Abstract
Purpose
To show the evolution of anterior chamber structures 6 years after cataract surgery in a case with Acanthamoeba keratitis (AK).
Methods
A 37-year-old woman with AK receiving long-term treatment with chlorhexidine, propamidine isethionate and steroids developed a white cataract and iris atrophy. Penetrating keratoplasty and cataract surgery were performed with subsequent intraocular pressure elevation requiring Molteno shunt implantation. Two years after the last surgery, endothelial decompensation developed and another penetrating keratoplasty was performed. Intraoperatively, the anterior and posterior capsules were completely transparent.
Results
Six years after cataract surgery, the intraocular lens was centered with clear anterior and posterior capsules without lens epithelial cells proliferation. No Soemmering's ring formation or posterior capsule opacification was found. Also, no zonular damage or pseudophacodonesis was observed.
Conclusions
This case suggests that AK infection and AK treatment not only cause white progressive cataract but also lens epithelial cell death. The capsules may be completely clear 6 years after cataract surgery, with a good quality of vision regardless of intraocular lens material or design.
Key words: Acanthamoeba keratitis, Lens epithelial cells, Penetrating keratoplasty, Glaucoma, Cataract, Iris atrophy
Introduction
Acanthamoeba keratitis (AK) is often misdiagnosed initially because of specific clinical signs in the early stage that mimic other types of corneal infection. Claerhout et al. [1] suggested that a diagnostic delay of more than 18 days between symptom onset and the start of antiamoebic treatment results in poor final visual acuity (VA). Recently, Chew et al. [2] found that after AK, 24% of patients rapidly developed mature cataracts and 6% suffered from iris atrophy. In addition, different reports on the toxicity of AK treatments have been published [1, 3]. For example, Ehlers and Hjortdal [3] reported 2 cases with a white cataract, secondary glaucoma, and iris atrophy after prolonged treatment with 0.02% chlorhexidine and 0.1% propamidine isethionate. Cataract, iris atrophy, and ischemic ocular inflammation are associated with chlorhexidine use [3, 4] or topical polyhexamethylene biguanide 0.02% [2] in patients with AK. Cationic compounds such as chlorhexidine can disrupt the lens surface, provoke lenticular oxidative or osmotic stress, and contribute to cataract formation by altering lipid membranes, damaging lens fibers, and inducing electrolyte imbalances [5]. Chew et al. [2] suggested that the presence of chlorhexidine or polyhexamethylene biguanide combined with the reactive inflammation in patients with severe stromal disease led to the decompensation of the lens capsule and subsequent rapid progression of mature cataracts.
We describe a patient with AK and adverse effects such as white cataract, glaucoma, and iris atrophy. The patient underwent phacoemulsification and intraocular lens (IOL) implantation, glaucoma drainage implant surgery, and penetrating keratoplasty (PKP). After 6 years of follow-up, no capsular opacification or zonular damage was observed, and the lens epithelial cells (LECs) were apparently destroyed. To our knowledge, no previous similar cases have been published.
Case Report
A 37-year-old woman with −6.0 diopters of myopia in the right eye wore a hydrogel contact lens. In August 2004, she reported intense ocular pain with punctate keratitis. The patient visited several doctors and finally, 2 months later, AK was diagnosed using confocal microscopy. Subsequently, a treatment with 0.02% chlorhexidine drops (three times/day), propamidine isethionate (Brolene; Rhône-Poulenc Rorer, Eastbourne, UK) (three times/day), and 0.25% fluorometholone drops (FML-Forte; Allergan, Irvine, Calif., USA) (twice/day) was started in October 2004.
In June 2005, the patient was referred to our department for PKP. The patient continued the treatment, and a white cataract, conjunctival hyperemia, corneal abscess, and iris atrophy developed (fig. 1). The best-corrected VA (BCVA) was light perception (3 logMar units) and the intraocular pressure (IOP) measured by pneumotonometry was 23 mm Hg. In July 2005, uneventful combined PKP and open sky cataract extraction was performed. A +14-diopter CZ-70-BD rigid IOL (Alcon Laboratories Inc., Fort Worth, Tex., USA) was implanted in the bag. The BCVA 3 months postoperatively was 0.2 logMar (+1.5–5 × 60°). The fundus was normal with a papilar cup/disc ratio of 0.6. However, the IOP was 25 mm Hg using timolol maleate and 0.15% brimonidine tartrate (Combigan; Allergan, Irvine, Calif., USA) (twice/day). Four months after PKP and cataract surgery, a double-plate Molteno shunt (Molteno Ophthalmic Ltd., Dunedin, New Zealand) was implanted in the superior quadrants, with the tube introduced into the nasal anterior chamber.
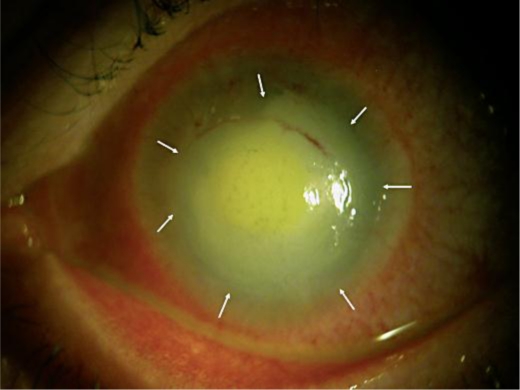
Fig. 1.
White cataract, conjunctival hyperemia, corneal abscess, and iris atrophy (arrows) secondary to AK.
In July 2007, the IOP was 16 mm Hg, but corneal stromal edema with endothelial decompensation developed and a second PKP was performed. A 7-mm-diameter PKP combined with posterior pars plana vitrectomy using a temporary keratoprosthesis was performed. The Molteno implant tube was moved from the anterior chamber to the vitreous cavity in order to increase the distance between the Molteno tube and the corneal button, thereby avoiding endothelial decompensation. During surgery, the anterior and posterior capsules were transparent, with no LEC proliferation or adherence of the capsules (fig. 2). The IOL was moved inside the capsular bag after the corneal button was removed, and no adherence between the capsules and IOL was observed. In July 2009, the BCVA was 0.05 logMar (+1.5–2.5 × 110°) and the corneal graft was clear (1,500 endothelial cells/mm2). The IOP was 17 mm Hg using 0.5% timolol maleate (twice/day) and fluorometholone (three times/day). The IOL was centered with clear anterior and posterior capsules without LEC proliferation (fig. 3). The IOL edge was clear; there was no zonular damage or pseudophacodonesis. The patient wore a brown cosmetic contact lens to treat the photophobia with a similar iris color to that of the left eye.
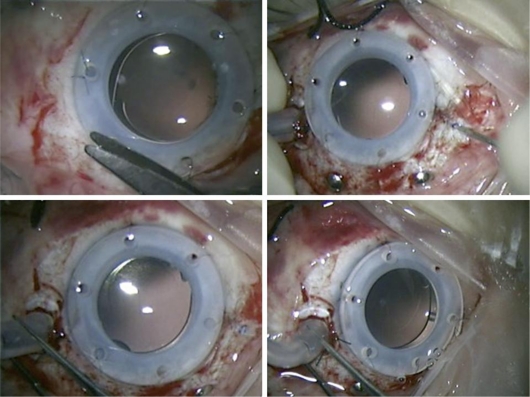
Fig. 2.
Clear capsules observed during vitrectomy using a Landis temporary keratoprosthesis. This procedure was performed 2 years after cataract surgery with IOL implantation.
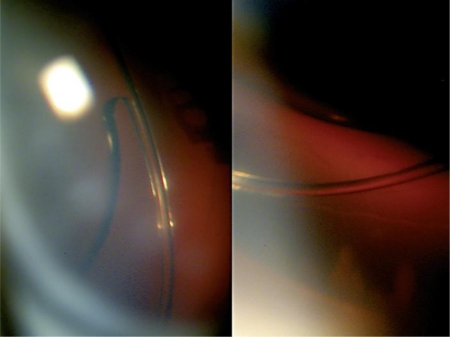
Fig. 3.
Haptic and optic (white arrows) of the CZ-70-BD rigid IOL. No LEC proliferation is observed. The capsules are clear 6 years after cataract surgery. Note the absence of Soemmering's ring formation outside the IOL optic.
Results and Discussion
The current case demonstrates the effects of AK infection and AK treatment on the anterior chamber structures after cataract surgery with a follow-up of 6 years. The clear PKP and the iris atrophy facilitate observation of the IOL, capsular bag, and the zonular fibers. Ehlers and Hjortdal [3] described 2 almost identical cases with similar characteristics, but with a follow-up of 6 months. No effects of AK infection and AK treatment on the LECs have been described. The intention of this report is to point out the apparent death of LECs without posterior capsule opacification (PCO) after 6 years of follow-up in a case with severe AK.
The apparent LEC death in the current case was associated with remarkable characteristics. First, both capsules were totally clear without adhesion between them, which could be important for evaluating the retinal periphery if needed. Second, the IOL haptics did not adhere to the capsules 2 years after cataract surgery (during the second PKP), and the IOL was moved inside the capsular bag without resistance, suggesting the possibility of an easy IOL exchange in late postoperative stages. In addition, the IOL was inside and centered in the capsular bag because the capsulorrhexis diameter was smaller than the diameter of the IOL optic. These factors indicate that the adhesion of the capsules to each other or between the capsules and the IOL may be unnecessary for centering the IOL and maintaining the IOL inside the bag. Although the IOL was large, no folds or wrinkles developed in the posterior capsule because no posterior capsule fibrosis had developed – an important factor in the quality of vision. No Soemmering's ring formation outside the IOL optic was found. No PCO was observed with this rounded-edge optic IOL, suggesting that the LEC destruction was the most important prevention factor in this case. Finally, no zonular damage or pseudophacodonesis was observed; thus, no apparent modification of the zonular fibers occurred in this case.
Indirectly, this case also showed that LEC death may completely prevent PCO. PCO remains the most frequent long-term complication of cataract surgery, with high rates in young adults and children [6]. PCO is caused by collagen deposition, proliferation and migration of residual LECs, epithelial-mesenchymal transition, and lens fiber generation [6]. This case also showed that completely clear capsules are possible 6 years after cataract surgery, with a good quality of vision independent of IOL material or designs. Selective LEC death resulting from administration of cytotoxic pharmacologic agents without endothelial or iris damage is not presently possible, except with the use of devices for protecting other tissues in the anterior chamber. Maloof et al. [7] reported on the Perfect Capsule device (Milvella Ltd., Sydney, N.S.W., Australia) that permits delivery of cytotoxic agents selectively into the capsular bag. Awasthi et al. [6] suggested that targeting residual LECs with therapeutic agents that have minimal or no effect on other ocular tissues is a highly desirable approach for eradicating PCO.
The current case suggests that rapid and progressive mature cataract formation in AK may be related to LEC destruction and PCO prevention 6 years after cataract surgery.
Disclosure Statement
None of the authors has a financial, commercial, or proprietary interest in any instrument or intraocular lens mentioned in this report.
References
- 1.Claerhout I, Goegebuer A, Van Den Broecke C, Kestelyn P. Delay in diagnosis and outcome of Acanthamoeba keratitis. Graefes Arch Clin Exp Ophthalmol. 2004;242:648–653. doi: 10.1007/s00417-003-0805-7. [DOI] [PubMed] [Google Scholar]
- 2.Chew HF, Yildiz EH, Hammersmith KM, Eagle RC, Jr, Rapuano CJ, Laibson PR, Ayres BD, Jin YP, Cohen EJ. Clinical outcomes and prognostic factors associated with acanthamoeba keratitis. Cornea. 2011;30:435–441. doi: 10.1097/ICO.0b013e3181ec905f. [DOI] [PubMed] [Google Scholar]
- 3.Ehlers N, Hjortdal J. Are cataract and iris atrophy toxic complications of medical treatment of acanthamoeba keratitis? Acta Ophthalmol Scand. 2004;82:228–231. doi: 10.1111/j.1600-0420.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- 4.Murthy S, Hawksworth NR, Cree I. Progressive ulcerative keratitis related to the use of topical chlorhexidine gluconate (0.02%) Cornea. 2002;21:237–239. doi: 10.1097/00003226-200203000-00026. [DOI] [PubMed] [Google Scholar]
- 5.Brandt JD. Does benzalkonium chloride cause cataract? Arch Ophthalmol. 2003;121:892–893. doi: 10.1001/archopht.121.6.892. [DOI] [PubMed] [Google Scholar]
- 6.Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol. 2009;127:555–562. doi: 10.1001/archophthalmol.2009.3. [DOI] [PubMed] [Google Scholar]
- 7.Maloof A, Neilson G, Milverton EJ, Pandey SK. Selective and specific targeting of lens epithelial cells during cataract surgery using sealed-capsule irrigation. J Cataract Refract Surg. 2003;29:1566–1568. doi: 10.1016/s0886-3350(03)00058-0. [DOI] [PubMed] [Google Scholar]