Abstract
Purpose:
To define the etiology of pneumonia, using a battery of serological tests, among patients presenting to physicians’ offices in Cumberland County, Nova Scotia from July 2, 1989 to July 1, 1990.
Methods:
Patients presenting to their physician’s office with symptoms suggestive of pneumonia were invited to participate in the study by completing a questionnaire, having a chest radiograph and providing acute and convalescent phase serum samples. These serum samples were tested for antibodies to Mycoplasma pneumoniae, Coxiella burnetii, Legionella pneumophila, adenovirus, and influenza viruses A and B. Some of the samples were tested for antibodies to Chlamydia pneumoniae.
Results:
Seventy-five of the inception cohort of 203 patients had a chest radiograph compatible with pneumonia, a completed questionnaire and acute and convalescent phase serum samples. There were 39 females and 36 males with a mean age of 41.7 years. Twenty-six (35%) were admitted to hospital. The mortality rate was 3%. Forty-five per cent had a diagnosis made by serology: M pneumoniae, 22 (29%); influenza A virus, five (7%); C burnetii, L pneumophila, adenovirus, two (3%) each.
Conclusions:
While it is not possible to generalize about these findings because of ascertainment bias, the data suggest that M pneumoniae is a common cause of pneumonia presenting to a physician’s office and that mortality is low in this group of patients.
Keywords: Coxiella burnetii, Epidemiology, Legionella, Mycoplasma, Pneumonia
Abstract
Objectif:
Définir l’étiologie de la pneumonie à l’aide d’une batterie de tests sérologiques chez les patients qui se présentèrent dans les cabinets médicaux du comté de Cumberland (Nouvelle-Écosse) entre juillet 1989 et le 1er juillet 1990.
Méthodes:
Des patients se présentant au cabinet de leur médecin avec des symptômes évocateurs de pneumonie ont été invités à participer à l’étude en remplissant un questionnaire, en subissant une radiographie pulmonaire et en fournissant des spécimens sériques durant la phase aiguë et la convalescence. Ces échantillons de sérum ont été analysés à l’égard des anticorps dirigés contre Mycoplasma pneumoniae, Coxiella burnettii, Legionella pneumophila, l’adénovirus et les virus de l’influenza A et B. Certains échantillons ont été analysés à l’égard des anticorps anti-Chlamydia pneumoniae.
Résultats:
Soixante-quinze pour cent de la cohorte d’inception de 203 patients qui présentaient des signes radiologiques de pneumonie ont rempli le questionnaire et soumis des échantillons de sérum durant la phase aiguë et la convalescence. Il y avait 39 femmes et 36 hommes, dont la moyenne d’âge était de 41,7 ans. Vingt-six (35 %) ont été admis à l’hôpital. Le taux de mortalité a été de 3 %. Quarante-cinq pour cent ont vu leur diagnostic confirmé par des analyses sérologiques: M pneumoniae, 22 (29 %); virus de l’influenza 5 (7 %); C burnetii, L pneumophila, adénovirus, 2 (3 %) chacun.
Conclusions:
Bien que l’on ne puisse généraliser, nos données suggèrent que M pneumoniae est une cause fréquente de pneumonie parmi les patients qui se présentent chez leur médecin et le taux de mortalité est faible dans ce groupe de patients.
Most studies of community-acquired pneumonia have dealt with the various aspects of this illness in patients requiring admission to hospital (1–17). There are very few studies which report on pneumonia as it presents to the physician’s office (18,19). The investigation of a presumed outbreak of pneumonia in a community in Nova Scotia gave us an opportunity to observe the features of pneumonia as it presents to the physician’s office.
MATERIALS AND METHODS
Background:
During the first week of July 1989, four seriously ill patients were transferred from Highland View Regional Hospital in Cumberland County, Nova Scotia to the Victoria General Hospital in Halifax. The initial diagnosis was pneumonia. One patient was eventually proven to have Mycoplasma pneumoniae pneumonia; a second patient had adenovirus myocarditis; a third had pulmonary emboli; and the fourth had viral myositis. These cases prompted an investigation of a possible outbreak of pneumonia by the Medical Officer of Health. Physicians in Cumberland County were asked to obtain chest radiographs and acute and convalescent serum samples on patients who had a clinical illness compatible with pneumonia (fever and cough). Nurses from the Department of Health and Fitness administered a questionnaire to patients who agreed to participate in the study. By the end of October 1989 it was apparent that there was no longer a need for the Department of Health intervention in that there was not a threat to public health. The Department of Health continued to collect data until December 31, 1989. From January 1, 1990 to June 30, 1990 physicians were asked to continue the study as a research project. Informed consent was obtained from the patients.
Case finding:
Family physicians identified 203 patients on the basis of clinical symptoms. If an opacity was present on chest radiograph, the radiologist notified a public health nurse, who contacted the patient to ensure that serum samples were collected and asked the patient to complete a questionnaire regarding his or her illness.
Each chest radiograph was reviewed independently by one of the investigators. Seventy-five patients had a chest radiograph compatible with pneumonia, paired serum samples and a completed questionnaire.
Serological testing:
The serum samples were tested for antibodies to Legionella pneumophila serogroup 1; M pneumoniae; Coxiella burnetii; and adenovirus. Samples collected during the period November 1 to March 31 were tested for antibodies to influenza A and B and 88 of the serum samples were tested for antibodies to Chlamydia pneumoniae. An indirect immunofluorescence test was used to detect antibodies to L pneumophila (20). All of the reagents used for this test were kindly supplied by the Centers for Disease Control (Atlanta). The legionella antigens were ether-killed suspensions of organisms grown on artificial medium and suspended in normal yolk sac in phosphate buffered saline. Serum specimens were screened at dilutions of 1:64 and 1:128. If fluorescence was present at the 1:128 dilution, further dilutions of this sample were prepared. A fourfold rise in antibody titre or a stable titre of 1:256 or greater was considered to be a positive result (21).
Antibodies to C burnetii were determined by an indirect microimmunofluorescence test with use of phase I and II antigens of strain Nine Mile (obtained from Dr JC Williams, Maryland). Antibodies to the remaining antigens were determined by use of a standard complement-fixation technique in microtitre plates. The adenovirus antigen was purchased from Flow Laboratories (Virgina); influenza A and B, and M pneumoniae antigens were from MA Bioproducts (Maryland). Antibodies to C pneumoniae were determined by Dr JT Grayston, University of Washington, Seattle, as previously described (22).
A fourfold rise or drop in antibody titre was considered to be a positive response. In addition, stable M pneumoniae titres of 1:512 or greater were considered to be positive.
A positive test for C pneumoniae was defined as a fourfold rise in immunoglobulin (Ig) G antibody or an IgM titre of 1:16 or greater.
RESULTS
During the study period July 2, 1989 to July 1, 1990, 203 patients who were considered by their physicians to have met the case definitions provided acute and convalescent serum samples. One hundred and seventy-one (84%) patients had a chest radiograph compatible with pneumonia. The 75 patients who had a chest radiograph compatible with pneumonia, acute and convalescent serum samples, and a completed questionnaire formed the basis of this study. There were 39 females and 36 males with a mean age of 41.7 years. Three (4%) died. The age distribution and the number in each age group who were hospitalized is shown in Table 1. The rate of hospitalization varied with age: 11 of 52 (21%) who were 65 years of age or less were hospitalized, compared with 15 of 23 (65%) of those who were more than 65 years old (P<0.0003). Overall, 26 of 75 (35%) were hospitalized. The symptoms that these patients complained of are listed in Table 2. Cough was the most common symptom followed by fatigue and fever. It is noteworthy that not all patients had cough and fever – features that were part of the case definition circulated to physicians. However, these symptoms represent those reported by the patients when the questionnaire was completed. Over half (55%) had another family member who was ill when pneumonia was diagnosed.
TABLE 1.
Number and percentage of 75 patients with pneumonia in each age group and number and percentage in that age group requiring hospitalization
| Age group (years) | Number (%) | Number hospitalized (%) |
|---|---|---|
| ≤5 | 2 (3) | 0 |
| 6–10 | 15 (20) | 1 (6) |
| 11–16 | 3 (4) | 1 (33) |
| 17–35 | 18 (24) | 2 (11) |
| 36–50 | 10 (13) | 5 (50) |
| 51–5 | 4 (5) | 2 (50) |
| >65 | 23 (31) | 15 (65) |
TABLE 2.
Symptoms of 75 patients with community-acquired pneumonia
| Symptom | Number with this symptom (%) |
|---|---|
| Cough | 66 (86) |
| Fatigue | 64 (85) |
| Fever | 56 (75) |
| Chills | 46 (61) |
| Chest pain | 45 (60) |
| Sweats | 41 (55) |
| Headache | 40 (53) |
| Myalgia | 40 (53) |
| Sore throat | 36 (48) |
| Nausea | 20 (27) |
| Vomiting | 18 (24) |
| Diarrhea | 13 (17) |
| Earache | 11 (15) |
| Rash | 2 (3) |
The entire inception cohort of 203 patients comprised 107 (53%) males and 95 (47%) females. The mean age was 47.4 years and 81 (40%) were hospitalized.
The antibiotics used to treat the pneumonia were: erythromycin, 45 (61%); penicillin/ampicillin, 11 (15%); cephalosporin, eight (11%); ciprofloxacin, eight (11%); tetracycline, five (7%); aminoglycoside, one (1.3%).
Information was obtained from 141 of 203 (70%) regarding antibiotic therapy. These included: erythromycin, 82 of 141 (58%); cephalosporins, 26 (18%); ampicillin/amoxicillin, 15 (11%); ciprofloxacin, eight (6%); tetracyclines, eight (6%); penicillin, one (0.7%); cloxacillin, one (0.7%); and aminoglycoside, one (0.7%).
Forty-five per cent of patients had an etiological diagnosis determined by serological studies (Table 3). M pneumoniae accounted for the majority of cases of unknown etiology: 22 of 34 (65%).
TABLE 3.
Causes of pneumonia in 75 patients with community-acquired pneumonia
| Agent | Number (%) |
|---|---|
| Mycoplasma pneumoniae | 22 (29) |
| Influenza A | 5 (7) |
| Coxiella burnetii | 2 (3) |
| Legionella pneumophila | 2 (3) |
| Adenovirus | 2 (3) |
| Chlamydia pneumoniae* | 1 of 19 |
| Total | 34 (45%) |
Only 19 of 75 patients had serum tested for antibodies to C pneumoniae
The results from testing all 203 serum pairs were: M pneumoniae, 33 (16%); C pneumoniae, seven of 88 (8%); influenza A, seven (3.4%); C burnetii, five (2.4%); L pneumophila, four (1.9%); adenovirus, three (1.4%); influenza B, one (0.5%).
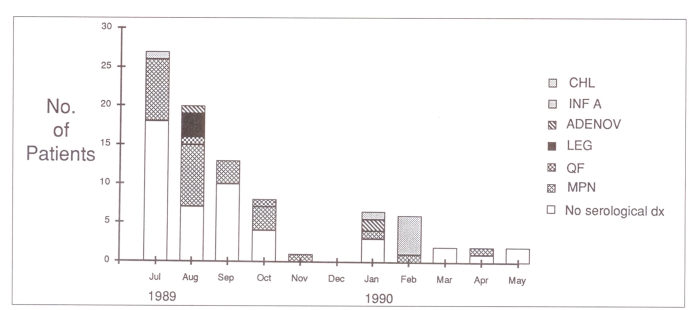
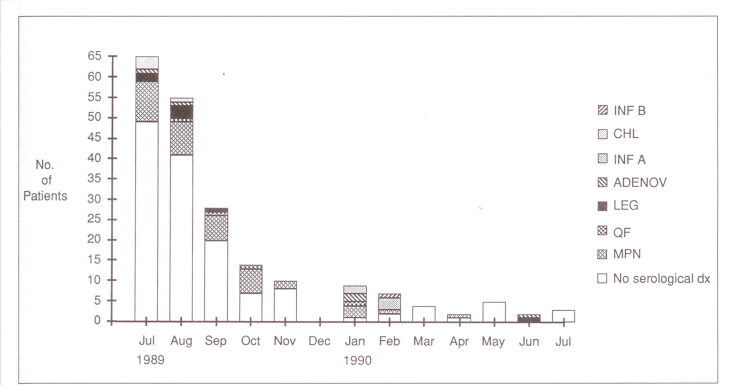
Figure 1 shows the monthly distribution of the 75 patients with pneumonia. Figure 2 gives the monthly distribution of the 203 patients who had acute and convalescent serum samples tested. Most of the patients were entered into the study from July to October, when there was a concern that an outbreak of pneumonia might be in progress.
Figure 1.
Month of onset of pneumonia for 75 patients. The number of patients with pneumonia due to each of the indicated agents is also shown. Chl Chlamydia pneumoniae; Inf A Influenza A virus; Adenov Adenovirus; Leg Legionella pneumophila; QF Coxiella burnetii – Q fever; MPN Mycoplasma pneumoniae; dx Diagnosis; the clear space represents the number of patients who had no antibody rise to the agents indicated above
Figure 2.
Month of onset of illness for 203 patients who had acute and convalescent serum samples collected. Chl Chlamydia pneumoniae; Inf A Influenza A virus; Adenov Adenovirus; Leg Legionella pneumophila; QF Coxiella burnetii – Q fever; MPN Mycoplasma pneumoniae; dx Diagnosis; the clear space represents the number of patients who had no antibody rise to the agents indicated above
DISCUSSION
The results of this study are not generalizable because of ascertainment bias. Only 75 of the 203 patients had all three elements: chest radiograph, acute and convalescent serum samples, and a completed questionnaire. Conclusions cannot be drawn about the attack rate for pneumonia and whether an outbreak occurred because of incomplete data collection. However, the results show that patients with pneumonia presenting to the physician’s office are considerably different from patients with community-acquired pneumonia who are hospitalized. Indeed, only 35% of the 75 patients were admitted to hospital. In a study of pneumonia in a prepaid medical care group in Seattle, Foy et al (18) found that 17% of those with pneumonia were admitted to hospital. The second major difference between these patients and hospitalized patients with community-acquired pneumonia is the much lower mortality rate: 4%, compared with 21% in our study of hospitalized patients (16). Hospitalization was age-related: 21% of those 65 years of age or less were hospitalized, compared with 65% of those older than 65 years of age. A practitioner-based study in Switzerland found that 14 of 161 (8.7%) patients with pneumonia required hospitalization and the mortality rate was 1.2% (23).
There was a high diagnostic yield of 45% from serological testing. This remains a valid finding no matter how the data are analyzed: 60 of the entire cohort of 203 (29.5%) had a diagnosis made serologically. Even if all the M pneumoniae cases are excluded (assuming they were all part of an outbreak), a diagnosis could be made serologically in 27 of 203 (13.3%). This compares with 39% in Foy’s study (18) and 43% in a study of 161 patients carried out over three years in Sweden (19). The Swiss study (23) found no etiology for the pneumonia in 47% of cases. Streptococcus pneumoniae accounted for 17 (10.5%) cases, Legionella species for three (1.8%), Chlamydia species for nine (5.5%), M pneumoniae for 28 (17%), C burnetii for three (1.8%), influenza for 19 (11.8%) and respiratory viruses other than influenza for seven (4.3%). There were four cases due to other bacteria.
The high rate of M pneumoniae, the 3% rate of L pneumophila and the presence of C pneumoniae suggest that erythromycin should be the antibiotic of choice in treating pneumonia in the physician’s office. It is noteworthy that 61% of the patients in the present study received erythromycin therapy. However, 11% received ciprofloxacin, an antibiotic that is not a first line choice in treating pneumonia (24).
This study demonstrates the necessity for well designed studies of patients with pneumonia presenting to their physician’s office. Several questions need to be answered. What are the causes of pneumonia in this population? Is M pneumoniae the most common cause, as demonstrated in this study? What are the factors that influence the physician to recommend hospitalization? Age seems (probably with attendant comorbidities) to have been one such factor in this study. What is an appropriate diagnostic workup? None of the patients in this study had sputum cultured before prescription of antibiotics. It is not cost-effective to carry out comprehensive serological studies on all patients with pneumonia. The best approach would be to have sentinal practices in each province for this purpose and to make the data available to all physicians on a timely basis.
Acknowledgments
This paper is dedicated to the late Dr A Slomic, Amherst, Nova Scotia, who helped us with the analysis of the chest radiographs. This research was supported by a grant-in-aid from National Health and Welfare, Canada. We thank Dr JT Grayston, University of Washington, Seattle for performing the serological testing for Chlamydia pneumoniae.
REFERENCES
- 1.Bath JCJL, Boissard GPB, Calder MA, Moffat MAJ. Pneumonia in hospital practice in Edinburgh 1960–1962. Br J Dis Chest. 1964;58:1–16. doi: 10.1016/s0007-0971(64)80017-6. [DOI] [PubMed] [Google Scholar]
- 2.Mufson MA, Chang V, Gill V, Wood SC, Romansky MJ, Chanock RM. The role of viruses, mycoplasmas and bacteria in acute pneumonia in civilian adults. Am J Epidemiol. 1967;86:526–44. doi: 10.1093/oxfordjournals.aje.a120763. [DOI] [PubMed] [Google Scholar]
- 3.Fekety FR, Jr, Caldwell J, Gump D, et al. Bacteria, viruses and mycoplasmas in acute pneumonia in adults. Am Rev Respir Dis. 1971;104:499–507. doi: 10.1164/arrd.1971.104.4.499. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan RJ, Jr, Dowdle WR, Marine WM, Hierholzer JD. Adult pneumonia in a general hospital: Etiology and host risk factors. Arch Intern Med. 1972;129:935–42. [PubMed] [Google Scholar]
- 5.Dorff GJ, Rytel MW, Farmer SG, Scanlon G. Etiologies and characteristic features of pneumonia in a municipal hospital. Am J Med Sci. 1973;266:349–58. doi: 10.1097/00000441-197311000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Garb JL, Brown RB, Garb JR, Tuthill RW. Differences in etiology of pneumonias in nursing home and community patients. JAMA. 1978;240:2169–72. [PubMed] [Google Scholar]
- 7.White RJ, Blainey AD, Harrison KJ, Clarke SKR. Causes of pneumonia presenting to a district general hospital. Thorax. 1981;36:566–70. doi: 10.1136/thx.36.8.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNabb WR, Shanson DC, Williams TDM, Lant AF. Adult community-acquired pneumonia in central London. J R Soc Med. 1984;77:550–5. doi: 10.1177/014107688407700705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacFarlane JT, Finch RG, Ward MJ, Macrae AD. Hospital study of adult community-acquired pneumonia. Lancet. 1982;ii:255–8. doi: 10.1016/s0140-6736(82)90334-8. [DOI] [PubMed] [Google Scholar]
- 10.Kerttula Y, Leinonen M, Koskela M, Makela PH. The etiology of pneumonia. Application of bacterial serology and basic laboratory methods. J Infect. 1987;14:21–30. doi: 10.1016/s0163-4453(87)90730-4. [DOI] [PubMed] [Google Scholar]
- 11.Larsen RA, Jacobson JA. Diagnosis of community-acquired pneumonia: Experience at a community hospital. Compr Ther. 1984;10:20–5. [PubMed] [Google Scholar]
- 12.Research Committee of the British Thoracic Society and the Public Health Laboratory Service Community-acquired pneumonia in adults in British hospitals in 1982–1983: A survey of aetiology, mortality, prognostic factors and outcome. Q J Med. 1989;62:195–220. [PubMed] [Google Scholar]
- 13.Mohamed ARE, Price Evans DA. The spectrum of pneumonia in 1983 at the Riyadh Armed Forces Hospital. J Infect. 1987;14:31–7. doi: 10.1016/s0163-4453(87)90756-0. [DOI] [PubMed] [Google Scholar]
- 14.Holmberg H. Aetiology of community-acquired pneumonia in hospital treated patients. Scand J Infect Dis. 1987;19:491–501. doi: 10.3109/00365548709032413. [DOI] [PubMed] [Google Scholar]
- 15.Levy M, Dromer F, Brion N, Leturdu F, Carbon C. Community-acquired pneumonia. Importance of initial noninvasive bacteriologic and radiographic investigations. Chest. 1988;92:43–8. doi: 10.1378/chest.93.1.43. [DOI] [PubMed] [Google Scholar]
- 16.Marrie TJ, Durant H, Yates L. Community-acquired pneumonia requiring hospitalization: 5-year prospective study. Rev Infect Dis. 1989;11:586–99. doi: 10.1093/clinids/11.4.586. [DOI] [PubMed] [Google Scholar]
- 17.Fang GD, Fine M, Orliff J, et al. New and emerging etiologies for community-acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases. Medicine (Baltimore) 1990;69:307–16. doi: 10.1097/00005792-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Foy HM, Wentworth B, Kenny GE, Kloeck JM, Grayston JT. Pneumococcal isolations from patients with pneumonia and control subjects in a prepaid medical care group. Am Rev Respir Dis. 1975;111:595–603. doi: 10.1164/arrd.1975.111.5.595. [DOI] [PubMed] [Google Scholar]
- 19.Berntsson E, Lagergard T, Strannegard O, Trollfors B. Etiology of community-acquired pneumonia in outpatients. Eur J Clin Microbiol. 1986;5:446–7. doi: 10.1007/BF02075702. [DOI] [PubMed] [Google Scholar]
- 20.McDade JE, Shepard CC, Fraser DW, Tsai TR, Redus MA, Dowdle WR, the Laboratory Investigative Team Legionnaires’ disease. Isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 21.Cordes LG, Fraser DW. Legionnaires’ disease; Pontiac Fever. Med Clin North Am. 1980;64:395–416. doi: 10.1016/s0025-7125(16)31600-5. [DOI] [PubMed] [Google Scholar]
- 22.Marrie TJ, Grayston JT, Wang S-P, Kuo C-C. Pneumonia associated with the TWAR strain of chlamydia. Ann Intern Med. 1987;106:507–11. doi: 10.7326/0003-4819-106-4-507. [DOI] [PubMed] [Google Scholar]
- 23.Erard PH, Moser F, Wenger A, Saghafi L, Bille J, Francioli P. Prospective study on community-acquired pneumonia diagnosed and followed up by private practitioners. Abstracts of the 1991 Interscience Conference on Antimicrobial Agents and Chemotherapy Abstract; p. 108. [Google Scholar]
- 24.Hooper DC, Wolfson JS. Fluoroquinolone antibiotics. N Engl J Med. 1991;324:384–94. doi: 10.1056/NEJM199102073240606. [DOI] [PubMed] [Google Scholar]