Abstract
Osteoarthritis (OA) is a disease of articular cartilage, with aging as the main risk factor. In OA, changes in chondrocytes lead to the autolytic destruction of cartilage. Transforming growth factor-β has recently been demonstrated to signal not only via activin receptor-like kinase 5 (ALK5)-induced Smad2/3 phosphorylation, but also via ALK1-induced Smad1/5/8 phosphorylation in articular cartilage. In aging cartilage and experimental OA, the ratio ALK1/ALK5 has been found to be increased, and the expression of ALK1 is correlated with matrix metalloproteinase-13 expression. The age-dependent shift towards Smad1/5/8 signalling might trigger the differentiation of articular chondrocytes with an autolytic phenotype.
Keywords: Aging, Bone morphogenetic protein, Osteoarthritis, Osteophyte, Signalling, Smad, Transforming growth factor-β
Introduction
Osteoarthritis (OA) is the most universal joint disease with an increasing prevalence in the Western world attributable to the aging population (Restrepo and Rozental 1994). The primary characteristic of OA is the destruction of articular cartilage but synovial inflammation, the formation of osteophytes and sclerosis of the subchondral bone are features of this common disease (Loeser 2010). Unfortunately, articular cartilage has a limited repair capacity and therefore damaged cartilage will not heal. As a result, loss of articular cartilage in OA is progressive and will ultimately result in loss of joint function. Pain, tenderness, joint stiffness, crepitus and synovial effusions are the most common clinical symptoms. However, early OA is usually not clinically noticed because of the absence of innervation in articular cartilage. Pharmalogical interventions are mainly focused at pain and no therapy that interferes with the disease process has as yet been developed. The ultimate “therapy” for OA is the replacement of damaged joints by artificial ones but artificial joints have only a limited time span and cannot be applied in all affected joints.
The cause of OA is, in most cases, unknown (primary OA) but a number of risk factors have been identified, such as gender and obesity. However, the main risk factor for primary OA is age (Zhang and Jordan 2010). In their study in the USA, Murphy et al. (2008) have estimated that nearly half of the adults will develop symptomatic knee OA by age 85 years. Many ideas have been put forward to explain the association between OA and aging. The oldest theory linking OA with aging is that the cartilage destruction observed in OA joints is just a result of simple wear and tear processes. Nevertheless, normal use has not been shown to increase the risk for OA (Marmor 1969). An alternative explanation is based on the clear changes that are observed in the extracellular matrix of aging cartilage. The size of the proteoglycan responsible for the pressure-resilient properties of cartilage, namely aggrecan, becomes shorter with age (Lark et al. 1995). Moreover, cross-linking between the collagen fibrils and glycation end products significantly increase in human aging cartilage, making the matrix more brittle and more vulnerable to mechanically induced damage (Verzijl et al. 2001; DeGroot et al. 2004).
The homeostasis of articular cartilage is totally dependent on the embedded cartilage cells, the chondrocytes, and therefore, we can anticipate that age-related changes in chondrocytes are involved in the OA disease process. Chondrocytes in OA cartilage show markers of apoptosis and this has been suggested to be related to OA (Blanco et al. 1998). However, human OA is an extremely slow progressive disease, probably taking decades to become clinically overt, and this is not in agreement with the high numbers of apoptotic cells reported in OA cartilage. Loss of chondrocyte energy supply by degeneration of mitochondrial function has also been suggested as a causative factor in the loss of cartilage homeostasis (Martin and Buckwalter 2003). Another mechanism involving mitochondrial activity is oxidative stress by reactive oxygen species, since the loss of insulin-like growth factor (IGF)-I receptor signalling, the main anabolic factor of articular chondrocytes, is observed during aging (Fortier and Miller 2006). Oxidative stress has been suggested to induce chondrocyte senescence and to inhibit IGF-I receptor signalling (Studer et al. 2000). Alteration in responses to growth factors other than IGF-I can play a role in the OA process. We have found that chondrocytes in aged mice have an altered response to the cytokine transforming growth factor-β (TGF-β) compared with chondrocytes in young mice and that this might be causally related to OA (Scharstuhl et al. 2002b; Fortier and Miller 2006; Blaney Davidson et al. 2005, 2006b). Therefore, the focus of the present review is on this age-related change in TGF-β signalling in OA.
TGF-β signalling via serine/threonine kinase receptors and Smads
TGF-β signals via heteromeric complexes of transmembrane serine/threonine type I and type II receptors. The type I receptors, also termed activin receptor-like kinases (ALKs), act downstream of type II receptors and determine receptor specificity (Heldin et al. 1997). TGF-β belongs to a large family of structurally related cytokines that also includes bone morphogenetic proteins (BMPs). TGF-β mainly signals via the broadly expressed type I receptor ALK5. In endothelial cells, TGF-β has been shown to signal via ALK1, a feature that recently has been found to be shared with chondrocytes (Blaney Davidson et al. 2009; Goumans et al. 2002, 2003b). BMPs signal via ALK1, -2, -3 and −6. Upon type I receptor activation, intracellular signalling is initiated by the phosphorylation of receptor-regulated (R) Smad proteins. Whereas ALK5 stimulates Smad2/3 phosphorylation, ALK1, -2, -3 and −6 mediate the activation of Smad1/5/8 (Blaney Davidson et al. 2009; Finnson et al. 2008). Phosphorylated R-Smads form heteromeric complexes with the common mediator (co)-Smad4. These heteromeric complexes accumulate in the nucleus where they, together with co-activators and repressors, control transcriptional responses (Heldin et al. 1997). Frequently, these two main intracellular Smad pathways are found to act opposing each other, even antagonizing each other, for example by forming so-called mixed Smad2/3-Smad1/5/8 complexes (Daly et al. 2008; Goumans et al. 2003a, 2003b, 2007). Both the Smad2/3 and Smad1/5/8 signalling cascade have been reported to control chondrocyte differentiation.
OA and chondrocyte differentiation
Cartilage can be divided into temporary and permanent cartilage. Articular cartilage is a permanent tissue, whereas cartilage in the growth plate is only present during childhood and early adolescence. Cartilage formation (chondrogenesis), as can be seen in the developing embryo, is a strictly regulated process (Lefebvre and Bhattaram 2010). Mesenchymal precursor cells condense and initiate chondrogenic differentiation. Differentiation of precursor cells into differentiated chondrocytes is characterized by cell proliferation and the deposition of cartilage-specific molecules, such as type II collagen and aggrecan. In the growth plate, the stage of differentiated chondrocytes is followed by chondrocyte terminal differentiation. This results in chondrocyte hypertrophy and the breakdown of cartilage and bone deposition. During terminal differentiation, chondrocytes enlarge, up to tenfold, and express type X collagen and matrix metalloproteinase 13 (MMP13). The latter enzyme is crucial during the degradation of the cartilage matrix in the growth plate, with loss of this enzyme in knockout mice resulting in a non-functional growth plate (Inada et al. 2004). Alternatively, in articular cartilage, chondrocyte terminal differentiation is inhibited, resulting in stable cartilage in the synovial joints. However, during OA development, chondrocytes in articular cartilage undergo phenotypic changes that bear a close resemblance to the alteration that occurs in terminally differentiating chondrocytes in the growth plates (Tchetina et al. 2005). Terminal differentiation of chondrocytes in the growth plate and changes in articular chondrocytes in OA cartilage cannot be considered as entirely identical processes, although alterations in these cells show a number of parallel characteristics (such as the high expression of MMP13), which are most likely controlled by similar mechanisms.
TGF-β is involved in all stages of chondrogenesis, from condensation to terminal differentiation. Data from in vitro studies of mesenchymal precursor cells indicate that TGF-β is the main initiator of chondrogenesis in these cells (Iwasaki et al. 1993; Mackay et al. 1998). Mesenchymal condensation, proliferation of chondroblasts and the deposition of cartilage-specific extracellular matrix molecules is strongly stimulated by TGF-β.
In the initial stages of chondrocyte differentiation, TGF-β acts primarily as a stimulator in the progression of chondrocyte differentiation. However, this is different from the role of TGF-β in the late stages of differentiation. TGF-β clearly blocks chondrocyte terminal differentiation (Serra et al. 1997; Yang et al. 2001). Mice knocked out for Smad3 express elevated numbers of hypertrophic chondrocytes in articular cartilage (Yang et al. 2001). Furthermore, TGF-β blocks the expression of the terminal differentiation marker type X collagen in cultures of primary mouse limb bud mesenchymal cells (Zhang et al. 2004). Smad2 and 3 are essential signalling molecules in the inhibitory effect of TGF-β on chondrocyte terminal differentiation and Smad3 appears to play a more prominent role than Smad2 (Alvarez and Serra 2004). These findings clearly demonstrate that TGF-β stimulates the early stages of chondrocyte differentiation but blocks chondrocyte terminal differentiation.
Whereas terminal differentiation is blocked by signalling via Smad2/3, we and others have shown that terminal differentiation strictly requires Smad1/5/8 signalling (Hellingman et al. 2011). In knockout mice, the loss of both Smad 1 and 5 has been shown to result in the obstruction of chondrocyte terminal differentiation and severe cartilage defects. In addition, the inhibitory Smad6 and Smurf1 and 2 are known inhibitors of mainly Smad1/5/8 signalling interfering with Smad signalling and accelerating proteosomal breakdown of phospho-Smads. Mice overexpressing either Smad6 or Smurf1 demonstrate inhibition of chondrocyte terminal differentiation (Horiki et al. 2004). Overexpression of Smurf2 in differentiating chicken chondrocytes stimulates chondrocyte terminal differentiation and maturation as a result of reduced Smad2/3 signalling (Wu et al. 2008b).
The transcription factor Runx2 plays a major role in the control of chondrocyte terminal differentiation. Mice that lack functional Runx2 also completely lack bone formation, because chondrocyte terminal differentiation is absent (Hecht et al. 2007). Runx2 is a central switch that integrates the signals of both Smad pathways, thereby controlling chondrocyte terminal differentiation (Javed et al. 2008, 2009; Miyazono et al. 2004; Lian et al. 2003; Leboy et al. 2001). Smads have been reported to modulate chondrocyte differentiation by a physical interaction with Runx2. Complex formation of Runx2 with Smad1 is vital for the function of Runx2, whereas the interaction of Smad3 with Runx2 inhibits Runx2 functioning (Hjelmeland et al. 2005; Kang et al. 2005; Javed et al. 2008, 2009; Zheng et al. 2007). Apparently, Runx2 can be switched on or off by different Smads, thus controlling chondrocyte terminal differentiation.
Chondrocyte terminal differentiation is thought to play a major role in OA development and Smad signalling regulates this process. However, other factors in addition to the Smad signalling routes can affect chondrocyte differentiation and play a potential role in OA development. Chondrocytes can be activated by inflammatory cytokines, by the Wnt signalling cascade or by extracellular matrix-derived triggers (Blom et al. 2004, 2007, 2009; Goldring et al. 2008; Xu et al. 2007; Kawaguchi 2009; Zhu et al. 2009). Of note, most of these factors also influence TGF-β and Smad signalling, modulating the stability and activity of the various Smad routes and hence adapting the effect of TGF-β on chondrocyte differentiation and OA development.
Genetic aspects of TGF-β and OA
A relationship between the genetic variants of TGF-β itself, TGF-β signalling and binding molecules and OA is reported in humans. Elevated TGF-β1 activity has been suggested to be associated with elevated bone mass and OA. Elevated bone mass is thought to be related to OA development, since the opposite, osteoporosis, appears to protect against OA (Livshits et al. 2010). The long bones of patients with Camurati-Engelmann disease show osteosclerosis. Camurati-Engelmann disease is associated with a mutation in the TGF-β1 gene, resulting in elevated TGF-β activity attributable to an altered binding of lamina-associated polypeptide-1 to mature TGF-β1 (Saito et al. 2001; Wu et al. 2006). Spinal osteophyte formation, an indication of OA development, is associated with variants of the TGF-β1 gene in Japanese women (Yamada 2000; Yamada et al. 2000). This variant also protects this population from osteoporosis, stressing the inverse relationship between low bone mass and OA. However, in a population of German women, no such relationship could be confirmed between this TGF-β1 variant and bone mass (Hinke et al. 2001).
Another genetic variant, in this case proposed to result in decreased TGF-β activity, is reported for asporin, a small leucine-rich proteoglycan. Asporin is a TGF-β inhibitor that is highly expressed in articular cartilage. In Asian populations, the so-called D-14 variant is associated with OA (Kizawa et al. 2005; Song et al. 2008; Jiang et al. 2006). This D-14 variant is a stronger TGF-β inhibitor than the common D-13 variant. Reduced TGF-β activity is proposed to result in decreased synthesis of cartilage-specific extracellular matrix molecules and thus leads to OA (Kizawa et al. 2005). However, in Caucasians, an association between the asporin D-14 variant and OA is lacking in most of the investigated populations (Atif et al. 2008; Rodriguez-Lopez et al. 2006).
Mutations and genetic variation in the Smad3 gene are associated with OA (Yao et al. 2003; van de Laar et al. 2011; Valdes et al. 2010). Increased expression of Smad3 and several key elements of the TGF-β signalling pathway have been found by van de Laar et al. (2011). A relationship between increased Smad3 signalling and OA is unexpected, since studies in transgenic mice have shown that the loss of Smad3 results in accelerated chondrocyte terminal differentiation and OA (Wu et al. 2008a; Yang et al. 2001). Moreover, overexpression of Smurf2 in mice, leading to the inhibition of Smad3 signalling, elicits cartilage damage in vivo (Wu et al. 2008a). This discrepancy can be explained on the basis that either too much or too little Smad3 activity will lead to OA or that different mechanism are involved in the various patient groups. Changes in connective tissues related to altered Smad3 signalling in patients with an aortic phenotype can indirectly result in OA attributable to altered joint stability. On the other hand, genetic variants that will result in decreased Smad3 signalling will produce OA by another mechanism, most probably related to changes in chondrocyte differentiation.
The above-described studies indicate that changes in TGF-β or TGF-β signalling components are related to OA development. Since primary OA is a highly common disease, subtle genetic variations can be expected to contribute to this aging-related disease but not to play a critical role. Moreover, genome-wide association studies have been unsuccessful in detecting genes that have a major contribution to OA in the general population (Panoutsopoulou et al. 2011). This indicates that gene variations might not play a major role in primary OA but that general aging processes might be at the root of the development of this degenerative disease. We propose that an age-related alteration in TGF-β signalling plays a crucial role in the OA disease process.
Shift in ALK1/ALK5 balance in ageing and OA
In our initial studies, comparing the counteracting effect of TGF-β on interleukin-1-induced cartilage damage in young and old mice, we observed that TGF-β was able to counteract the deleterious effect of interleukin-1 in young mice but not in old ones (Scharstuhl et al. 2002b; van Beuningen et al. 1994a). This finding encouraged us to investigate the differences in TGF-β signalling in the cartilage of young and old animals. An age-related loss of the TGF-β type I receptor ALK5 and phosphorylation of Smad2/3 in murine articular cartilage was one of the most striking findings (Blaney Davidson et al. 2006b). The expression of non-phosporylated Smad2 or Smad3 was no different in young and old animals. Since OA development and aging are highly correlated, we also studied the relationship between OA and alterations in TGF-β signalling components in two experimental models of OA. In both models used, namely the meniscus destabilization model and STR/ORT mice (spontaneous OA), OA development was associated with a striking loss of ALK5 expression (Blaney Davidson et al. 2006b).
In parallel in vitro studies, we have demonstrated that chondrocytes, like endothelial cells, also use ALK1 as a TGF-β type I receptor (Blaney Davidson et al. 2009; Finnson et al. 2008; Goumans et al. 2002). In chondrocytes, this results in the activation of the Smad1/5/8 route (Blaney Davidson et al. 2009; Finnson et al. 2008; Goumans et al. 2002). Interestingly, expression of this alternative TGF-β receptor does not diminish to a comparable extent as ALK5 during aging and experimental OA (Blaney Davidson et al. 2009). As a consequence of the sharp drop in ALK5 and only a small reduction in ALK1 expression, the ALK1/ALK5 ratio is strongly elevated in aged and OA articular chondrocytes. The increased ALK1/ALK5 ratio is reflected in an increased Id1/PAI1 expression ratio, indicating a shift from Smad2/3 to Smad1/5/8 signalling during aging and OA in murine cartilage (Blaney Davidson et al. 2009).
Keeping the regulation of chondrocyte differentiation in mind, we can anticipate that a shift in TGF-β signalling from ALK5 to ALK1 will affect chondrocyte differentiation.
A dominant expression of ALK5 will result in mainly Smad2/3 signalling, whereas ALK1 dominance will lead to the activation of the Smad1/5/8 pathway. The balance of these routes has been shown to control chondrocyte differentiation via Runx2 (see above).
We have further demonstrated that, in chondrocytes, the overexpression of constitutive active ALK5 (Smad2/3) results in increased aggrecan expression, whereas constitutive ALK1 (Smad1/5/8) expression leads to increased expression of MMP13 (Blaney Davidson et al. 2009). Moreover, inhibition of ALK5 expression by using short interfering RNA causes the elevated expression of MMP13, the major cartilage degrading enzyme in OA (Billinghurst et al. 1997). Thus, MMP13 expression is apparently determined by the balance in ALK1 (Smad1/5/8) and ALK5 (Smad2/3) signalling. Of note, the cartilage of human OA knee joints shows a significant correlation between ALK1 and MMP13 mRNA expression (Blaney Davidson et al. 2009). These observations indicate that ALK1 signalling stimulates changes in chondrocyte differentiation that are similar to the chondrocyte phenotype demonstrated in OA cartilage, a phenotype characterized by the highly elevated expression of MMP13 (Billinghurst et al. 1997; Reboul et al. 1996).
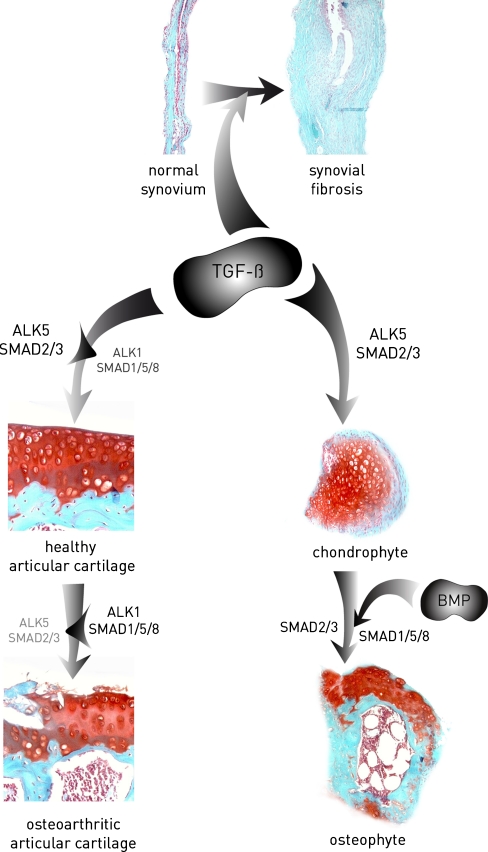
We postulate that, because of frequent sub-clinical micro-traumata to the cartilage matrix, TGF-β is released from the extracellular matrix. In articular chondrocytes of young articular cartilage, TGF-β acts as a repair factor, stimulates the synthesis of matrix molecules and keeps the cells in a quiescent state by the inhibitory effect of TGF-β, via Smad2/3, on the progression of chondrocyte differentiation. During the aging of chondrocytes and OA development via an until now unknown mechanism, signalling through ALK1 and Smad1/5/8 is favoured compared with signalling via ALK5 and Smad2/3. The dominant Smad1/5/8 signalling triggers the articular chondrocytes to leave their quiescent state. This stimulates the progression to a chondrocyte phenotype with characteristics comparable with those of hypertrophic chondrocytes normally only found in the growth plates. These hypertrophic chondrocytes are essential for the removal of the cartilage matrix, mainly by MMP13, to make bone deposition possible (Stickens et al. 2004). These chondrocytes have an autolytic phenotype degrading their surrounding cartilage matrix, as observed in OA cartilage (Fig. 1).
Fig. 1.
Transforming growth factor-β (TGF-β) plays a role in key characteristics of osteoarthritis (OA): cartilage damage, osteophyte formation and synovial fibrosis. TGF-β is required for the maintenance of healthy cartilage during which it signals primarily via Smad2/3. With age and OA, a shift in the activin receptor-like kinase 5 (ALK5):ALK1 ratio is established, favouring Smad1/5/8 signalling and leading to a loss of the cartilage protective role of Smad2/3. We suggest a role for this balance shift towards ALK1 in cartilage damage during OA development. In addition, TGF-β is a crucial factor in the onset of osteophyte development. In later stages of osteophyte development, bone morphogenetic proteins (BMP) might be equally important. TGF-β is a major player in fibrotic diseases and, during OA, it induces synovial fibrosis
TGF-β and osteophyte formation
We propose a role for TGF-β not only in the regulation of chondocyte behaviour and cartilage destruction, but also in another feature of OA, namely osteophyte formation. Osteophytes are outgrowths at the margins of the joint that originate from activated mesenchymal stem-cell-like periosteal cells lining the articulating joint surface and can lead to clinical problems (van der Kraan and van den Berg 2007). We have shown, in murine models, that the injection of TGF-β or BMPs results in osteophyte formation in the knee joint (van Beuningen et al. 1994b, 1998, 2000). Cartilaginous osteophytes can be seen within 1 week after injection and, after 1 month, these structure have ossified and are true osteophytes.
The osteophytes induced by BMPs or TGF-β injection are not identical. Osteophytes induced by TGF-β originate from the periosteal cells lining the joint, whereas in the case of BMP-2-induced osteophytes, cells in the, not yet closed, murine growth plate contribute to the formation of the osteophytes (van Beuningen et al. 1994b, 1998). During experimental OA, the pattern of osteophyte formation closely resembles that of TGF-β-induced osteophytes but is quite different from the pattern induced by BMP-2 (Blaney Davidson et al. 2007). Moreover, the outer layer of osteophytes developing during experimental OA strongly express TGF-β1 and phospho-Smad2/3. The latter is not only confined to the surface cells, but also shows strong positive cells in the deeper layer of the osteophytes. Smad1/5/8 signalling is mainly observed in hypertrophic chondrocytes in the late stages of osteophyte formation (own data, not shown).
To explore further the role of TGF-β in the process of osteophyte formation, we performed blocking studies using specific TGF-β inhibitors. TGF-β activity was blocked by means of a scavenging soluble TGF-β type II receptor extracellular domain, by intra-articular overexpression of LAP-1 or by intra-articular overexpression of Smad7. All three treatments resulted in a significant reduction in osteophyte formation compared with controls. Overexpression of adenoviral Smad6, targeting mainly the Smad1/5/8 route, was far less effective than Smad7 overexpression (Scharstuhl et al. 2002a, 2003). In addition, adenoviral overexpression of gremlin, a BMP inhibitor, fully blocked BMP-2-induced osteophyte formation. However, the blocking of BMP activity by gremlin neither inhibited TGF-β-induced nor experimental OA-associated osteophyte formation (Blaney Davidson et al. 2007). These observations clearly demonstrate that TGF-β plays a dominant role in the induction of osteophytes, at least in murine OA models, and that the role of BMPs is limited in the early stages. The latter finding does not exclude a role for BMPs during cartilage maturation, as is seen in developing osteophytes (Lories et al. 2005).
TGF-β is released, and partly activated, from damaged joint tissues during OA development or by inflammation accompanying the OA process. Mesenchymal stem cells in the periosteum are triggered, by the released TGF-β, to initiate chondrogenic differentiation, as has been shown in vitro by using periosteum explants (O'Driscoll et al. 1994). The mesenchymal cells go through a developmental process similar to that seen in the growth plates. Cartilage deposition is followed by chondrocytes maturation leading to chondrocyte hypertrophy and finally to replacement by bone. This indicates that TGF-β most likely not only plays a role in cartilage destruction, but also in the concomitant formation of new cartilage and bone, namely the osteophytes.
TGF-β and synovial fibrosis
Synovial fibrosis can be often observed in OA-affected joints (Revell et al. 1988). We have shown that both the injection and the adenoviral overexpression of TGF-β results in substantial synovial fibrosis characterized by fibroblast proliferation and collagen accumulation (Bakker et al. 2001; van Beuningen et al. 1994b). Moreover, the blocking of TGF-β itself or of TGF-β signalling results in a significant decrease in synovial fibrosis in murine experimental OA models (Blaney Davidson et al. 2006a; Scharstuhl et al. 2003). These findings indicate that TGF-β is an important driving-force for synovial fibrosis in OA and contributes to the characteristic stiffness of affected joints.
Concluding remarks and targets for therapy
We postulate that TGF-β plays a central role in OA development, being a protective factor initially but later playing a role in OA pathogenesis characterized by cartilage destruction, osteophyte formation and synovial fibrosis. In our view, the OA process is driven by the loss of the Smad2/3 block and acquirement of the Smad1/5/8 push on chondrocyte differentiation in articular chondrocytes leading to an advancement of chondrocyte differentiation and finally an autolytic phenotype. In addition, TGF-β released by tissue damage and inflammation triggers periosteal cells to form osteophytes and to stimulate a fibrotic reaction of the synovial fibroblasts.
Since this view does not regard OA as a simple and unavoidable result of wear and tear, pharmalogical interventions should be an option. OA is initially a focal process and not all chondrocytes will show an OA-like phenotype. Some cells will have an autolytic phenotype, whereas other cells will still be in a quiescent healthy state of differentiation. These cells could be targeted for therapy to block further progression of the OA process.
A disbalance of ALK1/ALK5 and loss of dominant Smad2/3 signalling is at the basis of the OA process, in our view. Specific ALK1 inhibitors that can penetrate cartilage or compounds specifically stimulating the Smad2/3 route should be developed and tested in pre-clinical models of OA. To date, no effective therapy has been developed for OA that interferes with disease progression. Painkillers and joint replacements are the only therapeutic options at the moment. The blocking of the Smad1/5/8 pathway or stimulation of smad2/3 signalling, combined with the use of MMP13 inhibitors to block the autolytic phenotype of fully OA chondrocytes, should be pursued as potential remedies. The proposed treatments would hit the OA process at its foundation, inhibiting the generation of chondrocytes with an autolytic phenotype.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
This work was supported by The Netherlands Organization for Scientific Research (MW-NWO and the Centre for Biomedical Genetics).
The authors declare no conflicts of interest.
References
- Alvarez J, Serra R. Unique and redundant roles of Smad3 in TGF-beta-mediated regulation of long bone development in organ culture. Dev Dyn. 2004;230:685–699. doi: 10.1002/dvdy.20100. [DOI] [PubMed] [Google Scholar]
- Atif U, Philip A, Aponte J, Woldu EM, Brady S, Kraus VB, Jordan JM, Doherty M, Wilson AG, Moskowitz RW, Hochberg M, Loeser R, Renner JB, Chiano M. Absence of association of asporin polymorphisms and osteoarthritis susceptibility in US Caucasians. Osteoarthritis Cartilage. 2008;16:1174–1177. doi: 10.1016/j.joca.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker AC, van de Loo FA, van Beuningen HM, Sime P, van Lent PL, van der Kraan PM, Richards CD, van den Berg WB. Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthritis Cartilage. 2001;9:128–136. doi: 10.1053/joca.2000.0368. [DOI] [PubMed] [Google Scholar]
- Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Blaney Davidson EN, Scharstuhl A, Vitters EL, van der Kraan PM, van den Berg WB. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;7:R1338–R1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson EN, Vitters EL, van den Berg WB, van der Kraan PM. TGF beta-induced cartilage repair is maintained but fibrosis is blocked in the presence of Smad7. Arthritis Res Ther. 2006;8:R65. doi: 10.1186/ar1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson EN, Vitters EL, van der Kraan PM, van den Berg WB. Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis. 2006;65:1414–1421. doi: 10.1136/ard.2005.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson EN, Vitters EL, van Beuningen HM, van de Loo FA, van den Berg WB, van der Kraan PM. Resemblance of osteophytes in experimental osteoarthritis to transforming growth factor beta-induced osteophytes: limited role of bone morphogenetic protein in early osteoarthritic osteophyte formation. Arthritis Rheum. 2007;56:4065–4073. doi: 10.1002/art.23034. [DOI] [PubMed] [Google Scholar]
- Blaney Davidson EN, Remst DF, Vitters EL, van Beuningen HM, Blom AB, Goumans MJ, van den Berg WB, van der Kraan PM. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Immunol. 2009;182:7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- Blom AB, van Lent PL, Holthuysen AE, van der Kraan PM, Roth J, van Rooijen N, van den Berg WB. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12:627–635. doi: 10.1016/j.joca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Blom AB, van der Kraan PM, van den Berg WB. Cytokine targeting in osteoarthritis. Curr Drug Targets. 2007;8:283–292. doi: 10.2174/138945007779940179. [DOI] [PubMed] [Google Scholar]
- Blom AB, Brockbank SM, van Lent PL, van Beuningen HM, Geurts J, Takahashi N, van der Kraan PM, van de Loo FA, Schreurs BW, Clements K, Newham P, van den Berg WB. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60:501–512. doi: 10.1002/art.24247. [DOI] [PubMed] [Google Scholar]
- Daly AC, Randall RA, Hill CS. Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol Cell Biol. 2008;28:6889–6902. doi: 10.1128/MCB.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroot J, Verzijl N, Wenting-van Wijk MJ, Jacobs KM, Van EB, Van Roermund PM, Bank RA, Bijlsma JW, TeKoppele JM, Lafeber FP. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004;50:1207–1215. doi: 10.1002/art.20170. [DOI] [PubMed] [Google Scholar]
- Finnson KW, Parker WL, ten Dijke P, Thorikay M, Philip A. ALK1 opposes ALK5/Smad3 signaling and expression of extracellular matrix components in human chondrocytes. J Bone Miner Res. 2008;23:896–906. doi: 10.1359/jbmr.080209. [DOI] [PubMed] [Google Scholar]
- Fortier LA, Miller BJ. Signaling through the small G-protein Cdc42 is involved in insulin-like growth factor-I resistance in aging articular chondrocytes. J Orthop Res. 2006;24:1765–1772. doi: 10.1002/jor.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring MB, Otero M, Tsuchimochi K, Ijiri K, Li Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis. 2008;67(Suppl 3):iii75–iii82. doi: 10.1136/ard.2008.098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med. 2003;13:301–307. doi: 10.1016/S1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12:817–828. doi: 10.1016/S1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, de Boer TP, Smits AM, van Laake LW, van Vliet P, Metz CH, Korfage TH, Kats KP, Hochstenbach R, Pasterkamp G, Verhaar MC, van der Heyden MA, de Kleijn D, Mummery CL, van Veen TA, Sluijter JP, Doevendans PA. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007;1:138–149. doi: 10.1016/j.scr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Hecht J, Seitz V, Urban M, Wagner F, Robinson PN, Stiege A, Dieterich C, Kornak U, Wilkening U, Brieske N, Zwingman C, Kidess A, Stricker S, Mundlos S. Detection of novel skeletogenesis target genes by comprehensive analysis of a Runx2(−/−) mouse model. Gene Expr Patterns. 2007;7:102–112. doi: 10.1016/j.modgep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hellingman CA, Davidson EN, Koevoet W, Vitters EL, van den Berg WB, van Osch GJ, van der Kraan PM. Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng Part A. 2011;17:1157–1167. doi: 10.1089/ten.tea.2010.0043. [DOI] [PubMed] [Google Scholar]
- Hinke V, Seck T, Clanget C, Scheidt-Nave C, Ziegler R, Pfeilschifter J. Association of transforming growth factor-beta1 (TGFbeta1) T29→C gene polymorphism with bone mineral density (BMD), changes in BMD, and serum concentrations of TGF-beta1 in a population-based sample of postmenopausal German women. Calcif Tissue Int. 2001;69:315–320. doi: 10.1007/s002230020024. [DOI] [PubMed] [Google Scholar]
- Hjelmeland AB, Schilling SH, Guo X, Quarles D, Wang XF. Loss of Smad3-mediated negative regulation of Runx2 activity leads to an alteration in cell fate determination. Mol Cell Biol. 2005;25:9460–9468. doi: 10.1128/MCB.25.21.9460-9468.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiki M, Imamura T, Okamoto M, Hayashi M, Murai J, Myoui A, Ochi T, Miyazono K, Yoshikawa H, Tsumaki N. Smad6/Smurf1 overexpression in cartilage delays chondrocyte hypertrophy and causes dwarfism with osteopenia. J Cell Biol. 2004;165:433–445. doi: 10.1083/jcb.200311015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, Krane SM. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci USA. 2004;101:17192–17197. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki M, Nakata K, Nakahara H, Nakase T, Kimura T, Kimata K, Caplan AI, Ono K. Transforming growth factor-beta 1 stimulates chondrogenesis and inhibits osteogenesis in high density culture of periosteum-derived cells. Endocrinology. 1993;132:1603–1608. doi: 10.1210/en.132.4.1603. [DOI] [PubMed] [Google Scholar]
- Javed A, Bae JS, Afzal F, Gutierrez S, Pratap J, Zaidi SK, Lou Y, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J Biol Chem. 2008;283:8412–8422. doi: 10.1074/jbc.M705578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed A, Afzal F, Bae JS, Gutierrez S, Zaidi K, Pratap J, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Specific residues of RUNX2 are obligatory for formation of BMP2-induced RUNX2-SMAD complex to promote osteoblast differentiation. Cells Tissues Organs. 2009;189:133–137. doi: 10.1159/000151719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Shi D, Yi L, Ikegawa S, Wang Y, Nakamura T, Qiao D, Liu C, Dai J. Replication of the association of the aspartic acid repeat polymorphism in the asporin gene with knee-osteoarthritis susceptibility in Han Chinese. J Hum Genet. 2006;51:1068–1072. doi: 10.1007/s10038-006-0065-6. [DOI] [PubMed] [Google Scholar]
- Kang JS, Alliston T, Delston R, Derynck R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. EMBO J. 2005;24:2543–2555. doi: 10.1038/sj.emboj.7600729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi H. Regulation of osteoarthritis development by Wnt-beta-catenin signaling through the endochondral ossification process. J Bone Miner Res. 2009;24:8–11. doi: 10.1359/jbmr.081115. [DOI] [PubMed] [Google Scholar]
- Kizawa H, Kou I, Iida A, Sudo A, Miyamoto Y, Fukuda A, Mabuchi A, Kotani A, Kawakami A, Yamamoto S, Uchida A, Nakamura K, Notoya K, Nakamura Y, Ikegawa S. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet. 2005;37:138–144. doi: 10.1038/ng1496. [DOI] [PubMed] [Google Scholar]
- Lark MW, Bayne EK, Lohmander LS. Aggrecan degradation in osteoarthritis and rheumatoid arthritis. Acta Orthop Scand Suppl. 1995;266:92–97. [PubMed] [Google Scholar]
- Leboy P, Grasso-Knight G, D'Angelo M, Volk SW, Lian JV, Drissi H, Stein GS, Adams SL. Smad-Runx interactions during chondrocyte maturation. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S15–S22. [PubMed] [Google Scholar]
- Lefebvre V, Bhattaram P. Vertebrate skeletogenesis. Curr Top Dev Biol. 2010;90:291–317. doi: 10.1016/S0070-2153(10)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JB, Stein JL, Stein GS, van Wijnen AJ, Montecino M, Javed A, Gutierrez S, Shen J, Zaidi SK, Drissi H. Runx2/Cbfa1 functions: diverse regulation of gene transcription by chromatin remodeling and co-regulatory protein interactions. Connect Tissue Res. 2003;44(Suppl 1):141–148. [PubMed] [Google Scholar]
- Livshits G, Ermakov S, Popham M, Macgregor AJ, Sambrook PN, Spector TD, Williams FM. Evidence that bone mineral density plays a role in degenerative disc disease: the UK Twin Spine study. Ann Rheum Dis. 2010;69:2102–2106. doi: 10.1136/ard.2010.131441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser RF. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med. 2010;26:371–386. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115:1571–1579. doi: 10.1172/JCI23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- Marmor D. The arthritides, a medicolegal review. Osteoarthritis (degenerative, hypertrophic, senescent, or "wear and tear" arthritis) Med Trial Tech Q. 1969;15:37–58. [PubMed] [Google Scholar]
- Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):106–110. doi: 10.2106/00004623-200300002-00014. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. Coordinate regulation of cell growth and differentiation by TGF-beta superfamily and Runx proteins. Oncogene. 2004;23:4232–4237. doi: 10.1038/sj.onc.1207131. [DOI] [PubMed] [Google Scholar]
- Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll SW, Recklies AD, Poole AR. Chondrogenesis in periosteal explants. An organ culture model for in vitro study. J Bone Joint Surg Am. 1994;76:1042–1051. doi: 10.2106/00004623-199407000-00013. [DOI] [PubMed] [Google Scholar]
- Panoutsopoulou K, Southam L, Elliott KS, Wrayner N, Zhai G, Beazley C, Thorleifsson G, Arden NK, Elliott KS, Carr A, Chapman K, Deloukas P, et al. Concise report: insights into the genetic architecture of osteoarthritis from stage 1 of the arcOGEN study. Ann Rheum Dis. 2011;70:864–867. doi: 10.1136/ard.2010.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996;97:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo HE, Rozental M. The social impact of aging populations: some major issues. Soc Sci Med. 1994;39:1323–1338. doi: 10.1016/0277-9536(94)90364-6. [DOI] [PubMed] [Google Scholar]
- Revell PA, Mayston V, Lalor P, Mapp P. The synovial membrane in osteoarthritis: a histological study including the characterisation of the cellular infiltrate present in inflammatory osteoarthritis using monoclonal antibodies. Ann Rheum Dis. 1988;47:300–307. doi: 10.1136/ard.47.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Lopez J, Pombo-Suarez M, Liz M, Gomez-Reino JJ, Gonzalez A. Lack of association of a variable number of aspartic acid residues in the asporin gene with osteoarthritis susceptibility: case-control studies in Spanish Caucasians. Arthritis Res Ther. 2006;8:R55. doi: 10.1186/ar1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Kinoshita A, Yoshiura K, Makita Y, Wakui K, Honke K, Niikawa N, Taniguchi N. Domain-specific mutations of a transforming growth factor (TGF)-beta 1 latency-associated peptide cause Camurati-Engelmann disease because of the formation of a constitutively active form of TGF-beta 1. J Biol Chem. 2001;276:11469–11472. doi: 10.1074/jbc.C000859200. [DOI] [PubMed] [Google Scholar]
- Scharstuhl A, Glansbeek HL, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Inhibition of endogenous TGF-beta during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J Immunol. 2002;169:507–514. doi: 10.4049/jimmunol.169.1.507. [DOI] [PubMed] [Google Scholar]
- Scharstuhl A, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Loss of transforming growth factor counteraction on interleukin 1 mediated effects in cartilage of old mice. Ann Rheum Dis. 2002;61:1095–1098. doi: 10.1136/ard.61.12.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharstuhl A, Vitters EL, van der Kraan PM, van den Berg WB. Reduction of osteophyte formation and synovial thickening by adenoviral overexpression of transforming growth factor beta/bone morphogenetic protein inhibitors during experimental osteoarthritis. Arthritis Rheum. 2003;48:3442–3451. doi: 10.1002/art.11328. [DOI] [PubMed] [Google Scholar]
- Serra R, Johnson M, Filvaroff EH, Laborde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YQ, Cheung KM, Ho DW, Poon SC, Chiba K, Kawaguchi Y, Hirose Y, Alini M, Grad S, Yee AF, Leong JC, Luk KD, Yip SP, Karppinen J, Cheah KS, Sham P, Ikegawa S, Chan D. Association of the asporin D14 allele with lumbar-disc degeneration in Asians. Am J Hum Genet. 2008;82:744–747. doi: 10.1016/j.ajhg.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P, Werb Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer RK, Levicoff E, Georgescu H, Miller L, Jaffurs D, Evans CH. Nitric oxide inhibits chondrocyte response to IGF-I: inhibition of IGF-IRbeta tyrosine phosphorylation. Am J Physiol Cell Physiol. 2000;279:C961–C969. doi: 10.1152/ajpcell.2000.279.4.C961. [DOI] [PubMed] [Google Scholar]
- Tchetina EV, Squires G, Poole AR. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J Rheumatol. 2005;32:876–886. [PubMed] [Google Scholar]
- Valdes AM, Spector TD, Tamm A, Kisand K, Doherty SA, Dennison EM, Mangino M, Tamm A, Kerna I, Hart DJ, Wheeler M, Cooper C, Lories RJ, Arden NK, Doherty M. Genetic variation in the SMAD3 gene is associated with hip and knee osteoarthritis. Arthritis Rheum. 2010;62:2347–2352. doi: 10.1002/art.27530. [DOI] [PubMed] [Google Scholar]
- van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. In vivo protection against interleukin-1-induced articular cartilage damage by transforming growth factor-beta 1: age-related differences. Ann Rheum Dis. 1994;53:593–600. doi: 10.1136/ard.53.9.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest. 1994;71:279–290. [PubMed] [Google Scholar]
- van Beuningen HM, Glansbeek HL, van der Kraan PM, van den Berg WB. Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage. 1998;6:306–317. doi: 10.1053/joca.1998.0129. [DOI] [PubMed] [Google Scholar]
- van Beuningen HM, Glansbeek HL, van der Kraan PM, van den Berg WB. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-beta injections. Osteoarthritis Cartilage. 2000;8:25–33. doi: 10.1053/joca.1999.0267. [DOI] [PubMed] [Google Scholar]
- van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007;15:237–244. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- van de Laar I, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, Hoedemaekers YM, Willemsen R, Severijnen LA, Venselaar H, Vriend G, Pattynama PM, Collee M, Majoor-Krakauer D, Poldermans D, Frohn-Mulder IM, Micha D, Timmermans J, Hilhorst-Hofstee Y, Bierma-Zeinstra SM, Willems PJ, Kros JM, Oei EH, Oostra BA, Wessels MW, Bertoli-Avella AM. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–126. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Bank RA, Bayliss MT, Bijlsma JW, Lafeber FP, Maroudas A, TeKoppele JM. Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol. 2001;20:409–417. doi: 10.1016/S0945-053X(01)00158-5. [DOI] [PubMed] [Google Scholar]
- Wu X, Ma J, Han JD, Wang N, Chen YG. Distinct regulation of gene expression in human endothelial cells by TGF-beta and its receptors. Microvasc Res. 2006;71:12–19. doi: 10.1016/j.mvr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Wu Q, Kim KO, Sampson ER, Chen D, Awad H, O'Brien T, Puzas JE, Drissi H, Schwarz EM, O'Keefe RJ, Zuscik MJ, Rosier RN. Induction of an osteoarthritis-like phenotype and degradation of phosphorylated Smad3 by Smurf2 in transgenic mice. Arthritis Rheum. 2008;58:3132–3144. doi: 10.1002/art.23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wang M, Zuscik MJ, Chen D, O'Keefe RJ, Rosier RN. Regulation of embryonic endochondral ossification by Smurf2. J Orthop Res. 2008;26:704–712. doi: 10.1002/jor.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Peng H, Glasson S, Lee PL, Hu K, Ijiri K, Olsen BR, Goldring MB, Li Y. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56:2663–2673. doi: 10.1002/art.22761. [DOI] [PubMed] [Google Scholar]
- Yamada Y. Association of a Leu(10)→Pro polymorphism of the transforming growth factor-beta1 with genetic susceptibility to osteoporosis and spinal osteoarthritis. Mech Ageing Dev. 2000;116:113–123. doi: 10.1016/S0047-6374(00)00131-7. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Okuizumi H, Miyauchi A, Takagi Y, Ikeda K, Harada A. Association of transforming growth factor beta 1 genotype with spinal osteophytosis in Japanese women. Arthritis Rheum. 2000;43:452–460. doi: 10.1002/1529-0131(200002)43:2<452::AID-ANR28>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JY, Wang Y, An J, Mao CM, Hou N, Lv YX, Wang YL, Cui F, Huang M, Yang X. Mutation analysis of the Smad3 gene in human osteoarthritis. Eur J Hum Genet. 2003;11:714–717. doi: 10.1038/sj.ejhg.5201034. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ziran N, Goater JJ, Schwarz EM, Puzas JE, Rosier RN, Zuscik M, Drissi H, O'Keefe RJ. Primary murine limb bud mesenchymal cells in long-term culture complete chondrocyte differentiation: TGF-beta delays hypertrophy and PGE2 inhibits terminal differentiation. Bone. 2004;34:809–817. doi: 10.1016/j.bone.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Zheng L, Baek HJ, Karsenty G, Justice MJ. Filamin B represses chondrocyte hypertrophy in a Runx2/Smad3-dependent manner. J Cell Biol. 2007;178:121–128. doi: 10.1083/jcb.200703113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O'Keefe RJ, Zuscik M, Chen D. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/jbmr.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]