Abstract
Toxoplasma gondii infections are prevalent in a wide range of mammalian hosts including humans. Infection in pregnant women may cause the transmission of parasite to the fetus that makes serious problems. IgM antibodies against Toxoplasma (Toxo-IgM) have been believed to be significant indicators for both recently acquired and congenital toxoplasmosis. So far, however, there has not been any recognized protein of T. gondii that specifically reacts to IgM antibodies. Here, an antigen exclusively for detection of IgM antibodies screened by two-dimensional electrophoresis and mass spectrometry has been reported. The study identified 13 Toxoplasma proteins probed by IgG antibodies and one (rhpotry protein 2 [ROP2]) by IgM antibodies with human sera of Toxo-IgM–-IgG+ and -IgM+-IgG–, respectively, which had been prescreened by Toxo-IgM and -IgG commercial kits from the suspected cases. Following cloning, expression, and purification of the fragment of ROP2186–533, an enzyme-linked immunosorbent assay with rROP2186–533 to measure IgM and IgG antibodies was developed. As a result, 100%(48/48) of sera with Toxo-IgM+-IgG–showed positive Toxo-IgM but none of them (0%) showed positive Toxo-IgG when rROP2186–533 was used as antigen. Neither Toxo-IgG nor Toxo-IgM antibodies were found when tested with 59 sera of Toxo-IgM–-IgG+. These results indicate that rROP2186–533 could be used as an antigen that specifically capture Toxo-IgM antibodies and may have a high potential in the serological diagnosis of both acute acquired and congenital toxoplasmosis.
Introduction
Toxoplasma gondii is an obligate intracellular parasite that can invade multiple cell types and cause infection and disease in diverse vertebrate species. Previous studies showed that early maternal infection (first and second trimesters) may result in severe congenital toxoplasmosis and death of the fetus in uterus and spontaneous abortion. Late maternal infection (third trimester), however, usually gives rise to normal appearing newborns (Montoya and Liesenfeld, 2004). Since women who acquired infection prior to pregnancy are essentially not at risk for delivering an infected infant, it is important to determine whether the pregnant woman has the acute infection during gestation. Unfortunately, this is the most frequent challenge to physicians the world over.
Serum IgM antibodies against Toxoplasma (Toxo-IgM) are believed as one of the markers for the diagnosis of acute or congenital Toxoplasma infection. Recently, Liang et al. (2011) identified several antigens for detection of IgM antibodies using protein microarray displaying the polypeptides products of Toxoplasmic exons with well-characterized sera. Usually, IgM antibodies are generated within a week after infection, reaching a peak, and then rapidly decrease. IgG antibodies against Toxoplasma (Toxo-IgG) appear within 1–2 weeks and persist even for life of patients. Positive serum IgG antibodies, however, only show that once infected with T. gondii, but could not differentiate between recent and distant infection, whereas negative serum IgM antibodies could basically exclude recent infection. At present in China, serological survey of Toxoplasma infection is one of the issues of screening teratogenic factors in pregnant women. The aim of this work is to find antigens which can be specifically recognized by IgM antibodies against T. gondii.
Materials and Methods
Parasites
The RH tachyzoites of T. gondii were maintained by mouse passage and the PRU strain was kept in the laboratory by oral passage of mice with the brain tissues.
Preparation of the tachyzoite lysates of T. gondii RH strain
Tachyzoites of RH strain were injected into BALB/c mice intraperitoneally and peritoneal exudates were collected 72 h after infection. Parasites obtained were digested with 0.25% trypsin solution and washed three times with phosphate-buffered saline (PBS, 10 mM sodium phosphate containing 0.15 M NaCl, pH 7.2). After repeated freezing and thawing, the parasite pellets were subjected to sonication (50 W, ultrasound 5 sec, interval 5 sec) for 5 min on the ice bath, followed by centrifugation at 100,000 g for 30 min at 4°C. The supernatant was harvested and stored at −80°C.
Sera from T. gondii-infected individuals
Forty-eight sera obtained from individuals with positive Toxo-IgM but negative Toxo-IgG (IgM+-IgG–) and 59 sera with positive Toxo-IgG but negative Toxo-IgM (IgM–-IgG+) were collected from the Provincial Reference Labs for Prenatal Screening of Toxoplasmosis corresponding to the Eugenic Birth Plan of China. Serum samples from 96 healthy donors, who had been Toxo-antibodies free by prescreening, were served as control to establish the full range of background values. All the sera were tested twice by the commercial serologic test kits (Toxo IgG and IgM enzyme immunoassay test kit; Biocheck).
Sera from other protozoa-infected humans
Twenty sera of patients infected with Plasmodium vivax and 25 with Leishmania spp. were collected. Serum samples were stored at −80°C for use.
Sera from toxoplasma-infected mice
Eight-week-old male BALB/c mice (specific pathogen free) were infected orally with 10 cysts of low virulent T. gondii PRU strain. Blood samples of the mice were obtained on days 3, 7, 14, and 28 postinfection to separate the sera. Sera from uninfected BALB/c mice of the same gender and age were served as negative control. Each of experimental group was comprised of five animals and the sera of each time point were pooled before testing.
Two-dimensional electrophoresis
Samples were further purified using a 2D clean-up kit (Bio-Rad). Following the manufacturer's protocol, the final pellets were dissolved in 125 μL rehydration buffer. Isoelectric focusing (IEF) was performed in 7 cm pH 3–10 IPG strips (Bio-Rad) using a Protean IEF Cell (Bio-Rad) with a surface temperature of 17°C and a maximum current of 50 μA/strip. The second dimension was performed on 10% SDS-PAGE using a Mini Protean cell (Bio-Rad). Proteins were separated for 30 min at 50 V and then at 110 V until the dye front reached the bottom of the gel. After separation, proteins were either visualized by CBB-staining for proteomic analysis or used for immunoblotting.
Immunoblot analysis
Proteins from two-dimensional electrophoresis (2-DE) gels were transferred onto three nitrocellulose membranes. The membranes were blocked in PBS with 5% skim milk powder for 2 h at room temperature and rinsed with washing buffer (PBS containing 0.05% Tween-20 [PBST]) for three times. Next, the membranes were incubated overnight at 4°C in pooled sera from the patients with IgM+-IgG–(1:190 dilution) and with Toxo-IgM–-IgG+ (1:280 dilution). The sera of control were diluted in 1:200. The blots were washed three times with PBST and then incubated with the corresponding goat anti-human IgM-horseradish peroxidase-labeled conjugate and goat anti-human IgG-horseradish peroxidase-labeled conjugate (diluted 1:10,000 and 1:17,600, respectively) for 2 h at room temperature. Following three additional washes, the membranes were developed with the enhanced chemiluminescent kit (Pierce).
Protein identification by liquid chromatography/tandem mass spectrometry (LC/MS-MS)
Twenty-four gel spots containing the matched antigenic proteins were manually excised from the 2-DE gel. The procedure was performed as previously described (Zhong et al., 2010).
Identification and production of recombinant ROP2186–533
In order to successfully express ROP2186–533 fragment, a pair of primers was designed to amplify the structural gene except the signal peptide. The primers of ROP2186–533 (accession No. Z36906) were designed (sense: 5′-AGG CATATG GAC ACG AAC CCT ATG T-3′, antisense: 5′- GAATTC TTA TTG CAA TGG GAG GAG G-3′) with NdeI and EcoRI restriction sites incorporated. The cDNA of T. gondii RH strain was used as templates for PCR. As the result, a DNA fragment with the length of 1044 bp could code for a peptide fragment of ROP2186–533 (from amino acids 186 to 533). Following subcloning into the plasmid pMD18-T vector, the ROP2186–533 was ligated to the expression vector pET-28a. The rROP2186–533 peptide was expressed in the Escherichia coli BL21 strain transformed with the recombinant plasmid of pET-28a-rROP2186–533 following induction of isopropyl-β-D-thiogalactoside. The cell lysates with the corresponding fusion peptide were subjected to 12% SDS-PAGE and transferred onto a nitrocellulose membrane. After blocking with 5% skim milk, the membrane was incubated in the presence of an anti-His-tag monoclonal antibody, and subsequently with secondary anti-mouse IgG antibodies conjugated with HRP. The films were developed as mentioned above.
Application of recombinant antigen of ROP2186–533
The recombinant antigen of ROP2186–533 was applied for diagnosis of both human and mouse serum samples using enzyme-linked immunosorbent assay (ELISA). Optimization of the protocol and dilution of reagents were determined by checkerboard titration. Briefly, the plates were coated with rROP2186–533 in 200 ng/well. The human sera were diluted in 1:10 and the mouse sera in 1:40 for both IgG and IgM antibodies detection. The working concentration of the reagents was prepared as per the manufacture's instruction. The whole soluble antigen of T. gondii (WSA) was used at concentration of 200 ng/well for detection of the mouse IgM, with the sera dilution of 1:40. After washing five times with PBST, the color reaction was developed by adding 3,3′ 5,5′-tetramethyl benzidine dihydrochloride substrate buffer. The absorbance was read at 450 nm using an auto ELISA reader (Bio-Tek).
Statistical analysis
The cut-off values for assays were defined as the higher of the following: The mean±2SD optical density values of the negative control samples. p<0.05 was considered statistically significant.
Results
Two-dimensional electrophoresis
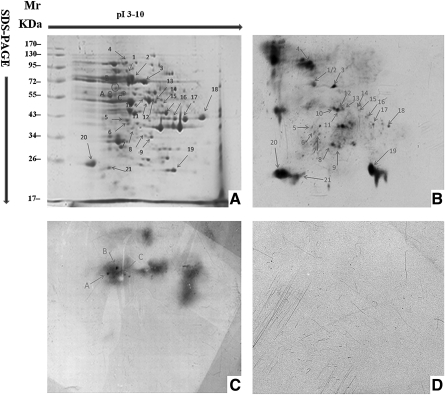
At least five batches of T. gondii soluble protein were analyzed with 2-DE and more than 200 spots were visualized in the gel (Fig. 1A). The protein maps were identical, showing the high reproducibility of this assay.
FIG. 1.
Two-dimensional electrophoresis (2-DE) and immunoblot patterns of the whole soluble protein of Toxoplasma gondii RH strain. (A) CBB-stained 2-DE pattern with pH 3–10 linear strips, points marked by numbers are the common protein spots between immunoblot pattern of IgM–-IgG+ and 2-DE pattern, points marked by letters are the common protein spots between immunoblot pattern of IgM+-IgG–and 2-DE pattern. (B) Immunoblot map probed by sera of patients with IgM–-IgG+. (C) Immunoblot map probed by patient sera with IgM+-IgG–. (D) Immunoblot map probed by normal control sera of healthy donors.
Immunoblot maps of WSA
The sera of patients with Toxo-IgM+-IgG– and -IgM–-IgG+ were used to screen IgM- and IgG-recognized proteins. Sera of the patients were pooled in order to eliminate individual differences. As shown in Fig. 1B, there were more than 50 spots that display strong reactivity to the pooled sera with Toxo-IgM–-IgG+, and 21 of them had a correspondence to the points on the 2-DE gel. The pooled sera with Toxo-IgM+-IgG–, however, showed seven spots with detectable immunoreactivity and three of them could be matched to the 2-DE gel perfectly (Fig. 1C). Sera from healthy controls showed no spots (Fig. 1D). The matched 24 protein spots were excised, digested by typsin, and further subjected to analysis with LC/MS-MS.
Identification of immunoreactive proteins by LC/MS-MS
Twenty one spots recognized by IgG antibodies were successfully identified by LC/MS-MS as corresponding to 13 different proteins (Table 1). Interestingly, three points were recognized by the sera of Toxo-IgM+-IgG–and only one of them, the rhpotry protein 2 (ROP2), could be identified and characterized by LC/MS-MS.
Table 1.
Immunoreactive Proteins Identified by LC/MS-MS
| Spot no. | Putative protein | Unique peptides | Coverage (%) | pI (Exp/Obs)a | Mr(Kda) (Exp/Obs)a | MASCOT score | Accession no. |
|---|---|---|---|---|---|---|---|
| 1 | Hsp70 | 84 | 63.65 | 5.3/5.4 | 73.4/72.0 | 830.28 | BAB20284 |
| 2 | Hsp70 | 49 | 47.48 | 5.3/5.6 | 73.4/72.0 | 480.26 | BAB20284 |
| 3 | Nucleoside triphosphate hydrolase 3 | 23 | 42.36 | 6.0/6.2 | 69.1/70.1 | 220.31 | AAC80188 |
| 4 | Importin β-1 subunit | 2 | 2.59 | 4.4/5.2 | 98.3/109.1 | 20.27 | CAJ20312 |
| 5 | Hsp70 | 18 | 25.22 | 5.3/5.4 | 73.4/41.5 | 190.27 | BAB20284 |
| 6 | Hsp70 | 18 | 18.40 | 5.3/5.5 | 73.4/40.1 | 190.32 | BAB20284 |
| 7 | Actin | 9 | 29.52 | 4.9/5.7 | 41.9/38.2 | 70.25 | CAJ20602 |
| 8 | Mitochondrial malate-dehydrogenase | 4 | 10.43 | 8.9/6.5 | 50.3/35.2 | 40.36 | ABU49220 |
| 9 | Mitochondrial malate-dehydrogenase | 13 | 34.68 | 8.9/6.8 | 50.3/34.1 | 130.24 | ABU49220 |
| 10 | Mitochondrial F1-ATP synthase beta subunit precursor | 33 | 58.93 | 6.4/6.2 | 59.9/54.5 | 320.23 | ABB17195 |
| 11 | AF 123457-1 enolase | 55 | 84.68 | 5.6/6.4 | 48.2/52.9 | 540.35 | AAG60329 |
| 12 | Hexokinase | 30 | 41.67 | 5.8/6.8 | 51.4/53.0 | 310.28 | BAB55664 |
| 13 | AF 123457-1 enolase | 14 | 49.32 | 5.6/6.9 | 48.2/52.1 | 140.24 | AAG60329 |
| 14 | AF 123457-1 enolase | 2 | 7.88 | 5.6/7.1 | 48.2/50.5 | 20.26 | AAG60329 |
| 15 | Phosphoglycerate kinase 1 | 54 | 83.17 | 6.3/7.4 | 44.5/45.0 | 540.35 | ABE76509 |
| 16 | Phosphoglycerate kinase 1 | 4 | 18.57 | 6.3/7.8 | 44.5/42.1 | 40.38 | ABE76509 |
| 17 | Elongation factor 1-α | 5 | 13.62 | 9.2/8.4 | 48.9/42.1 | 50.24 | CAJ20335 |
| 18 | Elongation factor 1-α | 3 | 8.04 | 9.2/9.2 | 48.9/42.5 | 30.28 | CAJ20335 |
| 19 | Glucose-6-phosphate-1-dehyrogenase | 17 | 43.57 | 7.4/7.7 | 62.7/23.5 | 160.36 | CAJ20381 |
| 20 | Serine-threonine phosophatase 2C | 29 | 71.00 | 5.3/4.0 | 36.9/25.4 | 290.27 | CAC86553 |
| 21 | Inflammatory profilin | 5 | 41.72 | 4.2/4.5 | 17.5/24.0 | 50.36 | CAJ20409 |
| C | B chain B, rop2 from Toxoplasma gondii | 2 | 7.34 | 6.5/5.1 | 42.5/57.5 | 20.28 | Z36906 |
Expected and observed molecular mass (Mr) and isoelectric point (pI).
LC/MS-MS, liquid chromatography/tandem mass spectrometry.
Cloning and expression of ROP2186–533
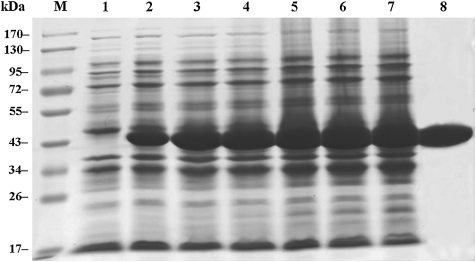
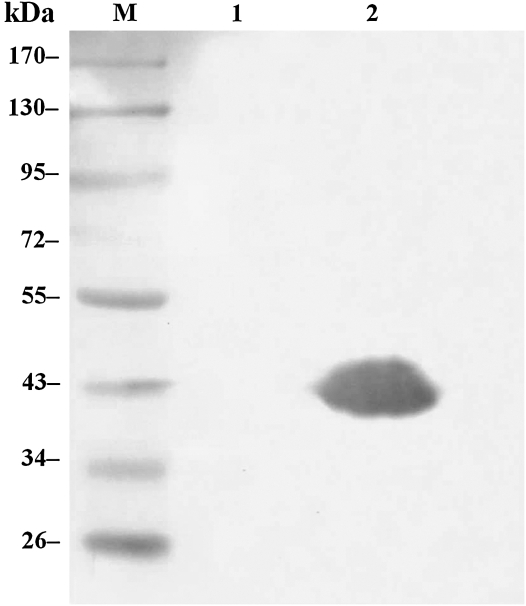
The recombinant ROP2186–533 were cloned and expressed successfully in E. coli (Fig. 2). The fusion expression of rROP2186–533 was validated in cell lysates by monoclonal antibody against His-tag. As shown in Fig. 3, a specific band was observed in the developed film. The concentration of purified rROP2186–533 was found to be 6.0 mg/mL.
FIG. 2.
SDS-PAGE analysis of rROP2186–533. Lane M: Prestained protein ladder. Lane 1: Cell lysates from pET-28a in Escherichia coli BL21 strain. Lane 2–7: Cell lysates from pET-28a-ROP2186–533 in E. coli BL21 strain induced with isopropyl-β-D-thiogalactoside (IPTG) for 1–6 h. Lane 8: Purification products of rROP2186–533.
FIG. 3.
Identification of rROP2186–533 by Western blotting probed with anti-His-tag monoclonal antibody. Cell lysates from pET28a-rROP2 in E. coli BL21 before (lane 1) and after (lane 2) IPTG induction. Lane M: Prestained protein ladder.
Assessment of rROP2186–533 for Toxo-antibodies detection
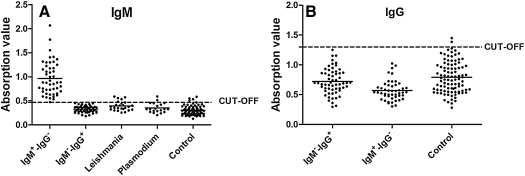
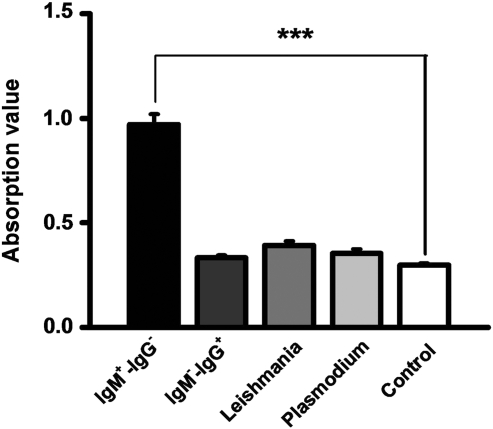
To evaluate the efficacy of rROP2186–533 for the diagnosis of toxoplasmosis, rROP2186–533 was used in ELISA for tests of sera from the suspected patients, who had been prescreened by Toxo-kits commercially available. The cut-off values were 0.469 for Toxo-IgM and 1.3 for Toxo-IgG (Fig. 4), which yielded a sensitivity of 100% for Toxo-IgM with sera of Toxo-IgM+-IgG–and 0% for Toxo-IgG with sera of Toxo -IgM–-IgG+. Cross-reactivity in 4 of 25 (16%) was found in those infected with Leishmania spp. and 2 of 20 (10%) with Plasmodium vivax (Fig. 4). There is a significant difference of OD values of Toxo-IgM between the sera of Toxo-IgM+-IgG–and control (p<0.0001) (Fig. 5). These data showed that the rROP2186–533 could capture specific Toxo-IgM and had a high efficacy for the detection of IgM antibodies against toxoplasmosis.
FIG. 4.
(A) Level of IgM antibodies against rROP2186–533 in sera of patients. (B) Level of IgG antibodies against rROP2186–533 in sera of patients. IgM+-IgG–: Sera of patients with Toxo-IgM antibodies only. IgM–-IgG+: Sera of patients with Toxo-IgG antibodies only. Leishmania: Sera of patients with Leishmania spp. Plasmodium: Sera of patients with tertian malaria. Control: healthy donors.
FIG. 5.
Comparison of the antibody titers of rROP2186–533 in each group. ***p<0.0001 compared with healthy donors. IgM+-IgG–: Sera of patients with Toxo-IgM antibodies only. IgM–-IgG+: Sera of patients with Toxo-IgG antibodies only. Leishmania: Sera of patients with Leishmania spp. Plasmodium: Sera of patients with tertian malaria. Control: healthy donors.
Comparison of rROP2186–533 and WSA in ELISA for diagnosis of toxoplasmosis
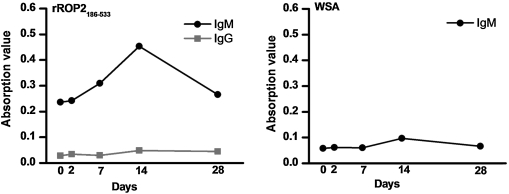
To further prove the results achieved, sera of the mice xinfected with PRU strain were detected with rROP2186–533 and WSA, respectively. The Toxo-IgM antibodies appeared on day seven postinfection (PI), peaked on 14 PI, and rapidly decreased on 28 PI (Fig. 6) when tested with rROP2186–533 antigen. The data showed that the kinetics of Toxo-IgM antibodies response was consistent with that generally accepted (Galvan-Ramirez et al., 2010; Habib et al., 2011). But Toxo-IgG antibodies were not detectable at any time points tested with the rROP2186–533. The results indicated that the rROP2186–533 was a promising candidate of diagnostic antigen exclusively for Toxo-IgM detection and could be superior to WSA in terms of specificity and sensitivity (Fig. 6).
FIG. 6.
The kinetics of IgG and IgM antibodies againt T. gondii with rROP2186–533 and whole soluble antigen of T. gondii in enzyme-linked immunosorbent assay.
Discussion
The detection of Toxo-IgM antibodies still is one of the practical methods for diagnosis of congenital Toxoplasma infection in some laboratories although the PCR-based techniques have been increasingly used. A negative IgM result virtually rules out recent infection unless the sera are tested so early after the acute infection that an antibody response has not yet occurred or is not yet detectable (Liesenfeld et al., 1997). In addition, the majority of studies on Toxo-IgM antibody tests have reported the exceedingly high numbers of false-positive and false-negative results (Verhofstede et al., 1989; Bobic et al., 1991), such as the six most commonly used commercial IgM kits in the United States (Wilson et al., 1997).
This work successfully screened out one antigen (ROP2) recognized by Toxo-IgM. ROP2 is a member of the prominent rhoptry protein family secreted from specialized apical organells of T. gondii during parasite invasion into host cells and participates in the penetration and formation of the parasitophorous vacuole membrane (Beckers et al., 1994). Previous study revealed three potential epitopes of ROP2 (cDNA sequences 197–216, 393–410, and 501–524) recognized by human T cells, the most frequently recognized in proliferation assays being the selected peptides 197 to 216 and 501 to 524 (45% and 36%, respectively) (Saavedra et al., 1996). The rhoptry protein ROP2 has been shown earlier to be a candidate antigen for the establishment of serological assays (van Gelder et al., 1993). Previous studies reported that the rROP2196–561 expression had positive reactions to both Toxo-IgG and -IgM antibodies (Martin et al., 1998). Here we found that the truncated fragment of ROP2186–533 had the capacity to specifically capture Toxo-IgM antibodies. This might be presumed that the reduced fragment with 28 aa from 534 to 561 could be associated with binding to Toxo-IgG antibodies.
The rROP2186–533 fragment, with 348 amino acids covering the three epitopes mentioned above and therefore retained its potential utility as a T-cell repertoire stimulator, was constructed and expressed. To further identify the serodiagnostic value of rROP2186–533, sera from suspected cases with T. gondii, which had been prescreened with commercial kits, were subjected to Toxo-IgM assay. The present results provided the evidence that the efficacy of rROP2186–533 goes in parallel with the Toxo-IgM detection kit commercially available for serodiagnosis of acute acquired and congenital Toxoplasma infection.
Cross-reactivity is an issue in serological diagnosis. In this study, the rROP2186–533 has cross-reaction to some extent with the sera from the patients with leishmaniasis (16%) and malaria (10%). Compared the sequence of rROP2186–533 amino acids with those from leishmanial and malarial parasites, the coverage rate is 5% and 4%, respectively. The rROP2186–533, although truncated, might share some similarity with homologue peptides of these apicomplaxan protozoa. These parasite infections, however, are normally distinguishable by their clinical features.
Conclusions
In summary, Toxo-IgM and -IgG antibodies in the sera of suspected Toxoplasma infection were examined and the antibody kinetics in mice infected with PRU strain of T. gondii with the rROP2186–533 antigen in ELISA were analyzed. The data showed that the rROP2186–533 was an efficient diagnostic antigen for detection of Toxo-IgM antibodies compared with WSA. It could be used as a specific antigen to capture Toxo-IgM antibodies for serodiagnosis of congenital toxoplasmosis and for differentiating between recent and latent infections.
Acknowledgments
This work was funded by the Natural Basic Research Program of China (Grant No. 2010CB530001), the Research Program of Anhui, China (Grant No. 08020303066), and the Hefei Scientific Research Program (Grant No. 2008–1005). We thank Prof. Xia Li, Prof. Jianping Chen, and Prof. Hui Xia for providing serum samples.
Disclosure Statement
No competing financial interests exist.
References
- Beckers CJ. Dubremetz JF. Mercereau-Puijalon O, et al. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol. 1994;127:947–961. doi: 10.1083/jcb.127.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobic B. Sibalic D. Djurkovic-Djakovic O. High levels of IgM antibodies specific for Toxoplasma gondii in pregnancy 12 years after primary toxoplasma infection. Case report. Gynecol Obstet Invest. 1991;31:182–184. doi: 10.1159/000293151. [DOI] [PubMed] [Google Scholar]
- Galvan-Ramirez ML. Madriz Elisondo AL. Rico Torres CP, et al. Frequency of Toxoplasma gondii in pork meat in Ocotlan, Jalisco, Mexico. J Food Prot. 2010;73:1121–1123. doi: 10.4315/0362-028x-73.6.1121. [DOI] [PubMed] [Google Scholar]
- Habib FS. Ali NM. El-Kadery AA, et al. Sequential recognition of antigenic markers of Toxoplasma gondii tachyzoite by pooled sera of mice with experimental toxoplasmosis. Parasitol Res. 2011;108:151–160. doi: 10.1007/s00436-010-2046-0. [DOI] [PubMed] [Google Scholar]
- Liang L. Doskaya M. Juarez S, et al. Identification of potential serodiagnostic and subunit vaccine antigens by antibody profiling of toxoplasmosis cases in Turkey. Mol Cell Proteomics. 2011;10:M110–006916. doi: 10.1074/mcp.M110.006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesenfeld O. Press C. Montoya JG, et al. False-positive results in immunoglobulin M (IgM) Toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J Clin Microbiol. 1997;35:174–178. doi: 10.1128/jcm.35.1.174-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin V. Arcavi M. Santillan G, et al. Detection of human Toxoplasma-specific immunoglobulins A, M, and G with a recombinant Toxoplasma gondii rop2 protein. Clin Diagn Lab Immunol. 1998;5:627–631. doi: 10.1128/cdli.5.5.627-631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JG. Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- Saavedra R. Becerril MA. Dubeaux C, et al. Epitopes recognized by human T lymphocytes in the ROP2 protein antigen of Toxoplasma gondii. Infect Immun. 1996;64:3858–3862. doi: 10.1128/iai.64.9.3858-3862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder P. Bosman F. de Meuter F, et al. Serodiagnosis of toxoplasmosis by using a recombinant form of the 54-kilodalton rhoptry antigen expressed in Escherichia coli. J Clin Microbiol. 1993;31:9–15. doi: 10.1128/jcm.31.1.9-15.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhofstede C. Van Renterghem L. Plum J. Comparison of six commercial enzyme linked immunosorbent assays for detecting IgM antibodies against Toxoplasma gondii. J Clin Pathol. 1989;42:1285–1290. doi: 10.1136/jcp.42.12.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. Remington JS. Clavet C, et al. Evaluation of six commercial kits for detection of human immunoglobulin M antibodies to Toxoplasma gondii. The FDA Toxoplasmosis Ad Hoc Working Group. J Clin Microbiol. 1997;35:3112–3115. doi: 10.1128/jcm.35.12.3112-3115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong ZR. Zhou HB. Li XY, et al. Serological proteome-oriented screening and application of antigens for the diagnosis of Schistosomiasis japonica. Acta Trop. 2010;116:1–8. doi: 10.1016/j.actatropica.2010.04.014. [DOI] [PubMed] [Google Scholar]