Abstract
The liver changes with age leading to an impaired ability to respond to hepatic insults and increased incidence of liver disease in the elderly. Therefore, there is critical need for rapid model systems to study aging-related liver changes. One potential opportunity is murine models of human progerias, or diseases of accelerated aging. Ercc1−/Δ mice model a rare human progeroid syndrome caused by inherited defects in DNA repair. To determine if hepatic changes that occur with normal aging occur prematurely in Ercc1−/Δ mice, we systematically compared liver from 5 month-old, progeroid Ercc1−/Δ mice to old (24–36 month) wild-type (WT) mice. Both displayed areas of necrosis, foci of hepatocellular degeneration and acute inflammation. Loss of hepatic architecture, fibrosis, steatosis, pseudocapillarization, and anisokaryosis were more dramatic in Ercc1−/Δ mice than in old WT mice. Liver enzymes were significantly elevated in serum of Ercc1−/Δ mice and old WT mice, while albumin was reduced, demonstrating liver damage and dysfunction. The regenerative capacity of Ercc1−/Δ liver following partial hepatectomy was significantly reduced. There was evidence of increased oxidative damage in Ercc1−/Δ and old WT liver, including lipofuscin, lipid hydroperoxides and acrolein as well as increased hepatocellular senescence. There was a highly significant correlation in genome-wide transcriptional changes between old WT and 16 but not 5 week-old Ercc1−/Δ mice emphasizing that the Ercc1−/Δ mice acquire an aging profile in early adulthood.
Conclusion
There are strong functional, regulatory and histopathological parallels between accelerated aging driven by a DNA repair defect and normal aging. This supports a role for DNA damage in driving aging and validates a murine model for rapidly testing hypotheses about causes and treatment for aging-related hepatic changes.
Keywords: DNA repair, DNA damage, progeria, hepatic fibrosis, mouse model
INTRODUCTION
Aging is characterized by the progressive loss of homeostatic reserve in all tissues leading to a decreased ability to respond to stress, functional decline and dramatically increased risk of morbidity and mortality(1). Chronic liver disease and cirrhosis are the twelfth leading cause of death in the United States, with 25,192 (1.1%) deaths each year(2). Mortality due to liver disease is not limited to the United States, as the World Health Organization (WHO) estimates that 20 million individuals have cirrhosis and/or liver cancer worldwide, and that 1–2 million die annually as a result of liver failure. Many liver diseases are more prevalent in the elderly, including alcohol-induced cirrhosis, viral hepatitis-induced cirrhosis, diabetic-associated chronic liver disease, hepatocellular carcinoma and biliary cirrhosis(3, 4), illustrating that aged liver is less able to cope with a variety of stressors.
Although there are no definitive biomarkers of aged liver, there are several hallmark structural changes. The volume of the liver decreases with age(5). This decrease is gender-specific, with an average decrease of 6.5% in males and 14.3% in females(6). Hepatocytes are quiescent cells, until stimulated to proliferate following injury or surgical resection(7). The capacity for hepatocyte regeneration following partial hepatectomy decreases with age(8). During the aging process, the nuclear size and shape of hepatocytes becomes altered(9). Nuclear invaginations and larger nuclei are commonly associated with age in liver samples(10). Nuclear polyploidy of hepatocytes increases with age, rising from 7–10% in young mice to approximately 30% in old mice(9). Finally, lipofuscin, insoluble oxidized proteins, accumulates in the cytoplasm of hepatocytes(11, 12).
Liver sinusoidal endothelial cells (LSEC) line the hepatic microvasculature, express little to no basement membrane, and exhibit numerous fenestrations necessary for filtering lipopoteins from the portal blood supply. A reduction in the number of these pores combined with the thickening of the endothelium, called pseudocapillarization, is commonly seen in aged livers(13), as is non-alcoholic fatty liver disease or steatosis(14). The underlying cause for fatty liver disease is not known, but some possibilities include diabetes mellitus, oxidative stress, mitochondrial dysfunction and metabolic and hormonal imbalances(15). All of these age-related liver changes in humans are recapitulated in the mouse(16–20).
Aging research is challenging because of the incredible variability in humans. Mice offer an attractive alternative because genetic and environmental variables can be controlled. Nevertheless, the time and expense required to generate and maintain mice >3 years of age is daunting. One approach to overcome this is to use mouse models of accelerated aging, or human progerias. Werner syndrome, Cockayne syndrome, XFE progeroid syndrome and trichothiodystrophy are examples of human diseases of accelerated aging(21). A common thread linking these disorders is that they all result from mutations in genes that are involved in genome maintenance. This strongly suggests that DNA damage can promote age-related degenerative changes.
ERCC1-XPF is a structure-specific endonuclease involved in the repair of several types of DNA lesions including interstrand crosslinks, double-strand breaks, and bulky, helix-distorting lesions(22, 23). ERCC1 and XPF are obligate binding partners and stabilize one another in vivo(24). Mutations in XPF that cause reduced expression of ERCC1-XPF cause a progeroid syndrome characterized by dramatically accelerated aging of most organ systems(24). Ercc1−/− and Xpf−/− mice have an identical phenotype to one another and mimic the human progeroid syndrome(25, 26). These mice die in the 4th week of life with aging-like degenerative changes, including osteoporosis, neurodegeneration, bone marrow hypoplasia, epidermal atrophy, sarcopenia, and liver and kidney dysfunction(24, 27). Liver dysfunction is life-limiting in Ercc1−/− mice(28). Mice with reduced but not ablated ERCC1-XPF expression live longer than Ercc1−/− mice (30 weeks vs. 4 weeks maxiumum lifespan)(29, 30) The Ercc1−/Δ mice are healthy into adulthood (8 weeks) then begin to show numerous progressive symptoms associated with aging(30). The aim of this study was to systematically compare the liver of 4–5 month-old Ercc1−/Δ mice to that of old WT mice (2+ yrs old) to determine if the progeroid mice offer an accurate model of natural aging that could be used to accelerate research on the biology of aging-related liver changes and therapeutic interventions to extend healthspan.
EXPERIMENTAL PROCEDURES
Animal Care and Experimentation
Experiments involving mice were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and in accordance with the NIH guidelines for humane care of animals. Ercc1−/Δ mice were bred and genotyped as previously described(23). All mice used in this study were in an f1 mixed genetic background (FVB/n:C57Bl/6).
Histological analyses
6–7 and 20–24 week old WT and Ercc1−/Δ mice, along with aged (26–36 month) WT mice were sacrificed by CO2 inhalation, and excised livers were fixed with 10% formalin and embedded in paraffin. Tissue sections (6µm) were cut and stained with hematoxylin and eosin (H&E) to detect changes in liver architecture and Masson’s trichrome to detect fibrosis, using standard procedures. Alternatively liver specimens were cryopreserved by fixation in 10% formalin for 2–4 hours, followed by incubation in 30% sucrose at 4°C overnight. Tissues were embedded in OCT and flash frozen prior to sectioning on a Microm cryostat.
Liver Perfusion and Processing for Ultrastructural Analysis
For scanning and transmission electron microscopy, the liver of euthanized animals was cleared of blood by perfusion with phosphate-buffered saline (PBS) at 3mL/min through the inferior vena cava as previously described(31). Samples of liver were fixed with glutaraldehyde and processed also as described previously(32). TEM images were captured with the JEOL JEM 1011 microscope and SEM images were taken with the JEOL JSM 6330F microscope (JEOL Ltd, Peabody, MA). Porosity was determined using MetaMorph imaging software. Fenestrations were manually predetermined and porosity expressed as percent of measured total sinusoid area.
Immunofluorescence Analyses
Staining for acrolein was performed on deparaffinized liver sections from 20 wk-old WT, Ercc1−/Δ mice and aged (24–36 mth-old) WT mice. Paraffin embedded tissue sections were subjected to heat induced-epitope retrieval by incubation in sodium citrate buffer (10mM, pH 6.0) for 10 min in a microwave, followed by 30 min cool-down. Sections were permeabilized with PBS-Tween (0.05%) and incubated with rabbit polyclonal to acrolein (1:500, ab37110, Abcam, Cambridge, MA), CD31 (1:500, 550274, BD Pharmingen, San Diego, CA), F4/80 (1:500, 552958, BD Pharmingen, San Diego, CA), α-SMA (1:500, #6198, Sigma, St. Louis, MO), and Desmin (1:250, ab15200, Abcam, Cambridge, MA) overnight at 4°C. The secondary antibody, Alexa Fluor 594 goat anti-rabbit IgG (1:1000, A11012, Molecular Probes, Eugene, OR), was allowed to incubate for 60 min in the dark. Nuclei were stained with Vectashield with DAPI mounting medium (Vector Laboratories, Inc., Burlingame, CA). Ten random images per sample were acquired using an Olympus BX51 fluorescent microscope. Acrolein fluorescence intensity was quantified using the Axio Vision imaging software (Carl Zeiss, Gottingen, Germany). Average fluorescence intensity is shown ± S.E.M. All p-values reported were derived using a standard Student’s t test with a two-tailed distribution. Differences were considered significant at the 95% confidence interval (p < 0.05).
Immunohistochemical analyses
Immunohistochemistry for Ki67 (rat anti-mouse Ki67 (TEC-3), Dako Cytomation, Carpinteria, CA) was performed on deparaffinized liver sections from 10 wk-old WT and Ercc1−/Δ mice that had undergone partial hepatectomy surgery. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. Tissue sections were subjected to heat induced-epitope retrieval by incubation in sodium citrate buffer (10mM, pH 6.0) for 30 min in a decloaker, followed by 30 min cool-down. Blocking was done with 5% rabbit serum for 20 min. Primary antibody was applied for 1 hour. Secondary antibody was applied for 30 minutes, followed by the label antibody (ABC Elite, Vector Laboratories, Burlingame, CA) for 30 min. DAB chromagen (Dako Cytomation, Carpinteria, CA) was applied for 6 min, followed by 2 rinse steps in distilled water. Hematoxylin was used as a counterstain. Brightfield images were collected using an Olympus BX51 fluorescent microscope, and Ki67 positive cells were quantified from 5 random fields of view from each of 4 mice per group. Mean percentages were plotted ± S.E.M.; asterisk indicates p<0.05 as calculated with the Student’s t test with a two-tailed distribution.
Detection of Lipids
Slides with frozen liver tissue were warmed to room temperature, fixed in 2% PFA for 10 min, rinsed with PBS, and incubated with LipidTox Red Neutral Lipid Stain (Invitrogen, Foster City, CA) for 30 min at room temperature. Tissues were rinsed in PBS and nuclei were counterstained with Hoechst dye for 1 min. Hoechst dye was rinsed off with 3 PBS washes and tissue was covered using gelvatol mounting medium. Image acquisition was done on an Olympus BX51 fluorescent microscope.
See Supplemental information for additional Experimental Procedures.
RESULTS
Histopathological changes in progeroid Ercc1−/Δ mouse liver mimic normal aging
To investigate structural changes in the liver of progeroid Ercc1−/Δ mice we compared liver tissue of 20–24 week-old Ercc1−/Δ mice to wild-type (WT) littermates and to 26–34 month old WT mice. The relative liver/body weight of Ercc1−/Δ mice was not significantly different from littermate controls at any age(30). The architecture of the Ercc1−/Δ liver was irregular, with mild to moderate variation in lobular size (Figure 1A). Pathologic changes detected in both Ercc1−/Δ mice and old WT mice include multifocal degeneration, necrosis and neutrophilic inflammation, a diffuse increase in sinusoidal lining cells, and karyomegaly, with intranuclear inclusions (Figure 1 and Supplemental Figure 1). None of these changes were detected in young adult (20–24 week-old) WT mice. The only abnormalities detected in juvenile (7 wk-old) Ercc1−/Δ mice were mild anisokaryosis and intranuclear inclusions. Liver from aged WT mice, like humans, displays mild portal fibrosis (Figure 1B) and increased neutral lipid accumulation (Figure 1C). Similar changes were detected in 20–24 wk-old Ercc1−/Δ mice, but not age-matched WT mice or 7 wk-old Ercc1−/Δ mice. This demonstrates that the Ercc1−/Δ mice undergo progressive degenerative processes, rather than having developmental liver abnormalities.
Figure 1. Premature onset of age-related pathology in Ercc1−/Δ mouse liver.
(A) Hematoxylin and eosin (H&E) stained liver sections from 7 wk, 23 wk, and 34 mth-old WT mice, and Ercc1−/Δ littermates (10X). The bottom right panel is a high magnification (40X) image of Ercc1−/Δ liver (23 wk) illustrating inflammatory infiltrate (black arrows), abnormal nuclei with inclusion (green arrows) and dead hepatocytes (blue arrows).
(B) Masson’s trichrome stained liver sections. Insets (40X) in the bottom right panel show perisinusoidal fibrosis in Ercc1−/Δ and old WT liver samples.
(C) LipidTOX™ stain of liver sections to detect neutral lipids (20X).
(D) Immunofluorescence detection of desmin and α-SMA, markers of hepatic stellate cells and their activation, respectively, in frozen liver sections (20X).
Stellate cells are pericytes that reside between hepatocytes and endothelial cells lining the sinusoids in the extracellular space of Disse. Stellate cells store fat and when activated produce extracellular matrix proteins, contributing to fibrosis. With aging in humans, stellate cell number, but not activity is reported to increase(35). Similar results were found in 3 year-old WT mice and progeroid Ercc1−/Δ mice, as measured by desmin and α-smooth muscle actin (SMA) staining, markers of stellate cells and their activation, respectively (Figure 1D). Kupffer cells, resident liver macrophages, are also reported to increase in number and phagocytic activity with aging in humans (36). However, we did not detect increased F4/80 immunostaining, a macrophage marker, in liver sections of old WT or progeroid mice (Supplemental Figure 2).
Pseudocapillarization and defenestration in progeroid Ercc1−/Δ and old WT mouse liver
Age-associated endothelial changes in the liver are prominent and contribute to age-related decline in liver function(37). Expression of CD31, a marker of endothelium, was dramatically increased in 5 month-old Ercc1−/Δ mice and 2.5 year-old WT mice compared to young adult WT mice (Figure 2A), indicative of pseudocapillarization. Pseudocapillarization encompasses many alterations including thickening of the sinusoidal endothelial cells (LSECs) and increased deposition of basement membrane proteins in the space of Disse(13, 38). Transmission electron microscopy of liver from old and progeroid mice revealed a thickening of the basement membrane (Figure 2B). This was confirmed by immunblot detection of the basement membrane protein laminin, which revealed a dramatically increased level of laminin in old WT and 20 week-old Ercc1−/Δ mouse liver compared to young adult WT mice (Figure 2C).
Figure 2. Age-related thickening of the sinusoidal endothelium in Ercc1−/Δ mouse liver.
(A) Immunofluorescence detection of CD31, a surface marker of endothelial cells in frozen liver sections (20X).
(B) Transmission electron micrographs (TEM; 12,000X) of liver illustrating thickening of the basement membrane in sinusoids of mutant and old WT mice compared to young WT. Insets show enlargement of a portion of the original image and arrows indicate deposition of basement membrane and pseudocapillarization of the sinusoidal endothelium.
(C) Immunoblot to detect the extracellular matrix protein laminin in liver lysates from 20 wk-old Ercc1−/Δ mice, control littermates and old WT (2–3 year) mice (n=3 per group). Densitometry was used for quantification and averages are listed as a ratio compared to 20 week-old WT mice.
With advanced age LSECs also lose their fenestrations, necessary for lipoprotein filtration, endocytosis, and immunological functions(37). Scanning electron microscopy of murine liver sections revealed a significant reduction in fenestration of the sinusoids in old (26–30 month-old) WT mice compared to young (20 week-old) WT animals (Figure 3A). Micrographs from 20 week-old Ercc1−/Δ mice looked more similar to those from the old WT mice than to their WT littermates, with significantly reduced fenestrations, decreased porosity, and loss of sieve plates (Figure 3A–3B).
Figure 3. Premature defenestration in Ercc1−/Δ mouse liver.
(A) Scanning electron micrograph (10,000X) of liver, illustrating defenestration in sinusoids of mutant and old WT mice. Insets show enlargement of a portion of the original image.
(B) Quantification of porosity shown in A. 3 SEM images per mouse (n=3 mice per group) were quantified, and the % open area of the sinusoid is represented ± S.E.M. Asterisks indicate significant differences.
(C) Serum cholesterol and (D) triglyceride levels in WT and Ercc1−/Δ mice of various ages. The average ± S.E.M. are plotted (n=6 for 7 wk-old mice, n=9 for 21 wk-old and old WT mice). Asterisks indicate significant differences as calculated by a paired Student’s t test.
Age-related LSEC defenestration can lead to impaired hepatic clearance of atherogenic lipoproteins, contributing to hypertriglyceridemia, hypercholesteremia and vascular disease(39, 40). Therefore we measured serum cholesterol levels in the mice. Cholesterol levels were normal in young (7 wk-old) Ercc1−/Δ mice (Figure 3C). By 21 weeks of age, when defenestration was apparent, serum cholesterol was significantly elevated in Ercc1−/Δ mice compared to WT littermates. In contrast, serum triglycerides were significantly lower in 21 week-old compared to littermate controls (Figure 3D). Serum lipoproteins were not significantly altered in old WT mice compared to young WT mice.
Functional changes in progeroid Ercc1−/Δ mouse liver mimic normal aging
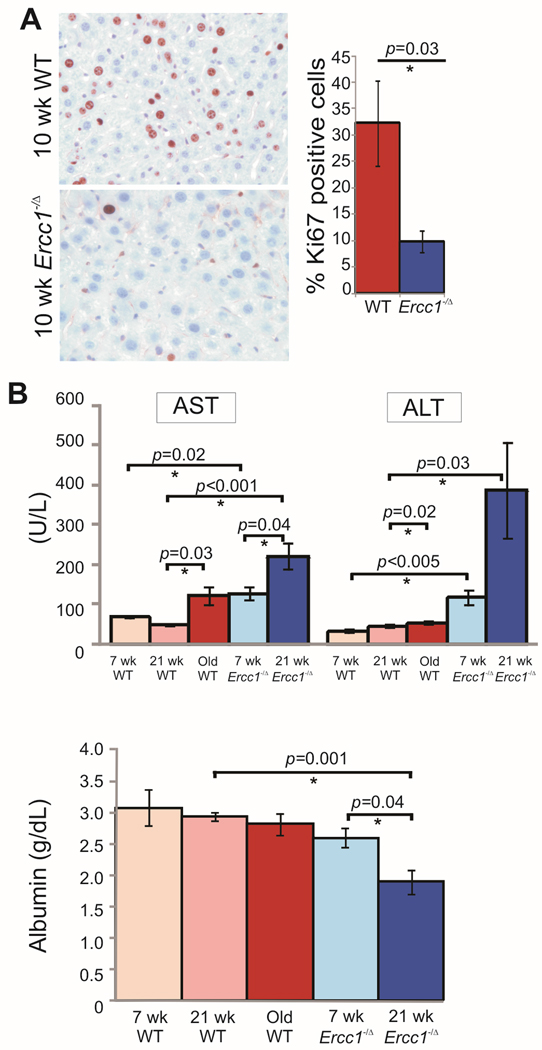
Under normal conditions hepatocytes are quiescent cells, but can be stimulated to proliferate in response to parenchymal damage(41). In an experimental setting, proliferation is induced by partial hepatectomy (PHx), which leads to compensatory hyperplasia. Hepatocyte proliferation in response to PHx is significantly decreased in aged rodents compared to young animals(8). Thirty-percent of the liver was surgically resected from 10 wk-old WT and Ercc1−/Δ mice. Ercc1−/Δ mice did not survive a standard PHx, which removes 70% of the liver. Thus the surgery was modified to only remove 1 lobe, or 30% of the liver. At 48 hours post-resection, the mice were euthanized and liver tissue collected for analysis. Previous experiments in which WT mice were euthanized every 12 hours post-partial hepatectomy, from 12–72 hours, indicated that 48 hours was the time point at which proliferation was the highest in 10 week-old WT mice (data not shown). Immunohistochemical staining for the proliferation-associated antigen Ki67 revealed a significant decrease in the number of proliferating cells in the liver from Ercc1−/Δ mice compared to WT littermates (Figure 4A). Therefore regenerative capacity of the liver is prematurely reduced in Ercc1−/Δ mice.
Figure 4. Premature loss of liver function in Ercc1−/Δ mice.
(A) Representative liver sections from 10 week-old WT and Ercc1−/Δ mice immunostained for the proliferation marker Ki67 48 hr post-partial hepatectomy. The fraction of proliferating hepatocytes is graphed (5 random fields of view analyzed from n=4 mice per genotype; asterisk indicates p<0.05, Student’s two tailed t-test).
(B) Serum chemistries reflecting liver function. Plotted are the average ± S.E.M. for 6 mice per age and genotype. WT animals are graphed in reds; Ercc1−/Δ mice in blues. Darker colors indicate increasing age. AST is aspartate transminase; ALT is alanine transaminase. Asterisks indicate significant differences.
To further monitor liver function and integrity, liver function tests were performed on plasma of Ercc1−/Δ and WT mice at multiple ages (Figure 4B). ALT was significantly elevated in old WT mice compared to young, as previously reported(42). ALT was also significantly elevated in Ercc1−/Δ mice compared to age-matched WT mice, even at 7 wks of age. ALT levels more than doubled as the Ercc1−/Δ mice aged. AST was also significantly elevated in old WT and 21 wk-old Ercc1−/Δ mice compared to young adult WT mice. Serum albumin is known to decrease with age in humans and rodents(43, 44). Albumin was significantly reduced in the plasma of 21 wk-old, but not 7 wk-old Ercc1−/Δ mice (Figure 4B). Thus, a very similar pattern of time-dependent changes in liver function tests, indicative of chronic, progressive tissue degeneration and loss of organ function, are observed in Ercc1−/Δ and WT mice. However, the changes are dramatically accelerated in the Ercc1−/Δ mice.
Increased cellular senescence in Ercc1−/Δ and old WT liver
To further investigate the mechanism driving the loss of regenerative capacity in Ercc1−/Δ and old WT mice, we asked if hepatocytes were undergoing cellular senescence. Liver sections were stained to detect senescence-associated β-galactosidase (SA-β gal) activity, a marker of cellular senescence (Figure 5A). SA-β Gal staining was increased in liver of 20 week-old Ercc1−/Δ and old WT mice compared to 20 week-old WT mice. p16INK4a is another established marker of senescent cells(45). p16INK4a expression was increased 1.4- and 2.0-fold in 20 wk-old Ercc1−/Δ and 2 yr-old WT mice, respectively, compared to young WT mice (Figure 5B). Increased cell size, nuclear size, and the nuclear:cytoplasmic ratio are also associated with cell senescence and old age(46–48). The nuclear size of hepatocytes was heterogeneous in Ercc1−/Δ and old WT mice compared to 7 and 23 wk-old WT mice (Figure 5C). Hepatocyte nuclei of Ercc1−/Δ mice were significantly larger than that of WT littermates at 7 wks of age, and increased by 21 wks of age (Figure 5C). Nuclear size was also significantly increased in old WT mice compared to young WT mice. Electron micrographs revealed a highly irregular surface area of the hepatocyte nuclei from Ercc1−/Δ and old, but not young, WT mice (Figure 5D).
Figure 5. Premature senescence of Ercc1−/Δ mouse liver.
(A) Senescence-associated (SA) β-galactosidase histochemical stain on flash frozen liver sections.
(B) Immunoblot detection of the senescence marker p16INK4a in liver lysates of a 20 week-old WT mouse and an Ercc1−/Δ littermate, and a 26 month-old WT mouse. Tubulin was used as a loading control to calculate the fold-increase in p16 expression relative to adult WT mice.
(C) DAPI staining of hepatocyte nuclei illustrating increased nuclear size and heterogeneity in old WT (34 months) and adult Ercc1−/Δ mouse liver (1000X). Quantification of nuclear size using Metamorph imaging software and images of DAPI-stained frozen liver sections. Plotted are the average nuclear areas ± S.E.M. calculated from 10 random fields from 5 mice per group. The asterisks indicate significant differences.
(D) Transmission electron micrographs of representative hepatocyte nuclei (12,000X).
Increased oxidative damage in Ercc1−/Δ liver is also found with natural aging
Oxidative stress has been implicated in driving age-dependent cellular senescence(49). To determine if oxidative stress is elevated in Ercc1−/Δ mice and with old age, we measured lipid peroxidation in liver. The level of lipid hydroperoxides, measured by ELISA, was elevated in the liver of 20 wk-old Ercc1−/Δ mice compared to WT littermates (Figure 6A). A similar elevation was seen in old WT mouse liver compared to young WT. This increase in lipid peroxidation was confirmed by immunodetection of acrolein, a stable product of lipid peroxidation. Ercc1−/Δ and old WT mice had significantly increased acrolein compared to adult WT mice (Figure 6B).
Figure 6. Increased oxidative damage in liver of old and progeroid mice.
(A) Immunodetection of lipid hydroperoxides, a by-product of lipid peroxidation, in liver lysates from 20 wk-old WT and Ercc1−/Δ littermate mice vs. old WT mice (26–34 months of age). The box plot represents the mean (in colored square), along with the 25th and 75th percentile for each group (n=5 mice). Average lipid hydroperoxide levels for groups were as follows: 20 wk WT (4.8µM/g liver), 20 wk Ercc1−/Δ (7.3µM/g liver; p=0.12, one-tailed Student’s t-test), and old WT (7.2µM/g liver).
(B) Immunofluorescence detection of acrolein, a by-product of lipid peroxidation. The staining intensity was quantified using AxioVision imaging software. Plotted are the averages ± S.E.M. determined from analysis of 10 random fields of liver sections from 2 mice per group. Asterisks indicate significant differences.
(C) Representative images illustrating lipofuscin accumulation in 20 week-old WT and Ercc1−/Δ mice and 26–34 month-old WT mouse liver. AxioVision software was used for quantification of 10 random fields measured from 5 mice per group. Plotted is the average fluorescence intensity for each group ± S.E.M. Asterisks indicate significant differences.
One of the most common markers of aged liver is the cytoplasmic accumulation of lipofuscin, which is highly oxidized lipid material(11). Lipofuscin granules, which fluoresce at 488nm, were 30-fold elevated in liver of 20 week-old Ercc1−/Δ mice compared to WT littermates (Figure 6C). A similar increase in lipofuscin was observed in old WT mouse liver compared to young WT mice.
Transcriptional changes in Ercc1−/Δ mouse liver parallels natural aging
Gene expression changes were measured by microarray in liver of Ercc1−/Δ mice and WT littermates at 5 and 16 weeks of age. Genes that were significantly up or downregulated compared to WT littermates are shown (Figure 7A). There is a progressive downregulation of genes associated with the IGF-1/GH axis and oxidative phosphorylation in Ercc1−/Δ mice as they age compared to WT littermates. Additionally, genes involved in DNA damage, oxidative stress response, cell cycle arrest, and apoptosis were significantly upregulated in 16 week-old Ercc1−/Δ mice compared to WT littermates. qPCR was used to confirm the microarray results (Figure 7B).
Figure 7. Age-associated transcriptional reprogramming in liver of Ercc1−/Δ mice.
(A) Significant gene expression changes (p<0.05, two tailed t-test) in the liver of 5- and 16-week old Ercc1−/Δ mutant livers as compared to littermate control animals. Note the progressive dampening in the expression of genes involved in oxidative metabolism and the GH/IGF1 axis along with the upregulation in the expression of genes involved in cell cycle arrest, DNA damage responses, oxidative stress and detoxification (red color: up-regulated; green color: down-regulated, FC: fold change, wks: weeks).
(B) qPCR measuremtn of mRNA levels of a subset of genes identified to be differentially expressed in the liver of 16-week old Ercc1−/Δ animals compared to wild-type littermates. For each gene, expression levels in the mutant tissue are plotted relative to those of controls (red dotted line). Error bars indicate S.E.M. between replicates (n ≥ 4).
(C) Spearman’s r correlation reflecting transcriptome similarities between the 5 or 16 week-old Ercc1−/Δ and the 130 week-old mouse livers where −1.0 is a perfect negative (inverse) correlation, 0.0 is no correlation, and 1.0 is a perfect positive correlation.
The transcriptional changes observed in 16 week-old Ercc1−/Δ mouse liver are comparable to old WT (130 week) mice (Figure 7C). Spearman’s r correlation indicates that as Ercc1−/Δ progeroid mice age from 5 weeks to 16 weeks, their gene expression profiles have increasing similarity to old WT mice.
DISCUSSION
The human liver undergoes numerous characteristic structural and functional changes with increasing age(13). Structural changes include loss of organ volume, nuclear polyploidy, anisokaryosis, and pseuodcapillarization(13, 42, 50). These changes lead to impaired liver function and loss of regenerative capacity. Not surprisingly, transplanted livers from late-age donors have decreased graft acceptance and function(51, 52). However, the underlying cause for these changes remains unknown.
Non-human primates have been used to study liver aging, but the commonly used species Macaca mulatta and Macaca nemestrina can live more than 20 years(53, 54). Mice are shorter-lived and importantly age-related changes characteristic of aged human liver are recapitulated in mice, including pseudocapillarization, increased polyploidy, decreased hepatocyte number, and increased nuclear size(18–20, 55). Thus, mice represent an accurate model system for studying age-associated liver changes in humans. However, the 3 year lifespan of mice still represents a substantial barrier to rapid testing of hypotheses about the causes and treatments of liver aging.
Herein we establish that the structural, functional and regulatory changes that occur in the liver of WT mice in their third year of life are recapitulated in the Ercc1−/Δ progeroid mouse strain within their 7-month lifespan. There is a highly significant correlation between the genome-wide expression profile of 4 month-old Ercc1−/Δ mice and 32 month-old WT mice (Figure 7). Both have elevated LFTs and reduced regenerative capacity, as well as focal necrosis, inflammation, anisokaryosis, and steatosis (Figures 1 and 4). Markers of cellular senescence (SA-βgal and p16) are elevated in livers of progeroid and old mice (Figure 5). This is supported by the strong upregulation of genes associated with cell cycle arrest (Figure 7) and the observation that livers of Ercc1−/Δ mice, like that of old WT mice(56–58) have reduced regenerative capacity following partial hepatectomy (Figure 4). Collectively, these data support the conclusion that age-related decrease of liver function is due to dysfunction of hepatocytes rather than their attrition. Interestingly, there is accumulation of oxidation products (lipofuscin and lipid peroxidation) in the livers of progeroid and old mice (Figure 6), suggesting that oxidative damage may drive hepatocyte dysfunction.
Defenestration and pseudocapillarization, which were significantly elevated in livers of Ercc1−/Δ and old WT mice (Figures 2 and 3), contribute to reduced regenerative capacity(58). Defenestration is also implicated in dyslipidemia due to impaired clearance of lipoproteins from the blood to hepatocytes(39). Serum cholesterol was significantly elevated in progeroid Ercc1−/Δ mice compared to littermate controls (Figure 3). In contrast, serum triglycerides were significantly lower in mutant animals compared to WT mice. Despite the fact that the extent of defenestration was similar in progeroid and old WT mice, serum lipid levels were within normal range in the naturally aged mice. Hence the dyslipidemia in the Ercc1−/Δ mice may reflect metabolic changes in the mice rather than a loss of liver transport mechanisms. Indeed, metabolic profiling of the Ercc1−/Δ mice revealed increased serum HDL but decreased LDL and VLDL and increased urinary ketone bodies(59). These changes mimic caloric restriction (60) and occur as a consequence genetic reprogramming rather than diminished nutrient intake in the Ercc1−/Δ mice (Figure 7 and(59, 61)). The growth hormone/insulin-like growth factor-1 axis is also attenuated in Ercc1−/Δ mice and old WT mice (Figure 7 and(24, 61)). This drives the metabolic shift and also contributes to impaired liver regeneration(62).
Ercc1−/Δ mice age rapidly as a consequence of an engineered mutation in the gene that encodes one subunit of the DNA repair endonuclease ERCC1-XPF (63), which is required for multiple DNA repair pathways (22, 23). XFE progeroid syndrome, which the ERCC1-deficient mice model, is characterized by accelerated aging of the hematopoietic, hepatobiliary, endocrine, musculoskeletal and neurological systems(24, 27). Werner syndrome, caused by mutations in WRN, which encodes a DNA repair helicase and exonuclease, is characterized by early onset cancer, cardiovascular disease and osteoporosis(64). Both Ercc1−/Δ and WrnΔhel/Δhel mice display early onset of dyslipidemia, steatosis, pseudocapillarization and defenestration in the liver(65). This demonstrates that DNA repair is critical for protecting against loss of liver homeostasis and function. Importantly, levels of 8-oxo-deoxyguanine, an endogenous oxidative DNA lesion, are greater in old WT mice than young(66–68), consistent with the hypothesis that unrepaired DNA damage may contribute to liver aging even in repair-proficient organisms.
The mice in this study were not exposed to environmental genotoxins. Thus the degenerative changes observed are attributed to the accumulation of oxidative damage arising as a consequence of normal metabolism. The fact that the onset of degenerative changes are accelerated in DNA repair-deficient Ercc1−/Δ mice compared to WT reveals that specifically endogenous DNA damage can drive liver degeneration, likely through driving hepatocyte senescence(69). In humans, environmental exposures as well as endogenously produced genotoxic by-products undoubtedly contribute to the accumulating burden of DNA damage and thereby age-related liver dysfunction.
It is important to note that many of the age-related endpoints measured were more severely affected in Ercc1−/Δ mice than in WT mice near the end of their respective lifespans. This includes LFTs, fibrosis, steatosis, pseudocapillarization, and anisokaryosis. A unifying explanation for why the liver of 4–5 month-old Ercc1−/Δ mice looks worse than 2–3 year-old WT mice is that the Ercc1−/Δ mice undergo activation of stress response pathways, similar to that induced by caloric restriction, which are protective, as the result of accumulated, unrepaired DNA damage. (Figure 7 and (24, 61)). Thus the Ercc1−/Δ mice survive longer than expected given their burden of damage and therefore have extreme aging, comparable to that of supercentenarians(30). Nevertheless, Ercc1−/Δ mice develop the majority of structural and funtional characteristics of old human(13) and mouse liver within a matter of five months, rather than years. The vast majority of these changes occur in adulthood (after 7 weeks of age), making the changes distinct from developmental abnormalities and degenerative in nature. Ercc1−/Δ mice therefore represent an accurate model system for rapidly testing hypotheses about the mechanism underlying age-related liver degeneration and pharmacological interventions aimed at delaying or ameliorating age-associated liver disease.
Supplementary Material
ACKNOWLEDGEMENTS
Financial Support: We thank Chelsea Feldman, Cheryl Clauson and members of the Niedernhofer lab for experimental support and critical reading of the manuscript. This work was supported by the National Institutes of Health grants ES016114 and -032 to L.J.N. and CA076541 to D.B.S., the Ellison Medical Foundation (AG-NS-0303-05; L.J.N.), and Pilot Projects from the University of Pittsburgh Claude B. Pepper Center (P30AG024827; PI: Studenski) and a Pilot the Pittsburgh Center for Kidney Research (P30DK079307; PI Kleyman). This project used the UPCI Cell and Tissue Imaging and Animal Facilities and was supported in part by award P30CA047904.
List of Abbreviations
- CDC
Center for Disease Control
- WHO
World Health Organization
- ERCC
excision repair cross complementation group 1
- LSEC
liver sinusoidal endothelial cells
- WT
Wild-type
- H&E
Haematoxylin & Eosin
- α-SMA
alpha smooth muscle actin
- SEM
Scanning electron microscopy
- TEM
Transmission electron microscopy
- AST
Aspartate transaminase
- ALT
Alanine transaminase
- SA β-gal
senescence-associated β-galactosidase
Contributor Information
Siobhán Q. Gregg, Email: greggs@upmc.edu.
Verónica Gutiérrez, Email: vgutierrez@fcien.edu.uy.
Andria Rasile Robinson, Email: robinsonar@upmc.edu.
Tyler Woodell, Email: twoodell@uw.edu.
Atsunori Nakao, Email: nakaoa@upmc.edu.
Mark A. Ross, Email: mross@pitt.edu.
George K. Michalopoulos, Email: michalopoulosgk@upmc.edu.
Lora Rigatti, Email: rigattil@pitt.edu.
Carrie E. Rothermel, Email: carrie.rothermel@gmail.com.
Irene Kamileri, Email: kamileri@imbb.forth.gr.
George Garinis, Email: garinis@imbb.forth.gr.
Donna Beer Stolz, Email: dstolz@pitt.edu.
Laura J. Niedernhofer, Email: niedernhoferl@upmc.edu.
REFERENCES
- 1.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. Services USDoHaH, editor. Hyattsville, MD: 2010. Deaths: Final Data for 2007. [PubMed] [Google Scholar]
- 3.del Olmo JA, Serra MA, Rodriguez F, Escudero A, Gilabert S, Rodrigo JM. Incidence and risk factors for hepatocellular carcinoma in 967 patients with cirrhosis. J Cancer Res Clin Oncol. 1998;124:560–564. doi: 10.1007/s004320050215. [DOI] [PubMed] [Google Scholar]
- 4.Frith J, Jones D, Newton JL. Chronic liver disease in an ageing population. Age Ageing. 2009;38:11–18. doi: 10.1093/ageing/afn242. [DOI] [PubMed] [Google Scholar]
- 5.Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, James OF. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology. 1989;9:297–301. doi: 10.1002/hep.1840090222. [DOI] [PubMed] [Google Scholar]
- 6.Popper H. Aging and the liver. Prog Liver Dis. 1986;8:659–683. [PubMed] [Google Scholar]
- 7.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucher NL, Swaffield MN, Ditroia JF. The Influence of Age Upon the Incorporation of Thymidine-2-C14 into the DNA of Regenerating Rat Liver. Cancer Res. 1964;24:509–512. [PubMed] [Google Scholar]
- 9.Chipchase MD, O'Neill M, Melton DW. Characterization of premature liver polyploidy in DNA repair (Ercc1)-deficient mice. Hepatology. 2003;38:958–966. doi: 10.1053/jhep.2003.50421. [DOI] [PubMed] [Google Scholar]
- 10.Andrew W. An electron microscope study of age changes in the liver of the mouse. Am J Anat. 1962;110:1–18. doi: 10.1002/aja.1001100102. [DOI] [PubMed] [Google Scholar]
- 11.Schmucker DL, Sachs H. Quantifying dense bodies and lipofuscin during aging: a morphologist's perspective. Arch Gerontol Geriatr. 2002;34:249–261. doi: 10.1016/s0167-4943(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 12.Jung T, Bader N, Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci. 2007;1119:97–111. doi: 10.1196/annals.1404.008. [DOI] [PubMed] [Google Scholar]
- 13.McLean AJ, Cogger VC, Chong GC, Warren A, Markus AM, Dahlstrom JE, Le Couteur DG. Age-related pseudocapillarization of the human liver. J Pathol. 2003;200:112–117. doi: 10.1002/path.1328. [DOI] [PubMed] [Google Scholar]
- 14.Kagansky N, Levy S, Keter D, Rimon E, Taiba Z, Fridman Z, Berger D, et al. Non-alcoholic fatty liver disease--a common and benign finding in octogenarian patients. Liver Int. 2004;24:588–594. doi: 10.1111/j.1478-3231.2004.0969.x. [DOI] [PubMed] [Google Scholar]
- 15.Adams LA, Angulo P. Recent concepts in non-alcoholic fatty liver disease. Diabet Med. 2005;22:1129–1133. doi: 10.1111/j.1464-5491.2005.01748.x. [DOI] [PubMed] [Google Scholar]
- 16.Warren A, Bertolino P, Cogger VC, McLean AJ, Fraser R, Le Couteur DG. Hepatic pseudocapillarization in aged mice. Exp Gerontol. 2005;40:807–812. doi: 10.1016/j.exger.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Hoare M, Das T, Alexander G. Ageing, telomeres, senescence, and liver injury. J Hepatol. 2010;53:950–961. doi: 10.1016/j.jhep.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Ito Y, Sorensen KK, Bethea NW, Svistounov D, McCuskey MK, Smedsrod BH, McCuskey RS. Age-related changes in the hepatic microcirculation in mice. Exp Gerontol. 2007;42:789–797. doi: 10.1016/j.exger.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtsubo K, Nomaguchi TA. A flow cytofluorometric study on age-dependent ploidy class changes in mouse hepatocyte nuclei. Mech Ageing Dev. 1986;36:125–131. doi: 10.1016/0047-6374(86)90013-8. [DOI] [PubMed] [Google Scholar]
- 20.Pieri C, Giuli C, Del Moro M, Piantanelli L. Electron-microscopic morphometric analysis of mouse liver. II. Effect of ageing and thymus transplantation in old animals. Mech Ageing Dev. 1980;13:275–283. doi: 10.1016/0047-6374(80)90040-8. [DOI] [PubMed] [Google Scholar]
- 21.Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–1359. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- 22.Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, de Wit J, et al. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad A, Robinson AR, Duensing A, van Drunen E, Beverloo HB, Weisberg DB, Hasty P, et al. ERCC1-XPF endonuclease facilitates DNA double-strand break repair. Mol Cell Biol. 2008;28:5082–5092. doi: 10.1128/MCB.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 25.McWhir J, Selfridge J, Harrison DJ, Squires S, Melton DW. Mice with DNA repair gene (ERCC-1) deficiency have elevated levels of p53, liver nuclear abnormalities and die before weaning. Nat Genet. 1993;5:217–224. doi: 10.1038/ng1193-217. [DOI] [PubMed] [Google Scholar]
- 26.Tian M, Shinkura R, Shinkura N, Alt FW. Growth retardation, early death, and DNA repair defects in mice deficient for the nucleotide excision repair enzyme XPF. Mol Cell Biol. 2004;24:1200–1205. doi: 10.1128/MCB.24.3.1200-1205.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasher JM, Lalai AS, Heijmans-Antonissen C, Ploemacher RE, Hoeijmakers JH, Touw IP, Niedernhofer LJ. Reduced hematopoietic reserves in DNA interstrand crosslink repair-deficient Ercc1−/− mice. EMBO J. 2005;24:861–871. doi: 10.1038/sj.emboj.7600542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selfridge J, Hsia KT, Redhead NJ, Melton DW. Correction of liver dysfunction in DNA repair-deficient mice with an ERCC1 transgene. Nucleic Acids Res. 2001;29:4541–4550. doi: 10.1093/nar/29.22.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Waard MC, van der Pluijm I, Zuiderveen Borgesius N, Comley LH, Haasdijk ED, Rijksen Y, Ridwan Y, et al. Age-related motor neuron degeneration in DNA repair-deficient Ercc1 mice. Acta Neuropathol. 2010;120:461–475. doi: 10.1007/s00401-010-0715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolle MET, Kuiper RV, Roodbergen M, Robinson J, de Vlugt S, Wijnhoven SWP, Beems RB, de la Fonteyne L, de With P, van der Pluijm I, Niedernhofer LJ, Hasty P, Vijg J, Hoeijmakers JHJ, Harry van Steeg Broad segmental progeroid changes in short-lived Ercc1−/Δ7 mice. Pathobiology of Aging and Age-related diseases. 2011;1:7219. doi: 10.3402/pba.v1i0.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 32.Wack KE, Ross MA, Zegarra V, Sysko LR, Watkins SC, Stolz DB. Sinusoidal ultrastructure evaluated during the revascularization of regenerating rat liver. Hepatology. 2001;33:363–378. doi: 10.1053/jhep.2001.21998. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–1170. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 34.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warren A, Cogger VC, Fraser R, Deleve LD, McCuskey RS, Le Couteur DG. The effects of old age on hepatic stellate cells. Curr Gerontol Geriatr Res. 2011;2011:439835. doi: 10.1155/2011/439835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilmer SN, Cogger VC, Le Couteur DG. Basal activity of Kupffer cells increases with old age. J Gerontol A Biol Sci Med Sci. 2007;62:973–978. doi: 10.1093/gerona/62.9.973. [DOI] [PubMed] [Google Scholar]
- 37.Le Couteur DG, Warren A, Cogger VC, Smedsrod B, Sorensen KK, De Cabo R, Fraser R, et al. Old age and the hepatic sinusoid. Anat Rec (Hoboken) 2008;291:672–683. doi: 10.1002/ar.20661. [DOI] [PubMed] [Google Scholar]
- 38.Le Couteur DG, Cogger VC, Markus AM, Harvey PJ, Yin ZL, Ansselin AD, McLean AJ. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology. 2001;33:537–543. doi: 10.1053/jhep.2001.22754. [DOI] [PubMed] [Google Scholar]
- 39.Le Couteur DG, Cogger VC, McCuskey RS, R DEC, Smedsrod B, Sorensen KK, Warren A, et al. Age-related changes in the liver sinusoidal endothelium: a mechanism for dyslipidemia. Ann N Y Acad Sci. 2007;1114:79–87. doi: 10.1196/annals.1396.003. [DOI] [PubMed] [Google Scholar]
- 40.Hilmer SN, Cogger VC, Fraser R, McLean AJ, Sullivan D, Le Couteur DG. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005;42:1349–1354. doi: 10.1002/hep.20937. [DOI] [PubMed] [Google Scholar]
- 41.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 42.Schmucker DL. Age-related changes in liver structure and function: Implications for disease ? Exp Gerontol. 2005;40:650–659. doi: 10.1016/j.exger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Kitani K. Liver and aging. Gastroenterol Jpn. 1992;27:276–285. doi: 10.1007/BF02777737. [DOI] [PubMed] [Google Scholar]
- 44.Vranckx R, Savu L, Lambert N, de Conchard GV, Grosse R, Mourey MS, Corman B. Plasma proteins as biomarkers of the aging process. Am J Physiol. 1995;268:R536–R548. doi: 10.1152/ajpregu.1995.268.2.R536. [DOI] [PubMed] [Google Scholar]
- 45.Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci U S A. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherwood SW, Rush D, Ellsworth JL, Schimke RT. Defining cellular senescence in IMR-90 cells: a flow cytometric analysis. Proc Natl Acad Sci U S A. 1988;85:9086–9090. doi: 10.1073/pnas.85.23.9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moraes AS, Guaraldo AM, Mello ML. Chromatin supraorganization and extensibility in mouse hepatocytes with development and aging. Cytometry A. 2007;71:28–37. doi: 10.1002/cyto.a.20356. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe T, Shimada H, Tanaka Y. Human hepatocytes and aging: a cytophotometrical analysis in 35 sudden-death cases. Virchows Arch B Cell Pathol. 1978;27:307–316. doi: 10.1007/BF02889003. [DOI] [PubMed] [Google Scholar]
- 51.Busquets J, Xiol X, Figueras J, Jaurrieta E, Torras J, Ramos E, Rafecas A, et al. The impact of donor age on liver transplantation: influence of donor age on early liver function and on subsequent patient and graft survival. Transplantation. 2001;71:1765–1771. doi: 10.1097/00007890-200106270-00011. [DOI] [PubMed] [Google Scholar]
- 52.Hoofnagle JH, Lombardero M, Zetterman RK, Lake J, Porayko M, Everhart J, Belle SH, et al. Donor age and outcome of liver transplantation. Hepatology. 1996;24:89–96. doi: 10.1002/hep.510240116. [DOI] [PubMed] [Google Scholar]
- 53.Maloney AG, Schmucker DL, Vessey DS, Wang RK. The effects of aging on the hepatic microsomal mixed-function oxidase system of male and female monkeys. Hepatology. 1986;6:282–287. doi: 10.1002/hep.1840060221. [DOI] [PubMed] [Google Scholar]
- 54.Sutter MA, Wood WG, Williamson LS, Strong R, Pickham K, Richardson A. Comparison of the hepatic mixed function oxidase system of young, adult, and old non-human primates (Macaca nemestrina) Biochem Pharmacol. 1985;34:2983–2987. doi: 10.1016/0006-2952(85)90025-5. [DOI] [PubMed] [Google Scholar]
- 55.Stuart GR, Oda Y, de Boer JG, Glickman BW. Mutation frequency and specificity with age in liver, bladder and brain of lacI transgenic mice. Genetics. 2000;154:1291–1300. doi: 10.1093/genetics/154.3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Quail E, Hung NJ, Tan Y, Ye H, Costa RH. Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proc Natl Acad Sci U S A. 2001;98:11468–11473. doi: 10.1073/pnas.201360898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Krupczak-Hollis K, Tan Y, Dennewitz MB, Adami GR, Costa RH. Increased hepatic Forkhead Box M1B (FoxM1B) levels in old-aged mice stimulated liver regeneration through diminished p27Kip1 protein levels and increased Cdc25B expression. J Biol Chem. 2002;277:44310–44316. doi: 10.1074/jbc.M207510200. [DOI] [PubMed] [Google Scholar]
- 58.Furrer K, Rickenbacher A, Tian Y, Jochum W, Bittermann AG, Kach A, Humar B, et al. Serotonin reverts age-related capillarization and failure of regeneration in the liver through a VEGF-dependent pathway. Proc Natl Acad Sci U S A. 2011;108:2945–2950. doi: 10.1073/pnas.1012531108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nevedomskaya E, Meissner A, Goraler S, de Waard M, Ridwan Y, Zondag G, van der Pluijm I, et al. Metabolic profiling of accelerated aging ERCC1 d/− mice. J Proteome Res. 2010;9:3680–3687. doi: 10.1021/pr100210k. [DOI] [PubMed] [Google Scholar]
- 60.Cruzen C, Colman RJ. Effects of caloric restriction on cardiovascular aging in non-human primates and humans. Clin Geriatr Med. 2009;25:733–743. ix–x. doi: 10.1016/j.cger.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schumacher B, van der Pluijm I, Moorhouse MJ, Kosteas T, Robinson AR, Suh Y, Breit TM, et al. Delayed and accelerated aging share common longevity assurance mechanisms. PLoS Genet. 2008;4:e1000161. doi: 10.1371/journal.pgen.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krupczak-Hollis K, Wang X, Dennewitz MB, Costa RH. Growth hormone stimulates proliferation of old-aged regenerating liver through forkhead box m1b. Hepatology. 2003;38:1552–1562. doi: 10.1016/j.hep.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 63.Weeda G, Donker I, de Wit J, Morreau H, Janssens R, Vissers CJ, Nigg A, et al. Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr Biol. 1997;7:427–439. doi: 10.1016/s0960-9822(06)00190-4. [DOI] [PubMed] [Google Scholar]
- 64.Muftuoglu M, Oshima J, von Kobbe C, Cheng WH, Leistritz DF, Bohr VA. The clinical characteristics of Werner syndrome: molecular and biochemical diagnosis. Hum Genet. 2008;124:369–377. doi: 10.1007/s00439-008-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Labbe A, Garand C, Cogger VC, Paquet ER, Desbiens M, Le Couteur DG, Lebel M. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for Werner syndrome. Gerontol A Biol Sci Med Sci. 2010;66:264–278. doi: 10.1093/gerona/glq184. [DOI] [PubMed] [Google Scholar]
- 66.Mikkelsen L, Bialkowski K, Risom L, Lohr M, Loft S, Moller P. Aging and defense against generation of 8-oxo-7,8-dihydro-2'-deoxyguanosine in DNA. Free Radic Biol Med. 2009;47:608–615. doi: 10.1016/j.freeradbiomed.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 67.Zahn RK, Zahn-Daimler G, Ax S, Reifferscheid G, Waldmann P, Fujisawa H, Hosokawa M. DNA damage susceptibility and repair in correlation to calendric age and longevity. Mech Ageing Dev. 2000;119:101–112. doi: 10.1016/s0047-6374(00)00170-6. [DOI] [PubMed] [Google Scholar]
- 68.Lopez-Diazguerrero NE, Luna-Lopez A, Gutierrez-Ruiz MC, Zentella A, Konigsberg M. Susceptibility of DNA to oxidative stressors in young and aging mice. Life Sci. 2005;77:2840–2854. doi: 10.1016/j.lfs.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 69.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.