherwise indicated.
Significant comparisons, assessed as p≤.05 nominal value.
Atypical differed from undifferentiated.
Metabolic, body composition, and bone mineral density measures
Women with undifferentiated features had more total fat, abdominal fat, and greater waist to hip ratio than controls (Table 3). Consistently, this group had a higher LDL, log triglycerides, and total cholesterol levels, as well as higher fasting glucose, insulin levels, and HOMA-IR compared to controls. After adjusting for whole body percent fat mass, the following remained significant: least squares adjusted means ± SD for undifferentiated vs. control, LDL, 123.6±27.0 vs. 108.0±27.1, p<0.05; log triglycerides, 4.7±0.50 vs. 4.3±0.51, p<0 .002; total cholesterol, 196.4±31.0 vs. 174.8±31.1, p<0.02; and fasting glucose, 93.5±9.5 vs. 88.1±9.5, p<0.05. Women with atypical features had more total body and abdominal fat, as well as greater waist/hip ratio than controls. Women with melancholic features did not differ from controls in their metabolic profiles.
Table 3. Metabolic, body composition, and bone mineral density measures.
| Depressive Subtype | ||||
| Characteristic | Healthy Controls (n = 44) | Undifferentiated (n = 22) | Atypical (n = 16) | Melancholic (n = 51) |
| Body Composition | ||||
| Whole Body Fat Mass (gm) | 21887±6574 | 28155±11896c | 30586±15164a | 24716±10996 |
| Whole Body Lean Mass (gm) | 47228±6271 | 47409±8476 | 50712±8122 | 46303±7575 |
| Whole Body % Fat | 31.2%±5.5 | 36.1±6.5c | 35.5%±9.3a | 33.4%±7.7 |
| Abdominal % Fat | 22.7%±7.5(n = 30) | 29.2%±9.6c(n = 15) | 29.6%±12.5a(n = 11) | 26.1%±10.1(n = 34) |
| Waist/Hip Ratio | 0.75±0.05(n = 41) | 0.81±0.05c(n = 19) | 0.79±0.07a(n = 13) | 0.77±0.05(n = 43) |
| Lipids | (n = 41) | (n = 19) | (n = 15) | (n = 46) |
| HDL (mg/dL) | 58.8±11.5 | 55.9±15.5 | 55.9±14.7 | 57.3±12.8 |
| LDL (mg/dL) | 106.0±22.6 | 126.1±33.0c | 118.8±31.9 | 116.2±28.4 |
| Log Triglycerides (mg/dL) | 4.25±0.40 | 4.76±0.69c | 4.44±0.61 | 4.56±0.78b |
| Total Cholesterol (mg/dL) | 173.4±25.7 | 198.1±35.8c | 186.9±32.7 | 181.8±32.8 |
| Glucose metabolism | (n = 41) | (n = 19) | (n = 15) | (n = 46) |
| Fasting Glucose (mg/dL) | 87.6±9.5 | 94.1±11.8c | 85.9±5.4 | 88.2±9.6 |
| Insulin (mcU/mL) | 6.06±4.28 | 9.72±8.16c | 9.64±5.63 | 7.70±5.12 |
| HOMA-IR | 1.46±1.13 | 2.37±2.16c | 2.03±1.11 | 1.72±1.29 |
| Percent prevalence of low BMD (T score<−1) | ||||
| AP Spine | 9.1 | 18.2 | 18.8 | 21.6 |
| Femoral Neck | 2.3 | 27.3c | 18.8 | 11.8 |
| Total Femur | 2.3 | 18.2 | 12.5 | 13.7 |
Values are reported as mean ± SD or percent.
Sample size in parenthesis, unless otherwise indicated.
Significant comparisons, assessed as p≤.05 nominal value.
Atypical differs from control.
Melancholic differs from control.
Undifferentiated differs from control.
Women with undifferentiated depression had a significantly higher prevalence of low BMD at the femoral neck than controls. Furthermore, GEE analysis indicated that low BMD at any of the three sites was more common in each subtype of depression than in controls. Odds ratios for low BMD were as follows: undifferentiated group: 6.81 (95% CI: 1.93–24.77, p<0.004); atypical group 5.83 (95% CI: 1.63–20.92, p<0.007); melancholic group: 4.22 (95% CI: 1.44–12.39, p<0.009). Higher mean 8 AM cortisol levels for all women were associated with low BMD (p<0.0001).
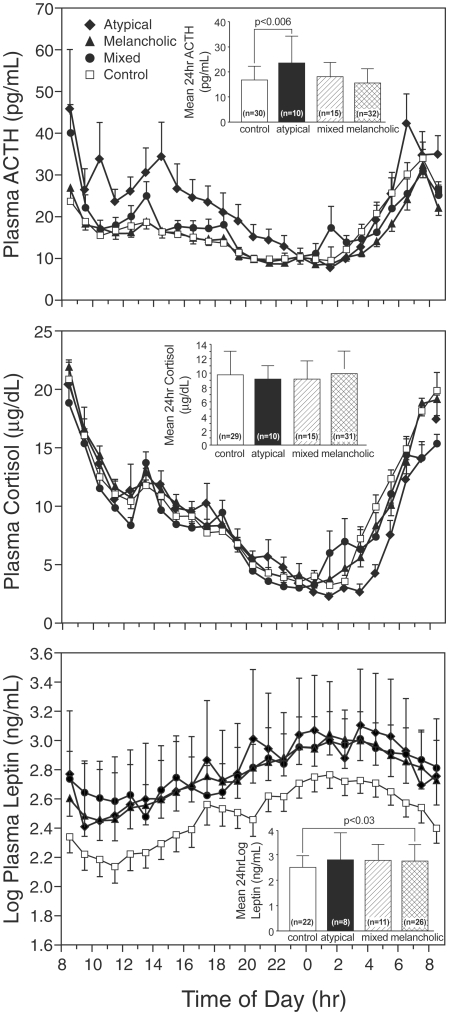
Circadian ACTH, cortisol and leptin profiles
Twenty-four hour plasma ACTH, cortisol, and leptin secretion are depicted in Figure 1. Mean 24-hour plasma ACTH concentrations were higher in the atypical subtype vs. the control group (F (1, 83) = 4.01, p<0.05), as well as in the melancholic vs. control group (F (1, 83) = 6.89, p = 0.01). In addition, a significant group by time interaction (F (33, 909) = 2.38, p<0.0001) revealed that ACTH was elevated in the atypical group only in the daytime (Figure 1), with the greatest differences between atypical and controls from 10 AM to 5 PM (all p<0.01). These differences remained significant after adjustment for BMI.
Figure 1. Circadian profiles of plasma ACTH, cortisol, and leptin.
No differences in mean 24-hour plasma cortisol values were observed. After adjustment for total body fat, the mean 24-hour adjusted log leptin value was elevated in the melancholic subgroup, as compared with controls (adjusted means = 2.84 melancholic vs. 2.63 control; F (1, 66) = 5.17, p<0.03).
Discussion
The three different phenotypes of depression exhibited distinct features compared to control subjects; women with atypical or undifferentiated MDD exhibited greater BMI, waist/hip ratio, whole body and abdominal fat mass, and higher mean 24-hour ACTH levels. (Table 4). Women with melancholic features had higher mean 24-hour leptin levels than controls. Finally, women with undifferentiated depression exhibited higher fasting lipids, glucose, and insulin levels; and had a greater prevalence of low BMD at the femoral neck than controls.
Table 4. Synopsis of significant findings in the three subtypes of depression vs. healthy controls.
| Undifferentiated | Atypical | Melancholic | |
| BMI | ⇑ | ||
| Weight | ⇑ | ||
| Percent married | ⇓ | ||
| Age at menarche | ⇓ | ||
| Physical conditioning (Cooper Test) | ⇓ | ||
| Months of symptoms of depression (Lifetime depression) | ⇓ | ||
| Waist circumference | ⇑ | ||
| Fat mass | ⇑ | ⇑ | |
| Abdominal fat mass | ⇑ | ||
| Fasting glucose | ⇑ | ||
| Fasting insulin | ⇑ | ||
| HOMA | ⇑ | ||
| LDL | ⇑ | ||
| Triglycerides | ⇑ | ⇑ | |
| Total cholesterol | ⇑ | ||
| Prevalence of low BMD | ⇑ (femoral neck) | ||
| ACTH (After adjustment for BMI) | ⇑ | ⇑ | |
| Leptin (after adjustment for body fat) | ⇑ |
Metabolic features
Both women with atypical or undifferentiated depression were heavier than controls. The greater BMI, waist circumference, and fat mass are consistent with the hyperphagia that distinguishes atypical depression. The NESDA Study, a large cohort study conducted in the Netherlands reported also higher body weight, a greater prevalence of metabolic syndrome, and more somatic symptoms in subjects with atypical depression [29]. Women with undifferentiated depression had significantly higher fasting glucose and insulin and HOMA index [30]. Specifically, the average HOMA index in this subgroup, 2.4, approached the accepted cut-off for insulin resistance, 2.6. In keeping with our findings, a recent meta-analysis reported a strong association between depression and type 2 diabetes, with a 60% increased risk in depressed subjects of developing diabetes over time [31]. We also examined lipid parameters: subjects with undifferentiated depression had higher LDL, tryglicerides and total cholesterol than controls. Some of the altered measures are part of the definition of metabolic syndrome;. MDD and metabolic syndrome co-occur, and may be related to each other in a bi-directional way, as they share common pathogenetic mechanisms, including elevated proinflammatory cytokines and glucocorticoids [32]. Whether women with undifferentiated depression have a greater risk for diabetes and cardiovascular disease than other clinical subtypes of depression, particularly after menopause when losing the protective effects of estrogens, remains to be determined in prospective studies.
Endocrine features
HPA Axis
We measured hourly plasma levels of ACTH and cortisol for 24 hours in different subtypes of depression. Alterations of the HPA axis in depression are a reliable finding in biological psychiatry. These abnormalities are however mostly observed during the depressive episodes and relate to clinical severity. The typical circadian profile of ACTH and cortisol, two pulsatile hormones, was preserved in the three clinical subtypes. This is consistent with the fact that most of these women were not depressed at the time of sampling and in clinical remission. We nevertheless observed some subtle differences compared to controls. Women with melancholic and atypical features of depression had higher plasma ACTH levels compared with controls. Higher plasma ACTH may be secondary to a dysregulation of the CRH hypothalamic neuron [33], reduced negative feedback of the pituitary corticotroph by cortisol, impaired response of the adrenals to ACTH, as well as a combination of all these factors. Although the majority of these patients were taking antidepressants, it is unlikely that higher plasma ACTH levels were due to pharmacological treatment as higher plasma ACTH has been reported in a cohort of women with untreated atypical features of depression [34]. A recent meta-analysis found no differences in ACTH levels in clinical subtypes [35]; however repeated sampling over 24 hours may unravel abnormalities not evident with single plasma sampling. Alterations in ACTH secretion have been reported in depression; with 10 min sampling over 24 hours Young et al. [36] demonstrated less “orderly” ACTH secretion, as indicated by increased entropy in non-medicated premenopausal women with depression.
Plasma leptin
Women with melancholic depression had higher leptin levels compared to controls. In a related study, we reported that 24-hour plasma leptin levels were about 30% higher in a subset of 23 women with depression compared to 23 BMI -matched controls [37]. Because leptin provides satiety cues to the brain [38], it may explain the lack of appetite in melancholic depression. Higher levels of plasma leptin in patients with melancholic depression may have been caused by increased sympathetic tone. Catecholamines stimulate leptin secretion [39] and CSF and plasma norepinephrine and epinephrine are increased in patients with melancholic depression [40]. A leptin hypothesis linking mood disorder and obesity, two largely overlapping conditions at the population level, has been formulated [41]. Extra-hypothalamic actions of leptin are beginning to emerge; these include antidepressant and mnemonic effects [42]. Inappropriately higher leptin levels for body weight in depressed subjects are reminiscent of inappropriately elevated leptin levels in obese subjects. Future studies should determine if subjects with depression develop a resistance to the antidepressant effects of leptin when gaining weight.
Reproductive history
Women with atypical depression had menarche approximately one year earlier (12 vs. 13) than control subjects. This age was, however, well within normative age, which is 12.5 in the US, based on the NHANES data [43]. In addition, given the study design, sampling bias may have contributed to the difference in age at menarche. Nevertheless, it should be noted that the risk of depression increases with decreasing age at menarche, as data from the Harvard Study of moods and cycle, a large population-based study, suggested using a nested case-control approach [44].
BMD features
The existence of an association between major depression and osteoporosis has been well documented [19], [45], and the endocrine and immune mechanisms at play have been summarized in recent reviews [24], [46]. In this study we analyzed the relationship between major depression and osteoporosis based on clinical subtypes. We found that osteopenia at the femoral neck was more prevalent in women with undifferentiated features than in controls. Higher morning cortisol levels were associated with low bone mass, but did not discriminate between the subtypes.
Study limitations and strengths
The following study limitations should be noted. Most women were on antidepressants, anxiolytics, and/or oral contraceptives; these medications may have affected some of the outcome measures. In addition, the frequency of blood sampling did not allow for hormone pulsatility analyses, and no dynamic endocrine testing was performed. Since the study was not specifically designed to assess phenotypic differences in clinical subtypes, the sample size was uneven among groups. The current analysis of phenotypic differences among clinical subtypes was of exploratory nature and the current findings should be regarded as hypothesis generating. On the other hand, this sample was prospectively assembled; homogeneous; carefully characterized in terms of clinical, endocrine, metabolic, and bone mass parameters; and a relatively large number of subjects underwent frequent sampling.
Overall clinical and pathophysiological relevance
The current findings suggest that among the three clinical subtypes, undifferentiated depression may carry the greatest cardiovascular morbidity and increased risk of diabetes and hip fractures. Historically, the construct of depressive subtypes was developed based on clinical symptoms and response to antidepressants. This study suggests that these subtypes may indeed be biologically rooted and have distinct medical consequences.
In summary, we found that different clinical subtypes of depression were associated with specific bone, metabolic, and endocrine features. Our findings lend support to the idea that depressive subtypes are not mere clinical entities but are rather distinct nosological entities, possibly underlying a differential dysregulation of the CRH hypothalamic neuron in depressive subtypes. Future studies should determine the extent to which these distinct features translate into differential medical morbidity, the genetic predisposition to different clinical phenotypes, and the stability of these traits over the lifetime.
Acknowledgments
We wish to thank: all the subjects participating in this study; Drs. David R. Rubinow and Mitchell A. Kling, NIMH, for useful comments upon reviewing the manuscript; Catherine Levy, RN and Angela Stuber, CNMT who performed the special analyses of abdominal fat in the DXA scans; Ms. Nancy Sebring, NIH CC for performing the anthropometric measures; Mr. Bart Drinkard, NIH CC for performing the Cooper test; Mrs. Kate Musallam nurse manager and all the other NIMH nurses who supported these studies; and Ms. Caitlin Toomey and Dr. Sima Mistry for careful editing of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was fully supported by the Intramural Program of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There were no current external funding sources for this study.
References
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benazzi F. Prevalence and clinical features of atypical depression in depressed outpatients: a 467-case study. Psychiatry Res. 1999;86:259–65. doi: 10.1016/s0165-1781(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 3.McGinn LK, Asnis GM, Rubinson E. Biological and clinical validation of atypical depression. Psychiatry Res. 1996;60:191–8. doi: 10.1016/0165-1781(96)02781-3. [DOI] [PubMed] [Google Scholar]
- 4.Liebowitz MR, Quitkin FM, Stewart JW, McGrath PJ, Harrison WM, et al. Antidepressant specificity in atypical depression. Arch Gen Psychiatry. 1988;45:129–37. doi: 10.1001/archpsyc.1988.01800260037004. [DOI] [PubMed] [Google Scholar]
- 5.Fountoulakis KN, Iacovides A, Gerasimou G, Fotiou F, Ioannidou C, et al. The relationship of regional cerebral blood flow with subtypes of major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2004;3:537–46. doi: 10.1016/j.pnpbp.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Quitkin FM, Rabkin JG, Stewart JW, McGrath PJ, Harrison W, et al. Sleep of atypical depressives. J Affect Disord. 1985;8:61–7. doi: 10.1016/0165-0327(85)90073-4. [DOI] [PubMed] [Google Scholar]
- 7.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatr. 2002;7:254–75. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 8.Lemus CZ, Asnis GM, Halbreich U, Harkavy Friedman JM, et al. Clinical variables and hypothalamic-pituitary-adrenal function in depression. The importance of mood reactivity. J Affect Disord. 1987;12:219–21. doi: 10.1016/0165-0327(87)90030-9. [DOI] [PubMed] [Google Scholar]
- 9.Withall A, Harris LM, Cumming SR. A longitudinal study of cognitive function in melancholic and non-melancholic subtypes of major depressive disorder. J Affect Disord. 2010;123:150–7. doi: 10.1016/j.jad.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Carragher N, Adamson G, Bunting B, McCann S. Subtypes of depression in a nationally representative sample. J Affect Disord. 2009;113:88–99. doi: 10.1016/j.jad.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Thase ME. Atypical depression: useful concept, but it's time to revise the DSM-IV criteria. Neuropsychopharmacology. 2009;34:2633–41. doi: 10.1038/npp.2009.100. [DOI] [PubMed] [Google Scholar]
- 12.Matza LS, Revicki DA, Davidson JR, Stewart JW. Depression with atypical features in the National Comorbidity Survey: classification, description, and consequences. Arch Gen Psychiatry. 2003;60:817–26. doi: 10.1001/archpsyc.60.8.817. [DOI] [PubMed] [Google Scholar]
- 13.Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, et al. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87:141–50. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Novick JS, Stewart JW, Wisniewski SR, Cook IA, Manev R, et al. Clinical and demographic features of atypical depression in outpatients with major depressive disorder: preliminary findings from STAR*D. J Clin Psychiatry. 2005;66:1002–11. doi: 10.4088/jcp.v66n0807. [DOI] [PubMed] [Google Scholar]
- 15.Perugi G, Akiskal HS, Lattanzi L, Cecconi D, Mastrocinque, et al. The high prevalence of “soft” bipolar (II) features in atypical depression. Compr Psychiatry. 1998;39:63–71. doi: 10.1016/s0010-440x(98)90080-3. [DOI] [PubMed] [Google Scholar]
- 16.Posternak MA, Zimmerman M. The prevalence of atypical features across mood, anxiety, and personality disorders. Compr Psychiatry. 2002;43:253–62. doi: 10.1053/comp.2002.33498. [DOI] [PubMed] [Google Scholar]
- 17.Asnis GM, McGinn LK, Sanderson WC. Atypical depression: clinical aspects and noradrenergic function. Am J Psychiatry. 1995;152:31–6. doi: 10.1176/ajp.152.1.31. [DOI] [PubMed] [Google Scholar]
- 18.Brown ES, Varghese FP, McEwen BS. Association of depression with medical illness: does cortisol play a role? Biol Psychiatry. 2004;55:1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- 19.Cizza G, Ravn P, Chrousos GP, Gold PW. Depression: a major, unrecognized risk factor for osteoporosis? Trends Endocrinol Metab. 2001;12:198–203. doi: 10.1016/s1043-2760(01)00407-6. [DOI] [PubMed] [Google Scholar]
- 20.Eskandari F, Martinez PE, Torvik S, Phillips TM, Sternberg EM, et al. Premenopausal, Osteoporosis Women, Alendronate, Depression (POWER) Study Group. Low bone mass in premenopausal women with depression. Arch Intern Med. 2007;167:2329–36. doi: 10.1001/archinte.167.21.2329. [DOI] [PubMed] [Google Scholar]
- 21.Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522–30. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- 22.Pearson S, Schmidt M, Patton G, Dwyer T, Blizzard L, et al. Depression and insulin resistance: cross-sectional associations in young adults. Diabetes Care. 2010;33:1128–33. doi: 10.2337/dc09-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158:1139–47. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 24.Cizza G. Major depressive disorder is a risk factor for low bone mass, central obesity, and other medical conditions. Dialogues Clin Neurosci. 2011;13:73–87. doi: 10.31887/DCNS.2011.13.1/gcizza. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID I/P), Version 2.0. 1997. Biometrics Research Department.
- 26.Eskandari F, Mistry S, Martinez PE, Torvik S, Kotila C, et al. POWER (Premenopausal, Osteopenia/Osteoporosis, Women, Alendronate, Depression) Study Group. Younger, premenopausal women with major depressive disorder have more abdominal fat and increased serum levels of prothrombotic factors: implications for greater cardiovascular risk. Metabolism. 2005;54:918–24. doi: 10.1016/j.metabol.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Pande RL, Perlstein TS, Beckman JA, Creager MA. Association of insulin resistance and inflammation with peripheral arterial disease: the National Health and Nutrition Examination Survey, 1999 to 2004. Circulation. 2008;118:33–41. doi: 10.1161/CIRCULATIONAHA.107.721878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agresti A. Categorical Data Analysis, 2nd ed. New York: John Wiley & Sons; 2002. [Google Scholar]
- 29.Lamers F, de Jonge P, Nolen WA, Smit JH, Zitman FG, et al. Identifying depressive subtypes in a large cohort study: results from the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry. 2010;71:1582–9. doi: 10.4088/JCP.09m05398blu. [DOI] [PubMed] [Google Scholar]
- 30.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, et al. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;12:3320–5. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 31.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;12:2383–90. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McIntyre RS, Rasgon NL, Kemp DE, Nguyen HT, Law CW, et al. Metabolic syndrome and major depressive disorder: co-occurrence and pathophysiologic overlap. Curr Diab Rep. 2009;9:51–9. doi: 10.1007/s11892-009-0010-0. [DOI] [PubMed] [Google Scholar]
- 33.Gold PW, Licinio J, Wong ML, Chrousos GP. Corticotropin releasing hormone in the pathophysiology of melancholic and atypical depression and in the mechanism of action of antidepressant drugs. Ann N Y Acad Sci. 1995;771:716–29. doi: 10.1111/j.1749-6632.1995.tb44723.x. [DOI] [PubMed] [Google Scholar]
- 34.Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Mol Psychiatry. 1999;4:182–8. doi: 10.1038/sj.mp.4000436. [DOI] [PubMed] [Google Scholar]
- 35.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73:114–26. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- 36.Young EA, Carlson NE, Brown MB. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology. 2001;25:267–76. doi: 10.1016/S0893-133X(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 37.Cizza G, Nguyen VT, Eskandari F, Duan Z, Wright EC, et al. Low 24-hour adiponectin and high nocturnal leptin concentrations in a case-control study of community-dwelling premenopausal women with major depressive disorder: the Premenopausal, Osteopenia/Osteoporosis, Women, Alendronate, Depression (POWER) study. J Clin Psychiatry. 2010;71:1079–87. doi: 10.4088/JCP.09m05314blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–86. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 39.Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic system. Hypertension. 2003;41:1072–1079. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 40.Gold PW, Wong ML, Goldstein DS, Gold HK, Ronsaville DS, et al. Cardiac implications of increased arterial entry and reversible 24-h central and peripheral norepinephrine levels in melancholia. Proc Natl Acad Sci USA. 2005;102:8303–8. doi: 10.1073/pnas.0503069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol. 2007;6:648–52. doi: 10.1016/j.coph.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banks WA. Extrahypothalamic effects of leptin: a therapeutic for depression and dementia? Endocrinology. 2011;152:2539–41. doi: 10.1210/en.2011-1161. [DOI] [PubMed] [Google Scholar]
- 43.Karapanou O, Papadimitriou A. Determinants of menarche. Reprod Biol Endocrinol. 2010;8:115. doi: 10.1186/1477-7827-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harlow BL, Cohen LS, Otto MW, Spiegelman D, Cramer DW. Early life menstrual characteristics and pregnancy experiences among women with and without major depression: the Harvard study of moods and cycles. J Affect Disord. 2004;79:167–76. doi: 10.1016/S0165-0327(02)00459-7. [DOI] [PubMed] [Google Scholar]
- 45.Cizza G, Primma S, Coyle M, Gourgiotis L, Csako G. Depression and osteoporosis: a research synthesis with meta-analysis. Horm Metab Res. 2010;42:467–82. doi: 10.1055/s-0030-1252020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cizza G, Primma S, Csako G. Depression as a risk factor for osteoporosis. Trends Endocrinol Metab. 2009;8:367–73. doi: 10.1016/j.tem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]