Abstract
An 80-year-old woman with rheumatoid arthritis had gait difficulties and frequent falls. MRI of the brain showed an extra-axial enhancing lesion overlying the right frontal–parietal cortex, that progressively extended to the contralateral side. This was accompanied by further decline in her functional status. We discuss the diagnostic and therapeutic approach of a pachy–leptomeningeal process in a rheumatoid patient.
Keywords: rheumatoid arthritis, brain biopsy, rheumatoid pachymeningitis, rheumatoid leptomeningitis
An 80-year-old woman presented to the neurology clinic with a history of unsteady gait and frequent falls and was admitted to our hospital for further evaluation. She had history of arterial hypertension, chronic obstructive pulmonary disease, and long-standing rheumatoid arthritis (RA) treated with prednisone and plaquenil. In her youth, she had pulmonary tuberculosis (TB) treated with streptomycin and para-aminosalicylic acid. Six months prior to her clinic visit, she was admitted to a local hospital for surgical treatment of a small bowel obstruction. Postoperatively, she developed atrial fibrillation. She received intravenous unfractionated heparin and then bridged to warfarin (target INR 2.0–3.0). While at the hospital, she fell and had a mild traumatic subarachnoid hemorrhage (SAH). She made a good recovery and was discharged home on warfarin. Four months following hospital discharge, she fell again and had a left femoral fracture requiring hip replacement.
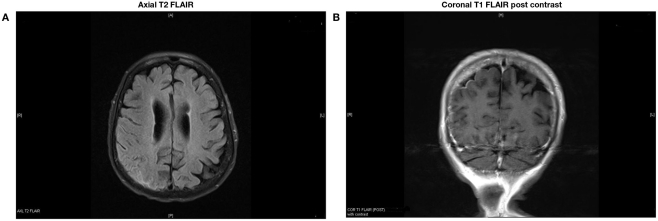
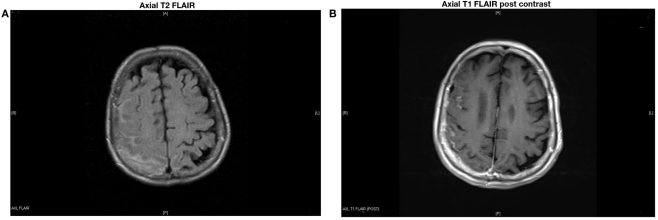
Neurological examination showed a broad based unsteady gait. A non-contrast head CT scan showed scattered dural and vascular calcifications. T2 weighted brain MRI showed an extra-axial high signal intensity lesion overlying the right parietal–occipital region with mild gyriform contrast enhancement (Figures 1A,B). There were no signal abnormalities on T1 or gradient echo sequences. A diagnosis of subacute post-traumatic SAH was made. She had successful cardioversion, and was discharged home on amiodarone. Five months later, she presented with worsening unsteadiness and left sided weakness. Neurological examination showed impaired left hand dexterity, mild weakness of the left leg, and a broad based gait. Non-contrast head CT showed gyriform hyperdensity along the lateral aspect of the right frontal cortex associated with sulcal effacement. Gadolinium enhanced brain MRI showed an enhancing hyperintense signal abnormality overlying the right parietal and posterior frontal lobes compatible with leptomeningeal and dural thickening (Figures 2A,B). Cerebrospinal fluid (CSF) analysis showed 62 red blood cells (RBCs), 2 white blood cells (WBCs), a glucose concentration of 60 mg/dl, and a protein content of 75 mg/dl. Cytology was negative for malignancy. HSV and VZV PCRs in CSF were negative. CSF Lyme titer and cryptococcal antigen were likewise negative. CSF VDRL was non-reactive. Viral, bacterial, acid fast bacilli (AFB), and fungal cultures were negative in CSF and blood. Serum C-reactive protein was 2.9 mg/dl, and erythrocyte sedimentation rate was 35 mm/h. Antinuclear antibodies, anti-Smith, anti-ribonucleoprotein, anti-SSa, anti-SSb, anti-histone, and SCL-70 were negative. Serum rheumatoid factor (RF) was <20. Serum angiotensin converting enzyme (ACE) level was 10 U/L. Contrast CT scan of the chest, abdomen, and pelvis showed low density lesions throughout the spleen, and 5 mm nodules in lungs.
Figure 1.
Extra-axial high signal intensity overlying the right parietal–occipital region with mild gyriform contrast enhancement.
Figure 2.
Hyperintense signal abnormalities overlying the right parietal and posterior frontal lobes with contrast enhancement, compatible with dural and leptomeningeal thickening.
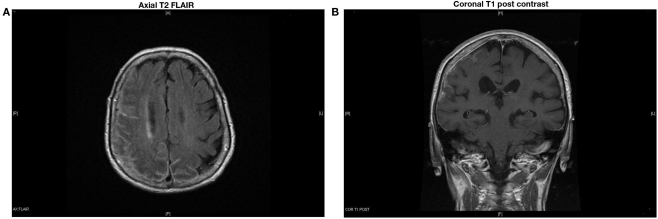
Symptoms progressed, and a follow-up gadolinium enhanced MRI showed worsening of the right-sided leptomeningeal and dural thickening as well as progression of the leptomeningeal enhancement over the left posterior parietal and occipital lobes (Figures 3A,B). A second lumbar puncture was performed. CSF was clear with an opening pressure of 8 cm of H2O. There were 9 RBCs and 12 WBCs (78% lymphocytes). CSF glucose was 60 mg/dl, and the protein content was 77 mg/dl. Cytology was again negative for malignant cells. Flow phenotyping showed mixed lymphoid population, predominantly T-cells and occasional B-cells, with no evidence of clonality. Bacterial, fungal, and AFB cultures were negative in blood and CSF. Quantiferon gold testing for TB was positive.
Figure 3.
Increased right-sided leptomeningeal and dural thickening with worsening of leptomeningeal enhancement over the left posterior parietal and occipital lobes.
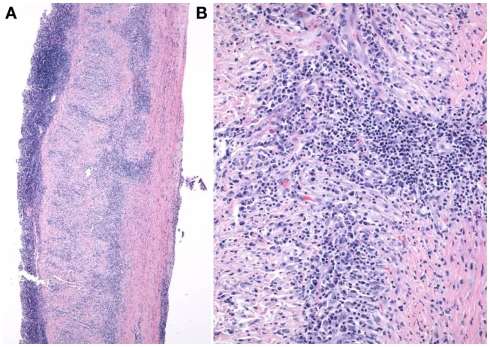
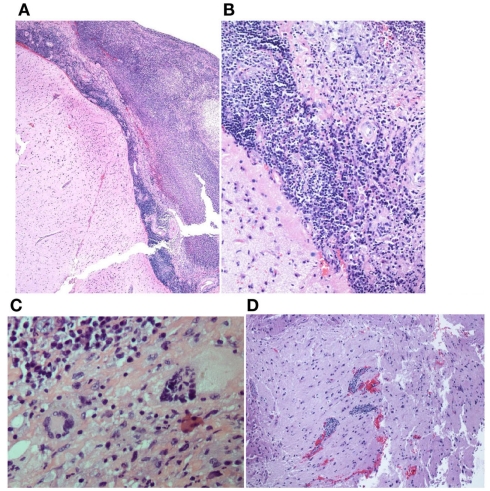
A brain/meningeal biopsy was done which showed a chronic inflammatory process with numerous plasma cells with Russell bodies and granulomatous reaction of the leptomeninges and dura. Findings were consistent with rheumatoid pachy and leptomeningitis (Figures 4A,B). In addition, there was an acute and chronic leptomeningitis with focal giant cell reaction with secondary parenchymal perivascular lymphocytic infiltrates with reactive gliosis (Figures 5A–D). The leptomeninges contained a focal abscess formation with suppurative inflammation (Figures 6A,B). Special stains including GMS, AFB, FITE, Gram, and cultures were negative. Sections of the pachymeningitis were sent for mycobacterium TB DNA PCR, and were also negative.
Figure 4.
(A,B) Dura matter with chronic pachymeningitis.
Figure 5.
(A,B) Acute and chronic leptomenigitis. (C) Acute and chronic leptomeningitis with giant cells. (D) Acute and chronic leptomeningitis with secondary parenchymal perivascular lymphocytic infiltrates.
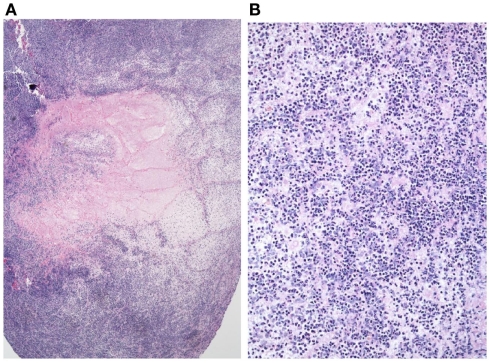
Figure 6.
(A,B) Focal abscess formation.
Discussion
Direct CNS involvement by inflammatory cells in RA, including rheumatoid meningitis, is extremely rare (Bathon et al., 1989; Chang and Paget, 1993; Voller et al., 2001; Bruggemann et al., 2010).
Bathon et al. (1989) reviewed 19 patients (10 men and 9 women) with inflammatory rheumatoid CNS disease. Most cases were diagnosed at autopsy. No MRI was performed. Most patients had long-standing RA, with mean duration of illness of 14 years at onset of neurological symptoms. Although rheumatoid CNS involvement may occur during the phase of acute synovitis, less than half of these patients had active synovitis when neurological symptoms developed. The authors concluded that RA duration and activity were unreliable indicators to diagnose rheumatoid CNS involvement. However, rheumatoid meningitis may occur early in the course of the disease and may even precede synovitis (Jones et al., 2006; Starosta and Brandwein, 2007).
In Bathon’s review, the clinical presentation of rheumatoid meningitis was characterized by altered mental status in 47% of cases, cranial neuropathies in 26%, hemiparesis/paraparesis in 21%, seizures in 21%, and headaches in 11%. Inflammatory rheumatoid CNS disease may produce pachymeningitis and leptomeningitis, as in the case of our patient (Bathon et al., 1989; Kato et al., 2003; Jones et al., 2006; Shimada et al., 2009). If the dura matter is predominantly affected, these patients present with pachymeningitis, characterized by headaches and cranial neuropathies caused by inflammation or dural fibrosis. If the leptomeninges are predominantly affected, mental status changes, gait imbalance, memory loss, depression, seizures, or paresis are more common (Kupersmith et al., 2004).
Along with rheumatoid meningitis, Bathon identified other extra-articular manifestations like subcutaneous nodules in 67% of cases, visceral nodules in 47%, and both in 40%. Our patient had lung and spleen nodules; these may have been a manifestation of RA, or related to her previous TB.
Cerebrospinal fluid findings in rheumatoid meningitis are variable and often non-diagnostic (Kupersmith et al., 2004). Lymphocytic pleocytosis is not always present. An increased protein level is frequently observed. However, sampling at points distant from the lesion might produce pseudo negative results (Kato et al., 2003; Kupersmith et al., 2004; Shimada et al., 2009). New specific markers are being identified. Determination of RF in CSF is often used as a diagnostic marker because strongly positive results specifically indicate the disease (Kato et al., 2003). Inflammatory cytokines, including TNF-α, IL-1β, and IL-6, play a central role in RA pathogenesis and in some cases high CSF levels have been identified in patients with rheumatoid meningitis, but their significance is still unknown (Kato et al., 2003; Shimada et al., 2009). Gadolinium enhanced MRI is quite useful. Diffuse or patchy enhancing extra-axial high intensity lesions in FLAIR and DWI, are considered characteristics of RA meningitis (Jones et al., 2006; Starosta and Brandwein, 2007; Shimada et al., 2009).
The final diagnosis is ultimately histopathological. In Bathon’s review, rheumatoid meningitis diagnosis was not achieved until autopsy in 17 of 19 patients. In two patients diagnosis was made by open brain biopsy. On gross examination, thickened meninges and nodules or plaques were observed frequently. Microscopic pathological examination demonstrated three abnormal patterns: rheumatoid nodules, non-specific meningeal inflammation, and vasculitis. Nodules were the most common finding (68%) and were histologically identical to subcutaneous rheumatoid nodules. Nodules were located in the cranial meninges (92%) and in the choroid plexus (15%). There were two cases affecting the spinal meninges. Nodules were absent in the brain parenchyma or spinal cord. Non-specific inflammatory infiltrates in the leptomeninges or pachymeninges, with mononuclear cells, particularly plasma cells, and less frequently necrosis and multinucleated giant cells, was found in 63% of cases. Reasons for a meningeal predilection, particularly the dura, rather than brain parenchyma, by invading inflammatory cells are unclear. Autoimmunity to collagen, a major component of the dura but not brain parenchyma, may play a role (Bathon et al., 1989). Vasculitis was identified in 37% of cases and involved the brain, spinal cord parenchyma, as well as the meninges. Vessel wall infiltrates consisted of lymphocytes and plasma cells. The authors concluded that CNS rheumatoid nodules might be considered specific for CNS rheumatoid disease. In instances of non-specific chronic inflammatory infiltration by vasculitis or meningitis the preponderance of plasma cells may distinguish CNS rheumatoid disease from other connective tissue disorders such as systemic lupus erythematosus (SLE) or Sjögren syndrome. Among patients diagnosed at autopsy, the most common findings are rheumatoid nodules. Among patients diagnosed by biopsy, it is a non-specific inflammatory infiltration, probably secondary to limited tissue sampling (Bathon et al., 1989; Jones et al., 2006; Starosta and Brandwein, 2007; Li and Kuzuhara, 2009; Shimada et al., 2009). In our patient, biopsy showed chronic inflammatory infiltration rich in plasma cells in both the pachymeninges and the leptomeninges. There was also a small focal abscess even though cultures were negative. In Bathon’s report, polymorphonuclear infiltrates were found in three patients (25%). Although our patient had a positive quantiferon TB gold test, likely due to her previous TB, AFB stains, cultures, and mycobacterium TB DNA PCR were negative in the meningeal samples.
There are no clear guidelines for the treatment of rheumatoid meningitis. Cyclophosphamide, azathioprine, and methotrexate in combination with corticosteroids have been recommended (Kato et al., 2003; Kupersmith et al., 2004; Jones et al., 2006; Starosta and Brandwein, 2007; Li and Kuzuhara, 2009; Shimada et al., 2009). Improvement has been obtained with corticosteroids alone. This suggests that immunosuppressants may not always be necessary in the induction phase of treatment, although they may be required to achieve tapering or cessation of corticosteroids to avoid potential long-term adverse effects (Shimada et al., 2009). Recurrence of rheumatoid meningitis in a patient treated with infliximab (Chou et al., 2006) and pachymeningitis occurring after administration of adalimumab have been reported (Ahmed et al., 2006).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahmed M., Luggen M., Hernan J. H. (2006). Hypertrophic pachymeningitis in rheumatoid arthritis after adalimumab administration. J. Rheumatol. 33, 2344–2346 [PubMed] [Google Scholar]
- Bathon J. M., Moreland L. W., Dibartolomeo A. G. (1989). Inflammatory central nervous system involvement in rheumatoid arthritis. Semin. Arthritis Rheum. 18, 258–266 10.1016/0049-0172(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Bruggemann N., Gottschalk S., Holl-Ulrich K. (2010). Cranial pachymeningitis: a rare neurological syndrome with heterogeneous etiology. J. Neurol. Neurosurg. Psychiatry 81, 294–298 10.1136/jnnp.2008.160457 [DOI] [PubMed] [Google Scholar]
- Chang D., Paget S. (1993). Neurologic complications of rheumatoid arthritis. Rheum. Dis. Clin. North Am. 19, 955–973 [PubMed] [Google Scholar]
- Chou R. C., Henson J. W., Tian D. (2006). Successful treatment of rheumatoid meningitis with cyclophosphamide but not infliximab. Ann. Rheum. Dis. 65, 1114–1116 10.1136/ard.2005.046847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. E., Belsley N. A., Mc Loud T. C. (2006). Rheumatoid meningitis: radiologic and pathologic correlation. AJR Am. J. Roentgenol. 186, 1181–1183 10.2214/AJR.05.0859 [DOI] [PubMed] [Google Scholar]
- Kato T., Hoshi K., Sekijima Y. (2003). Rheumatoid meningitis: an autopsy report and review of the literature. Clin. Rheumatol. 22, 475–480 10.1007/s10067-003-0788-0 [DOI] [PubMed] [Google Scholar]
- Kupersmith M. J., Martin V., Heller G. (2004). Idiopathic hypertrophic pachymeningitis. Neurology 62, 686–694 [DOI] [PubMed] [Google Scholar]
- Li Y., Kuzuhara S. (2009). Rheumatoid cranial pachymeningitis successfully treated with long term corticosteroids. Rheumatol. Int. 29, 583–585 10.1007/s00296-008-0708-3 [DOI] [PubMed] [Google Scholar]
- Shimada K., Matsui T., Kawakami M. (2009). Diffuse chronic leptomeningitis with seropositive rheumatoid arthritis: report of case successfully treated as rheumatoid leptomeningitis. Mod. Rheumatol. 19, 556–562 10.1007/s10165-009-0186-9 [DOI] [PubMed] [Google Scholar]
- Starosta M. A., Brandwein S. R. (2007). Clinical manifestations and treatment of rheumatoid pachymeningitis. Neurology 68, 1079–1080 10.1212/01.wnl.0000257824.72457.91 [DOI] [PubMed] [Google Scholar]
- Voller B., Vass K., Wanswchitz J. (2001). Hypertrophic chronic pachymeningitis as a localized immune process in the craniocervical region. Neurology 56, 107–109 [DOI] [PubMed] [Google Scholar]