Abstract
A case of primary pneumococcal lung abscess in a five-year-old child is described. Secondary anaerobic infection as a cause of cavitation was excluded by bronchoscopic culture of the cavity. Streptococcus pneumoniae is a rare but recognized cause of lung abscess in healthy children.
Keywords: Lung abscess, Pneumococcus
RÉSUMÉ
Un cas d’abcès pulmonaire pneumococcique primaire observé chez un enfant de cinq ans est décrit ici. L’infection anaérobique secondaire comme cause d’abcès a pu être écartée par une culture bronchoscopique de la cavité. Streptococcus pneumoniae est une cause rare mais reconnue d’abcès pulmonaire chez les enfants par ailleurs sains.
Streptococcus pneumoniae was a rare but recognized cause of primary lung abscess in the pre-antibiotic era (1). Now Staphylococcus aureus and polymicrobic anaerobic bacteria are the predominant causes of lung abscesses, and S pneumoniae is not usually considered in the differential diagnosis of this condition (2–5). When it occurs, primary S pneumoniae lung abscess is viewed as an unusual complication of severe or untreated pneumococcal disease in immunocompromised patients (4–6). Otherwise, lung abscess formation associated with pneumococcal pneumonia is ascribed to secondary infection with anaerobic bacteria (7). This report documents a healthy immunocompetent child with primary lung abscess due to S pneumoniae following a mild respiratory illness.
PATIENT DESCRIPTION
A five-year-old Caucasian girl was admitted in January 1991 to the Children’s Hospital in Winnipeg, Manitoba after three weeks of nocturnal and early morning coughing spells following a febrile respiratory illness in December 1990. The cough was productive but sputum was swallowed and not examined. She had associated anorexia, lethargy and a 2.3 kg weight loss. She was previously well without underlying cardiovascular, neurological or respiratory disease and she had no history of skin, soft tissue, respiratory or gastrointestinal infections suggesting abnormal humoral or cell mediated immunity. There was no known tuberculosis exposure nor history compatible with foreign body aspiration. The family had no pets nor had there been recent travel outside the province of Manitoba. She had not received any antibiotic therapy prior to hospital admission. There was no family history of hemaglobinopathy or disorders of complement or immunoglobulins, nor was there any family history of recurrent illness consistent with any known states of inherited or acquired immunodeficiency.
Upon physical examination, the child was quiet and pale, in no obvious distress, with a temperature of 36°C, respiratory rate of 24/min and a heart rate of 112 beats/min. Height was 117 cm and weight was 21.5 kg (95th and 90th percentile, resectively, for the patient’s age). The patient had normal dentition without obvious dental carries or periodontitis. Bronchovesicular breath sounds were heard over the right posterior upper lung zone, otherwise, the examination of the chest was unrevealing. No other abnormalities were detected on physical examination.
Results of a complete blood count were: leukocyte count of 24.8×109/L with 64% mature neutrophils, no immature granulocytes, 29% lymphocytes, and 6% monocytes; hemoglobin of 106 g/L; and platelet count of 949×109/L. White blood cell morphology was normal. No sputum was available for evaluation. Mantoux skin testing for tuberculosis was negative while an energy screen consisting of intradermal skin testing to trychophyton, mumps and Candida antigen was reactive. The immunoglobulin levels were: IgG, 1470 mg/dL (normal range 800 to 1800 mg/dL), IgA, 232 mg/dL (normal range 90 to 450 mg/dL); and IgM, 337 mg/dL (normal range 60 to 280 mg/dL). Complement levels were: C3, 151 mg/dL (normal range 55 to 120 mg/dL); and C4, 53 mg/dL (normal range 20 to 50 mg/dL). These findings are consistent with a normal response to acute infection.
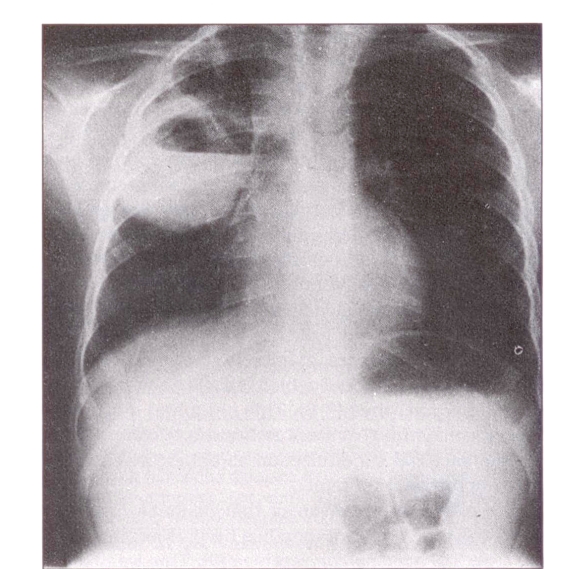
A large abscess in the right upper lobe with an air-fluid level was seen on her chest radiograph (Figure 1).
Figure 1.
Chest radiograph at diagnosis
Prior to antibiotic therapy, bronchoscopy was performed. No foreign body or endobronchial lesions were seen. A large amount of non-foul smelling purulent material was suctioned from the abscess cavity. Specimens were transported immediately under anaerobic conditions to the laboratory for culture. Anaerobic cultures were planted within 15 to 30 mins after the collection of specimen. Moderate numbers of neutrophils and Gram-positive diplococci were seen by Gram stain and S pneumoniae type 14 was isolated in pure culture. The organism was sensitive to oxacillin, erythromycin, tetracycline and vancomycin by the Kirby Bauer disc diffusion method. The minimum inhibitory concentration to penicillin was 0.08 mg/L. Anaerobic, fungal and mycobacterial cultures were negative. Blood cultures for aerobic and anaebrobic organisms were negative.
The patient was placed on intravenous penicillin G for seven days with dramatic improvement in her constitutional symptoms and reduction in the abscess size on the chest radiograph. She was continued on oral penicillin V 50 mg/kg/day until there was complete resolution of x-ray changes (four weeks).
DISCUSSION
Primary lung abscess is a well-recognized but uncommon problem in children. Unfortunately, studies of primary lung abscess are retrospective case series only, which makes the interpretation of the role of S pneumoniae difficult. Mark et al (4) examined 25 cases of lung abscess in children collected over 20 years. The patients had aerobic ‘pharyngeal cultures’ performed and 13 of 25 had bronchoscopic abscess aspiration for aerobic bacterial cultures. S aureus was identified in 11 of 25, Haemophilus influenzae type b in three, Streptococcus viridans in one, mixed aerobic organisms in five and S pneumoniae in only two. Unfortunately, the source of each culture was not noted and anaerobic cultures were not performed.
Recent controversy has emerged in the literature examining adult patients with lung abscesses. Debate has centred on whether most of the uncommon ‘pneumococcal’ lung abscesses are actually anaerobic lung infections occurring post pneumococcal pneumonia (7). Leatherman et al (7) reviewed 24 cases of bacteremic pneumococcal pneumonia and found four cases of cavitation. Three were associated with putrid sputum and poor dentition, two showed pulmonary gangrene, and one patient taken to bronchoscopy grew Bacteroides fragilis. They criticized previous studies attributing cavitation in pneumococcal pneumonia to S pneumoniae because of lack of anaerobic cultures, and cited experimental animal models which have failed to demonstrate cavitation. They postulated that infection with pneumococcus creates a microenvironment with a lowered redox potential which allows the growth of aspirated anaerobea (7–8). This child had a primary S pneumoniae lung abscess and not a secondary anaerobic bacterial superinfection as anaerobic cultures were appropriately obtained.
The causative organism of this patient’s lung abscess was type 14. Cavitation has been associated with the type 3 S pneumoniae serotype (9). This was based on post mortem studies which are of limited value in establishing the etiology of infection (10). A recent review of cavitating pneumonia by Yanco (1) does not show a predominance of type 3 S pneumoniae.
This patient was young, previously healthy and immunocompetent in marked contrast to the usual clinical presentation in adult patients who are usually alcoholic or elderly individuals, often with prior lung disease (1,7)
Mark et al (4) found that fever, anorexia and malaise occurred in all their pediatric cases of lung abscess regardless of etiology. Significant cough occurred in 16 of 25. This patient had all these symptoms, however, they are not pathognomonic for lung abscess and a chest radiograph was necessary for diagnosis. The nocturnal nature of her cough and the right upper lobe location of the abscess suggests that the patient had airway irritation from postural drainage of the abscess which freely communicated with the bronchus on bronchoscopy.
Traditionally, many lung abscesses in children are treated empirically with antistaphylococcal or anaerobic agents. Given the sensitivity of S pneumoniae in this case to oxacillin, this patient likely would have responded to this empiric therapy. Indeed, one wonders how many empirically treated lung abscesses are actually pneumococcal.
In summary, this case illustrates that primary pneumococcal lung abscess occurs in immunocompetent pediatric patients. There were no clinical findings which would have led to the diagnosis of pneumococcal abscess without collection of abscess material at bronchoscopy. In retrospect, the lack of specific risk factors for other known etiologies of lung abscesses was significant.
REFERENCES
- 1.Yanco BG, Deresinski SC. Necrotizing or cavitating pneumonia due to Streptococcus pneumoniae: Report of four cases and review of the literature. Medicine. 1980;59:449–57. doi: 10.1097/00005792-198011000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Brook I, Finegold SM. Bacteriology and therapy of lung abscess in children. J Pediatr. 1979;94:10–2. doi: 10.1016/s0022-3476(79)80341-8. [DOI] [PubMed] [Google Scholar]
- 3.Mark PH, Turner JA. Lung abscess in childhood. Thorax. 1968;23:216–20. doi: 10.1136/thx.23.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan K, Weinstein L. Abscess of the lung. In: Feigin RD, Cherry JD, editors. Textbook of Pediatric Infectious Diseases. 2nd edn. Philadelphia: Saunders; 1987. p. 354. [Google Scholar]
- 5.Ashner MI, Spier S, Coates AL, et al. Primary lung abscess in childhood: The long term outcome of conservative management. Am J Dis Child. 1982;136:491–4. doi: 10.1001/archpedi.1982.03970420015002. [DOI] [PubMed] [Google Scholar]
- 6.Purdy GD, Cullen M, Yedlin S, et al. An unusual neonatal case presentation: Streptococcus pneumoniae pneumonia with abscess and pneumatocele formation. J Perinatol. 1987;7:378–81. [PubMed] [Google Scholar]
- 7.Leatherman JW, Iber C, Davies SF. Cavitation in bacteremic pneumococcal pneumonia. Am Rev Respir Dis. 1984;129:317–21. [PubMed] [Google Scholar]
- 8.Gorbach SL, Bartlett JG. Anaerobic infections. N Engl J Med. 1974;290:1177–84. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- 9.Finland M, Sutlift WD. Infections with pneumococcus type III and type VIII: Characterization of pneumonia caused by pneumococcus type III and that associated with a biologically closely related organism pneumococcus type VIII. Arch Intern Med. 1934;53:481–94. [Google Scholar]
- 10.Wilson WR, Dolan CT, Washington JA, II, et al. Clinical significance of postmortem cultures. Arch Pathol. 1972;94:245. [PubMed] [Google Scholar]