Abstract
Many studies have demonstrated that women are substantially underrepresented in cardiovascular trials, but few have considered that women develop cardiovascular disease at older ages than men. The extent to which observed gender enrollment inequalities persist after accounting for age-gender differences in disease prevalence is unknown. The purpose of the study was to compare observed rates of women participating in cardiovascular clinical trials with expected rates of female participation based on age- and gender-specific population disease prevalence. Publications between 1997 and 2009 in the three leading medical journals were included to calculate observed women's enrollment rates. Population-based data in Canada were used to determine the expected enrollment rates of women. Multicenter, randomized cardiovascular clinical trials that enrolled both men and women were analyzed. Two reviewers independently extracted data on women's enrollment and important clinical trial characteristics. The female enrollment rate was 30% in the included 325 trials, which ranged from 27% in trials of coronary artery disease, 27% in heart failure, 31% in arrhythmia, to 45% in primary prevention. Increased female enrollment correlated strongly with increasing age at recruitment in cardiovascular clinical trials (P < 0.001). After accounting for age- and gender-specific differences in disease prevalence, gaps in female enrollment were much lower than the expected enrollment rates estimated by 5% in coronary artery disease, 13% in heart failure, 9% in arrhythmia, and 3% in primary prevention. Only cardiovascular trials were evaluated in our study. Female underrepresentation in cardiovascular clinical trials is smaller than conventionally believed after accounting for age- and gender-specific population disease prevalence. Our findings suggest that greater representation of women in cardiovascular clinical trials can be achieved through the recruitment of older populations.
KEY WORDS: female representation, age bias, randomized clinical trials, cardiovascular disease
INTRODUCTION
Although cardiovascular disease is one of the leading causes of morbidity and mortality among women, uncertainties remain regarding the safety and efficacy of many cardiovascular treatments in women.1–3 This is partly because women have been substantially underrepresented in randomized clinical trials,4–8 which are considered to be the most reliable form of scientific evidence that influences clinical practice.9 For example, in the landmark Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) trial, more than 40,000 patients were enrolled to evaluate the safety and effectiveness of fibrinolytic therapy, but women constituted only 25% of the trial participants.10 The recognition of female underrepresentation led to the National Institutes of Health Revitalization Act in 1993, which aimed to increase enrollment of women and minorities in clinical trials.11 Despite this and numerous public initiatives to raise awareness about women and heart disease, recent data continue to show that women's enrollment lags substantially behind that of men.4–8
Indeed, the reason for the persistent pattern of female underrepresentation in cardiovascular trials is largely unknown. It is well documented that patients enrolled in clinical trials are substantially different from the general population.12–15 For example, older patients with multiple chronic conditions are less likely to be enrolled in clinical trials than their younger and healthier counterparts.12 This enrollment pattern, coupled with the fact that women develop cardiovascular disease at older ages, may have a profound impact on enrollment rates of women in cardiovascular trials. To our knowledge, the extent to which observed gender enrollment inequalities persist after accounting for age- and gender-differences in population disease prevalence is unknown.
An improved understanding in women's enrollment patterns is essential for developing strategies to ensure greater generalizability of clinical trial results. This in turn will have a positive impact on the care of women with cardiovascular disease. Accordingly, we performed a systematic review of landmark cardiovascular clinical trials to address the following objectives: first, we evaluated enrollment rates of women in landmark cardiovascular clinical trials over the past decade; second, we determined the association between female representation and age of recruitment in trials; finally, we compared observed and expected enrollment rates of women based on population prevalence of cardiovascular disease.
METHODS
Study Selection
We limited our systematic review to studies published in the Journal of the American Medical Association (JAMA), The Lancet, and the New England Journal of Medicine (NEJM). These three medical journals were chosen because they had the highest impact factor, and published articles are most likely to have a significant impact on clinical practice. A similar methodology of focusing on the highest impact journals has been employed previously.16 We identified major cardiovascular trials using the search term “cardiovascular disease” in PubMed/National Library of Medicine, and limited the search to randomized clinical trials. Our search included studies that were published between January 1, 1997 and December 31, 2009. We excluded clinical trials performed in a single institution and trials enrolling fewer than 100 patients because they were often pre-clinical studies or early phases of clinical trials. We also excluded clinical trials that restricted enrollment to only men or only women to be consistent with prior studies that evaluated female enrollment patterns.4,6,7
Data Abstraction
Data were abstracted independently by two of the authors (WT, TZ). Only the original published reports were considered in the data abstraction process. Abstracted data included patient demographics (age, gender) and trial characteristics (inclusion and exclusion criteria, year of publication, number of study sites, trial location, intervention type, study population, and funding source). Intervention type was categorized as drugs, devices (e.g., coronary stents, implantable cardioverter defibrillators), or other. The study population was categorized as coronary artery disease, heart failure, arrhythmia, primary cardiovascular prevention, and other. Funding source was grouped as (1) not-for-profit, financed exclusively by federal, state, or other not-for-profit foundations; (2) for-profit, financed exclusively by pharmaceutical or device manufacturers; (3) not-for-profit and for-profit, financed jointly by not-for-profit and for-profit organizations; and (4) not stated.
Statistical Analysis
We first calculated the overall observed enrollment rate of women in cardiovascular clinical trials in the included studies. Observed enrollment rates were then stratified by study population. The expected enrollment rate of women in clinical trials was estimated using age- and gender-specific prevalence of cardiovascular disease in the Canadian general population using several data sources.17,18 Although Canadian data were used as opposed to data from the US, we have previously demonstrated similar patient demographics and co-morbidities among myocardial infarction and heart failure patients between these countries.19–21 Furthermore, these data were unselected and population-based, which allowed an accurate estimation of gender-specific disease prevalence.
Population data for coronary artery disease and heart failure were estimated using hospitalization records in Canada.17 This was appropriate because many of the included trials evaluated hospitalized patients with acute coronary syndrome and heart failure. Expected enrollment rate of arrhythmia trials was estimated using hospitalization records for atrial fibrillation in Ontario, Canada. Atrial fibrillation was chosen rather than all cardiac arrhythmia because the majority of the selected clinical trials enrolled patients with atrial fibrillation. Population data for primary prevention were estimated using data from the Canadian Community of Health Survey, cycle 4.1 (2007).18
To compare the observed rates of women participating in landmark cardiovascular clinical trials with expected rates of women's participation based on age- and gender-specific population disease prevalence, we estimated the difference between observed and expected in each trial and evaluated whether this difference was significant using a one-sample t-test. We also repeated the analyses using linear regression to regress observed rates on expected rates, with an indicator variable for each trial. Average annual change in female enrollment rates was evaluated using linear regression, with the proportion of women enrolled as the dependent variable and the publication year of the trials as the independent variable. The association between enrollment rates of women and age at recruitment into cardiovascular clinical trials was assessed using Pearson’s correlation and linear regression analysis.
Statistical models were created using R version 2.9.0 (2009, The R Foundation for Statistical Computing). P values of < 0.05 were considered statistically significant.
RESULTS
Female Representation in Cardiovascular Clinical Trials
A total of 325 landmark cardiovascular trials that enrolled more than 1.2 million patients were eligible for inclusion in the systematic review. The mean age at recruitment was 64 years, with the most common study population being trials of coronary artery disease (Table 1). The majority of the trials (66%) evaluated drug interventions, and for-profit companies sponsored 60% of the trials. The overall observed enrollment rate of women was 30% in the landmark cardiovascular trials. Enrollment rates of women ranged from 27% in trials of coronary artery disease, 27% in heart failure, 31% in arrhythmia, to 45% in primary prevention. Enrollment rates of women in different characteristics of trials are shown in Table 1. All the trials included in our systematic review had had a minimum age of enrollment of 18 years; 69 trials (21%) had an upper age exclusion criteria, which was on average 80 years of age.
Table 1.
Characteristics of the 325 Trials Included in the Systematic Review
| Number of trials, n (%) | Mean age at recruitment (years) | Enrollment rates of women (%) | |
|---|---|---|---|
| Overall | 325 (100) | 64 | 30 |
| Journal | |||
| JAMA | 82 (25) | 64 | 30 |
| NEJM | 145 (45) | 64 | 28 |
| The Lancet | 98 (30) | 63 | 32 |
| Number of enrolled patients | |||
| 100–499 | 76 (23) | 62 | 29 |
| 500–999 | 42 (13) | 62 | 25 |
| 1,000–4,999 | 129 (40) | 64 | 29 |
| 5,000–9,999 | 41 (13) | 65 | 31 |
| >10,000 | 37 (11) | 63 | 34 |
| Trial population* | |||
| Coronary artery disease | 182 (56) | 63 | 27 |
| Heart failure | 50 (15) | 66 | 27 |
| Arrhythmia | 47 (15) | 70 | 31 |
| Prevention | 42 (13) | 64 | 45 |
| Othera | 4 (1) | 62 | 31 |
| Interventional type† | |||
| Drug | 217 (67) | 64 | 30 |
| Device | 90 (28) | 64 | 28 |
| Other | 20 (6) | 66 | 32 |
| Funding source | |||
| Not-for-profit | 56 (17) | 64 | 30 |
| For-profit | 195 (60) | 64 | 30 |
| Jointly funded | 61 (19) | 64 | 28 |
| None stated | 13 (4) | 63 | 25 |
| Country of origin of enrolled patients | |||
| US | 193 (59) | 64 | 29 |
| Others | 132 (41) | 64 | 30 |
| Publication year | |||
| 1997–2000 | 75 (23) | 62 | 30 |
| 2001–2004 | 100 (31) | 64 | 30 |
| 2005–2009 | 217 | 64 | 29 |
*Trial population included in the other category included cardiac transplants or valvular heart disease studies
†Interventional type in the other category included behavioral, dietary, and nondevice surgical interventions. Two trials enrolled drug and device
Abbreviations: JAMA, Journal of the American Medical Association; NEJM, New England Journal of Medicine; n, number
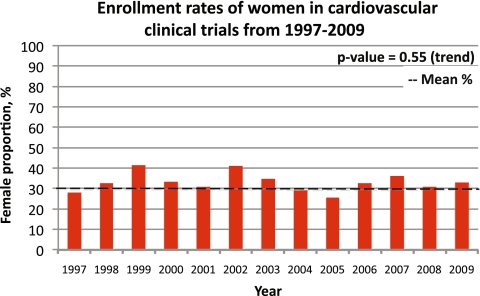
In 1997, the enrollment rate of women in cardiovascular trials was 27%, and in 2009, the proportion of female enrollment was 32% (Fig. 1). Over this period, no significant change in enrollment rates of women into cardiovascular trials was observed (P = 0.55). As well, enrollment rates of women did not differ substantially in trials when factors such as funding sources, study sample sizes, locations of recruitment, interventional types, or publication year were examined.
Figure 1.
Enrollment rates of women in cardiovascular clinical trials from 1997 to 2009.
Association of Women's Enrollment Rates and Age at Recruitment
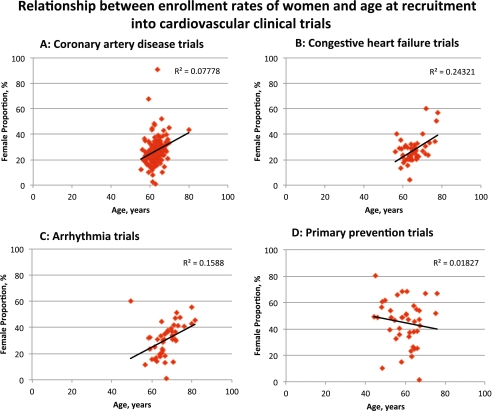
Figure 2a to d illustrates the relationship between enrollment rates of women and mean age at recruitment in trials of coronary artery disease, heart failure, arrhythmia, and primary prevention. Progressively higher female enrollment rates were observed with increasing age at recruitment in trials of coronary artery disease, heart failure, and arrhythmia (all P < 0.001), but not for primary prevention trials (P = 0.40). For every 5 years increased in enrollment age, we estimated an increase in women's enrollment of 4.8%, 4.2%, and 4.1% in trials of coronary artery disease, heart failure, and arrhythmia, respectively.
Figure 2.
Relationship between enrollment rates of women and age at recruitment into cardiovascular clinical trials. a Coronary artery disease trials. b Heart failure trials. c Arrhythmia trials. d Primary prevention trials. Y-axis shows enrollment rates of women in percentages; X-axis shows mean age at recruitment in years. Regression line with R2 values is reported using linear regression and Pearson’s correlations.
Observed and Expected Enrollment Rates of Women in Cardiovascular Trials
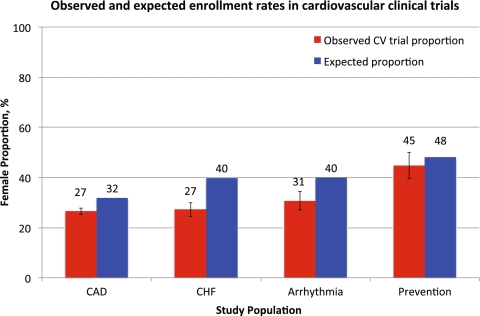
The observed enrollment rates of women in cardiovascular clinical trials and the expected enrollment rate of women that accounted for gender differences in cardiovascular disease prevalence are shown in Figure 3. After accounting for age- and gender-specific differences in disease prevalence using population data, observed clinical trial enrollment rates of women were significantly lower than expected, but by only 5% in coronary artery disease trials, 13% in heart failure trials, and 9% in arrhythmia trials compared with expected rates (all P < 0.001). The difference between observed and expected enrollment rates in primary prevention trials was 3%, but this difference did not achieve statistical significance (P = 0.23).
Figure 3.
Observed and expected enrollment rates in cardiovascular clinical trials.
DISCUSSION
In this systematic review, we estimated that approximately one in three participants in cardiovascular clinical trials were women. Enrollment rates of women did not change significantly in our study period from 1997 to 2009. We further evaluated reasons to account for female underrepresentation and found several new insights. First, we observed significant correlations between enrollment rates of women and age at recruitment. In patients with coronary artery disease, heart failure, and arrhythmia, trials that recruited older patients were associated with increased female participation. Second, after accounting for age- and gender-specific differences in expected disease prevalence based on population data, enrollment rates of women were only 3–13% lower in clinical trials than otherwise expected. These findings suggest gender enrollment inequalities in cardiovascular trials are not primarily due to a bias towards enrolling women. Instead, underrepresentation of women is largely driven by an epiphenomenon where clinical trial recruitment is focused on patients who are predominantly male, because of the preference towards enrolling younger patients. While issues affecting female enrollment in clinical trials are multi-factorial, findings from our study suggest increased female enrollment in clinical trials may be achieved through an increased emphasis on enrolling older patients with cardiovascular disease.
Our observed trends in women's enrollment rates in cardiovascular trials are consistent with other findings demonstrating that enrollment rates of women are substantially lower than in men, which has persisted for more than the past 2 decades. In 2000, Harris and Douglas evaluated cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute (NHLBI) and found the female enrollment rate to be 38% in trials that included both genders.7 In 2008, Kim and colleagues updated this analysis by examining NHLBI-funded trials from 1997 and 2006 and found the enrollment rate of women to be 27%.5 Our systematic review, which was not limited to federally funded studies, demonstrated that low enrollment rates of women are pervasive in cardiovascular trials, regardless of funding sources. As well, previously published studies in this area have focused on specific subpopulations such as NIH prevention trials, heart failure trials, or acute coronary artery disease trials, and did not examine the entire spectrum of cardiovascular clinical trials.4,6,8 Our paper has included trials examining different populations and has established that age plays a significant role in the underrepresentation of women regardless of the study population.
Many experts have raised concerns that underrepresentation of women in cardiovascular clinical trials has undermined the ability of patients and physicians to generalize research findings to clinical practice and undermined the care of women with cardiovascular disease.22,23 In the early 1990s, the underlying assumption of female underrepresentation was that women were not asked to participate in clinical trials. The National Institutes of Health responded by creating the Revitalization Act that encouraged female recruitment by mandating proportional representation, which only met with limited success.5,7 Indeed, our findings suggest that greater representation of women in cardiovascular trials can be achieved by the recruitment of older populations because the investigators would be able to draw on a larger pool of women with cardiovascular disease.
Our premise that women's enrollment patterns are explained by the complex relationships between age and population disease prevalence is supported by several observations. First, it is well recognized that the prevalence of women with cardiovascular disease varies with age because women develop cardiovascular disease at older ages than men.24 Therefore, fewer women with cardiovascular disease are eligible for recruitment into trials than men in younger age groups. Indeed, we found progressively higher women's enrollment rates with increasing age at recruitment into trials of coronary artery disease, heart failure, and arrhythmia. This finding was unlikely a result of the exclusion criteria set by the trials because only a minority of studies had an upper age limit for enrollment, and exclusion of these trials did not alter our overall findings. This relationship was not observed in trials of primary prevention, which also had the highest enrollment rates of women among different study populations because investigators can enroll women and men equally for primary prevention in all age groups. Previous studies have also shown that older patients are less likely to be enrolled in clinical trials because of various factors compared to their younger counterparts.25–27 Their impact is evident as the gaps between observed and expected enrollment rates of women were significantly reduced after accounting for age- and gender-specific differences in expected disease prevalence.
Even accounting for these factors, there was a gap between observed and expected enrollment rates of women of 5% in coronary artery disease trials, 9% in arrhythmia trials, and 13% in heart failure trials. Although we were unable to determine the exact reasons to explain these findings, several characteristics of the clinical trials (sample size, funding sources, location of trials, types of interventions) could be discounted, as they were not predictive of female enrollment. Prior evidence suggests women were 15% less willing to participate in clinical trials as compared to men because of concerns about treatment-related side effects.28 However, a gender difference in willingness to participate does not fully explain why variation existed between different cardiovascular conditions.
Several limitations of this study merit discussion. First, we used Canadian population data to determine the expected proportion of women, which could have underestimated or overestimated the expected prevalence of women with cardiovascular disease in other countries. However, these data were unselected, population-based, and represented patients with cardiovascular disease in Canada. Studies have suggested a similar prevalence of cardiovascular between the US and Canada. For example, self-reported rates of coronary vascular disease were 3.6% in the US and 4.2% in Canada.29,30 Other studies have also suggested hospitalized patients with myocardial infarction and heart failure have similar demographics, co-morbidities, and outcomes in the US comparative studies.19,20 Second, our results may be subject to publication bias because we only included trials published in the three most prominent medical journals. However, this search strategy has allowed us to draw conclusions from landmark cardiovascular clinical trials, which are the most likely to influence clinical practice. Third, results from our study may not be generalized to other areas of medicine as we focused our efforts to cardiovascular clinical trials. Finally, we evaluated a limited number of factors to explain enrollment patterns of women in clinical trials. Clearly, additional efforts are needed to understand the relative contribution of patient, physician, and regional factors.
In conclusion, the persistently low enrollment rates of women in cardiovascular clinical trials is largely driven by an epiphenomenon of age-recruitment bias where enrollment is drawn on cohorts of patients with cardiovascular disease that are predominantly male. Strategies to recruit older populations in clinical trials will also translate to a greater representation of women in cardiovascular clinical trials.
Acknowledgments
Funding/Support This analysis of the study was funded in part by a Canadian Institutes of Health Research (CIHR) operating grant—MOP 102487. Drs. Tsang and Wijeysundera are supported by CIHR Fellowship Awards. Dr. Alter is supported by a Career Investigator Award from the Heart and Stroke Foundation of Ontario. Dr. Ko is supported by a CIHR New Investigator Award.
Role of the Sponsors The results and conclusions are those of the authors, and should not be attributed to any of the funding or sponsoring agencies. The design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript were made independent of the funding agencies.
Conflict of Interest None disclosed.
References
- 1.Alexander KP, Chen AY, Newby LK, et al. Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) initiative. Circulation. 2006;114(13):1380–7. doi: 10.1161/CIRCULATIONAHA.106.620815. [DOI] [PubMed] [Google Scholar]
- 2.Berger JS, Sanborn TA, Sherman W, Brown DL. Influence of sex on in-hospital outcomes and long-term survival after contemporary percutaneous coronary intervention. Am Heart J. 2006;151(5):1026–31. doi: 10.1016/j.ahj.2004.05.062. [DOI] [PubMed] [Google Scholar]
- 3.Lansky AJ, Mehran R, Cristea E, et al. Impact of gender and antithrombin strategy on early and late clinical outcomes in patients with non-ST-elevation acute coronary syndromes (from the ACUITY trial) Am J Cardiol. 2009;103(9):1196–203. doi: 10.1016/j.amjcard.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162(15):1682–8. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 5.Kim ES, Carrigan TP, Menon V. Enrollment of women in National Heart, Lung, and Blood Institute-funded cardiovascular randomized controlled trials fails to meet current federal mandates for inclusion. J Am Coll Cardiol. 2008;52(8):672–3. doi: 10.1016/j.jacc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 6.Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001;286(6):708–13. doi: 10.1001/jama.286.6.708. [DOI] [PubMed] [Google Scholar]
- 7.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med. 2000;343(7):475–80. doi: 10.1056/NEJM200008173430706. [DOI] [PubMed] [Google Scholar]
- 8.Melloni C, Berger JS, Wang TY, et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes. 2010;3(2):135–42. doi: 10.1161/CIRCOUTCOMES.110.868307. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Haynes RB, Jaeschke RZ, Evidence-Based Medicine Working Group et al. Users' guides to the medical literature: XXV. Evidence-based medicine: principles for applying the Users' Guides to patient care. JAMA. 2000;284(10):1290–6. doi: 10.1001/jama.284.10.1290. [DOI] [PubMed] [Google Scholar]
- 10.Weaver WD, White HD, Wilcox RG, GUSTO-I investigators et al. Comparisons of characteristics and outcomes among women and men with acute myocardial infarction treated with thrombolytic therapy. JAMA. 1996;275(10):777–82. doi: 10.1001/jama.1996.03530340041027. [DOI] [PubMed] [Google Scholar]
- 11.National Institutes of Health Revitalization Act of 1993 Pub. L. No. 103-43 (June 10, 1993). In: Congress US, ed.
- 12.Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233–40. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- 13.Bahit MC, Cannon CP, Antman EM, et al. Direct comparison of characteristics, treatment, and outcomes of patients enrolled versus patients not enrolled in a clinical trial at centers participating in the TIMI 9 Trial and TIMI 9 Registry. Am Heart J. 2003;145(1):109–17. doi: 10.1067/mhj.2003.43. [DOI] [PubMed] [Google Scholar]
- 14.Hannaford PC, Kay CR, Ferry S. Agism as explanation for sexism in provision of thrombolysis. BMJ. 1994;309(6954):573. doi: 10.1136/bmj.309.6954.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jha P, Deboer D, Sykora K, Naylor CD. Characteristics and mortality outcomes of thrombolysis trial participants and nonparticipants: a population-based comparison. J Am Coll Cardiol. 1996;27(6):1335–42. doi: 10.1016/0735-1097(96)00018-6. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Torres J. Reported outcomes in major cardiovascular clinical trials funded by for-profit and not-for-profit organizations: 2000–2005. JAMA. 2006;295(19):2270–4. doi: 10.1001/jama.295.19.2270. [DOI] [PubMed] [Google Scholar]
- 17.Tu JV, Nardi L, Fang J, et al. National trends in rates of death and hospital admissions related to acute myocardial infarction, heart failure and stroke, 1994–2004. CMAJ. 2009;180(13):E118–25. doi: 10.1503/cmaj.081197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu M, Austin PC, Manuel DG, Tu JV. Comparison of cardiovascular risk profiles among ethnic groups using population health surveys between 1996 and 2007. CMAJ. 2010;182(8):E301–10. doi: 10.1503/cmaj.091676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko DT, Krumholz HM, Wang Y, et al. Regional differences in process of care and outcomes for older acute myocardial infarction patients in the United States and Ontario, Canada. Circulation. 2007;115(2):196–203. doi: 10.1161/CIRCULATIONAHA.106.657601. [DOI] [PubMed] [Google Scholar]
- 20.Ko DT, Tu JV, Masoudi FA, et al. Quality of care and outcomes of older patients with heart failure hospitalized in the United States and Canada. Arch Intern Med. 2005;165(21):2486–92. doi: 10.1001/archinte.165.21.2486. [DOI] [PubMed] [Google Scholar]
- 21.Tu JV, Pashos CL, Naylor CD, et al. Use of cardiac procedures and outcomes in elderly patients with myocardial infarction in the United States and Canada. N Engl J Med. 1997;336(21):1500–5. doi: 10.1056/NEJM199705223362106. [DOI] [PubMed] [Google Scholar]
- 22.Jneid H, Fonarow GC, Cannon CP, et al. Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008;118(25):2803–10. doi: 10.1161/CIRCULATIONAHA.108.789800. [DOI] [PubMed] [Google Scholar]
- 23.Kim AM, Tingen CM, Woodruff TK. Sex bias in trials and treatment must end. Nature. 2010;465(7299):688–9. doi: 10.1038/465688a. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 25.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 26.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22(22):4626–31. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 27.Trimble EL, Carter CL, Cain D, et al. Representation of older patients in cancer treatment trials. Cancer. 1994;74(7 Suppl):2208–14. doi: 10.1002/1097-0142(19941001)74:7+<2208::AID-CNCR2820741737>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Ding EL, Powe NR, Manson JE, Sherber NS, Braunstein JB. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: a randomized study of cardiovascular prevention trials. Arch Intern Med. 2007;167(9):905–12. doi: 10.1001/archinte.167.9.905. [DOI] [PubMed] [Google Scholar]
- 29.Public Health Agency of Canada. 2009 "Tracking Heart Disease and Stroke in Canada" http://www.phac-aspc.gc.ca/publicat/2009/cvd-avc/pdf/cvd-avs-2009-eng.pdf . (accessed June 7, 2011).
- 30.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]