Abstract
The ultrastructural changes in diabetic and idiopathic gastroparesis are not well studied and it is not known whether there are different defects in the two disorders. As part of the Gastroparesis Clinical Research Consortium, full thickness gastric body biopsies from 20 diabetic and 20 idiopathic gastroparetics were studied by light microscopy. Abnormalities were found in many (83%) but not all patients. Among the common defects were loss of interstitial cells of Cajal (ICC) and neural abnormalities. No distinguishing features were seen between diabetic and idiopathic gastroparesis. Our aim was to provide a detailed description of the ultrastructural abnormalities, compare findings between diabetic and idiopathic gastroparesis and determine if patients with apparently normal immunohistological features have ultrastructural abnormalities. Tissues from 40 gastroparetic patients and 24 age- and sex-matched controls were examined by transmission electron microscopy (TEM). Interstitial cells of Cajal showing changes suggestive of injury, large and empty nerve endings, presence of lipofuscin and lamellar bodies in the smooth muscle cells were found in all patients. However, the ultrastructural changes in ICC and nerves differed between diabetic and idiopathic gastroparesis and were more severe in idiopathic gastroparesis. A thickened basal lamina around smooth muscle cells and nerves was characteristic of diabetic gastroparesis whereas idiopathic gastroparetics had fibrosis, especially around the nerves. In conclusion, in all the patients TEM showed abnormalities in ICC, nerves and smooth muscle consistent with the delay in gastric emptying. The significant differences found between diabetic and idiopathic gastroparesis offers insight into pathophysiology as well as into potential targeted therapies.
Keywords: electron microscopy, smooth muscle, enteric nerves, interstitial cells of Cajal
Introduction
Gastroparesis is a disorder characterized by chronic delay in gastric emptying in the absence of obstruction. Gastroparesis is most commonly associated with diabetes (both type 1 and type 2) or is of unknown cause (idiopathic) [1, 2]. Idiopathic and diabetic gastroparesis are assumed to have very different pathophysiology but presently there are no known cellular abnormalities that can separate the two disorders.
Most of our understanding of the cellular basis for gastroparesis has come from animal studies focused on diabetic gastroparesis [11-21]. The lack of understanding of the cellular aetiology of gastroparesis is a significant limitation to developing targeted therapy. In response to the limited information known about gastroparesis and the limited therapies currently available, the National Institutes of Health established a Gastroparesis Clinical Research Consortium (GpCRC). As part of this consortium 40 full thickness gastric body biopsies (20 diabetic, 20 idiopathic) were collected from patients with diabetic and idiopathic gastroparesis and 20 site-, age- and sex-matched controls and then studied by immunohistochemistry [22]. The main finding of that study was that 83% of these patients had a cellular abnormality. The most common findings were loss of interstitial cells of Cajal (ICC), changes in enteric nerves and an immune cell infiltrate. None of the cellular abnormalities differentiated diabetic and idiopathic gastroparesis. Also in 17% of gastroparetics, no abnormality was found by light microscopy. In the same study, TEM examination was conducted on a limited number of the patients chosen because of their ICC and nerve abnormalities which confirmed the immunohistochemical findings [22].
The aim of the present study was to use TEM to provide a detailed description of the ultrastructural abnormalities in patients with diabetic and idiopathic gastroparesis, determine if there are distinguishing features at the ultrastructural level between diabetic and idiopathic gastroparesis and determine if patients with apparently normal immunohistological features have ultrastructural abnormalities.
Materials and methods
We used tissue from 60 full-thickness gastric biopsies, 58 of which had been previously studied by immunohistochemistry [22]. In the previous study, we did not have tissue processed for electron microscopy on two patients and therefore two new patients for whom we had both immunohistochemistry and electron microscopy processed tissue were added. Tissue was collected from the anterior aspect of the stomach, midway between the greater and lesser curvatures where the gastroepiploic vessels meet. The anatomy of individual stomachs varies but, in general, the region where the gastroepiploic arteries meet is about 9 cm proximal to the pylorus. Tissues were obtained from 20 diabetic and 20 idiopathic gastroparetic patients undergoing surgery for placement of a gastric electrical stimulator and from 20 age- and sex-matched patients undergoing gastric bypass surgery for obesity (Table 1) following IRB approved protocols. A technician in the operating room ensured that ischaemic time was minimized and the tissue properly cut, stored and transported. The control tissue was selected from patients that did not have diabetes, did not report gastrointestinal symptoms and had a screening H and E slide read as normal. To determine if the 20 controls used were different from non-obese controls, thus causing any significant histological changes, we prospectively collected full thickness gastric biopsy specimens from 4 non-obese, non-gastroparetic subjects [gastro-esophageal junction cancer with no previous weight loss (<5 lbs), and no previous chemotherapy or radiation therapy] and compared histological changes with the above control group.
Table 1.
Age, sex of patients and immunohistochemical abnormalities
| Controls | Diabetic gastroparesis | Idiopathic gastroparesis | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Age | Sex | IHC abnormalities | Age | Sex | IHC abnormalities | |||||||||||||
| 1 | 25 | F | 19 | F | ICC, nerves | 19 | F | Nerves, fibrosis | ||||||||||||
| 2 | 26 | M | 20 | F | Nerves, immune cells | 20 | F | Immune cells | ||||||||||||
| 3 | 28 | M | 25 | M | Muscle, fibrosis, immune cells | 20 | F | None | ||||||||||||
| 4 | 33 | M | 32 | F | None | 26 | M | ICC, nerves | ||||||||||||
| 5 | 34 | F | 34 | M | ICC, nerves, immune cells | 27 | F | ICC, nerves, muscle | ||||||||||||
| 6 | 36 | F | 37 | M | Immune cells | 29 | F | None | ||||||||||||
| 7 | 36 | M | 40 | F | Nerves, immune cells | 30 | F | Muscle, nerves, immune cells, ICC | ||||||||||||
| 8 | 37 | F | 41 | F | None | 31 | F | ICC, nerves | ||||||||||||
| 9 | 37 | F | 42 | F | Nerves, immune cells | 33 | F | ICC, nerves, immune cells | ||||||||||||
| 10 | 38 | M | 46 | F | ICC, nerves | 33 | F | None | ||||||||||||
| 11 | 40 | F | 48 | M | None | 35 | F | Muscle, nerves, immune cells | ||||||||||||
| 12 | 43 | F | 22 | F | ICC, nerves, fibrosis | 37 | F | ICC, nerves, immune cells | ||||||||||||
| 13 | 44 | F | 50 | M | ICC, nerves, immune cells | 40 | F | Nerves, fibrosis | ||||||||||||
| 14 | 48 | F | 55 | F | ICC, nerves, immune cells | 45 | F | Nerves, fibrosis | ||||||||||||
| 15 | 50 | F | 56 | F | ICC, nerves | 46 | M | Muscle, nerves, immune cells | ||||||||||||
| 16 | 51 | F | 58 | M | Nerves | 50 | F | ICC, nerves, immune cells | ||||||||||||
| 17 | 58 | F | 37 | F | None | 52 | M | ICC, nerves | ||||||||||||
| 18 | 64 | F | 60 | F | ICC, nerves, muscle, immune cells | 55 | M | ICC, nerves | ||||||||||||
| 19 | 64 | M | 66 | F | ICC | 64 | M | ICC, | ||||||||||||
| 20 | 70 | F | 68 | M | ICC, muscle | 75 | F | Muscle, nerves, immune cells | ||||||||||||
ICC: interstitial cells of Cajal.
For electron microscopy, four strips for each patient, 1 mm χ 10 mm long and containing the muscularis propria plus a small portion of the tunica submucosa, were immediately cut after the full thickness biopsy was obtained and fixed for 6 hrs in a solution of 2% glutaraldehyde 0.1M in cacodylate buffer, pH 7.4. After four rinses in the cacodylate-buffered solution containing 0.22M sucrose, the strips were post-fixed for 1 hr in 1% OsO4 in 0.1M phosphate buffer. After a rinse for 30 min. in ddH2O, the strips were en bloc stained in 2% uranyl acetate for 30 min. at 55°C and rinsed again in ddH2O for 10 min. Dehydration was carried out in graded ethanol and the strips then embedded in Spurr using flat moulds to obtain full-thickness sections with the circular muscle cut in cross-section. Semi-thin sections, obtained with an LKB NOVA ultramicrotome (Stockholm, Sweden), were stained with a solution of toluidine blue in 0.1M borate buffer and then observed under a light microscope. Circular muscle and myenteric plexus rich areas away from the strips’ edges with no apparent signs of mal-fixation or processing artefacts were selected. Ultra-thin sections of these selected areas were obtained with the LKB NOVA ultramicrotome using a diamond knife and stained with a saturated solution of uranyl acetate in methanol (50:50) per 12 min. at 45°C, followed by an aqueous solution of concentrated bismuth subnitrate per 10 min. at room temperature. At least 10–20 ultra-thin sections from all four strips of each patient were examined under a JEOL 1010 electron microscope (JEOL Ltd., Tokyo, Japan) and photographed.
Results
The ultra-structural features of age- and sex-matched patients undergoing gastric bypass surgery used as controls were similar to those seen in the non-obese patients undergoing gastric surgery for carcinoma and those reported in literature as normal features [23, 24] (Fig. 1A–E). Conversely, all of the gastroparetic patients had ultrastructural abnormalities, both those with at least one immunohistochemical abnormality and those with no apparent abnormalities on immunohistochemistry (Table 1).
Fig 1.
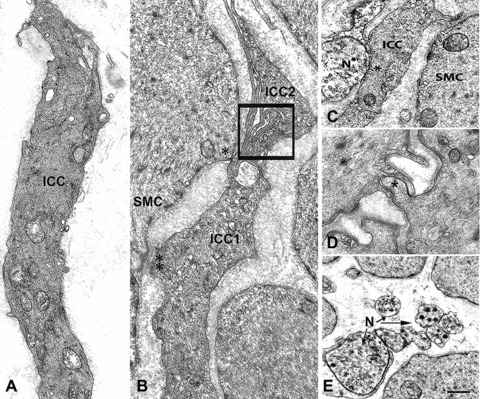
Interstitial cells of Cajal, smooth muscle cells and nerves in control patients. (A) An Interstitial cells of Cajal (ICC) with normal features: several caveolae are located along the cell contour, bundles of intermediate filaments and cisternae of smooth and rough endoplasmic reticulum are present in the cytoplasm. Bar = 1.6 μm. (B) Two ICC (ICC1 and ICC2) whose thin cell projections are in contact to each other (square). The ICC1 is also in contact (two asterisks: a desmosome-like junction; one asterisk: a gap junction) with a smooth muscle cell (SMC). Bar = 1 μm. (C) A close contact between one nerve ending (N) and an ICC (ICC). Nearby a smooth muscle cell (SMC). Bar = 1 μm. (D) A gap junction (asterisk) between two smooth muscle cells. Bar = 0.45 μm. (E) Six nerve endings (N, arrows) most of which are free in the stroma, i.e. have not a glial sheath. (A, C and D) are from obese controls, (B and E) from patients operated for gastric carcinoma. Bar = 1 μm.
Diabetic gastroparesis
As compared to controls, 17 of the 20 (85%) diabetic gastroparetics had ultrastructural changes in ICC morphology. Morphological abnormalities consisted of the presence of several intracytoplasmic vacuoles (Fig. 2A), mitochondria with a clear matrix (Fig. 2B) and an extended rough endoplasmic reticulum (Fig. 2C). All of these abnormalities were not always all present in the same cell and normal ICC were also present in some of these patients, together with the altered ones. Apoptotic features (compact chromatin filling the entire nucleus or as dark clumps close to remnants of the nuclear envelope, cytoplasm either completely empty or containing few swollen mitochondria, increased lysosomes) were also observed in all of the patients with ultrastructural changes (Fig. 3A). In contrast to controls, for all diabetic gastroparetics with ultrastructural changes, none of the ICC visualized were in contact with nerve endings and rarely had cell-to-cell contact with smooth muscle cells (Fig. 2A) and other ICC. Amounts of flocculent and filamentous material, similar to a basal lamina, were occasionally found around ICC (Fig. 2B and C). Both, intramuscular ICC and ICC in the myenteric plexus region had similar ultrastructural changes.
Fig 2.
Interstitial cells of Cajal abnormalities in diabetic gastroparesis. (A) An interstitial cell of Cajal (ICC) with normal features, apart from a small intracytoplasmatic vacuole (asterisk, patient #4). The arrow indicates a gap junction between two smooth muscle cells (SMC). Bar = 0.6 μm. (B) An ICC with mitochondria with clear matrix and short cristae in contact (arrow) with a smooth muscle cell (SMC, patient #14). The ICC nucleus has an irregular contour so the cell appears as having two nuclei. Bar = 0.8 μm. (C) Two ICC with an extended rough endoplasmic reticulum (rer) encased in a stroma rich in collagen fibrils (patient #14). In (B and C), the ICC have thick amounts of basal lamina-like material (asterisks). Bar = 1 μm.
Fig 3.
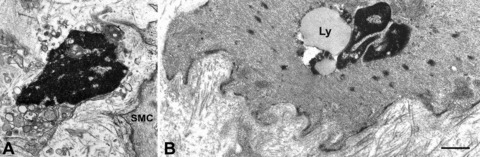
Altered interstitial cells of Cajal (ICC) and smooth muscle in diabetic gastroparesis. (A) A presumed ICC with apoptotic features: clumps of compacted chromatin filling the entire nucleus, a cytoplasm containing swollen mitochondria and lysosomes. SMC: smooth muscle cell (patient #4). Bar = 0.8 μm. (B) A smooth muscle cell with a large lipofuscin body (Ly) near the nucleus. Basal lamina is patchily thickened and the stroma rich in collagen fibrils (patient #11). Bar = 0.8 μm.
In all of the patients, the majority of the smooth muscle cells appeared normal and only a few (no more than 2–3 cells/section) showed morphological alterations, such as vacuoles, abundant lipofuscin bodies (Fig. 3B), swollen mitochondria (Fig. 4A), lamellar bodies, disordered arrangement of myofilaments, with the myofilaments no more parallel to each other or to the major axis of the cell (Fig. 4B). Gap junctions (Fig. 4C) were present in both normal and abnormal cells, although the connective tissue was filled with thin collagen fibrils that distanced the smooth muscle cells form each other (Figs 4B and 5A). Except for one patient (# 10), the basal lamina was patchily (Fig. 5B) or continuously (Fig. 5A and C) markedly thickened (up to 0.6 μm versus the 0.06 μm of controls) in all diabetic gastroparetics.
Fig 4.
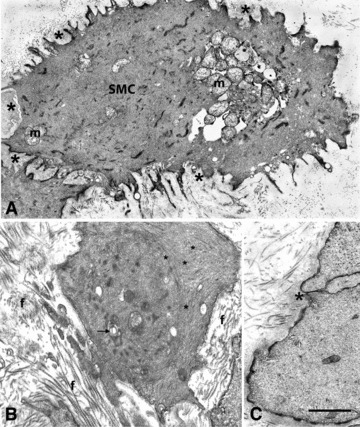
Smooth muscle cells changes in diabetic gastroparesis. (A) A smooth muscle cell (SMC) with clustered and swollen mitochondria (m) and a patchily thickened basal lamina (asterisks, patient #10). Bar = 0.8 μm. (B) A smooth muscle cell encased in a stroma rich in collagen fibrils (f) and with lamellar bodies (arrow) and chaotically arranged myofilaments (asterisks, patient #2). Bar = 0.5 μm. (C) Detail of a gap junction (asterisk, patient #1) between two smooth muscle cells with normal features. Bar = 0.4 μm.
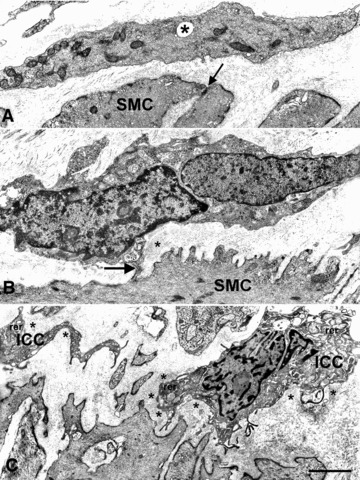
Fig 5.
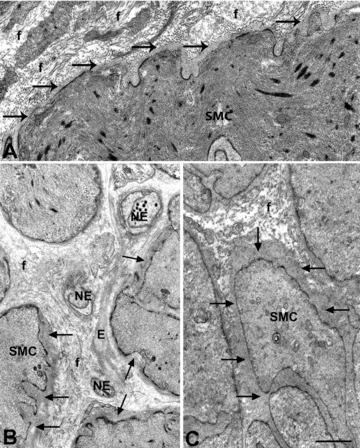
Basal lamina of smooth muscle cells in diabetic gastroparesis. (A–C) The smooth muscle cells are normal featured but their basal lamina is markedly thickened. (A and C), the basal lamina (arrows) around the smooth muscle cells (SMC) is continuously thickened and in (B) patchily. In (A), the stroma is particularly rich in collagen fibrils. Bar = 0.5 μm. In (B), note elastic fibres (E) connecting nerve endings (NE), all of which have a thick basal lamina and one (upper side) contains some synaptic vesicles. Collagen fibrils (f). (A) is from patient #2, (B) from patient #6 and (C) from patient #5. Bar = 0.6 μm.
In most of the subjects (except #6, that had only a few altered nerve endings), in each section, the nerve bundles contained several altered nerve endings. Abnormal nerve endings were large and either empty (Fig. 6A) or, especially in the patient 10, filled with filaments and microtubules (Fig. 6B). In the latter patient, the basal lamina had a normal thickness and elastic fibres attached the nerve endings to the smooth muscle cells (Fig. 6B) which was a finding in patients with idiopathic gastroparesis (see below) but not in controls. All other patients had a thick basal lamina and/or a thick sheath made by collagen fibrils (Figs 5B and 6C) around nerve fibres that appeared to be characteristic for diabetic gastroparesis. Free nerve endings (Fig. 6D), although few, were present and some of them, as in controls, were in close contact with smooth muscle cells.
Fig 6.
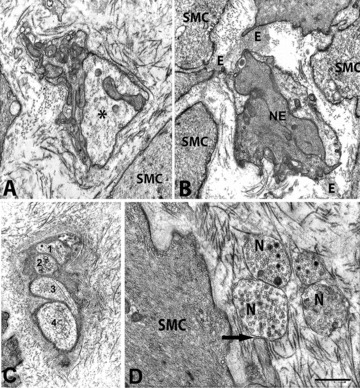
Nerve endings in diabetic gastroparesis. (A) A nerve bundle encased in a fibrillar stroma. One of the nerve endings (asterisk) is very large and empty (patient #2). SMC: smooth muscle cell. Bar = 0.5 μm. (B) Two nerve endings (NE) filled with neurofilaments and attached to smooth muscle cells (SMC) by elastic fibres (E, patient #10). Bar = 0.5 μm. (C) A small intramuscular nerve bundle with four nerve endings. The nerve bundle is surrounded by a very thick basal lamina and numerous collagen fibrils. The nerve endings did not contain synaptic vesicles (patient #1). Bar = 0.8 μm. (D) Free nerve endings (N) containing synaptic vesicles and one of which is in close contact (arrow) with a smooth muscle cell (SMC, patient #2). The stroma is rich in collagen fibrils. Bar = 0.4 μm.
The connective stroma always had an abnormal appearance. In particular, in many of the patients, even in the youngest ones, there was a large quantity of collagen fibrils not assembled in bundles, and the basal lamina was always either patchily or continuously thickened. Mast cells were frequently seen.
Idiopathic gastroparesis
There was a marked dropout of ICC in all idiopathic gastroparetics. Ultrastructural damage to ICC was more marked than in diabetic gastroparesis. Except for patients 4, 14 and 15 that had few (no more than 2–3 cells in 10 sections examined) patchily distributed normal residual ICC (Fig. 7A) all others had abnormal ICC features. The remaining cells were often hard to recognize as ICC as they had few mitochondria which was in contrast to normal ICC and all had a clear matrix and few cristae, many lamellar bodies and extremely large vacuoles that filled the cytoplasm (Fig. 7B and C). As in diabetic gastroparesis, none of the ICC was in contact with nerve endings and rarely with each other and the smooth muscle cells (Fig. 7A). Also, ICC with apoptotic features similar to those seen in diabetic gastroparetics were seen in all patients with idiopathic gastroparesis. Both, intramuscular ICC and ICC in the myenteric plexus region had similar ultrastructural changes. The majority of the smooth muscle cells were normal, gap junctions were always present and some showed caveolae arranged in long rows (Fig. 8A). Only a few smooth muscle cells (again 2–3cells/section) showed morphological alterations (Fig. 8B) and these findings were similar to those observed in diabetic gastroparesis.
Fig 7.
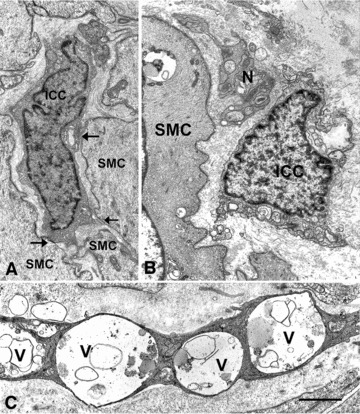
Interstitial cells of Cajal abnormalities in idiopathic gastroparesis. (A) An interstitial cell of Cajal (ICC) with apparently normal features in contact (arrows) with normal featured smooth muscle cells (SMC, patient #14). Bar = 1 μm. (B) A presumptive ICC (ICC) with clear mitochondria and intracytoplasmatic lamellar bodies near nerve endings (N, patient #16). SMC: smooth muscle cell with a large vacuole containing dense bodies. Bar = 1 μm. (C) A presumptive ICC showing evidence of severe injury (patient #1). The cytoplasm is dark and very large vacuoles (V) occupy large portions of the cell. Bar = 0.6 μm.
Fig 8.
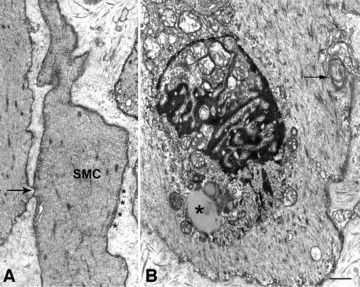
Smooth muscle cells changes in idiopathic gastroparesis. (A) Two smooth muscle cells, one of which (SMC) has particularly numerous caveolae (asterisks) aligned all along the plasma membrane (patient #10). The arrow indicates a gap junction between the two smooth muscle cells. Bar = 0.6 μm. (B) A smooth muscle cell with swollen mitochondria, lipofuscinic bodies (asterisk) and lamellar bodies (arrow, patient #10). Bar = 0.6 μm.
Nerve structures in all patients showed markedly altered morphology (Fig. 9A–D). In neuronal cell bodies, mitochondria had cristae with abnormal shape and orientation (Fig. 9A). Neurofilaments were chaotically arranged (no more oriented parallel to each other and to the major axis of the axon and often forming whorls) in both interganglial and intramuscular axons (Fig. 9A and D). Some of the intramuscular nerve endings were large and empty (Fig. 9C) and others contained lamellar bodies and synaptic vesicles sequestered in membranous envelopes (Fig. 9B and C). Nerve bundles containing normal nerve endings as well as free nerve endings were rarely seen. Glial cells ensheathing axons were also altered, with cytoplasm filled with lysosomes, and vacuoles (Fig. 9B–D).
Fig 9.
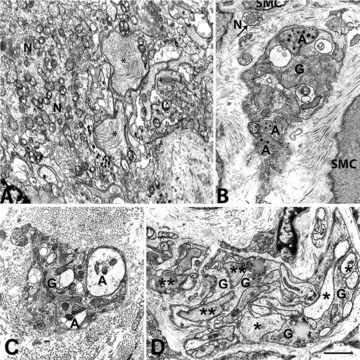
Nerve cell bodies and endings in idiopathic gastroparesis. (A) A myenteric ganglion with severely altered axons and neurons (patient #10). Two axons (asterisks) are enlarged and contain chaotically arranged neurofilaments. The mitochondria of the neuron (N) had abnormal cristae. Bar = 0.6 μm. (B) A small intramuscular nerve bundle immersed in a stroma rich in collagen fibrils (patient #10). The nerve endings (A) contain lamellar bodies and synaptic vesicles sequestered by a membranous envelope. Arrow: a free nerve ending (N) containing synaptic vesicles. SMC: smooth muscle cell; G: glial cell. Bar = 0.6 μm. (C) A small intramuscular nerve bundle encased in a thick fibrillar sheath (patient #15). Nerve endings (A) are empty or contain sequestered synaptic vesicles. G: glial cell. Bar = 0.6 μm. (D) A large nerve bundle with altered nerve endings. Some axons are empty (asterisks) and others are filled with filaments (two asterisks, patient #6). G: glial cells. In (A–C), the cytoplasm of the glial cells contains lamellar bodies. Bar = 1 μm.
The connective stroma showed fibrosis with a marked increase in collagen fibrils, which were particularly abundant around the nerve structures (Fig. 9C). Conversely, the basal lamina had a normal thickness everywhere which distinguished patients with idiopathic gastroparesis from diabetic gastroparesis with the exception of 2 patients (4 and 18) with a diagnosis of idiopathic gastroparesis who had a thick basal lamina patchily distributed around smooth muscle cells, ICC and nerve endings. Mast cells were seen but less commonly than in diabetic gastroparesis.
Discussion
There are two main findings that emerge from this study. The first is that TEM can identify cellular changes in all patients studied with diabetic and idiopathic gastroparesis. Even in the patients with no apparent immunohistological changes on light microscopy, ultrastructural evidence for cellular damage was present. TEM therefore offers an additional tool to study these patients and can provide additional information over light microscopy. The finding that 100% of patients with delayed gastric emptying have cellular changes in the muscle wall of the stomach suggests that these cellular changes may be responsible for the electrical and contractile abnormalities associated with gastroparesis. The second finding to emerge from this study is that there are significant differences between the ultrastructural defects in diabetic and idiopathic gastroparesis as highlighted in Table 2.
Table 2.
Differences in severity of various ultrastructural changes in diabetic and idiopathic gastroparesis
| Diabetic gastroparesis | Idiopathic gastroparesis | |
|---|---|---|
| ICC | ||
| Ultrastructural damage | ++ | +++ |
| ⇒ Poor ICC-ICC and ICC-smooth muscle contacts | ++ | ++ |
| ⇒ Loss of ICC-nerve endings contacts | +++ | +++ |
| ⇒ Apoptotic features | + | + |
| Smooth muscle | ||
| ⇒ Altered morphology | + | + |
| ⇒ Patchily and/or continuously thickened basal lamina | +++ | + |
| Nerves | ||
| ⇒ Altered neuronal bodies | − | +++ |
| ⇒ Altered nerve endings | + | ++ |
| ⇒ Thick connective sheath | +++ | +++ |
| Connective tissue stroma | ||
| ⇒ Fibrosis (marked increase in collagen fibrils) | ++ | +++ |
| ⇒ Thickened basal lamina | +++ | + |
(−) indicates no abnormalities. (+, ++, +++) indicates increasing severity of abnormality.
All tissues examined in this study had ICC, nerve or smooth muscle cell changes suggestive of injury consistent with the diagnosis of gastroparesis with documented delayed gastric emptying. These abnormalities, however, had a different appearance between the two disorders. Nineteen of 20 diabetic gastroparesis patients had a thickened basal lamina around smooth muscle cells and nerves. In contrast, tissues from 18 of 20 patients with idiopathic gastroparesis did not have the thickened basal lamina around smooth muscle cells and nerves but had more intense fibrosis than those from diabetic gastroparesis. Nerve damage was much more prominent in idiopathic gastroparesis with both nerve cell bodies and nerve fibres affected to a greater degree. Unlike in diabetic gastroparesis, glial cells were also abnormal in idiopathic gastroparesis.
Interstitial cells of Cajal were affected in both diabetic and idiopathic gastroparesis. Loss of ICC, already described in delayed gastric emptying associated with diabetes in mice, rats and human light microscopy studies [11, 13–19, 21], was confirmed and the findings extended to idiopathic gastroparesis. Moreover, TEM allowed the determination that residual ICC were rarely in contact with each other and smooth muscle cells and never to nerve endings. This is in sharp contrast to what is seen in control tissues. Given the role ICC play in the control of gastric motility [25] we can reasonably hypothesize that these findings will have a significant impact on the coordinated motor activity in both types of disorders.
Transmission electron microscopy also confirmed previous findings of abnormalities of nerve tissue in both disorders; the loss and/or alterations of synaptic vesicles we presently observed are in agreement with the loss of expression of several neurotransmitters and the neuronal nitric oxide synthase, previously demonstrated [14–16, 19] in diabetic and/ or idiopathic gastroparesis [11, 13, 14, 17, 21]. As outlined above, neuronal cell body, nerve fibres and glial cells were markedly altered in idiopathic gastroparesis, while only nerve ending abnormalities were seen in the diabetic patients. Smooth muscle cell abnormalities were not commonly seen in diabetic and idiopathic gastroparesis and gap junctions were maintained in all patients studied. However, contractile activity of the muscle coat may still be compromised because the smooth muscle cells were encased in a less elastic stroma. The TEM findings of markedly increased thin, scattered collagen fibrils that were not organized in bundles may explain why under light microscopy fibrosis (presence of thick bundles of fibrils) was rarely found [26]. The separation of nerves from ICC, and ICC and nerves from smooth muscle seen on TEM in both disorders suggests that there may be also impaired neurotransmission and transmission of the ICC electrical signal to smooth muscle affecting contractility. Furthermore, the marked changes in the basal lamina seen in diabetic gastroparesis, with the basal lamina either continuously or patchily thickened was similar to what has been described in other organs affected by diabetes such as blood vessels, the retina and the kidney. Its functional implication is a likely decreased ability to exchange metabolites leading to cell damage.
Similarly, the fibrillar sheath observed in idiopathic gastroparesis around smooth muscle cells, ICC and nerves may also compromise smooth muscle cell metabolism and cause cell sufferance (presence of vacuoles, lipofuscinic and lamellar bodies, disordered arrangement of myofilaments).
In conclusion, in this large ultrastructural study of prospectively collected tissue from both diabetic and idiopathic gastroparesis multiple cellular abnormalities were seen. TEM not only confirmed the light microscopy findings but also allowed two new observations to be made. All 40 patients with gastroparesis had cellular abnormalities when the tissue is examined under TEM. Also, while light microscopy does not differentiate well between diabetic and idiopathic gastroparesis, TEM does. In particular, a thick basal lamina was strongly associated with diabetic gastroparesis, while more severe damage to ICC and neurons was associated with idiopathic gastroparesis. The known fairly rapid turnover of ICC [25, 27, 28] and the sparing of synaptic vesicles offer hope that diabetic gastroparesis may be reversible with therapy. This is borne out by animal studies that have shown the reversibility of the global cellular and neuromuscular or motility changes [3, 4, 29, 30]. Conversely, in idiopathic gastroparesis, the severe nerve tissue (neuronal cell bodies, nerve endings and glial cells) impairment may play a larger role in development of gastroparesis. These insights should help differentiate the two disorders and also provide direction for the development of therapy targeted to each disorder.
Acknowledgments
We give thanks to Dr. Sean Harbison, Megan Garrity-Park, Shelly Gray, Valerie McNair, Gary Stoltz, Peter Strege and Kristy Zodrow for excellent technical and secretarial assistance, and to Daniele Guasti for his precious and excellent technical support for the electron microscope study. The Gastroparesis Clinical Research Consortium (GpCRC) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK073983, U01DK073975, U01DK073985, U01DK074007, U01DK073974, U01DK074008). This work was also supported by DK57061, DK84567 and PO1 DK68055.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Authors’ contributions
Henry Parkman, Thomas Abell, William Snape, Pankaj J. Pasricha, Maria Simonetta Faussone-Pellegrini, Madhusudan Grover, Gianrico Farrugia: acquisition of tissue and drafting of manuscript, critical review of the manuscript for important intellectual content, study concept and design, analysis and interpretation of data, critical revision of the data for important intellectual content, obtained funding.
Matthew Lurken, Cheryl Bernard, Thomas Smyrk: study concept and design, analysis and interpretation of data, critical revision of the data for important intellectual content.
William Hasler, Aynur Unalp-Arida, Linda Nguyen, Kenneth Koch, Jorges Calles, Linda Lee, James Tonascia, Frank Hamilton: analysis, interpretation of data, critical revision of the manuscript for important intellectual content.
Members of the Gastroparesis Clinical Research Consortium as of April 2011
Clinical Centers
Stanford University, Stanford, CA, USA: Pankaj Jay Pasricha, MD (Principal Investigator); Linda Nguyen, MD; Nighat Ullah, MD
California Pacific Medical Center, San Francisco, CA, USA: William Snape, MD (Principal Investigator); Robin Bishop (2008); Nata DeVole, RN; Mary Greene, MS; Sue Louiseau; Amy Marincek, RN, BSN (2008); Shelly Parker, RN MSN ANP-C ANP; Eve Pillor, RN MSN FNP; Courtney Ponsetto, RN; Katerina Shetler, MD
Mayo Clinic College of Medicine, Rochester, MN, USA: Gianrico Farrugia, MD (Principal Investigator); Madhusudan Grover, MD; Cheryl Bernard; Matt Lurken (2007–2009); K. Robert Shen, MD; Michael Sarr, MD; Michael Kendrick, MD
Temple University, Philadelphia, PA, USA: Henry P. Parkman, MD (Principal Investigator); Siva Doma, MD (2006–2007); Javier Gomez, MD (2008–2009); Steven Kantor; Vanessa Lytes, CRNP; Amiya Palit, MD; Zeeshan Ramzan, MD (2007–2008); Priyanka Sachdeva, MD; Kellie Simmons, RN; Sean Harbison, MD
Texas Tech University Health Sciences Center: Richard W. McCallum, MD (Principal Investigator); Reza Hejazi, MD; Irene Sarosiek, MD; Denise Vasquez; Natalia Vega
University of Michigan, Ann Arbor, MI, USA: William Hasler, MD (Principal Investigator); Michelle Atkinson, CSC; Radoslav Coleski, MD (2007–2008)
University of Mississippi Medical Center, Jackson, MS, USA: Thomas Abell, MD (Principal Investigator); JoAnne Fordham; Olivia Henry, RD; Archana Kedar, MD; Valerie McNair, LPN II; Susanne Pruett, RN (2007–2008); Margaret Smith, RN; Danielle Spree, CNP
Wake Forest University, Winston-Salem, NC, USA: Kenneth Koch, MD (Principal Investigator); Lynn Baxter; Jorge Calles, MD; Samantha Culler; Judy Hooker, RN; Paula Stuart, PA
Resource Centers
National Institute of Diabetes, Digestive and Kidney Diseases, Bethesda, MD, USA: Frank Hamilton, MD, MPH (Project Scientist); Steven James, MD; Rebecca Torrance, RN, MSN; Rebekah Van Raaphorst, MPH
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD, USA: James Tonascia, PhD (Principal Investigator); Patricia Belt; Ryan Colvin, MPH; Michele Donithan, MHS; Mika Green, MA; Milana Isaacson; Wana Kim; Linda Lee, MD; Alison Lydecker, MPH (2006–2008); Pamela Mann, MPH (2008–2009); Laura Miriel; Alice Sternberg, ScM; Aynur Ünalp-Arida, MD, PhD; Mark Van Natta, MHS; Ivana Vaughn, MPH; Laura Wilson, ScM; Katherine Yates, ScM
References
- 1.Farrell FJ, Keeffe EB. Diabetic gastroparesis. Dig Dis. 1995;13:291–300. doi: 10.1159/000171509. [DOI] [PubMed] [Google Scholar]
- 2.Stanciu GO. Gastroparesis and its management. Rev Med Chir Soc Med Nat Iasi. 2001;105:451–6. [PubMed] [Google Scholar]
- 3.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–64. doi: 10.1053/j.gastro.2008.09.003. , 2064 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horvath VJ, Vittal H, Lorincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of Cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–70. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Ordog T, Takayama I, Cheung WK, et al. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–9. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 6.Spangeus A, El-Salhy M. Myenteric plexus of obese diabetic mice (an animal model of human type 2 diabetes) Histol Histopathol. 2001;16:159–65. doi: 10.14670/HH-16.159. [DOI] [PubMed] [Google Scholar]
- 7.Spangeus A, Suhr O, El-Salhy M. Diabetic state affects the innervation of gut in an animal model of human type 1 diabetes. Histol Histopathol. 2000;15:739–44. doi: 10.14670/HH-15.739. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T, Nakamura K, Itoh H, et al. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535–44. doi: 10.1053/gast.1997.v113.pm9352855. [DOI] [PubMed] [Google Scholar]
- 9.Vittal H, Farrugia G, Gomez G, et al. Mechanisms of disease: the pathological basis of gastroparesis-a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336–46. doi: 10.1038/ncpgasthep0838. [DOI] [PubMed] [Google Scholar]
- 10.Wang XY, Huizinga JD, Diamond J, et al. Loss of intramuscular and submuscular interstitial cells of Cajal and associated enteric nerves is related to decreased gastric emptying in streptozotocin-induced diabetes. Neurogastroenterol Motil. 2009;21:1095–e92. doi: 10.1111/j.1365-2982.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 11.Battaglia E, Bassotti G, Bellone G, et al. Loss of interstitial cells of Cajal network in severe idiopathic gastroparesis. World J Gastroenterol. 2006;12:6172–7. doi: 10.3748/wjg.v12.i38.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ejskjaer NT, Bradley JL, Buxton-Thomas MS, et al. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med. 1999;16:488–95. doi: 10.1046/j.1464-5491.1999.00086.x. [DOI] [PubMed] [Google Scholar]
- 13.Forster J, Damjanov I, Lin Z, et al. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102–8. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Harberson J, Thomas RM, Harbison SP, et al. Gastric neuromuscular pathology in gastroparesis: analysis of full-thickness antral biopsies. Dig Dis Sci. 2010;55:359–70. doi: 10.1007/s10620-009-1071-2. [DOI] [PubMed] [Google Scholar]
- 15.He CL, Soffer EE, Ferris CD, et al. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–34. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki H, Kajimura M, Osawa S, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076–87. doi: 10.1007/s00535-006-1909-8. [DOI] [PubMed] [Google Scholar]
- 17.Lin Z, Sarosiek I, Forster J, et al. Association of the status of interstitial cells of Cajal and electrogastrogram parameters, gastric emptying and symptoms in patients with gastroparesis. Neurogastroenterol Motil. 2010;22:56–61. doi: 10.1111/j.1365-2982.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 18.Miller SM, Narasimhan RA, Schmalz PF, et al. Distribution of interstitial cells of Cajal and nitrergic neurons in normal and diabetic human appendix. Neurogastroenterol Motil. 2008;20:349–57. doi: 10.1111/j.1365-2982.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- 19.Pasricha PJ, Pehlivanov ND, Gomez G, et al. Changes in the gastric enteric nervous system and muscle: a case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 2008;8:21. doi: 10.1186/1471-230X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokol H, Lavergne-Slove A, Mikol J, et al. Severe isolated myopathic gastroparesis: a case report with pathological findings. Gut. 2006;55:1662. doi: 10.1136/gut.2006.105742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarate N, Mearin F, Wang XY, et al. Severe idiopathic gastroparesis due to neuronal and interstitial cells of Cajal degeneration: pathological findings and management. Gut. 2003;52:966–70. doi: 10.1136/gut.52.7.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–85. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faussone-Pellegrini MS, Pantalone D, Cortesini C. An ultrastructural study of the smooth muscle cells and nerve endings of the human stomach. J Submicrosc Cytol Pathol. 1989;21:421–37. [PubMed] [Google Scholar]
- 24.Faussone-Pellegrini MS, Pantalone D, Cortesini C. An ultrastructural study of the interstitial cells of Cajal of the human stomach. J Submicrosc Cytol Pathol. 1989;21:439–60. [PubMed] [Google Scholar]
- 25.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20(Suppl 1):54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida MM, Schuffler MD, Sumi SM. There are no morphologic abnormalities of the gastric wall or abdominal vagus in patients with diabetic gastroparesis. Gastroenterology. 1988;94:907–14. doi: 10.1016/0016-5085(88)90546-x. [DOI] [PubMed] [Google Scholar]
- 27.Gibbons SJ, De Giorgio R, Pellegrini MS, et al. Apoptotic cell death of human interstitial cells of Cajal. Neurogastroenterol Motil. 2009;21:85–93. doi: 10.1111/j.1365-2982.2008.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tharayil VS, Wouters MM, Stanich JE, et al. Lack of serotonin 5-HT2B receptor alters proliferation and network volume of interstitial cells of Cajal in vivo. Neurogastroenterol Motil. 2010;22:462–9. doi: 10.1111/j.1365-2982.2009.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi KM, Kashyap PC, Dutta N, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138:2399–409. doi: 10.1053/j.gastro.2010.02.014. , 2409 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins CC, Sawa A, Jaffrey S, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:373–84. doi: 10.1172/JCI8273. [DOI] [PMC free article] [PubMed] [Google Scholar]


