Abstract
A fundamental question in G protein coupled receptor biology is how a single ligand acting at a specific receptor is able to induce a range of signaling that results in a variety of physiological responses. We focused on Type 1 cannabinoid receptor (CB1R) as a model GPCR involved in a variety of processes spanning from analgesia and euphoria to neuronal development, survival and differentiation. We examined receptor dimerization as a possible mechanism underlying expanded signaling responses by a single ligand and focused on interactions between CB1R and delta opioid receptor (DOR). Using co-immunoprecipitation assays as well as analysis of changes in receptor subcellular localization upon co-expression, we show that CB1R and DOR form receptor heteromers. We find that heteromerization affects receptor signaling since the potency of the CB1R ligand to stimulate G-protein activity is increased in the absence of DOR, suggesting that the decrease in CB1R activity in the presence of DOR could, at least in part, be due to heteromerization. We also find that the decrease in activity is associated with enhanced PLC-dependent recruitment of arrestin3 to the CB1R-DOR complex, suggesting that interaction with DOR enhances arrestin-mediated CB1R desensitization. Additionally, presence of DOR facilitates signaling via a new CB1R-mediated anti-apoptotic pathway leading to enhanced neuronal survival. Taken together, these results support a role for CB1R-DOR heteromerization in diversification of endocannabinoid signaling and highlight the importance of heteromer-directed signal trafficking in enhancing the repertoire of GPCR signaling.
Introduction
Cannabinoid receptor signaling is involved in a variety of physiological processes including proliferation and migration, neurite elongation and guidance, synaptogenesis, and cell survival [1]–[4]. The molecular mechanisms that enable a single type of GPCR to achieve such a broad range of functions are of great physiological and clinical relevance, but to date are poorly understood.
CB1R is part of the endocannabinoid system that comprises the cannabinoid receptors, their endogenous ligands (the endocannabinoids), the enzymes that produce and inactivate the endocannabinoids, and the endocannabinoid transporters. The two major endocannabinoids, anandamide and 2-arachidonoylglycerol, are lipid-derived messengers generated by the metabolism of arachidonic acid, that acting as retrograde messengers, regulate neuritogenesis and neurite outgrowth [5]. In addition, a recent study reported longer hemopressins as peptide ligands capable of binding to CB1R and activating a distinct signal transduction pathway [6]. It is generally accepted that the endocannabinoid system is responsible for shaping the temporal and spatial diversity of cellular responses and hence likely to be involved in adaptive processes and plasticity [1], [5].
CB1R belongs to the family A of GPCRs and couples to Gi/o subtypes of heterotrimeric G proteins. CB1R activation usually results in the inhibition of adenylyl cyclase activity, inhibition of calcium channels [7], and activation of potassium channels [8]. CB1R activation also results in the activation of p42/44 MAP kinase (pERK), downstream of PLCβ [4], [9]. Finally, CB1R activation has been shown to lead to recruitment of GPCR kinase 3 and arrestin3, resulting in receptor desensitization [10]. Hence, cannabinoid receptors share a number of common characteristics with opioid receptors, and interactions between these two receptors appear to mutually modulate their activity [11]–[14].
The majority of studies examining interactions between CB1R and opioid receptors have focused on the mu opioid receptor (MOR) [15], [16], and relatively few studies have explored the interaction between CB1R and DOR. At the cellular level, in vitro studies demonstrate cross-desensitization between CB1R and DOR at various steps along the signal transduction pathway, including G protein activation and inhibition of adenylyl cyclase activity [17]–[21]. Functional interaction between CB1R and DOR has been proposed in vivo by studies showing that a DOR antagonist could block the anxiolytic activity of a low dose of the CB1R agonist Δ9tetrahydrocannabinol (THC) [22] and that mice lacking DOR show a significant increase in CB1R activity in several brain regions, as demonstrated by the [35S]GTPγS binding assay [23], [24]. These studies support the concept that CB1R and DOR interact, and that these interactions impact on CB1R activity.
In this study we characterize the direct interaction between CB1R and DOR and investigate its consequences on receptor function. We find that CB1R and DOR associate form receptor heteromers. Stimulation of CB1R within the CB1R-DOR heteromer leads to changes in CB1R signaling, including recruitment of arrestin3 to the CB1R-DOR complex and promotion of an arrestin3-mediated signaling pathway and enhanced neuronal survival. This, in turn, leads to the activation of anti-apoptotic signaling pathways. Taken together, we propose that heteromer-directed signaling leads to the diversification of endocannabinoid signaling by activating distinct signaling pathways with important physiological outcomes such as regulation of cell proliferation and apoptosis.
Materials and Methods
Materials
Neuro2A cells endogenously expressing CB1R (N2ACB1R) were obtained from ATCC. F11 cells were a gift from Dr. D. Felsenfeld (Mount Sinai School of Medicine). Monoclonal anti-phosphoERK, polyclonal anti-ERK, monoclonal anti-myc, polyclonal anti-phosphoDOR(S363), monoclonal anti-phosphoSTAT3 (Ser-727), polyclonal anti-phospho-p90rsk, polyclonal anti-STAT3, polyclonal anti-phosphop70S6K, polyclonal anti-BAD, polyclonal anti-lamin A/C and monoclonal anti-phosphoBAD antibodies were from Cell Signaling Technology Inc. Rabbit anti C-terminal CB1R antibody was from Cayman Chemicals. The polyclonal anti-calnexin and anti-FLAG antibodies and pertussis toxin were from Sigma. The anti AP-3δ (anti-delta SA4) monoclonal antibody was from the Developmental Studies Hybridoma Bank, University of Iowa. The monoclonal anti-AP-2α antibody was from BD Biosciences. Rabbit anti C-terminal CB1R and goat anti N-terminal CB1R polyclonal antibodies were gifts from Dr. Ken Mackie (University of Indiana). The mouse anti-arrestin3 antibody was from Abcam. The rat anti-DOR antibody was generated as described previously [25] and showed no specific signal in ELISA, Western Blot and immunocytochemistry assays with DOR −/− brains (see Figure S1A–C). Monoclonal anti-GAPDH antibody was from Novus Biological. The anti-pericentrin antibody was from Abcam. IRDye 680-labeled anti-rabbit or mouse and IRDye 800-labeled anti-mouse antibodies were from Li-COR. The Alexa 488-conjugated anti-goat, mouse or rabbit, Alexa 594-conjugated anti-rat, goat, mouse or rabbit and Alexa 647-conjugated anti-rabbit antibodies were from Invitrogen. HRP-conjugated anti-rabbit and anti-rat IgG antibodies were from GE Healthcare. Rabbit polyclonal anti-CB1R (C-terminal) antibody coupled to agarose beads, rabbit polyclonal anti-myc antibodies and siRNA to arrestin3 were from Santa Cruz Biotechnology. Hu210, U73122, and edelfosine were from Tocris Bioscience. Wild-type mouse DOR and DORΔ15 plasmids were characterized as described previously [26], [27]. The CellTiter-Glo® Luminescent Cell Viability Assay was from Promega. U2OS cells co-expressing prolink/enzyme donor-tagged human DOR and enzyme activator-tagged arrestin3 fusion protein and the PathHunter detection kit were from DiscoveRx.
Cell Lines and transfections
Neuro2A cells endogenously expressing CB1R (N2ACB1R) were maintained in DMEM containing 10% FBS and penicillin-streptomycin at 37°C in a humidified 5% CO2 incubator. Neuro2A cells stably expressing either myc-tagged DOR (N2ACB1RDOR) or Flag-tagged DORΔ15 (N2ACB1RDORΔ15) or transiently expressing the metalloprotease, endothelin converting enzyme-2 (N2ACB1RECE2) were grown in DMEM containing 10% FBS, penicillin-streptomycin and 500 µg/ml G418. F11 cells were grown in F12 media containing 2 mM L-glutamine, 15% FBS, HAT supplement and penicillin-streptomycin. U2OS cells co-expressing ProLink/Enzyme Donor (PK)-tagged human DOR and the Enzyme Activator (EA)-tagged β-arrestin fusion protein (a gift from DiscoveRx), were grown in MEM-alpha (Invitrogen) containing 10% fetal bovine serum, penicillin-streptomycin, 500 µg/ml geneticin and 250 µg/ml hygromycin. Transfections with plasmids and siRNAs were carried out as described [28]. For experiments with primary cortical or striatal neurons, cerebral cortices or striata were dissected from wild-type and DOR lacking (DOR−/−) mice (P1 or P2 mice pups). After trypsin treatment and mechanical trituration, neurons were seeded into poly-L-Lysine coated coverslips (cortical neurons) or 24 well plates (striatal neurons). Cells were grown for 14 days in Neurobasal-B27 media, supplemented with L-glutamine (growth media). On the third day (DIV3) after plating 10 µM cytosine arabinoside was added to inhibit glial growth.
Generation of shRNA lentiviral vector to DOR
GFP-tagged lentiviral shRNA to murine DOR was generated as described [29] by targeting 364–384 base pairs relative to the start codon of DOR. Infectious particles were produced by transient transfection of 293T cells using Effectene (Qiagen, Carlsbad, CA, USA). After determining the dose at which the virus was able to efficiently infect F11 cells without killing them (as visualized for EGFP fluorescence) we used this dose (5 µl) that also reduces DOR levels by >70% (Rozenfeld and Devi, unpublished) to examine the effect of down-regulation of DOR expression on ERK phosphorylation as described below.
Animals
DOR +/− embryos (Oprd1tm1Dgen; lacking the first exon of DOR [30]) were purchased from the Mutant Mouse Regional Resource Center (MMRRC). DOR −/− mice were generated at the Mount Sinai Mouse Genetics Research Facility by implantation of the embryos into C56/BL6J mice and two successive breeding cycles with wild-type mice from the same background (C56/BL6J). The genotypes of DOR−/− and littermate controls were confirmed by PCR analysis (not shown) and by immunofluorescence, ELISA and Western blotting with the DOR antibody (Figure S1A–C). All protocols complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Mount Sinai School of Medicine Institutional Animal Care and Use Committee (Permit # 02-0805). Brain regions from 3 month old male DOR−/− and their wild-type littermates were collected and membranes prepared as described [31]–[33].
[35S]GTPγS binding assays
Assays were carried out essentially as described [31]–[33]. Briefly, membranes from N2ACB1R and N2ACB1RDOR cells, and wild type and DOR −/− mice cortices were prepared as described [31]–[33]. The membranes (10 µg) were incubated with increasing concentrations (0–1 µM) of Hu210 in the presence of 100 µM GDP and 0.1 nM [35S]GTPγS for one hour at 30°C. Basal binding was determined in the presence of GDP and absence of agonist and cold GTPγS. Non-specific binding was determined by adding 10 µM GTPγS to a parallel set of tubes. Membrane bound radioactivity collected by filtration was detected using a scintillation counter. In experiments examining the effect of the CB1R antagonist, SR141716, cells were preincubated with SR141716 (1 µM) for 1 h prior to carrying out the assay.
Radiolabeled binding assays
Whole cell binding assays were carried out using N2ACB1R and N2ACB1RDOR cells as described previously [31]–[33] using [3H]SR141716A as a radiolabeled ligand for CB1R and [3H]DPDPE as a radiolabeled ligand for DOR. CB1R levels were 666.3±21.7 fmoles/mg protein in N2ACB1R cells and 637±48 fmoles/mg protein in N2ACB1RDOR cells. DOR levels were 1357±28.5 fmoles/mg protein in N2ACB1RDOR cells.
Cell fractionation
N2ACB1R or N2ACB1RDOR cells were grown in 6-well plates, serum starved for 6 hours, and stimulated with 100 nM Hu210 for 5 min. Nuclear and cytoplasmic fractions were prepared as described [34]. Immunoblotting for pERK and ERK were performed as described under Western blotting. The purity of the nuclear and cytoplasmic fractions was assessed by immunoblotting for the nuclear protein lamin A/C and the cytoplasmic protein GAPDH as described under Western blotting.
Coimmunoprecipitation
N2ACB1R, N2ACB1RDOR or N2ACB1RDORΔ15 cells were lysed for 1 h in lysis buffer (1% Triton, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, and 50 mM Tris-Cl, pH 7.4) containing protease inhibitor cocktail (Sigma). Cell lysates (100–200 µg of protein) were incubated with 1 µg of either rabbit polyclonal anti-CB1R (C-terminal) antibody coupled to agarose beads, anti-FLAG or anti-myc antibodies overnight at 4°C. The beads were washed three times with lysis buffer and once with the same buffer without detergent. Proteins were eluted in 60 µL of 2× Laemmli buffer containing 1% 2-mercaptoethanol, resolved by 10% SDS-PAGE, and immunoblotted for the specified antibodies as described under Western blotting.
Enzyme-linked immunosorbent assay (ELISA)
ELISA to quantify CB1R expression in either U2OS cells co-expressing ProLink/Enzyme Donor (PK)-tagged human DOR, Enzyme Activator (EA)-tagged β-arrestin fusion protein and myc-tagged mouse CB1R and to quantify DOR expression in wild-type, CB1R−/− or DOR−/− cortical membranes was carried out as described [25], [33], [35] using rabbit anti-myc (1∶1000), rat anti-DOR (1∶500), and HRP-conjugated anti-rabbit (1∶2000) or anti-rat antibodies (1∶1000). ELISA to quantify, total CB1R levels in N2ACB1R, N2ACB1RDOR or N2ACB1RECE2 cells was carried out in cells permeabilized with ice-cold methanol for 5 min while cell surface CB1R levels were determined in non-permeabilized cells using anti-CB1R (1∶500) and HRP-conjugated anti-rabbit (1∶1000) antibodies.
Confocal microscopy
Confocal microscopy imaging was carried out as described [28]. Primary cortical cells (14DIV) from wild-type or DOR−/− mice, N2ACB1R and N2ACB1RDOR cells transfected without or with a plasma membrane marker (a GFP fused with a prenylation site; Mb-GFP) or overexpressing arrestin3-eGFP (and treated without or with 100 nM Hu210 for 5 min) or F11 cells infected with scramble or DOR shRNA lentivirus were fixed with either 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) or with methanol and permeabilized with 0.1% Triton-X-100. The following antibodies were used as primary antibodies as described in the figure legends: goat anti-N-terminal CB1R (1∶500), rabbit anti-C-terminal CB1R (1∶500), rat anti-DOR (1∶500), mouse anti-myc (1∶1000), rabbit anti-phospho ERK (1∶1000), mouse anti-pericentrin (1∶1000), mouse anti-AP-3δ (1∶1000), mouse anti-AP-2α (1∶1000). The following antibodies were used as secondary antibodies: Alexa 488-conjugated anti-goat, rabbit or mouse (1∶1000), Alexa 594-conjugated anti-rat, mouse, goat or rabbit (1∶1000), Alexa 647-conjugated anti- rabbit (1∶1000). Slides were visualized with a Leica TCS SP5 confocal microscope. Images were acquired with an ×63/1.32 PL APO objective lens, and analyzed in sequential scanning mode. For studies examining colocalization of CB1R with the membrane marker Mb-GFP, colocalization was examined in horizontal and vertical sections of the cells. Metamorph software (Molecular Devices) was used for quantification of colocalization in multiple horizontal sections of 8 individual cells/group. The percentage of CB1R pixels colocalized with Mb-GFP was calculated in each section and the average for each cell represented in a graph (Figure S2C).
Phospho-ERK assays
Phospho-ERK assays were carried out as described in [28], [36]. Briefly, N2ACB1R, N2ACB1RDOR or N2ACB1RDORΔ15 cells alone or transiently transfected with control or arrestin3-targeting siRNA, or F11 cells infected with scramble or DOR shRNA lentivirus (∼40,000 cells/well) were seeded on 24-well plates. The next day, cells were starved for at least 4–6 h in serum-free medium prior to stimulation with Hu210 for the indicated times at the indicated concentrations. In some cases cells were preincubated for 30 min with indicated kinase inhibitors, followed by treatment with Hu210 in the presence of these inhibitors. In experiments examining the effect of pertussis toxin (PTX) pretreatment, cells were preincubated for 16 h with PTX (15 ng/ml) prior to carrying out the phospho-ERK assay. Cells were solubilized by directly adding 1× SDS buffer pre-warmed to 65°C, followed by sonication with a microtip for 5 sec and subjected to Western Blotting as described below.
Western blotting analyses
Western blots were carried out as described in [28], [36]. Briefly, proteins in cell lysates (30 µg protein; Laemmli buffer containing 1% 2-mercaptoethanol) were resolved in 10% SDS-PAGE gels and subjected to Western blot analysis. The following antibodies were used as primary antibodies: rabbit anti-phosphoERK (1∶1000), mouse anti-ERK (1∶1000), rabbit anti-CB1R (C-terminal; 1∶500), mouse anti-arrestin3 (1∶500), rabbit anti-phosphoDOR Ser 363 (1∶1000), mouse anti-myc (1∶1000), rabbit anti-lamin A/C (1∶2000), mouse anti-GAPDH (1∶2000), rabbit anti-STAT3 (1∶1000), mouse anti-phosphoSTAT3 (1∶1000), rabbit anti-phospho p70S6K (1∶1000), rabbit anti-phospho-p90rsk (1∶1000), rabbit anti-phosphoBAD (1∶1000), mouse anti-BAD (1∶1000), rabbit anti-calnexin (1∶1000). In experiments examining the specificity of rat anti-DOR antibodies, cortical membranes from wild-type and DOR−/− mice (30 µg protein) were solubilized in Laemmli buffer containing 1% 2-mercaptoethanol, followed by sonication with a microtip for 5 sec and subjected to Western Blotting using rat anti-DOR (1∶1000) antibodies. The following antibodies were used as secondary antibodies: IRDye 680-labeled anti-rabbit (1∶10,000), IRDye 800-labeled anti-mouse (1∶10,000) and IRDye 680-labeled anti-rat (1∶10,000). Blotting, imaging and band intensity measurements were performed using the Odyssey imaging system (LI-COR, Lincoln, NE) according to the manufacturer's protocols.
Arrestin recruitment assay
U2OS cells co-expressing ProLink/Enzyme Donor (PK)-tagged human DOR and the Enzyme Activator (EA)-tagged β-arrestin fusion protein, were transfected with myc-tagged mouse CB1R using FuGene as per manufacturer's protocol (Roche Diagnostics). Expression of myc-CB1R was determined by ELISA as described above. The day after transfection, cells were seeded in 96 well plates (20,000/well) in growth media. The following day media was removed and replaced with 90 µl growth media without antibiotics. Hu210 (0–10 µM in 10 µl) was added to the wells and incubated for 30 min. β-arrestin recruitment was determined using the PathHunter Detection Kit, as described in the manufacturer's protocol. Samples were read on a luminescence plate reader (Perkin-Elmer).
Survival and caspase-3 assays
N2ACB1R and N2ACB1RDOR cells (20,000 cells/well) were plated in complete media (+FBS) on poly-L-lysine coated 24-well plates. Next day, cells were placed in starving media (media containing 0.1% FBS). The cells were treated with 1 µM Hu210. Control cells were not subjected to any drug treatment. Fresh ligands were added daily without changing the medium. The number of live cells was determined by the Trypan Blue exclusion method. For the caspase-3 assay, N2ACB1R and N2ACB1RDOR cells (1×106 cells/well) were plated in complete media on 6-well plates. Next day, cells were placed in starving media and treated with 1 µM Hu210. Control cells were not subjected to any drug treatment. Fresh ligands were added daily without changing the medium. After 3 and 8 days, the medium was removed, wells washed with PBS, cells collected and assayed for caspase-3 activity using a colorimetric CaspACE TM assay system (Promega Corporation, Madison,WI) as per manufacturer's protocol.
For experiments involving the survival of striatal neurons, the CB1R receptor antagonist, AM251 (10 µM) was added to the growth media at DIV7 and cellular viability was assessed at DIV10 by monitoring intracellular ATP using the CellTiter-Glo® Luminescent Cell Viability Assay as described in the manufacturer's protocol.
Data Analysis
Data were analyzed (and EC50 and Emax values were determined) using Prism 4.0 (Graph Pad, San Diego, CA, U.S.A.). Student's t-test was used to determine statistical significance.
Results and Discussion
Increased CB1R signaling in the absence of DOR
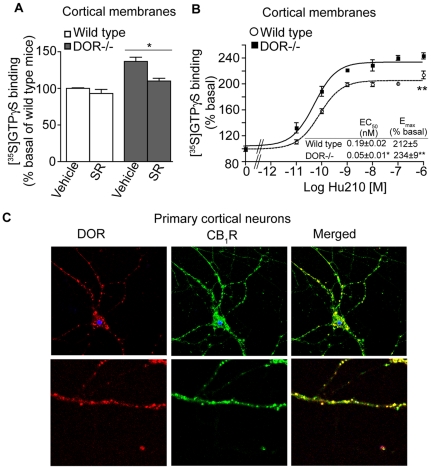
In order to examine the extent to which DOR would affect CB1R signaling, we compared classic G protein-mediated signaling in cortical membranes from wild type mice to signaling in membranes from mice lacking DOR (DOR−/−), using the [35S]GTPγS binding assay. We found that in the absence of DOR there was a small but significant increase in basal [35S]GTPγS binding; this increase could be partially blocked by treatment with the CB1R inverse agonist SR141716 (Fig. 1A). Absence of DOR also led to a ∼3-fold increase in the potency of Hu210 (a CB1R agonist) to induce [35S]GTPγS binding (Fig. 1B). These findings suggest that the presence of DOR regulates CB1R-mediated G-protein activation. This could be due to a functional interaction between CB1R and DOR arising either from an indirect intercellular regulatory mechanism (with the two receptors interacting within the same neuronal circuit) or from direct intracellular cross-talk (such as altered G protein coupling and/or physical interaction).
Figure 1. Increased CB1R activity in the absence of DOR.
A, Basal [35S]GTPγS binding was measured in cortical membranes from wild-type and DOR −/− mice. Membranes from cortices were prepared as described [31]–[33], treated with vehicle or 1 µM SR141716 (SR) for 1 hour and subjected to [35S]GTPγS binding as described in Methods. Basal [35S]GTPγS binding/10 µg protein in vehicle treated membranes is taken as 100%. Data represent Mean ± SEM (n = 3 individual animals in triplicate). Statistically significant differences between vehicle and SR141716 treatment are indicated *p<0.05, (t test). B, [35S]GTPγS binding assay in cortical membranes from wild-type and DOR −/− mice. Membranes were treated with increasing concentrations of the CB1R agonist Hu210 (0–1 µM) and [35S]GTPγS binding was measured as described in Methods. EC50 and Emax values were calculated using GraphPad Prism software. Data represent Mean ± SEM (n = 3 individual animals in triplicate). *p<0.05; **p<0.01 for DOR−/− vs wild-type (t test). C, Localization of endogenous CB1R and DOR in mouse primary cortical cells, 14DIV. Cells fixed with 4%PFA in PBS and permeablized with 0.1% Triton, were immunostained with the goat polyclonal anti-CB1R(N-terminal) antibody (1∶500; green) and rat polyclonal anti-DOR antibody (1∶500; red) and visualized using Alexa 488-conjugated anti-goat (1∶1000) and Alexa 594-conjugated anti-rat (1∶1000) secondary antibodies using confocal microscopy as described in Methods. Representative figure from 3 independent experiments shown.
CB1R and DOR co-localize in primary cortical neurons
Since the presence of intracellular cross-talk would require co-expression of these two receptors, we examined expression and localization of CB1R and DOR in primary cortical neurons. Using an antibody that selectively recognizes DOR (as evident from confocal microscopy analysis, ELISA and Western blotting assays either with cells expressing one or both receptors or membranes from DOR−/− mice and their littermate controls; Figure S1A–C) we found that DOR is expressed in primary cortical neurons (that also expresses CB1R) and that there is a large degree of co-localization between CB1R and DOR within neuronal cell bodies and in the processes (Fig. 1C). These findings suggest the possibility of intracellular interactions between CB1R and DOR in cells that express both receptors.
CB1R and DOR form interacting complexes
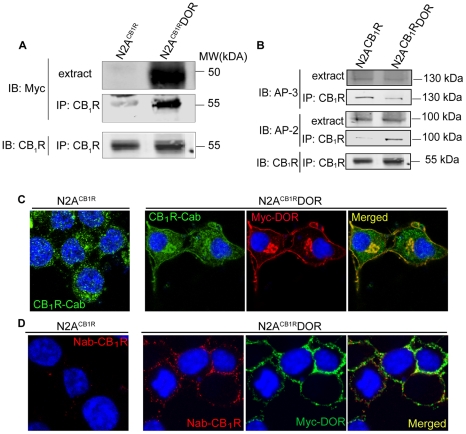
Next, we examined if CB1R and DOR exist in an interacting complex using co-immunoprecipitation analysis of membranes from Neuro2A cells that endogenously express CB1R (N2ACB1R) and from Neuro2A cells stably expressing myc-DOR (N2ACB1RDOR). We found that myc-DOR could be detected in CB1R immunoprecipitates from N2ACB1RDOR cells but not from N2ACB1R cells (Fig. 2A). Conversely, CB1R could be detected in myc-DOR immunoprecipitates from N2ACB1RDOR cells but not from N2ACB1R cells (Figure S1D). These results support a direct interaction between DOR and CB1R and are in agreement with our previous study using a bioluminescence resonance energy transfer (BRET) assay that showed that CB1R and DOR exist in close proximity for interaction in live cells [15]. In a previous study we had reported that CB1R, when expressed alone, associates with the adaptor protein AP-3 (involved in sorting to the endolysosomes) [28]. Here we find that the level of AP-3 associated with CB1R-DOR is significantly lower as compared to CB1R (Fig. 2B). In contrast, we find that the level of the adaptor protein AP-2 (involved in GPCR endocytosis [37]) associated with CB1R-DOR is substantially higher as compared to CB1R (Fig. 2B). These results, showing that the association of DOR with CB1R results in differential recruitment of adaptor proteins, suggest that CB1R-DOR interactions would lead to altered localization of CB1R.
Figure 2. Association between CB1R and DOR alters CB1R localization.
A, Lysates (100–200 µg) from N2ACB1R and N2ACB1RDOR cells were subjected to immunoprecipitation with 1 µg of anti-CB1R (C-terminal) antibody, the immunoprecipitates were resolved on 10% SDS-PAGE and probed for the presence of myc-DOR using mouse monoclonal anti-myc antibody (1∶1000) and for CB1R using rabbit polyclonal anti-CB1R (C-terminal) antibody (1∶500) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative of 3 independent experiments shown. B, CB1R-DOR complexes exhibit greater interaction with AP-2 than AP-3. Lysates (100–200 µg) from N2ACB1R and N2ACB1RDOR cells were subjected to immunoprecipitation using 1 µg of an anti-CB1R (C-terminal) antibody as described in Methods. The immunoprecipitates were resolved on 10% SDS-PAGE and probed for the presence of AP-3 (1∶1000), AP-2 (1∶1000) and CB1R (C-term) (1∶500) using specific antibodies as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative of 3 independent experiments shown. C, Localization of endogenous CB1R in N2ACB1R and of CB1R and DOR in N2ACB1RDOR cells. Cells fixed with 4%PFA in PBS and permeablized with 0.1% Triton, were stained with the rabbit polyclonal anti-CB1R (C-terminal) antibody (1∶500; green) and the mouse monoclonal anti-myc antibody (1∶1000; red) and visualized using Alexa 488-coupled anti-rabbit or Alexa 594-coupled anti-mouse secondary antibodies (1∶1000) using confocal microscopy as described in Methods. Representative of 3 independent experiments shown. D, Cell surface staining of endogenous CB1R and stably expressed DOR in N2ACB1RDOR cells. N2ACB1R and N2ACB1RDOR cells were stained with a goat polyclonal anti-CB1R (N-terminal) antibody (1∶500) and mouse monoclonal anti-myc antibodies (1∶1000) prior to fixation of the cells to label cell surface receptors, as described [28]. After fixation, cells were visualized with Alexa 594-coupled anti-goat and Alexa 488-coupled anti-mouse secondary antibodies (1∶1,000) using confocal microscopy as described in Methods. Representative of 3 independent experiments shown.
Association with DOR affects CB1R localization
Given the differential recruitment of adaptor proteins by CB1R-DOR, and the finding that when expressed alone, CB1R is mostly present in intracellular vesicles [37], [38], whereas DOR is found primarily at the cell surface, we examined changes in the subcellular localization of CB1R in the presence of DOR. Consistent with previous findings, we observed that in N2ACB1R cells, endogenous CB1R is not detected at the plasma membrane, but is mostly localized in intracellular compartments (Fig. 2C, left). In contrast, in N2ACB1RDOR cells, endogenous CB1R is localized at the plasma membrane (Fig. 2C, right). This DOR-induced plasma membrane localization of CB1R was confirmed by incubating non-permeabilized living N2ACB1R and N2ACB1RDOR cells with primary antibodies raised against extracellular epitopes of CB1R and myc-DOR (Fig. 2D). Under these conditions, there was virtually no CB1R staining in N2ACB1R cells (Fig. 2D, left) whereas both CB1R and DOR were readily detected at the plasma membrane of N2ACB1RDOR cells (Fig. 2D, right).
We also quantified CB1R levels at the plasma membrane in N2ACB1R and N2ACB1RDOR cells by measuring the extent of colocalization with a plasma membrane marker, a GFP fused with a prenylation site (Mb-GFP) (Figure S2A–C). We found <5% of CB1R colocalized with Mb-GFP in N2ACB1R cells, whereas >40% of CB1R staining colocalized with the plasma membrane marker in N2ACB1RDOR cells. In order to determine the specificity of the change in CB1R localization, we measured the changes in cell surface CB1R immunoreactivity in Neuro2A cells stably expressing the metalloprotease ECE2, a type II transmembrane protein that is transported to the plasma membrane via the secretory pathway (Gagnidze and Devi, unpublished). While the presence of DOR led to a significant increase in cell surface CB1R immunoreactivity, the presence of ECE2 did not affect CB1R plasma membrane levels (Figure S2D), confirming the specificity of CB1R-DOR interaction in modulating CB1R cell surface expression. We also examined the effect of decreasing endogenous DOR expression (by shRNA-expressing lentivirus) in F11 cells (that express both DOR [39] and CB1R [40]) on the localization of endogenous CB1R. We found that in cells transduced with a DOR shRNA-expressing lentivirus, there was no detectable CB1R at the cell surface (Figure S2E) indicating that expression of DOR modulates the localization of CB1R. These results are consistent with the notion that DOR associates with CB1R and facilitates its cell surface localization.
Co-expression of CB1R and DOR affects CB1R-mediated ERK signaling
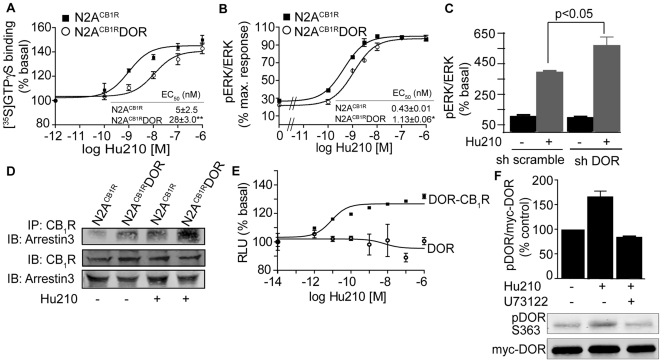
We examined if the association with DOR altered CB1R signaling by examining CB1R-mediated G protein coupling in N2ACB1RDOR cells. The presence of DOR led to a ∼10-fold decrease in the potency of Hu210 (a CB1R agonist) to induce [35S]GTPγS binding (Fig. 3A). We also examined the presence of DOR on CB1R-mediated modulation of pERK levels; pERK is a known downstream effector of CB1R. We find that the presence of DOR led to a 3-fold decrease in the potency of Hu210 to phosphorylate ERK (Fig. 3B & Figure S3A). In order to confirm that CB1R signal modulation was solely due to DOR co-expression, we downregulated DOR using shRNA expressing lentivirus in F11 cells that endogenously co-express CB1R and DOR and we examined pERK levels in response to CB1R activation. We find that downregulation of DOR leads to enhancement in pERK levels as compared to scrambled shRNA treatment (Fig. 3C & Figure S3B). Taken together these results are consistent with the idea that association with DOR leads to reduction in CB1R signaling; this could be due to recruitment of distinct sets of signaling molecules.
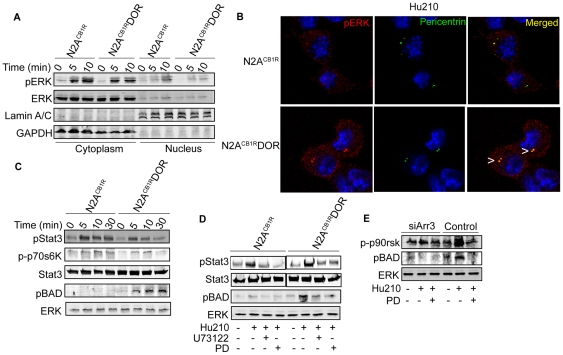
Figure 3. PLC-dependent arrestin3 association with CB1R-DOR complex.
A, [35S]GTPγS binding assay in membranes from N2ACB1R and N2ACB1RDOR cells. Membranes (10 µg) were treated with indicated concentrations of the CB1R agonist Hu210. [35S]GTPγS binding was measured as described in Methods. EC50 and Emax values were calculated using GraphPad Prism software. Data represent Mean ± SEM (n = 3 independent experiments in triplicate). B, Dose-response of Hu210-mediated ERK phosphorylation in N2ACB1R and N2ACB1RDOR cells. Starved N2ACB1R and N2ACB1RDOR cells seeded in 24 well-plates were treated with indicated concentrations of Hu210 for 5 minutes. Cell lysates (30 µg protein) were analyzed by Western blotting and probed for the levels of pERK (1∶1000) and ERK (1∶1000) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). EC50 and Emax values were calculated using GraphPad Prism software. Data represent Mean ± SEM (n = 3 independent experiments). *p<0.05 for N2ACB1RDOR vs N2ACB1R (t test). C, Effect of DOR down-regulation on ERK phosphorylation. F11 cells transduced with the DOR shRNA expressing lentivirus were starved for 4–6 h and treated with Hu210 (100 nM) for 5 min. Cell lysates (30 µg protein) were analyzed by Western blotting and probed for the levels of pERK (1∶1000) and ERK (1∶1000) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Data from 3 independent experiments is shown. *p<0.05 (t test). D, Examination of arrestin3 interaction with CB1R after Hu210 treatment. N2ACB1R and N2ACB1RDOR, starved for 4 hours were stimulated with 100 nM Hu210 for 5 minutes and cell lysates prepared as described in Methods. Lysates (30 µg protein) were subjected to either Western blotting using rabbit anti-CB1R (C-terminal 1∶500) and mouse anti-arrestin 3 antibodies (1∶500) or to immunoprecipitation using 1 µg of agarose-coupled anti-CB1R (C-terminal) antibody. Immunoprecipitates were probed for arrestin3 levels by Western blot using the mouse anti-arrestin 3 antibody. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative of 3 independent experiments shown. E, Effect of Hu210 on arrestin recruitment. U2OS cells co-expressing ProLink/Enzyme Donor (PK)-tagged DOR and the Enzyme Activator (EA)-tagged arrestin3 fusion protein without or with CB1R were treated with indicated concentrations of Hu-210. Arrestin3 recruitment was determined using the PathHunter Detection Kit as described in Methods. Data represent Mean ± SEM (n = 4). F, Effect of PLC inhibitor (U73122) on DOR phosphorylation at serine 363 after Hu210 treatment. N2ACB1RDOR cells were starved for 4–6 hours, and incubated with vehicle (DMSO) or U73122 (1 µM) for 30 minutes, then stimulated with 100 nM Hu210 for 5 minutes. Cell lysates (30 µg protein) were subjected to Western blotting using rabbit polyclonal phosphoDOR Ser 363 (1∶1000), mouse monoclonal anti-myc (1∶1000) antibodies and IR Dye 680 anti-rabbit and IR Dye 800 anti-mouse secondary antibodies (1∶10,000) as described in Methods. Data represent Mean ± SEM (n = 3).
CB1R activation leads to arrestin3 recruitment in cells co-expressing CB1R and DOR
We hypothesized that reduced CB1R signaling in N2ACB1RDOR cells could be due to altered arrestin3 recruitment to CB1R, as previous studies had demonstrated arrestin recruitment to opioid receptor heteromers [36]. We tested this by co-immunoprecipitation and found that the levels of arrestin3 associated with CB1R in the presence of DOR are higher than in the absence of DOR (Fig. 3D). Interestingly, activation of CB1R leads to a further increase (>2-fold higher in N2ACB1RDOR cells as compared to N2ACB1R cells) in arrestin3 recruitment (Fig. 3D). Further support for this comes from studies using the PathHunter arrestin assay (DiscoveRx). In this assay, DOR is fused to one fragment of β-galactosidase (β-gal), and arrestin3 is fused to its complementary fragment. Upon arrestin recruitment to DOR the complementation of the two β-gal fragments result in the restoration of enzyme activity. When CB1R (untagged) is expressed in these cells, we find that Hu210 treatment leads to a dose-dependent increase in β-gal activity; this is seen only in cells co-expressing CB1R and DOR and not in cells only expressing DOR (Fig. 3E) confirming activity dependent recruitment of arrestin3 to the heteromer. We directly examined enhanced arrestin3 recruitment to the CB1R-DOR heteromer by confocal microscopy analysis of N2ACB1RDOR cells expressing arrestin3-eGFP. We found that Hu210 treatment induces arrestin3 translocation to the cell membrane to a greater extent in N2ACB1RDOR cells as compared to N2ACB1R alone (Figure S2F). These findings suggest that the presence of DOR enhances arrestin3 binding to CB1R in cells co-expressing CB1R and DOR.
Next, we tested if DOR phosphorylation at Ser363 is differentially affected by CB1R-DOR interactions since this site is reported to be involved in desensitization and recruitment of arrestin3 [41]. We find that in N2ACB1RDOR cells, CB1R stimulation induced an increase in DOR Ser363 phosphorylation (Fig. 3F). Interestingly, PLC inhibitors, U73122 or edelfosine, completely block this effect (Fig. 3F & Figure S3C). These results suggest that the presence of DOR in N2ACB1RDOR cells leads to phosphorylation of DOR C-tail resulting in arrestin3 recruitment to the CB1R-DOR complex and PLC activity appears to be involved in this process.
Promotion of arrestin3-dependent signaling in cells co-expressing CB1R and DOR
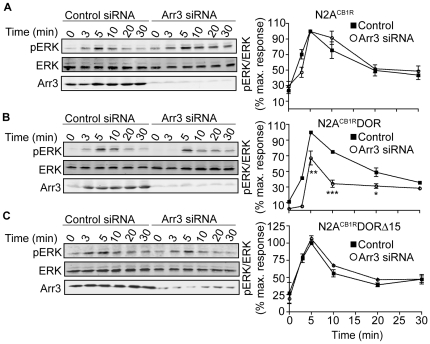
Next we examined arrestin3-dependent signaling by focusing on MAPK phosphorylation since previous studies have reported arrestin3 to mediate G protein-independent ERK activation [36], [42]. Interestingly, arrestin3 downregulation by RNAi led to a significant decrease in pERK levels in N2ACB1RDOR, but failed to do so in N2ACB1R cells (Fig. 4A & 4B) underscoring a role for arrestin3 in ERK signaling. We next examined the contribution of DOR C-tail to arrestin3-mediated signaling. We find that in N2ACB1RDORΔ15 cells (in which the C-term tail of DOR is deleted, but still forms heteromers with CB1R, see Figure S1D), arrestin3 down-regulation did not affect Hu210-mediated ERK phosphorylation (Fig. 4C). Since these 15 amino acids in the C-terminus contain residues important for receptor phosphorylation and downregulation [26], these results indicate that the C-terminal tail of DOR is required for recruiting and facilitating arrestin-dependent signaling by the heteromer. Taken with our previous observations that CB1R activation leads to phosphorylation of DOR at the C-terminal tail Ser363 (Fig. 3F), these results elucidate a possible mechanism for CB1R-DOR heteromer-mediated signaling that involves recruitment of novel, heteromer-specific signalosomes by DOR C-tail. Interestingly, we find that Hu210-mediated ERK phosphorylation is blocked by pretreatment with pertussis toxin in both in N2ACB1R and in N2ACB1RDOR cells (Figure S3D). This suggests that, in these cells, Hu210 treatment leads to arrestin3 mediated G-protein dependent ERK activation. The finding that cannabinoid stimulation leads to DOR phosphorylation-dependent arrestin3 recruitment indicates that direct interaction with DOR contributes to the desensitization of CB1R. In addition, in contrast to a single PLCβ-mediated signaling pathway, CB1R in the presence of DOR engages both PLC- and arrestin3-mediated pathways to phosphorylate ERK.
Figure 4. Engagement of arrestin3-dependent signaling in N2ACB1RDOR.
Time course of Hu210-mediated ERK phosphorylation in A, N2ACB1R; B, N2ACB1RDOR; and C, N2ACB1RDORΔ15 cells transfected with control or arrestin3-targeting siRNA. N2ACB1R alone or stably expressing either DOR or DORΔ15, transfected with a control or arrestin3-targeting siRNA, were starved for 4 hours, then stimulated with 100 nM Hu210 for the indicated times. Cell lysates (30 µg protein) were subjected to Western blotting for the levels of ERK (1∶1000), pERK (1∶1000), and arrestin3 (1∶500) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Data represent Mean ± SEM (n = 3); *p<0.05; **p<0.01; ***p<0.001, for control vs Arr3 siRNA (t test).
Novel signaling pathways activated in cells co-expressing CB1R and DOR
We have previously shown that GPCR heteromerization can lead to changes in the localization of pERK [36]. We examined the location of pERK in N2ACB1RDOR and N2ACB1R cells following CB1R activation and found a small but consistent increase in pERK in the nucleus of N2ACB1R but not in N2ACB1R DOR cells (Fig. 5A). However, upon further examination of N2ACB1RDOR cells, it was clear that pERK exhibited a distinct punctate distribution in a juxtanuclear compartment and this was not seen in N2ACB1R cells (Fig. 5B). This pERK enriched compartment in N2ACB1RDOR cells was identified as centrosomes using the centrosome marker, pericentrin (Fig. 5B). This is interesting since previous studies have reported the presence of arrestin3 and associated signaling molecules in centrosomes [43], [44]. This, taken with the reports that arrestin3 can regulate the phosphorylation of BAD and thereby activate anti-apoptotic signaling pathways [45] led us to predict that pERK substrates involved in cell survival would be differentially phosphorylated in N2ACB1RDOR vs. N2ACB1R cells. To test this we examined the phosphorylation levels of BAD, STAT3 and p70S6K as potential pERK substrates [4], [9]. We found that CB1R activation leads to substantially enhanced phosphorylation of BAD and reduced phosphorylation of STAT3 and p70S6K in N2ACB1RDOR cells as compared N2ACB1R cells (Fig. 5C & D). Furthermore, inhibition of PLC and MEK or downregulation of arrestin3 leads to a substantial decrease in phosphoBAD levels (Fig. 5D & 5E; Figure S4) supporting the involvement of the arrestin-pERK pathway in mediating BAD phosphorylation in N2ACB1RDOR cells (Fig. 6 D & E). The activation of this pathway, which has been implicated in blockade of apoptosis [46], suggests a role for heteromer-directed signaling in cannabinoid-mediated cell survival and differentiation.
Figure 5. Role of arrestin3 and ERK substrates in cannabinoid signaling by the CB1R-DOR heteromer.
A–B, Effect of CB1R-DOR heteromerization on subcellular localization of pERK. A, N2ACB1R and N2ACB1RDOR cells were treated with Hu210 (100 nM; 0, 5 or 10 min), cytoplasmic and nuclear extracts were prepared as described in Methods and analyzed (30 µg protein) by Western blotting with pERK (1∶1000), ERK (1∶1000), lamin A/C (1∶2000), and GAPDH (1∶2000) antibodies. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). B, N2ACB1R and N2ACB1RDOR cells treated with Hu210 (100 nM; 5 min) were immunostained with pERK (1∶1000, red) and pericentrin (1∶1000, green) antibodies and visualized using Alexa 488-conjugated anti-rabbit (1∶1000) and Alexa 594-conjugated anti-mouse (1∶1000) secondary antibodies using confocal microscopy as described in Methods. Representative of 3 independent experiments shown. C, Time course of phosphorylation of pERK substrates. Lysates (30 µg protein) from N2ACB1R and N2ACB1RDOR cells treated with Hu210 (100 nM; 0, 5, 10 or 30 min) were analyzed by Western blotting with STAT3 (1∶1000), phosphoSTAT3 (1∶1000), phospho-p70S6K (1∶1000), phospho-p90rsk (1∶1000) and phosphoBAD (1∶1000) antibodies. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). ERK (1∶1000) is used as a loading control. Representative of 3 independent experiments shown. D, Involvement of PLC and MEK in the phosphorylation of STAT3 and BAD. Lysates (30 µg protein) from N2ACB1R and N2ACB1RDOR cells treated with 100 nM Hu210 for 5 min in the absence or presence of U73122 (1 µM) or PD98059 (PD, 10 µM) were analyzed by Western blotting with STAT3 (1∶1000), phosphoSTAT3 (1∶1000), and phosphoBAD (1∶1000) antibodies. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). ERK (1∶1000) is used as a loading control. Representative of 3 independent experiments shown. E, Involvement of arrestin3 in BAD phosphorylation. Arrestin3 was down-regulated in N2ACB1RDOR cells by transfection with a siRNA. These cells were stimulated with Hu210 (100 nM) for 5 min, in the absence or presence of PD (10 µM). Lysates (30 µg protein) were analyzed by Western blotting with phospho-p90rsk (1∶1000) and phosphoBAD (1∶1000) antibodies. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). ERK (1∶1000) is used as a loading control. Representative of 3 independent experiments shown.
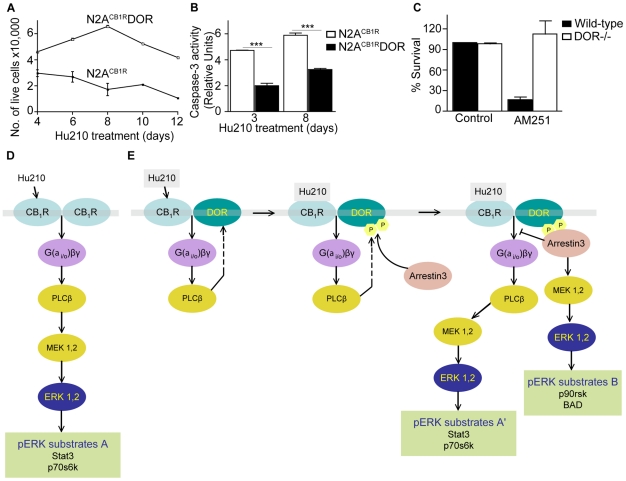
Figure 6. CB1R-DOR heteromerization promotes cell survival.
A, Hu210-treated N2ACB1RDOR cells exhibit increased survival compared to N2ACB1R cells. N2ACB1R or N2ACB1RDOR cells were treated with 1 µM Hu210 for the indicated days and survival measured by trypan blue exclusion as described in Methods. Data represent Mean ± SEM (n = 4 in triplicate). B, Hu210-treated N2ACB1RDOR cells exhibit lower apoptosis as compared to N2ACB1R cells. Apoptosis of N2ACB1R or N2ACB1RDOR treated for 3 or 8 days with 1 µM Hu210 was measured using caspase-3 activity assay as described in Methods. Data represent Mean ± SEM (n = 4 in triplicate); ***p<0.001 N2ACB1RDOR vs N2ACB1R (t test). C, CB1R antagonist treatment decreases neuronal survival of striatal neurons from wild-type but not DOR−/− mice. Primary striatal neurons from wild-type or from DOR−/− mice were prepared as described in Methods. The CB1R antagonist AM251 (10 µM) was added to the growth media at DIV7 and cellular viability assessed at DIV10 as described in Methods. Data represent Mean ± SEM (n = 2–4) D–E, A schematic of the signaling pathways emanating from CB1R in N2ACB1R (D) and N2ACB1RDOR (E) cells. Activation of CB1R in N2ACB1RDOR cells leads to differential activation of signaling molecules and phosphorylation of ERK substrates.
Cell survival is enhanced and apoptosis is decreased in cells co-expressing CB1R and DOR
We directly examined Hu210-mediated cell survival in N2ACB1RDOR cells, and compared it to N2ACB1R cells. We found a ∼2–4-fold increase in cell survival of N2ACB1RDOR compared to N2ACB1R cells (Fig. 6A). We also found that the enhanced survival of N2ACB1RDOR cells was due to decreased apoptosis, since the level of caspase-3 activity was reduced by ∼2-fold in N2ACB1RDOR compared to N2ACB1R cells (Fig. 6B). Next we tested the involvement of CB1R-DOR interactions in cell survival of primary cortical neurons from wild-type and compared that to the survival of neurons from DOR−/− mice. We find that treatment with the CB1R antagonist, AM251 leads to a substantial and significant decrease in survival of wild-type cortical neurons; this treatment did not affect survival of neurons from DOR −/− mice (Fig. 6C). This effect of the CB1R antagonist AM251, largely absent in neurons lacking DOR, supports the idea that activation of CB1R-DOR in the context of the heteromer leads to unique signaling involved in neuronal survival. These observations are relevant in light of recent studies reporting a role for cannabinoid signaling in neuronal development [5]. Therefore our results support the notion that DOR provides CB1R with a platform for recruiting new signaling molecules, leading to new levels of receptor regulation and fine-tuning of physiologically important signaling.
In this study, we show that the functional properties of CB1R are modulated by co-expression and heteromerization with DOR. We show that: (i) CB1R and DOR interacting complexes can be immunoprecipitated from cells expressing these receptors; (ii) CB1R receptors exhibit cell surface localization only in the presence of DOR; (iii) arrestin3 is recruited in a PLC dependent fashion to CB1R-DOR after Hu210 stimulation in cells expressing both receptors; and (iv) arrestin3 recruitment leads to an alternate pathway for pERK activation, which in turn leads to enhanced cell survival. These results support the notion that CB1R and DOR form heteromers, facilitating the promotion of distinct signaling pathways. A schematic summarizing this is shown in Fig. 6 D & E. In N2ACB1R cells, Hu210-mediated activation leads to PLC-dependent pERK activation via the classical G protein-PLC pathway, leading to the phosphorylation of ERK substrates STAT3 and p70S6 kinase (substrates “A”). In N2ACB1RDOR cells, Hu210 treatment leads to the PLC-dependent phosphorylation of DOR and recruitment of arrestin3 to the CB1R-DOR complex; activation of the arrestin3 pathway can lead to ERK activation and phosphorylation of additional ERK substrates p90rsk and BAD (substrates “B”). Enhanced arrestin3 recruitment to the CB1R-DOR complex may be associated with increased CB1R desensitization resulting in reduced, G protein-dependent-ERK activation via the classical pathway (leading to phosphorylation of substrates “A”).
The results of this study, together with other recent work, suggest a special role for arrestin3 in directing signals from interacting GPCRs [36]. We have previously shown that direct interaction between MOR and DOR leads to distinct signaling through recruitment of arrestin3 and promotion of arrestin-mediated signaling which, in turn, leads to the phosphorylation of distinct pERK substrates and activation of downstream transcription factor activity [36]. These previous findings, along with our current findings suggest that arrestin3 may play a pivotal role in orchestrating fine tune signaling regulation of GPCR heteromers.
In this study we show that a single cannabinoid agonist (Hu210) is able to elicit distinct signaling responses by binding and activating the same receptor, CB1R, based on its association with another GPCR, in this case DOR. We also show that the new pathway activated by CB1R in the presence of DOR is involved in the regulation of cell survival, suggesting that GPCR heteromerization contributes to the promotion of context-specific cellular responses, which occur only upon co-expression of the two receptors. Thus, the presence of DOR can influence CB1R activity either by direct interactions where DOR could serve as a chaperone helping to target CB1R to the cell surface and induce the recruitment of novel signaling molecules to the CB1R-DOR heteromer or by indirectly modulating signaling pathways activated by CB1R. In the GPCR field, it has recently been established that one GPCR can elicit multiple types of signaling/biological responses when stimulated by different types of ligands; this is termed ‘ligand-directed signaling specificity’ [47], [48]. Thus some agonists have been shown to preferentially activate G protein-mediated pathways while other agonists for the same receptor preferentially activate an arrestin-mediated pathway [49]. In this context, our current findings establish a key role for receptor heteromerization in directing signal specificity in response to a single ligand which we term as ‘heteromer-directed signaling specificity’. We identify the biochemical pathways by which heteromers facilitate distinct signaling cascades and show that activation of a heteromer-specific signaling pathway by a single agonist can induce distinct biological effects.
Supporting Information
A–C, Specificity of rat polyclonal DOR antibody: A, Immunofluorescence with mouse primary cortical cells, 14DIV. Cells from wild type or DOR −/− mice were fixed with 4% PFA in PBS and permeablized with 0.1% Triton, then stained with 1∶500 dilution of rat polyclonal anti-DOR antibody (red) and 1∶1000 dilution of Alexa Fluor 594 goat anti-rat secondary antibodies. Representative of 3 independent experiments shown. B, ELISA with cortical membranes from wild type, CB1R −/−, and DOR −/− mice. ELISA was carried essentially as described [25], [33], [35] with cortical membranes from wild type, CB1R −/−, and DOR −/− mice prepared as described [31]–[33] and 1∶500 dilution of rat polyclonal anti-DOR antibody and 1∶1000 dilution of HRP-conjugated anti-rat secondary antibody. Data represent Mean ± SEM (n = 3 animals/group). C, Western blot with cortical membranes from DOR −/− and wild-type mice. Cortical membranes (∼30 µg) from DOR −/− and wild-type mice prepared as described [31]–[33] were subjected to Western blotting using 1∶1000 dilution of rat polyclonal anti-DOR and rabbit polyclonal anti-calnexin (CNX) antibodies and 1∶10,000 dilution of IRDye 680-labeled anti-rat and IRDye 800-labeled anti-rabbit antibodies as described in Methods. (n = 2 animals/group). D, CB1R and DOR form interacting complexes. Lysates were prepared from N2A cells endogenously expressing CB1R (N2ACB1R) or stably transfected with either myc tagged DOR (N2ACB1RDOR) or Flag tagged DORΔ15 (N2ACB1RDORΔ15). (Left panel) Lysates (∼30 µg) were subjected to Western blotting with anti-CB1R (1∶500) or anti-calnexin (1∶1000) antibodies followed by incubation with IRDye 680-labeled anti-rabbit antibody (1∶10,000) as described in Methods. (Right panel) Lysates (100–200 µg) were subjected to immunoprecipitation using either anti-myc or anti-Flag antibodies (1 µg) to pulldown myc tagged DOR or Flag tagged DORΔ15 immunoprecipitates respectively. The immunoprecipitates were resolved on 10% SDS-PAGE and probed for the presence of CB1R (1∶500) as described in Methods. Representative of 3 independent experiments shown.
(TIF)
A, N2ACB1R or B, N2ACB1RDOR cells were transfected with a plasma membrane marker (Mb-GFP, green), and the cells were stained with the rabbit polyclonal anti-C-ter CB1R antibody (1∶500; magenta) and the mouse monoclonal anti-myc antibody (1∶1000; red). The secondary antibodies used were Alexa 594-coupled goat anti-mouse and Alexa 647-coupled goat anti-rabbit antibodies (1∶1,000). Colocalization of CB1R with the plasma membrane marker was examined in horizontal (left panel) and vertical (xzy) (right panel) sections of the cells. The position of xzy section is indicated by a white line. Representative of 3 experiments shown. C, Quantification of CB1R localized at plasma membrane in N2ACB1R or N2ACB1RDOR cells. Using the metamorph software (Molecular Devices), multiple horizontal cross-sections of 8 individual cells per group were used for quantification, and the average of all the values obtained per cell is plotted. Briefly, the percentage of CB1R pixels colocalized with Mb-GFP was calculated in each section, and the average for each cell is represented in the graph. ***p<0.001 N2ACB1R vs N2ACB1RDOR cells. In colocalization studies we find <5% of CB1R colocalize with Mb-GFP in N2ACB1R cells alone, whereas, >40% colocalize with the plasma membrane marker in N2ACB1RDOR cells. D, Cell surface localization of CB1R in N2ACB1R stably expressing DOR or ECE2. Non-permeabilized and permeabilized N2ACB1R stably expressing either DOR or ECE-2 were used to quantify the cell surface and total CB1R levels by ELISA as described in Methods. Increase in plasma membrane CB1R is seen in N2ACB1RDOR cells but not in cells stably expressing a type II transmembrane protein, the metalloprotease endothelin converting enzyme 2 (ECE2), confirming the specificity of DOR in the alteration of CB1R localization. Data represents Mean ± SE (n = 3). E, Effect of DOR down-regulation on CB1R cell surface localization. F11 cells transduced with the DOR shRNA expressing lentivirus, were stained with an anti-N-ter CB1R antibody (1∶500) prior to fixation of the cells to label cell surface receptors and visualized using Alexa 594 anti-rabbit antibodies (1∶1000) as described in Methods. Among the cells visualized, the green ones are transduced with the shRNA expressing virus (visualized as green, due to the concurrent expression of nuclear-targeted eGFP). CB1R could not be detected at the cell surface of the transduced cells (see arrowheads), whereas CB1R was present at the cell surface of untransduced cells (see arrows). F, Localization of arrestin3 in N2ACB1R and N2ACB1RDOR cells. N2ACB1R and N2ACB1RDOR cells transfected with arrestin3-eGFP were stimulated with 100 nM Hu210 for 5 min. Cells were fixed with methanol and incubated with DAPI for nuclear staining (blue). Representative of 3 experiments shown.
(TIF)
A, Dose-response of Hu210-mediated ERK phosphorylation in N2ACB1R and N2ACB1RDOR cells. Starved N2ACB1R and N2ACB1RDOR cells seeded in 24 well-plates were treated with indicated concentrations of Hu210 for 5 minutes. Cell lysates (30 µg protein) were analyzed by Western blotting and probed for the levels of pERK (1∶1000) and ERK (1∶1000) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative blot from 3 independent experiments shown. B, Effect of DOR down-regulation on ERK phosphorylation. F11 cells transduced with the DOR shRNA expressing lentivirus were starved for 4–6 h and treated with Hu210 (100 nM) for 5 min. Cell lysates (30 µg protein) were analyzed by Western blotting and probed for the levels of pERK (1∶1000) and ERK (1∶1000). IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative blot from 3 independent experiments shown. C, Effect of PLC inhibitor (edelfosine) on DOR phosphorylation at serine 363 after Hu210 treatment in N2ACB1RDOR cells. N2ACB1RDOR cells were starved for 4–6 hours, and incubated with vehicle (DMSO) or edelfosine (1 µM) for 30 minutes, then stimulated with 100 nM Hu210 for 5 minutes. Cell lysates (30 µg protein) were subjected to Western blotting and probed for the levels of phospho-DOR Ser 363 (1∶1000) and myc-DOR (1∶1000) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Data represent Mean ± SEM (n = 3). D, Effect of pertussis toxin on Hu210-mediated ERK phosphorylation in N2ACB1R and N2ACB1RDOR cells. Starved N2ACB1R and N2ACB1RDOR cells pretreated with pertussis toxin as described in Methods were treated with increasing concentrations of Hu210 (0–100 nM) for 5 minutes. Cell lysates (30 µg protein) were analyzed by Western blotting and probed for the levels of pERK (1∶1000) and ERK (1∶1000). IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Quantitation of data obtained with 100 nM Hu210 shown. Data represent Mean ± SEM (n = 3 independent experiments).
(TIF)
A, Effect of PLC inhibitor (edelfosine) on ERK phosphorylation. N2ACB1R and N2ACB1RDOR cells were starved for 4–6 hours, and incubated with vehicle (DMSO) or edelfosine (1 µM) for 30 minutes, then stimulated with 100 nM Hu210 for 5 minutes. Cell lysates (30 µg protein) were subjected to Western blotting and probed for the levels of pERK (1∶1000) and ERK (1∶1000) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative blot from 3 independent experiments shown. B, Effect of PLC inhibitor (edelfosine) on STAT3 phosphorylation. N2ACB1R and N2ACB1RDOR cells were starved for 4–6 hours, and incubated with vehicle (DMSO) or edelfosine (1 µM) for 30 minutes, then stimulated with 100 nM Hu210 for 5 minutes. Cell lysates (30 µg protein) were subjected to Western blotting and probed for the levels of pSTAT3 (1∶1000) and STAT3 (1∶1000) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative blot from 3 independent experiments shown. C, Effect of PLC inhibitor (edelfosine) on BAD phosphorylation. N2ACB1R and N2ACB1RDOR cells were starved for 4–6 hours, and incubated with vehicle (DMSO) or edelfosine (1 µM) for 30 minutes, then stimulated with 100 nM Hu210 for 5 minutes. Cell lysates (30 µg protein) were subjected to Western blotting and probed for the levels of pBAD (1∶1000) and BAD (1∶1000) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative blot from 3 independent experiments shown.
(TIF)
Acknowledgments
We thank DiscoveRx Corporation for their kind gifts of U2OS cells co-expressing tagged human DOR along with tagged arrestin3 fusion protein and the PathHunter Detection Kit. We thank Dr. K. Mackie for the rabbit anti-C-terminal CB1R and goat anti-N-terminal CB1R polyclonal antibodies and for the CB1R−/− brains and Dr. D. Felsenfeld for the gift of F11 cells.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Institutes of Health grants DA08862, DA19521 to LD and 1P50GM071558-01A27398 (Systems Biology Center of New York) to LD and AM, P20AA017067 to RR, GM54508 to RB and R24 CA095823 to MSSM-Microscopy Shared Resource Facility. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 2.Harkany T, Keimpema E, Barabas K, Mulder J. Endocannabinoid functions controlling neuronal specification during brain development. Mol Cell Endocrinol. 2008;286:S84–90. doi: 10.1016/j.mce.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Guzman M, Sanchez C, Galve-Roperh I. Cannabinoids and cell fate. Pharmacol Ther. 2002;95:175–184. doi: 10.1016/s0163-7258(02)00256-5. [DOI] [PubMed] [Google Scholar]
- 4.Bromberg KD, Ma'ayan A, Neves SR, Iyengar R. Design logic of a cannabinoid receptor signaling network that triggers neurite outgrowth. Science. 2008;320:903–909. doi: 10.1126/science.1152662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harkany T, Guzman M, Galve-Roperh I, Berghuis P, Devi LA, et al. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Gomes I, Grushko JS, Golebiewska U, Hoogendoorn S, Gupta A, et al. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 2009;23:3020–3029. doi: 10.1096/fj.09-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma'ayan A, Jenkins SL, Barash A, Iyengar R. Neuro2A differentiation by Galphai/o pathway. Sci Signal. 2009;2:cm1. doi: 10.1126/scisignal.254cm1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin W, Brown S, Roche JP, Hsieh C, Celver JP, et al. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J Neurosci. 1999;19:3773–3780. doi: 10.1523/JNEUROSCI.19-10-03773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cichewicz DL, Cox ML, Welch SP, Selley DE, Sim-Selley LJ. Mu and delta opioid-stimulated [35S]GTP gamma S binding in brain and spinal cord of polyarthritic rats. Eur J Pharmacol. 2004;504:33–38. doi: 10.1016/j.ejphar.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 12.Manzanares J, Corchero J, Fuentes JA. Opioid and cannabinoid receptor-mediated regulation of the increase in adrenocorticotropin hormone and corticosterone plasma concentrations induced by central administration of delta(9)-tetrahydrocannabinol in rats. Brain Res. 1999;839:173–179. doi: 10.1016/s0006-8993(99)01756-4. [DOI] [PubMed] [Google Scholar]
- 13.Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, et al. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002;22:1146–1154. doi: 10.1523/JNEUROSCI.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 15.Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bushlin I, Rozenfeld R, Devi LA. Cannabinoid-opioid interactions during neuropathic pain and analgesia. Curr Opin Pharmacol. 10:80–86. doi: 10.1016/j.coph.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dill JA, Howlett AC. Regulation of adenylate cyclase by chronic exposure to cannabimimetic drugs. J Pharmacol Exp Ther. 1988;244:1157–1163. [PubMed] [Google Scholar]
- 18.Law PY, Koehler JE, Loh HH. Comparison of opiate inhibition of adenylate cyclase activity in neuroblastoma N18tG2 and neuroblastoma x glioma NG108-15 hybrid cell lines. Mol Pharmacol. 1982;21:483–491. [PubMed] [Google Scholar]
- 19.Shapira M, Gafni M, Sarne Y. Independence of, and interactions between, cannabinoid and opioid signal transduction pathways in N18TG2 cells. Brain Res. 1998;806:26–35. doi: 10.1016/s0006-8993(98)00697-0. [DOI] [PubMed] [Google Scholar]
- 20.Rubovitch V, Gafni M, Sarne Y. The involvement of VEGF receptors and MAPK in the cannabinoid potentiation of Ca2+ flux into N18TG2 neuroblastoma cells. Brain Res Mol Brain Res. 2004;120:138–144. doi: 10.1016/j.molbrainres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Korzh A, Keren O, Gafni M, Bar-Josef H, Sarne Y. Modulation of extracellular signal-regulated kinase (ERK) by opioid and cannabinoid receptors that are expressed in the same cell. Brain Res. 2008;1189:23–32. doi: 10.1016/j.brainres.2007.10.070. [DOI] [PubMed] [Google Scholar]
- 22.Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2002;163:111–117. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- 23.Berrendero F, Mendizabal V, Murtra P, Kieffer BL, Maldonado R. Cannabinoid receptor and WIN 55 212-2-stimulated [35S]-GTPgammaS binding in the brain of mu-, delta- and kappa-opioid receptor knockout mice. Eur J Neurosci. 2003;18:2197–2202. doi: 10.1046/j.1460-9568.2003.02951.x. [DOI] [PubMed] [Google Scholar]
- 24.Uriguen L, Berrendero F, Ledent C, Maldonado R, Manzanares J. Kappa- and delta-opioid receptor functional activities are increased in the caudate putamen of cannabinoid CB1 receptor knockout mice. Eur J Neurosci. 2005;22:2106–2110. doi: 10.1111/j.1460-9568.2005.04372.x. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Decaillot FM, Gomes I, Tkalych O, Heimann AS, et al. Conformation-state sensitive antibodies to G-protein-coupled receptors. J Biol Chem. 2007;282:5116–5124. doi: 10.1074/jbc.M609254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cvejic S, Trapaidze N, Cyr C, Devi LA. Thr353, located within the COOH-terminal tail of the delta opiate receptor, is involved in receptor down-regulation. J Biol Chem. 1996;271:4073–4076. doi: 10.1074/jbc.271.8.4073. [DOI] [PubMed] [Google Scholar]
- 27.Decaillot FM, Befort K, Filliol D, Yue S, Walker P, et al. Opioid receptor random mutagenesis reveals a mechanism for G protein-coupled receptor activation. Nat Struct Biol. 2003;10:629–636. doi: 10.1038/nsb950. [DOI] [PubMed] [Google Scholar]
- 28.Rozenfeld R, Devi LA. Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. Faseb J. 2008;22:2311–2322. doi: 10.1096/fj.07-102731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fedorova E, Battini L, Prakash-Cheng A, Marras D, Gusella GL. Lentiviral gene delivery to CNS by spinal intrathecal administration to neonatal mice. J Gene Med. 2006;8:414–424. doi: 10.1002/jgm.861. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 31.Gomes I, Filipovska J, Devi LA. Opioid receptor oligomerization. Detection and functional characterization of interacting receptors. Methods Mol Med. 2003;84:157–183. doi: 10.1385/1-59259-379-8:157. [DOI] [PubMed] [Google Scholar]
- 32.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, et al. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, et al. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith ER, Smedberg JL, Rula ME, Xu XX. Regulation of Ras-MAPK pathway mitogenic activity by restricting nuclear entry of activated MAPK in endoderm differentiation of embryonic carcinoma and stem cells. J Cell Biol. 2004;164:689–699. doi: 10.1083/jcb.200312028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta A, Devi LA. The use of receptor-specific antibodies to study G-protein-coupled receptors. Mt Sinai J Med. 2006;73:673–681. [PubMed] [Google Scholar]
- 36.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. Faseb J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 38.Rozenfeld R. Type I cannabinoid receptor trafficking: all roads lead to lysosome. Traffic. 12:12–18. doi: 10.1111/j.1600-0854.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- 39.Fan SF, Shen KF, Scheideler MA, Crain SM. F11 neuroblastoma x DRG neuron hybrid cells express inhibitory mu- and delta-opioid receptors which increase voltage-dependent K+ currents upon activation. Brain Res. 1992;590:329–333. doi: 10.1016/0006-8993(92)91116-v. [DOI] [PubMed] [Google Scholar]
- 40.Coutts AA, Anavi-Goffer S, Ross RA, MacEwan DJ, Mackie K, et al. Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J Neurosci. 2001;21:2425–2433. doi: 10.1523/JNEUROSCI.21-07-02425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong MH, Xu C, Wang YJ, Ji JL, Tao YM, et al. Role of Src in ligand-specific regulation of delta-opioid receptor desensitization and internalization. J Neurochem. 2009;108:102–114. doi: 10.1111/j.1471-4159.2008.05740.x. [DOI] [PubMed] [Google Scholar]
- 42.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 43.Shankar H, Michal A, Kern RC, Kang DS, Gurevich VV, et al. Non-visual arrestins are constitutively associated with the centrosome and regulate centrosome function. J Biol Chem. 2010;285:8316–8329. doi: 10.1074/jbc.M109.062521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molla-Herman A, Boularan C, Ghossoub R, Scott MGH, Burtey A, et al. Targeting of beta-arrestin 2 to the centrosome and primary cilium: role in cell proliferation control. PlosOne. 2008;3:e3728. doi: 10.1371/journal.pone.0003728. doi: 10.1371/journal.pone.0003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. beta -arrestin2 mediates anti-apoptotic signaling through regulation of bad phosphorylation. J Biol Chem. 2009 doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergmann A. Survival signaling goes BAD. Dev Cell. 2002;3:607–608. doi: 10.1016/s1534-5807(02)00328-3. [DOI] [PubMed] [Google Scholar]
- 47.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 48.Rozenfeld R, Devi LA. Exploring a role for heteromerization in GPCR signalling specificity. Biochem J. 433:11–18. doi: 10.1042/BJ20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, et al. beta-arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A–C, Specificity of rat polyclonal DOR antibody: A, Immunofluorescence with mouse primary cortical cells, 14DIV. Cells from wild type or DOR −/− mice were fixed with 4% PFA in PBS and permeablized with 0.1% Triton, then stained with 1∶500 dilution of rat polyclonal anti-DOR antibody (red) and 1∶1000 dilution of Alexa Fluor 594 goat anti-rat secondary antibodies. Representative of 3 independent experiments shown. B, ELISA with cortical membranes from wild type, CB1R −/−, and DOR −/− mice. ELISA was carried essentially as described [25], [33], [35] with cortical membranes from wild type, CB1R −/−, and DOR −/− mice prepared as described [31]–[33] and 1∶500 dilution of rat polyclonal anti-DOR antibody and 1∶1000 dilution of HRP-conjugated anti-rat secondary antibody. Data represent Mean ± SEM (n = 3 animals/group). C, Western blot with cortical membranes from DOR −/− and wild-type mice. Cortical membranes (∼30 µg) from DOR −/− and wild-type mice prepared as described [31]–[33] were subjected to Western blotting using 1∶1000 dilution of rat polyclonal anti-DOR and rabbit polyclonal anti-calnexin (CNX) antibodies and 1∶10,000 dilution of IRDye 680-labeled anti-rat and IRDye 800-labeled anti-rabbit antibodies as described in Methods. (n = 2 animals/group). D, CB1R and DOR form interacting complexes. Lysates were prepared from N2A cells endogenously expressing CB1R (N2ACB1R) or stably transfected with either myc tagged DOR (N2ACB1RDOR) or Flag tagged DORΔ15 (N2ACB1RDORΔ15). (Left panel) Lysates (∼30 µg) were subjected to Western blotting with anti-CB1R (1∶500) or anti-calnexin (1∶1000) antibodies followed by incubation with IRDye 680-labeled anti-rabbit antibody (1∶10,000) as described in Methods. (Right panel) Lysates (100–200 µg) were subjected to immunoprecipitation using either anti-myc or anti-Flag antibodies (1 µg) to pulldown myc tagged DOR or Flag tagged DORΔ15 immunoprecipitates respectively. The immunoprecipitates were resolved on 10% SDS-PAGE and probed for the presence of CB1R (1∶500) as described in Methods. Representative of 3 independent experiments shown.
(TIF)
A, N2ACB1R or B, N2ACB1RDOR cells were transfected with a plasma membrane marker (Mb-GFP, green), and the cells were stained with the rabbit polyclonal anti-C-ter CB1R antibody (1∶500; magenta) and the mouse monoclonal anti-myc antibody (1∶1000; red). The secondary antibodies used were Alexa 594-coupled goat anti-mouse and Alexa 647-coupled goat anti-rabbit antibodies (1∶1,000). Colocalization of CB1R with the plasma membrane marker was examined in horizontal (left panel) and vertical (xzy) (right panel) sections of the cells. The position of xzy section is indicated by a white line. Representative of 3 experiments shown. C, Quantification of CB1R localized at plasma membrane in N2ACB1R or N2ACB1RDOR cells. Using the metamorph software (Molecular Devices), multiple horizontal cross-sections of 8 individual cells per group were used for quantification, and the average of all the values obtained per cell is plotted. Briefly, the percentage of CB1R pixels colocalized with Mb-GFP was calculated in each section, and the average for each cell is represented in the graph. ***p<0.001 N2ACB1R vs N2ACB1RDOR cells. In colocalization studies we find <5% of CB1R colocalize with Mb-GFP in N2ACB1R cells alone, whereas, >40% colocalize with the plasma membrane marker in N2ACB1RDOR cells. D, Cell surface localization of CB1R in N2ACB1R stably expressing DOR or ECE2. Non-permeabilized and permeabilized N2ACB1R stably expressing either DOR or ECE-2 were used to quantify the cell surface and total CB1R levels by ELISA as described in Methods. Increase in plasma membrane CB1R is seen in N2ACB1RDOR cells but not in cells stably expressing a type II transmembrane protein, the metalloprotease endothelin converting enzyme 2 (ECE2), confirming the specificity of DOR in the alteration of CB1R localization. Data represents Mean ± SE (n = 3). E, Effect of DOR down-regulation on CB1R cell surface localization. F11 cells transduced with the DOR shRNA expressing lentivirus, were stained with an anti-N-ter CB1R antibody (1∶500) prior to fixation of the cells to label cell surface receptors and visualized using Alexa 594 anti-rabbit antibodies (1∶1000) as described in Methods. Among the cells visualized, the green ones are transduced with the shRNA expressing virus (visualized as green, due to the concurrent expression of nuclear-targeted eGFP). CB1R could not be detected at the cell surface of the transduced cells (see arrowheads), whereas CB1R was present at the cell surface of untransduced cells (see arrows). F, Localization of arrestin3 in N2ACB1R and N2ACB1RDOR cells. N2ACB1R and N2ACB1RDOR cells transfected with arrestin3-eGFP were stimulated with 100 nM Hu210 for 5 min. Cells were fixed with methanol and incubated with DAPI for nuclear staining (blue). Representative of 3 experiments shown.
(TIF)
A, Dose-response of Hu210-mediated ERK phosphorylation in N2ACB1R and N2ACB1RDOR cells. Starved N2ACB1R and N2ACB1RDOR cells seeded in 24 well-plates were treated with indicated concentrations of Hu210 for 5 minutes. Cell lysates (30 µg protein) were analyzed by Western blotting and probed for the levels of pERK (1∶1000) and ERK (1∶1000) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative blot from 3 independent experiments shown. B, Effect of DOR down-regulation on ERK phosphorylation. F11 cells transduced with the DOR shRNA expressing lentivirus were starved for 4–6 h and treated with Hu210 (100 nM) for 5 min. Cell lysates (30 µg protein) were analyzed by Western blotting and probed for the levels of pERK (1∶1000) and ERK (1∶1000). IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative blot from 3 independent experiments shown. C, Effect of PLC inhibitor (edelfosine) on DOR phosphorylation at serine 363 after Hu210 treatment in N2ACB1RDOR cells. N2ACB1RDOR cells were starved for 4–6 hours, and incubated with vehicle (DMSO) or edelfosine (1 µM) for 30 minutes, then stimulated with 100 nM Hu210 for 5 minutes. Cell lysates (30 µg protein) were subjected to Western blotting and probed for the levels of phospho-DOR Ser 363 (1∶1000) and myc-DOR (1∶1000) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Data represent Mean ± SEM (n = 3). D, Effect of pertussis toxin on Hu210-mediated ERK phosphorylation in N2ACB1R and N2ACB1RDOR cells. Starved N2ACB1R and N2ACB1RDOR cells pretreated with pertussis toxin as described in Methods were treated with increasing concentrations of Hu210 (0–100 nM) for 5 minutes. Cell lysates (30 µg protein) were analyzed by Western blotting and probed for the levels of pERK (1∶1000) and ERK (1∶1000). IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Quantitation of data obtained with 100 nM Hu210 shown. Data represent Mean ± SEM (n = 3 independent experiments).
(TIF)
A, Effect of PLC inhibitor (edelfosine) on ERK phosphorylation. N2ACB1R and N2ACB1RDOR cells were starved for 4–6 hours, and incubated with vehicle (DMSO) or edelfosine (1 µM) for 30 minutes, then stimulated with 100 nM Hu210 for 5 minutes. Cell lysates (30 µg protein) were subjected to Western blotting and probed for the levels of pERK (1∶1000) and ERK (1∶1000) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative blot from 3 independent experiments shown. B, Effect of PLC inhibitor (edelfosine) on STAT3 phosphorylation. N2ACB1R and N2ACB1RDOR cells were starved for 4–6 hours, and incubated with vehicle (DMSO) or edelfosine (1 µM) for 30 minutes, then stimulated with 100 nM Hu210 for 5 minutes. Cell lysates (30 µg protein) were subjected to Western blotting and probed for the levels of pSTAT3 (1∶1000) and STAT3 (1∶1000) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative blot from 3 independent experiments shown. C, Effect of PLC inhibitor (edelfosine) on BAD phosphorylation. N2ACB1R and N2ACB1RDOR cells were starved for 4–6 hours, and incubated with vehicle (DMSO) or edelfosine (1 µM) for 30 minutes, then stimulated with 100 nM Hu210 for 5 minutes. Cell lysates (30 µg protein) were subjected to Western blotting and probed for the levels of pBAD (1∶1000) and BAD (1∶1000) as described in Methods. IRDye 680 anti-rabbit and IRDye 800 anti-mouse were used as secondary antibodies (1∶10,000). Representative blot from 3 independent experiments shown.
(TIF)