Abstract
Mitochondria are central to the execution of apoptosis, and the Bcl-2 protein family of pro- and anti-apoptotic proteins interacts with mitochondria to regulate apoptosis. Using bioinformatics we predicted that miR-181, a microRNA expressed in brain, could target the 3′UTRs of Bcl-2 family members Bcl-2-L11/Bim, Mcl-1, and Bcl-2. Using the luciferase reporter assay we confirmed these targets. We used mimic and inhibitor to alter miR-181a levels in primary astrocyte cultures and found miR-181a reduction was associated with increased Bcl-2 and Mcl-1 protein levels. Decreased miR-181a levels reduced glucose deprivation induced apoptosis, mitochondrial dysfunction, and loss of mitochondrial membrane potential in astrocytes.
Keywords: Bcl-2, Mcl-1, Bim, astrocyte, miR-181, mitochondria
1. Introduction
Mitochondria are known to play a leading role in the decision between cell death and cell survival via control of signaling to induce apoptosis. A key family in the regulation of apoptosis via the mitochondrial pathway is the Bcl-2 family consisting of about 20 pro- and anti-apoptotic proteins divided into three groups. The anti-apoptotic multidomain members, such as the prototype B cell lymphoma-2 (Bcl-2), contain BH1-4 domains. The pro-apoptotic multidomain proteins often referred to as the Bax subfamily contain domains BH1-3. The BH3-only group is pro-apoptotic (Adams and Cory, 2007). These proteins control apoptosis by regulating opening of the mitochondrial membrane permeability pore. In addition to their role in regulating mitochondrial permeability, recent work indicates a role for these proteins in control of mitochondrial fission and fusion (Rolland and Conradt, 2010) and in cellular homeostasis with respect to metabolism, calcium signaling, endoplasmic reticulum function, and autophagy (Danial et al., 2010). Bcl-2 decreases after brain ischemia (Martinez et al., 2007) and overexpression of pro-survival Bcl-2 family proteins protects against cerebral ischemia in vivo (Kitagawa et al., 1998; Zhao et al., 2003) and in vitro (Xu et al., 1999). The neuroprotective mechanism involves maintaining mitochondrial function (for review see (Ouyang and Giffard, 2004a). However, the regulation of the Bcl-2 family following cerebral ischemia is not fully understood.
Tissue and cell type specific expression of microRNAs (miRNAs) is now appreciated to provide an additional level of control of protein expression by silencing specific messengerRNAs (mRNAs) to which the miRNA binds with sequence specificity, usually to a sequence within the mRNA 3′UTR. This results either in mRNA silencing or degradation. Because the sequence recognition site or seed in the miRNA is generally only 6 bp long, each of the several hundred miRNAs has many targets, and each mRNA can be targeted by multiple miRNAs. miR-181 has been studied particularly in the setting of immune cell differentiation and leukemia (Chen et al., 2004; Li et al., 2007; Zimmerman et al., 2010). These prior studies have identified several targets for miR-181 including phosphatases SHP-2, PTPN22, DUSP5, and DUSP6, and Bcl-2 family member Mcl-1. In addition Kazenwadel and colleagues identified a key role of miR-181 in controlling Prox 1 levels and thereby determining endothelial cell fate as either lymphatic or blood phenotype (Kazenwadel et al., 2010).
These earlier studies found that the miR-181 family, especially miR-181a and miR-181b, are enriched in brain (Chen et al., 2004; Miska et al., 2004) and their aberrant expression has been associated with brain diseases. hsa-miR-181a and hsa-miR-181b are reduced in human gliomas and glioma cell lines, and expression is negatively correlated with tumor grade (Shi et al., 2008). miR-181a sensitizes human malignant glioma cells to radiation (Chen et al., 2010). While miR-181 is enriched in brain, we do not yet know the cell type specific expression patterns. Our prior results show that in brain cortex, Bcl-2 expression decreases with age, being highest from embryonic day 14 to postnatal day 0, then declining (Xu et al., 2004). Bcl-2 expression was much higher in cultured astrocytes than in cultured neurons, so this study was performed in astrocytes.
Despite their abundance and functional importance, very few studies to date have evaluated the role of miRNAs in ischemic brain damage and in particular in the regulation of cell death and mitochondrial function. Profiling studies of miRNAs following brain ischemia showed changes in miR-181 (Jeyaseelan et al., 2008; Yuan et al., 2010). Due to the high expression of miR-181 in brain, its broad conservation through evolution and the observation that its expression changes after cerebral ischemia we decided to investigate the role of miR-181 in brain cell response to ischemic stress.
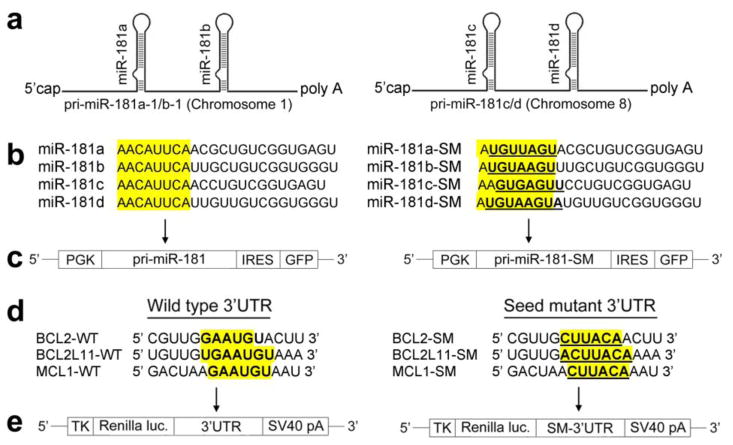
At least three RNA species, long primary miRNA (pri-miRNA), ~70-nucleotide (nt) precursor miRNA (pre-miRNA), and ~22-nt mature miRNA, are made from miRNA genes through transcription and sequential endonucleolytic maturation steps (Kim, 2005). The miR-181 family consists of four members (miR-181a, miR-181b, miR-181c, and miR-181d) that map to two distinct genomic loci in clusters (Fig. 1a) (mirbase.org). In this study, we first identified putative apoptosis related targets of the broadly conserved miR-181 using computational prediction algorithms to identify potential target sequences in the mRNA 3′UTRs of three Bcl-2 family members. Using the dual luciferase reporter assay we validated the targets. We then examined the relationship between miR-181 expression and Bcl-2 family protein expression in primary astrocytes. Lastly we verified that mitochondrial function was improved by decreasing miR-181 in stressed astrocytes, as we previously found by directly increasing expression of Bcl-2 (Ouyang and Giffard, 2004a).
Fig. 1.
pri-miR-181, 3′UTRs of Bcl-2 family and vectors. (a) Schematic representation of the genomic organization of the mouse miR-181. (b) Mature wild type (WT) and seed mutated (SM) miR-181a, miR-181b, miR-181c, and miR-181d sequences. (c) Vector MWX-PGK-IRES-GFP for miR-181. (d) Wild type (WT) and seed mutated (SM) 3′UTRs of Bcl-2, Bcl-2-L11, and Mcl-1. (e) Renilla luciferase reporter vector phRL-TK.
2. Materials and methods
2.1 pri-miRNA, miRNA, 3′UTRs, Luciferase reporter assay
Since pri-miR-181a and b or pri-miR-181c and d are only about 100 nt apart (Fig. 1a), we made pri-miR-181a/b and pri-miR-181cd constructs. Wild-type mature miR-181a–d and their seed mutant sequences are shown in Fig. 1b. DNA fragments containing the pri-miR-181ab or pri-miR-181cd hairpin (or their seed mutants) and ~250 nt flanking sequence on each side were cloned downstream of the PGK promoter in MWX-PGK-IRES-GFP (Fig. 1c); MWX-PGK-IRES-GFP was a kind gift from Dr. Chang-Zheng Chen at Stanford University. Mouse Bcl-2, Bcl-2-L11, and Mcl-1 3′UTRs (Fig. 1d) were cloned into the Renilla luciferase reporter vector phRL-TK (Fig. 1e, Promega, Madison, WI, USA). The primer sets used to generate specific 3′UTR fragments are shown in Table 1. We generated mutant 3′UTRs of the Bcl-2, Bcl-2-L11, and Mcl-1 gene with 6 base substitutions; mutated nucleotides are indicated in bold and underlined (Fig. 1b and d). Both wild type and mutant inserts were confirmed by sequencing. Fluorescent-tagged miRNA transfection control, negative control, miR-181a mimic, and inhibitor were purchased from Thermo Scientific via Dharmacon (Lafayette, CO, USA); catalogue numbers are listed in Table 2.
Table 1.
Primer sets used to generate 3′UTRs of Bcl-2 family genes
| 3′UTR | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| BCL2 | TCAGGCAAATGGTCGAATC | CAAGAAGCAGCTTGAAGGAG |
| BCL2L11 | GTCATGTCCCTCTCTTGGTG | GTCCTACCAAAAACGAGAGAG |
| MCL1 | GAACATAAGAGGGAAGTCTTGGA | GGGTTATTTAGGTTCAAAGTTTTG |
Table 2.
Materials bought from companies with catalog numbers
| Name | Catalog Number | Company |
|---|---|---|
| miR-181a Primer | 000480 | Applied Bioscience (Foster City, CA, USA) |
| miR-181b Primer | 001098 | |
| miR-181c Primer | 000482 | |
| miR-181d Primer | 001099 | |
| U6 snRNA Primer | 001973 | |
| miR-181a Mimic | C-310435-05-0005 | Thermo Scientific via Dharmacon (Chicago, IL, USA) |
| miR-181a Inhibitor | C-310435-07-0005 | |
| Positive Control | CP-004500-01-05 | |
| Negative Control | CN-001000-01-05 |
BOSC 23 cells were harvested 48 hr post-transfection and assayed using the Dual-Luciferase system (E1960, Promega). Results are expressed as relative luciferase activity by first normalizing to the firefly luciferase transfection control, then to the Renilla/firefly value of the empty control vector and finally to the corresponding seed mutant reporter control.
2.2 Reverse Transcription Quantitative real-time Polymerase Chain Reaction (RT-qPCR)
Total RNA was isolated with TRIzol® (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed using the TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA). Equal amounts of total RNA (200 ng) were reverse-transcribed with 1.3 mM dNTPs (with dTTP), 50 U reverse transcriptase, 10 U RNase inhibitor, and specific miRNA reverse transcriptase primers (Applied Biosystems) at 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min. PCR reactions were then conducted using the TaqMan® MicroRNA Assay Kit (Applied Biosystems) at 95°C for 10 min, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 min. Each reaction contained 0.75 μl of the RT reaction product, 5 μl TaqManR 2×Universal PCR Master Mix (Applied Biosystems) in a total volume of 10 μl using the 7900HT (Applied Biosystems). Predesigned primer/probes (Applied Biosystems) for miRNAs and mouse U6 were from Applied Biosystems; catalogue numbers are listed in Table 1. The expression of miR-181a/b/c/d was normalized using U6 as the internal control. Measurements were normalized to U6 (ΔCt) and comparisons calculated as the inverse log of ΔΔCT to give the relative fold change for all miRNA levels (Livak and Schmittgen, 2001). Liu et al. have validated U6 as not changing in cerebral ischemia (Liu et al., 2010). The PCR experiments were repeated 3 times, each using separate sets of samples.
2.3 Cell cultures and transfection
Primary astrocyte cultures were prepared from postnatal day 1–3 Swiss Webster mice (Charles River, Wilmington, MA, USA) as described previously (Ouyang et al., 2006). Briefly, neocortices were dissected, treated with trypsin, and plated as a single-cell suspension. BOSC 23 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in Dulbecco’s Modified Eagle Medium (DMEM, #11995, Gibco, Carlsbad, CA, USA) supplemented with 10% FBS and 100 μg/ml penicillin/streptomycin. Astrocyte primary cultures in 24-well plates were transfected with miR-181a mimic, or inhibitor, or their controls using FuGeneHD (Roche, Branford, CT, USA) according to the manufacturer’s instructions.
2.4 Injury paradigms, assessment of cell death, and live cell imaging
Glucose deprivation (GD) was performed on primary astrocyte cultures as described previously (Ouyang et al., 2011; Ouyang et al., 2006). Cell injury was quantified after GD by microscopic evaluation and cell counting after Hoechst 33342 (5 μM) and propidium iodide (PI, 5 μM) staining. PI stains dead cells but does not cross intact plasma membranes. Hoechst dye is a cell-permeant nucleic acid stain that labels all nuclei. Mitochondrial membrane potential was monitored using tetramethylrhodamine methyl ester (TMRE) and reactive oxygen species were monitored using hydroethidine (HEt) as previously described (Ouyang et al., 2011) using a Zeiss Axiovert 200M fluorescence microscope (Zeiss, Jena, Germany).
2.5 Immunoblotting
Immunoblotting was performed as previously described (Han et al., 2009). Briefly, equal amounts (75 μg) of protein were separated on a polyacrylamide gel (Invitrogen), and electrotransferred to Immobilon polyvinylidene fluoride membrane (Millipore Corp., Billerica, MA, USA). Membranes were blocked and incubated overnight with primary antibody against Bcl-2 (1:1000, #2870, Cell Signaling, Danvers, MA, USA), Bim (1:500, ALX-804-527, Enzo, Plymouth Meeting, PA, USA), Mcl-1 (1:1000, 600-401-394, Rockland, Gilbertsville, PA, USA), and β-actin (1:1000, 926–42210, LiCOR Bioscience, Lincoln, NE, USA), washed and incubated with 1:15000 anti-rabbit antibody (926–32221, LiCOR Bioscience) and anti-mouse antibody (926–32220, LiCOR Bioscience). Immunoreactive bands were visualized using the LICOR Odyssey infrared imaging system according to the manufacturer’s protocol. Densitometric analysis was performed using ImageJ software (NIH). Band intensities were normalized to β-actin.
2.6 Statistics
All data reported represent at least 3 independent experiments for n=3–6 cultures in each experiment. Data reported are means ± SD. Statistical difference was determined using T test for comparison of two groups or ANOVA followed by Mann-Whitney test for experiments with >2 groups using Prism 5.0a software (Graphpad, La Jolla, CA, USA). P < 0.05 was considered significant.
3. Results
3.1 miR-181 targets the 3′UTRs of three Bcl-2 family members
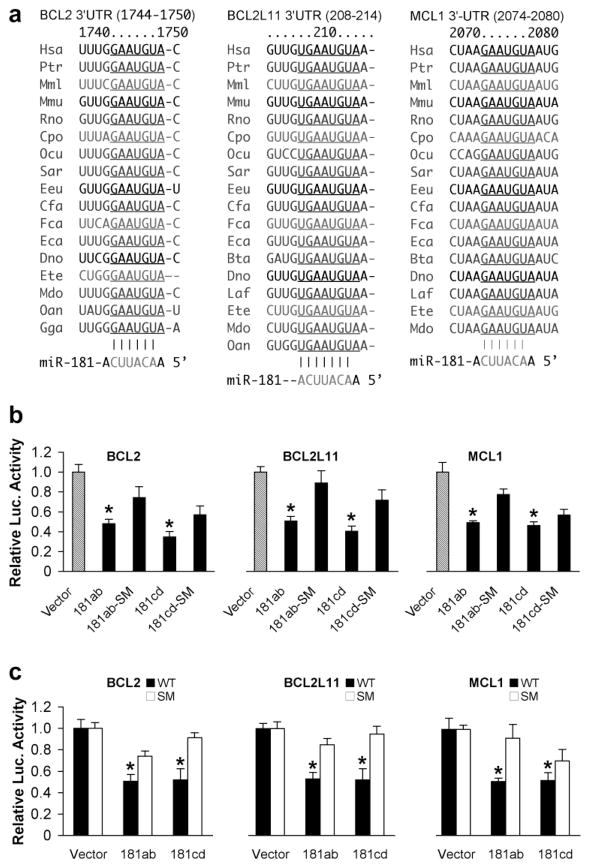
Using computational miRNA target prediction algorithms, as detailed at TargetScan (http://targetscan.org, Release 5.1) and Microcosm Targets (http://www.ebi.ac.uk/enright-srv/microcosm), we identified three members of the Bcl-2 family with mRNA 3′UTRs that were potential targets: Bcl-2 and Mcl-1 in the pro-survival subgroup and Bim/Bcl-2-like 11 (Bcl-2-L11) a BH3-only pro-apoptotic protein. Interestingly, the seed sequences of all these 3′UTRs targeted by miR-181 are highly evolutionarily conserved (Fig 2a), suggesting a critical role in normal physiology. The common names corresponding to the scientific names in the figure is listed in Table 3. To validate these 3′UTRs as targets of miR-181, we cotransfected cells with constructs of luciferase control reporter, luciferase target reporter containing the 3′UTR of Bcl-2, Bcl-2-L11/Bim, or Mcl-1 (wild type, WT) and pri-miR-181 (WT or pri-miR-181 seed mutant (SM)). As frequently observed, there is some difference in luciferase activity with the addition of even the pri-miR-181 SM compared to the vector control. These differences generally reflect non-specific effects; in this case the BOSC23 cells used do not normally express miR-181. Others have seen similar changes with miRNA SM (Arnold et al., 2011). We also tested additional negative controls for miR-181, scrambled RNAi and an irrelevant miRNA mmu-let-7b and saw little effect on luciferase activity with any of the three 3′UTRs (data not shown). As shown in Fig. 2b, both pri-miR-181ab and pri-miR-181cd repress all three 3′UTRs compared to their pri-miR-181 mutant controls. To exclude off-target effects we also mutated the seed sequences of the 3′UTRs of Bcl-2, Bcl-2-L11, and Mcl-1 as shown in Fig. 1d. We cotransfected cells with constructs of luciferase control reporter, luciferase target reporter containing the 3′UTR of wild type or its mutant, and miR-181ab or cd WT. These results validate Bcl-2, Bcl-2-L11, and Mcl-1 as targets of both miR-181ab and miR-181cd (Fig. 2c).
Fig. 2.
miR-181 targets 3 members of the Bcl-2 family. (a) The seed sequences of Bcl-2, Bcl-2-L11, and Mcl-1 3′UTRs targeted by miR-181 are highly conserved across species (from TargetScan). (b) Dual luciferase activity assays performed in BOSC23 cells co-transfected with the plasmid containing luciferase followed by the Bcl-2 or Bcl-2-L11 or Mcl-1 3′UTR (WT) and plasmids encoding either pri-miR-181ab or pri-miR-181cd or their seed mutants (SM) demonstrates that miR-181 recognizes all of these 3′UTRs. (c) The same assay performed with the wild type 3′UTRs of Bcl-2, Bcl-2-L11, or Mcl-1 (WT) or their seed mutants (SM) shows that miR-181ab and cd both reduce luciferase activity. Luciferase assays were performed 3 times in triplicate. *P<0.01 compared to the SM group.
Table 3.
List of scientific and common names
| Scientific name | Common name | |
|---|---|---|
| Mmu | Mus musculus | House mouse |
| Rno | Rattus norvegicus | Rat |
| Has | Homo sapiens | Human |
| Ptr | Pan Troglodytes | Chimpanzee |
| Mml | Macaca mulatta | Rhesus macaque |
| Cpo | Cambarellus Patzcuarensis (var. orange) | Orange Dwarf Crayfish |
| Ocu | Oncorhynchus | Salmon |
| Sar | Scatophagus argus | Spotted Scat (fish) |
| Eeu | Euonymus europaeus | European Spindle Tree |
| Cfa | Cannis Familiaris | Dog |
| Eca | Eqqus Caballus | Horse |
| Bta | Bos taurus | Oxen |
| Dno | Dendragapus obscurus | Grouse |
| Laf | Larus californicus | California gull |
| Ete | Embiotocidae | Surf perch |
| Mdo | Monodelphis domestica | Gray short-tailed opossum |
| Fca | Felis catus | Cat |
| Oan | Ornithorhynchus anatinus | Platypus |
| Gga | Gallus gallus | Chicken |
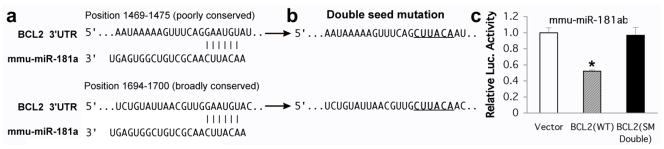
We initially only mutated the miR-181 binding site that is predicted to be conserved across species. This led to an increase in luciferase activity, but did not reach the level seen in vector control. In the case of the mouse BCL2 3′UTR, a second binding site (Position 1480–1486, 220bp before the conserved target site) exists. When both BCL2 3′UTR binding sites are mutated full luciferase activity is restored (Fig. 3), suggesting that this second site may also be active.
Fig. 3.
(a) The mouse BCL2 3′UTR has two miR-181 binding sites. One is broadly conserved among vertebrates and another is poorly conserved. (b) Mutation of both binding sites at the same time. (c) The double mutant completely abolishes miR-181ab repression activity compared to vector control. *P<0.01.
3.2 miR-181 alters pro-survival Bcl-2 family protein levels in astrocytes
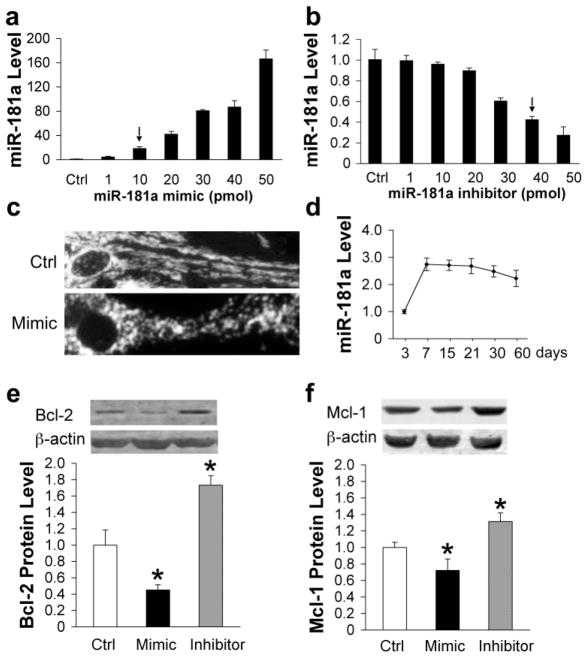
Since miR-181 is highly expressed in brain, we studied its effects on these proteins in astrocytes. Our preliminary results showed that in mouse brain miR-181a was present at the highest level, with miR-181b about 10%, miR-181c about 34%, and miR-181d about 8% of miR-181a levels (data not shown). We chose to focus on the most highly expressed family member, miR-181a. We titrated mimic and inhibitor and measured levels of miR-181a (Fig. 4a and b). We noted that with 50 pmol mimic the configuration of mitochondria in the cells changed from the usual filamentous pattern, upper panel, to a fragmented pattern (Fig. 4c), so we chose a lower concentration of mimic for the subsequent studies. We used transfection with either 10 pmol mimic or 40 pmol inhibitor, which resulted in an increase of 16 fold in miR-181a level with mimic (Fig. 4a) or a decrease of 58% with inhibitor (Fig. 4b). We previously found that the ability to undergo apoptosis decreased with time in culture for astrocytes (Xu et al., 2004), so we assessed miR-181a levels in astrocytes with time in culture, using RT-qPCR (Fig. 4d). Levels increased between day 3 and 7, and then were fairly stable through 60 days. If changes in miR-181a are biologically relevant they should result in changes in protein levels. We examined the protein levels of Bcl-2, Bim, and Mcl-1 by Western blot after transfection of astrocytes with either mimic or inhibitor. Protein levels of Mcl-1 and Bcl-2 changed significantly in transfected cells, decreasing in the presence of mimic and increasing after transfection with inhibitor (Fig. 4e,f), but effects were stronger for Bcl-2, decreasing more than 50% with mimic, while Mcl-1 decreased about 30%. Bim protein was not detectable in our primary cultured astrocytes using commercially available antibodies.
Fig. 4.
Expression of miR-181 and Bcl-2 family proteins after miR-181a mimic or inhibitor transfection of astrocytes. Dose-response of miR-181a levels to transfection with increasing amounts of miR-181a mimic (a) or inhibitor (b) in primary cultures of astrocytes. (c) Mitochondrial morphology changes from a threadlike network (upper) to fragmented round dots (lower) after transfection with 50 pmol miR-181a mimic. Micrographs were taken after staining cells with tetramethylrhodamine methyl ester. (d) Relative miR-181a levels in astrocytes after different durations in vitro. Bcl-2 (e) and Mcl-1 (f) protein expression in primary astrocyte cultures is significantly decreased by transfection with miR-181a mimic and significantly increased by transfection with inhibitor, N=3. All experiments were performed 3 times in triplicate. *P<0.01 compared to Ctrl.
3.3 miR-181 influences apoptosis, mitochondrial function, and oxidative stress in glucose deprived astrocytes
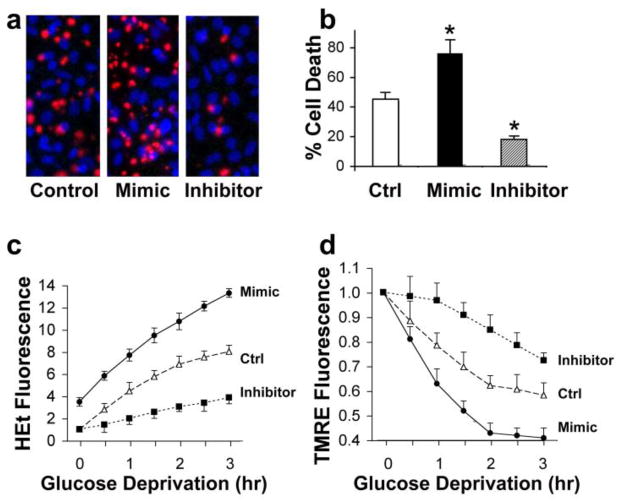
We previously observed that overexpression of anti-apoptotic Bcl-2 family members could reduce cell death, reduce oxidative stress, and protect mitochondrial membrane potential when astrocytes were stressed with glucose deprivation (Ouyang et al., 2002; Papadopoulos et al., 1998). Cells were transfected with miR-181a mimic or inhibitor 1 d before GD. We examined the effects of changing miR-181a levels on astrocyte survival of 24 hr glucose GD. As demonstrated in Fig. 5a and b, elevated expression of miR-181a reduced cell survival by 31%, whereas knockdown of endogenous miR-181a levels increased survival by 27% compared to control cells. Increased levels of miR-181a were associated with increased numbers of cells showing condensed nuclear morphology (Fig. 1a, mimic), typical of apoptosis in astrocytes (Xu et al., 2004). Reactive oxygen species generation was then assessed during glucose deprivation using hydroethidine. They increased with mimic and decreased with inhibitor relative to control astrocytes (Fig. 5c). Note the higher starting level of ROS compared with the control in miR-181a mimic treated cells (Fig. 5c). Mitochondrial membrane potential was assessed with tetramethylrhodamine during GD and more rapid depolarization was observed in cells transfected with mimic, and slower depolarization in cells transfected with inhibitor (Fig. 5d). Thus reduction of miR-181a is associated with reduced cell death, reduced oxidative stress, and preserved mitochondrial function while increased miR-181a levels have the opposite effects (Table 4).
Fig. 5.
Effect of miR-181a mimic and inhibitor on astrocyte ischemia-like injury in vitro. (a) miR-181a mimic aggravates and miR-181a inhibitor reduces injury induced by 24 hr glucose deprivation (GD) in astrocytes. Representative micrographs of cultures stained with propidium iodide (red, dead cells) and Hoechst dye (blue, live cells), are shown. (b) Bar graph shows quantitation of cell death by cell counting. * indicates significantly different compared to control (Ctrl) cultures subjected to the same injury. (c) Transfection with miR-181a mimic or inhibitor affects the time course of reactive oxygen species (ROS) generation. Increasing ROS is detected as increasing HEt fluorescence due to GD stress. (d) Increased miR-181a mimic and inhibitor alter the time course of change in mitochondrial membrane potential induced by GD as assessed by TMRE fluorescence. Mitochondrial depolarization is indicated by decreased fluorescence. All experiments were performed 3 times in triplicate. *P<0.01
Table 4.
Summary of related changes in miR-181a and target protein levels, cell survival and mitochondrial function
| Transfection | miR-181a level | Bcl-2 or Mcl-1 levels | Cell survival | Mitochondrial function |
|---|---|---|---|---|
| miR-181a mimic | ↑ | ↓ | ↓ | ↓ |
| miR-181a inhibitor | ↓ | ↑ | ↑ | ↑ |
4. Discussion
This is the first investigation of the regulation of Bcl-2 family members in astrocytes by miRNA. Apoptosis is controlled at many levels, often reflecting the specific cell type and tissue involved. Recent work has begun to define ways in which apoptosis may also be controlled by changes in miRNA, though much of it has been in cancer, and some in development. To date research on miRNAs in brain ischemia has focused on profiling expression changes, with several studies employing different models of cerebral ischemia (Dharap et al., 2009; Jeyaseelan et al., 2007; Liu et al., 2010; Tan et al., 2009; Yuan et al., 2010). Yuan and colleagues (Yuan et al., 2010) reported that hippocampal miR-181a was upregulated at 30 min of reperfusion with a further increase after 24 hrs. In this paper we validate three targets of miR-181a in the Bcl-2 family, Bcl-2, Mcl-1, and Bcl-2-L11/Bim. The first two are anti-apoptotic while the last is pro-apoptotic. We examined the effects of altering miR-181a levels in primary culture astrocytes, and observed effects on Bcl-2 and Mcl-1, but were unable to detect Bim protein in our cells. The possibility that one miRNA controls both pro- and antiapoptotic members of the Bcl-2 family clearly raises issues about how specificity is achieved, and in the case of astrocytes it appears that differential expression of the proteins leads to increased vulnerability with increased miR-181a and decreased vulnerability when it is reduced, consistent with changes in protein levels of Bcl-2 and Mcl-1. Since control of mRNA by miRNA is combinatorial, it is likely that the complement of miRNA expressed at a given time in a given cell will determine the overall changes in mRNA function.
The genomic context of the miR-181 binding site in the Bcl-2 3′UTR will affect its ability to regulate the mRNA. Interestingly, an endogenous antisense transcript (cDNA: AK085305), starting approx. 380 bp from the miR-181 binding site is present in the UCSC Genome browser. Natural antisense transcript-mediated inhibition of mRNA function has been reported by Faghihi et al (Faghihi et al., 2010). According to this report long natural antisense RNAs may be pertinent to miRNA-based regulation of mRNAs. In addition, alternative polyadenylation may occur in some species and some tissue settings. If the mRNA terminates before the miR-181 binding site those mRNA would not be regulated by miR-181. In our case, the 3′ UTRs were cloned from murine astrocytes so in this brain cell type the miR-181 binding sites are present.
Overexpression of any of the five pro-survival members protects cells against apoptosis induced by a variety of cytotoxic stimuli (Cory et al., 2003). Gene targeting studies, however, reveal some specificity in vivo, including differences in relative expression by cell types and tissue (Cory et al., 2003; Ranger et al., 2001). Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage (Arbour et al., 2008). Mcl-1 deficiency results in peri-implantation embryonic lethality (Rinkenberger et al., 2000), the most severe phenotype among knockouts of the anti-apoptotic Bcl-2 family members.
The reduction of hsa-miR-181a and b in gliomas, negative correlation with tumor grade (Shi et al., 2008), and ability to sensitize to radiation (Chen et al., 2010) are consistent with the ability found here for reduction of miR-181 to increase resistance to apoptosis in normal astrocytes. In ischemia we seek to increase resistance to apoptosis, the opposite of the desired effect in cancer. Bcl-2 has previously been studied as a protein that can protect from cerebral ischemia, including in vivo and in vitro ischemia (Lawrence et al., 1996; Zhao et al., 2003). A prior report demonstrated a correlation between miR-181 levels and Bcl-2 levels, but did not use a luciferase assay to validate the putative target sequence (Chen et al., 2010). In the case of Mcl-1, Zimmerman and colleagues (Zimmerman et al., 2010) reported positive luciferase results for human miR-181b against Mcl-1, and further observed regulation of miR-181 by Lyn kinase in leukemia. We confirmed their results with mouse miR-181a.
The results reported here suggest that miR-181 regulation of Bcl-2 and Mcl-1 contributes to mitochondrial dysfunction observed with in vitro ischemic stress, in this case glucose deprivation, in astrocytes. We and others have observed that Bcl-2, Bcl-xL and Mcl-1, when overexpressed, lead to a controlled increase in oxidative stress and antioxidant defense, with increased levels of superoxide dismutase and glutathione peroxidase (Kowaltowski et al., 2004; Papadopoulos et al., 1998). The observed effects of altered levels of miR-181 are consistent with prior observations on the effects of overexpression of Bcl-2 or Bcl-xL alone, namely a decrease in oxidative stress and preserved mitochondrial membrane potential (Kowaltowski et al., 2004; Ouyang and Giffard, 2004a, b; Papadopoulos et al., 1998).
In summary, Bcl-2, Mcl-1, and Bim are targets of mouse miR-181. Protein levels of Bcl-2 and Mcl-1 change inversely with changes in miR-181a. Reduction of miR-181a levels is associated with reduced cell death, reduced oxidative stress and preserved mitochondrial function in astrocytes.
Highlights.
miR-181a targets multiple Bcl-2 family members
Reducing miR-181a protects neural cells from ischemic stress
Reducing miR-181a protects mitochondrial function
Acknowledgments
This work was supported in part by NIH grants NS053898 and GM49831 to RGG. The authors would like to thank Chang-Zheng Chen for plasmid and technical consulting and William Magruder for help preparing the manuscript. The authors have no conflicting financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Current Opinion in Immunology. 2007;19:488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, Cheung ECC, Kelly MA, MacKenzie AE, Park DS, Opferman JT, Slack RS. Mcl-1 Is a Key Regulator of Apoptosis during CNS Development and after DNA Damage. The Journal of Neuroscience. 2008;28:6068–6078. doi: 10.1523/JNEUROSCI.4940-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold CP, Tan R, Zhou B, Yue SB, Schaffert S, Biggs JR, Doyonnas R, Lo MC, Perry JM, Renault VM, Sacco A, Somervaille T, Viatour P, Brunet A, Cleary ML, Li L, Sage J, Zhang DE, Blau HM, Chen C, Chen CZ. MicroRNA programs in normal and aberrant stem and progenitor cells. Genome Research. 2011;21:798–810. doi: 10.1101/gr.111385.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P, Hu W. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol Rep. 2010;23:997–1003. doi: 10.3892/or_00000725. [DOI] [PubMed] [Google Scholar]
- Cory S, Huang DCS, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- Danial NN, Gimenez-Cassina A, Tondera D. Homeostatic functions of BCL-2 proteins beyond apoptosis. Advances in Experimental Medicine and Biology. 2010;687:1–32. doi: 10.1007/978-1-4419-6706-0_1. [DOI] [PubMed] [Google Scholar]
- Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab. 2009;29:675–687. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihi MA, Zhang M, Huang J, Modarresi F, Van der Brug M, Nalls M, Cookson M, St-Laurent G, Wahlestedt C. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biology. 2010;11:R56. doi: 10.1186/gb-2010-11-5-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han RQ, Ouyang YB, Xu L, Agrawal R, Patterson AJ, Giffard RG. Postischemic brain injury is attenuated in mice lacking the beta2-adrenergic receptor. Anesth Analg. 2009;108:280–287. doi: 10.1213/ane.0b013e318187ba6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaseelan K, Herath WB, Armugam A. MicroRNAs as therapeutic targets in human diseases. Expert Opin Ther Targets. 2007;11:1119–1129. doi: 10.1517/14728222.11.8.1119. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- Kazenwadel J, Michael MZ, Harvey NL. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood. 2010;116:2395–2401. doi: 10.1182/blood-2009-12-256297. [DOI] [PubMed] [Google Scholar]
- Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tsujimoto Y, Ohtsuki T, Kuwabara K, Matsushita K, Yang G, Tanabe H, Martinou JC, Hori M, Yanagihara T, Chan PH. Amelioration of Hippocampal Neuronal Damage After Global Ischemia by Neuronal Overexpression of BCL-2 in Transgenic Mice Editorial Comment. Stroke. 1998;29:2616–2621. doi: 10.1161/01.str.29.12.2616. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Fenton RG, Fiskum G. Bcl-2 family proteins regulate mitochondrial reactive oxygen production and protect against oxidative stress. Free Radical Biology and Medicine. 2004;37:1845–1853. doi: 10.1016/j.freeradbiomed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Lawrence M, Ho D, Sun G, Steinberg G, Sapolsky R. Overexpression of Bcl-2 with herpes simplex virus vectors protects CNS neurons against neurological insults in vitro and in vivo. The Journal of Neuroscience. 1996;16:486–496. doi: 10.1523/JNEUROSCI.16-02-00486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QJ, Chau J, Ebert PJR, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen CZ. miR-181a Is an Intrinsic Modulator of T Cell Sensitivity and Selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, Sharp FR. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30:92–101. doi: 10.1038/jcbfm.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-[Delta][Delta]CT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Martinez G, Musumeci G, Loreto C, Carnazza M. Immunohistochemical changes in vulnerable rat brain regions after reversible global brain ischaemia. Journal of Molecular Histology. 2007;38:295–302. doi: 10.1007/s10735-007-9102-9. [DOI] [PubMed] [Google Scholar]
- Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5:R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Carriedo SG, Giffard RG. Effect of Bcl-XL overexpression on reactive oxygen species, intracellular calcium, and mitochondrial membrane potential following injury in astrocytes. Free Radical Biology and Medicine. 2002;33:544–551. doi: 10.1016/s0891-5849(02)00912-7. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Giffard RG. Cellular neuroprotective mechanisms in cerebral ischemia: Bcl-2 family proteins and protection of mitochondrial function. Cell Calcium. 2004a;36:303–311. doi: 10.1016/j.ceca.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Giffard RG. Changes in astrocyte mitochondrial function with stress: effects of Bcl-2 family proteins. Neurochemistry International. 2004b;45:371–379. doi: 10.1016/j.neuint.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Xu LJ, Emery JF, Lee AS, Giffard RG. Overexpressing GRP78 influences Ca(2+) handling and function of mitochondria in astrocytes after ischemia-like stress. Mitochondrion. 2011;11:279–286. doi: 10.1016/j.mito.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Xu LJ, Sun YJ, Giffard RG. Overexpression of inducible heat shock protein 70 and its mutants in astrocytes is associated with maintenance of mitochondrial physiology during glucose deprivation stress. Cell Stress Chaperones. 2006;11:180–186. doi: 10.1379/CSC-182R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Koumenis IL, Xu L, Giffard RG. Potentiation of murine astrocyte antioxidant defence by bcl-2: protection in part reflects elevated glutathione levels. European Journal of Neuroscience. 1998;10:1252–1260. doi: 10.1046/j.1460-9568.1998.00134.x. [DOI] [PubMed] [Google Scholar]
- Ranger AM, Malynn BA, Korsmeyer SJ. Mouse models of cell death. Nat Genet. 2001;28:113–118. doi: 10.1038/88815. [DOI] [PubMed] [Google Scholar]
- Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes & Development. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- Rolland SG, Conradt B. New role of the BCL2 family of proteins in the regulation of mitochondrial dynamics. Current Opinion in Cell Biology. 2010;22:852–858. doi: 10.1016/j.ceb.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, You Y. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res. 2008;1236:185–193. doi: 10.1016/j.brainres.2008.07.085. [DOI] [PubMed] [Google Scholar]
- Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K. Expression profile of MicroRNAs in young stroke patients. PLoS One. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Chock V, Yang EY, Giffard RG. Susceptibility to apoptosis varies with time in culture for murine neurons and astrocytes: changes in gene expression and activity. Neurological Research. 2004;26:632–643. doi: 10.1179/016164104225017587. [DOI] [PubMed] [Google Scholar]
- Xu L, Lee JE, Giffard RG. Overexpression of bcl-2, bcl-xL or hsp70 in murine cortical astrocytes reduces injury of co-cultured neurons. Neuroscience Letters. 1999;277:193–197. doi: 10.1016/s0304-3940(99)00882-4. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Wang JY, Xu LY, Cai R, Chen Z, Luo BY. MicroRNA expression changes in the hippocampi of rats subjected to global ischemia. J Clin Neurosci. 2010;17:774–778. doi: 10.1016/j.jocn.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. Journal of Neurochemistry. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman EI, Dollins CM, Crawford M, Grant S, Nana-Sinkam SP, Richards KL, Hammond SM, Graves LM. Lyn Kinase-Dependent Regulation of miR181 and Myeloid Cell Leukemia-1 Expression: Implications for Drug Resistance in Myelogenous Leukemia. Molecular Pharmacology. 2010;78:811–817. doi: 10.1124/mol.110.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]