Abstract
A case-control study was undertaken to identify and quantify antimicrobial and nonantimicrobial drug risk factors associated with a sustained outbreak of Clostridium difficile diarrhea on two medical (teaching and nonteaching) units and an oncology unit. In total, 80 cases associated with an endemic clone of toxigenic C difficile were compared with controls. Eighty controls were selected from a group of 290 controls randomly chosen from the outbreak period. The controls were matched to cases according to age, admitting diagnosis and unit of admission. Seventy (88%) patients in the case group received at least one antibiotic before diarrhea, compared with 37 (46%) patients in the control group. Major risk factors implicated in the development of C difficile diarrhea in hospitalized patients were the following antimicrobial agents: ceftazidime (adjusted odds ratio [aor]=26.01, 95% ci 5.67 to 119.19, P=0.0001); cefuroxime (aor=5.17, ci 1.86 to 14.36, P=0.005); ciprofloxacin (aor=3.81, ci 1.05 to 13.79, P=0.04); and clindamycin (aor=15.16, ci 2.93 to 78.44, P=0.004). This is the first time that the use of ciprofloxacin has been linked to the development of C difficile diarrhea. Use of gastrointestinal drugs (ranitidine, famotidine, cimetidine, omeprazole and sucralfate) was also an added risk (aor=3.20, ci 1.39 to 7.34, P=0.01); however, antineoplastic therapy was not significant (P<0.53). Recognition of the specific high risk drugs may spur more restricted use of these agents, which may help in controlling C difficile diarrhea in hospitalized patients.
Keywords: Clostridium difficile diarrhea, Logistic regression, Risk factors, Selected drugs
RÉSUMÉ :
Une étude de cas a été entreprise afin d’identifier et de mesurer les facteurs de risque liés aux médicaments, antimicrobiens et autres, associés à une épidémie soutenue de diarrhée à Clostridium difficile dans deux unités médicates (d’enseignement et non d’enseignement) et dans une unité d’oncologie. En tout, 80 cas associés à un clone endémique de C. difficile toxigénique ont été comparés avec des témoins. Quatre-vingt témoins ont été choisis à partir d’un groupe de 290 témoins sélectionnés au hasard durant la période de 1’épidémie. Les témoins ont été assortis aux cas selon l’âge, selon le diagnostic à l’admission et l’unité d’hospitalisation. Soixante-dix patients (88 %) du groupe atteint ont reçu au moins un antibiotique avant de présenter de la diarrhée, contre 37 patients (46 %) du groupe-témoin. Les facteurs de risque d’importance impliqués dans les épisodes de diarrhée à C. difficile chez les patients hospitalisés étaient les agents antimicrobiens suivants : ceftazidime (risque relatif ajusté [rra] = 26,01, 95 % ic 5,67 à 119,19, P=0,0001); céfuroxine (rra = 5,17, ic 1,86 à 14,36, P=0,005), ciprofloxacine (rra = 3,81, ic 1,05 à 13,79, P=0,04) et clindamycine (rra = 15,16 ic 2,93 à 78,44, P=0,004). C’est la première fois que le recours à la ciprofloxacine est associé à l‘installation de diarrhée à C. difficile. L’utilisation de médicaments gastro-intestinaux (ranitidine, famotidine, cimétidine, oméprazole et sucralfate) a également correspondu à un risque accru (rra = 3,20, ic 1,39 à 7,34, P=0,01). Toutefois, le traitement antinéoplasique ne s’est pas révélé significatif (P<0,53). La reconnaissance des médicaments spécifiques liés à un risque élevé pourrait entraîner un usage plus restrictif de ces agents, qui contribuera à maîtriser la diarrhée à C. difficile chez les patients hospitalisés.
Clostridium difficile is a major cause of gastrointestinal infections associated with a wide spectrum of clinical manifestations, from asymptomatic intestinal carriage to mild self-limited diarrhea to severe, potentially fatal pseudomembranous colitis (1). The diagnosis of C difficile diarrhea rests on the detection of the specific cytotoxin in stool filtrates and/or the demonstration of the toxigenic organism in culture, accompanied by the clinical symptoms of diarrhea (2).
Antibiotic use has been implicated in the pathogenesis of C difficile disease, especially colitis (1). The role of prior antibiotic use has been clinically recognized (3) and confirmed by laboratory studies involving antibiotic-treated hamsters (4,5). The association between the use of clindamycin and C difficile diarrhea is well known (3,6–9). Other antimicrobial agents (eg, amoxicillin, cephalosporins, cotrimazole, aminoglycosides, vancomycin and neomycin) have been linked to C difficile diarrhea (10–16). As a result of the frequent use of broad spectrum antimicrobial agents, C difficile diarrhea has emerged as a major nosocomial problem (10,12,13,15,17–20). The role of nonantimicrobial drugs, however, has not been clearly delineated (12–14,21,22).
Since early 1990 there has been an apparent increase in the number of hospitalized patients with diarrhea due to toxigenic C difficile in our institution. While cases were initially sporadic in various nursing units of the hospital, increases in the frequency of cases have subsequently been documented in the medical and oncology service areas. Using the numerical analysis of whole cell protein patterns, we have deduced the epidemiology of an evolving C difficile outbreak in our institution (23). We have also observed the presistent transmission of a toxigenic clone of C difficile among our in-patients, predominantly on four units (24). In the present investigation we have undertaken a controlled study to identify and quantify specific antimicrobial and nonantimicrobial drugs predisposing patients to C difficile diarrhea.
PATIENTS AND METHODS
Case definition:
Henderson General Hospital is a 465-bed tertiary care university-affiliated teaching hospital primarily serving adults. The hospital has an out-patient cancer clinic on campus and houses two oncology (medical and hematological) nursing units for cancer patients from the region. The hospital also has an active medical teaching unit. Demographic characterisitics of the patient population on the medical and oncology units have not changed over the past 10 years. Laboratory-based surveillance of infections is routinely performed hospital-wide. This routine surveillance, initiated before the C difficile outbreak, involves an ongoing, systematic recording, analysis and interpretation of accurate microbiological results essential to the planning, implementation and evaluation of the infection control practice, and is closely integrated with the timely dissemination of these data to the infection control committee.
The endemic spread of a toxigenic clone of C difficile (electrophoretic type 1) in the hospital units has been previously reported (24). There has been a marked increase in electrophoretic type 1 cases since October 1991. There was an apparent decrease in the number of new cases beyond May 1993, which was the peak month during this outbreak. The present case-control investigation covers a period of 20 months, from October 1991 through May 1993. C difficile diarrhea cases were evaluated based on the medical charts and microbiolgical results of stool examinations undertaken on in-patients. A C difficile diarrhea case included the following criteria: first, an adult patient hospitalized for more than three days; second, a patient identified by a health care provider as having at least five episodes of diarrhea in 24 h for three or more days; third, a patient with loose stools in which cytotoxin (C difficile toxin B) and enterotoxin (C difficile toxin A) were detected; and fourth, toxigenic C difficile was the only enteric pathogen isolated as the causative agent of diarrhea.
The date of the first detection of toxin or isolation of toxigenic C difficile was considered to be the date of disease onset because it was often impossible to determine the onset of diarrhea from a retrospective review of the patient chart. Therapy for suspected C difficile diarrhea or colitis included discontinuation of any antimicrobial agents and gastrointestinal drugs used before the onset of diarrhea and institution of oral metronidazole. There were no follow-up sigmoidoscopic or colonoscopic examinations performed on elderly inpatients with C difficile diarrhea.
Case-control study:
A retrospective case-control analysis was undertaken using 80 cases associated with the endemic type 1 C difficile. The cases were chosen from a sampling frame of 170 patients hospital-wide. The cases were on a medical teaching unit, a nonteaching medical unit and a hematological oncology unit, and were in the age group of 58 to 88 years (mean age ±15). Two hundred and ninety controls without a previous history of fecal toxin detection and/or toxigenic C difficile isolation, and a negative stool culture of C difficile and its toxins between October 1, 1991 and May 31, 1993 were randomly selected from all adult patients admitted to these three units. Eighty of these controls were matched to the cases according to age, admitting diagnosis and unit of admission.
The patient charts and laboratory-based surveillance records were reviewed, and the following information was recorded for both cases and controls: age; sex; diagnosis on admission; dates of admission and discharge; and date of first detection of C difficile toxin or first isolation of toxigenic C difficile (in cases). Pharmacy records were reviewed to examine the medication profiles of the cases and matched controls. The names and dates of antimicrobial agents prescribed before and after the onset of diarrhea; antineoplastic drugs; and other medications known to affect the gastrointestinal system (H2-blockers, antacids, cytoprotective agents and laxatives) were recorded. Antimicrobial agents used in fewer than four (5%) cases compared were excluded from the analyses. Antineoplastic drugs analysed were: cytarabine, cyclophosphamide, doxorubicin, vincristine and etoposide. The gastrointestinal drugs included in the analyses were: ranitidine, famotidine, cimetidine, omeprazole, alginic acid, sucralfate and aluminium/magnesium salts. No other gastro-intestinal agents were studied. For the case group, all medications given six weeks before the date of onset of diarrhea were recorded. For the control group, all medications and the length of treatment during the patient’s stay on the affected unit were recorded.
Statistical analyses:
All statistical analyses were performed using SAS statistical software (version 6.07; SAS Institute, Inc, North Carolina). Univariate analysis was performed initially and the variables were subsequently entered in the stepwise logistic function regression (slfr) model. Demographic and exposure variables were coded as dichotomous (0 ‘absent’ and 1 ‘present’) variables. Crude odds ratios (cor) and ci for odds ratios were calculated by the standard methods. Fisher’s exact test was used to measure the statistical significance. For the slfr analysis, variables were entered into and removed from the model according to the following selection process. First, the adjusted χ2 statistic for all the variables not yet in the model were analyzed. The variable corresponding to the most significant χ2 statistic was entered into the model if its significance level was less than 0.4. Second, a variable was removed from the model if it was the least significant variable in the model or the level of significance of the maximum likelihood estimate was greater than 0.2. The selection process terminated if more variables could not be entered by the forward selection process or if the last variable entered into the model was the only variable subsequently removed, cors from the univariate analysis and adjusted odds ratios from the slfr model were considered significant if the 95% ci did not include 1.0.
RESULTS
Description of the outbreak:
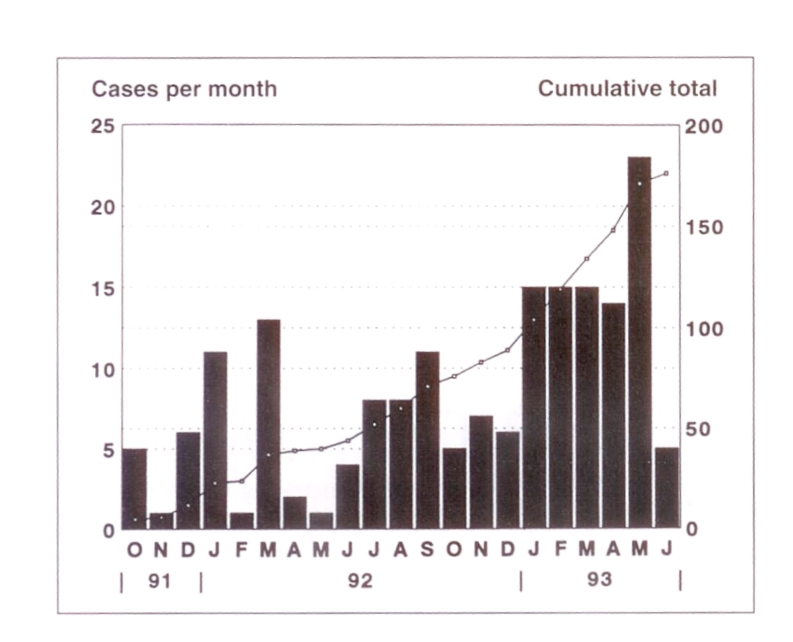
An epidemic curve representing the monthly number of new C difficile cases and the cumulative total over the period from October 1991 through June 1993 is shown in Figure 1. A total of 170 cases of C difficile diarrhea were identified during the study period from October 1991 through May 1993, which was the sampling frame for the selection of cases in the present investigation.
Figure 1.
Epidemic curve of a sustained outbreak of Clostridium difficile infection. Cases occurred on 13 of 24 nursing units in the hospital. More than 65% of cases occurred on a medical teaching unit, a nonteaching medical unit and a hematological oncology unit
The hospital-wide attack rate increased from 0.15/100 discharges in 1991 to 1.36/100 discharges in 1993. The earliest recorded incidence rate, in 1988, was 0.013. Cases were identified from 13 of 24 units in the hospital over the study period. Three units had only one patient each with C difficile. Among the remaining 10 units were six where at least two cases were identified within four weeks of each other. A sharp increase in attack rate was recorded for the medical teaching and nonteaching medical units and a hematological oncology unit. The increase was most dramatic in the first five months of 1993, with a rate of 6.09 for the nonteaching medical unit; 2.49 for the medical teaching unit; and 1.81 for the hematological oncology unit. To ensure that this outbreak was not simply a laboratory phenomenon, it was documented that the laboratory procedures of toxin assays or microbiological identification during the period of this study were unchanged.
Before October 1991, the cases were sporadic in nature on seven units in the hospital, as described previously (23). An initial outbreak of diarrhea with a cluster of nine cases on the medical teaching unit within a period of eight weeks was believed to have started on November 20, 1991. From this time, subsequent cases were identified more frequently on the same unit. Once established on this unit, toxigenic C difficile spread to cause further secondary outbreaks successively through a hematological oncology unit, a nonteaching medical unit and, eventually, the intesive care unit (24).
Case-control comparisons:
To determine the risk factors for C difficile diarrhea, a case-control investigation was conducted focusing on patients housed on the three most frequently affected units: a medical teaching unit, a nonteaching medical unit and a hematological oncology unit. The in-patients selected for the case group had the following diagnoses on admission: 24 patients had pneumonia, 19 hematological malignancy, 14 carcinoma, 13 heart disease, five diabetes (cellulitis) and five urinary tract infection. Matching was performed based on age, admitting diagnosis and unit of admission. There were 23 oncology patients with secondary pneumonia or line-related sepsis in the case group who were matched to 23 oncology patients with similar secondary infections in the control group. The cases and matched controls were distributed throughout the outbreak period of October 1991 through May 1993. The cases and controls were similar with respect to mean age (cases, 72.6; controls, 70.9), and male:female ratio (cases, 1:1.05; controls, 1:0.66). There were no significant differences in numbers of previous admissions, length of stay at the present admission and recent surgical intervention (P>0.05). No diarrhea or any other symptomatic C difficile infection was recorded for patients in the control group either on admission or during stay in the hospital.
A more intensive comparison of recent medications administered was performed. Seventy (88%) patients in the case group received at least one antibiotic, compared with 37 (46%) in the control group. Twenty-one cases received one antibiotic; 30 cases received two antibiotics; 16 cases received three antibiotics; and three cases received four or more antibiotics, compared with nine, 22, six and none, respectively, for the controls (Mantel-Haenszel χ2=23.975, P<0.0001).
Table 1 lists 11 variables tested by univariate analysis. Case patients were more likely than controls to have been exposed to ceftazidime, cefuroxime and clindamycin. In the univariate analysis of cases and matched controls, significant risk factors identified were: ceftazidime (cor=10.36, 95% ci 2.97 to 36.18), cefuroxime (cor=2.22, 95% ci 1.10 to 4.48) and clindamycin (cor= 15.74, 95% ci 3.57 to 69.46). Antineoplastic therapy using cytarabine, cyclophosphamide, doxorubicin, vincristine and etoposide was not a significant risk factor (P<0.53). However, there was a significant difference between cases and controls in the use of gastrointestinal drugs (ranitidine, famotidine, cimetidine, omeprazole and sucralfate) (cor=2.64, 95% ci 1.39 to 5.00). The gastrointestinal drugs alginic acid and aluminium/magnesium salt relaxatives were associated with only four cases and three controls.
TABLE 1.
Drugs used in cases and matched controls and relative risks in the univariate analysis and SLFR model
| Variable | Cases (n=80) | Controls (n=80) | Crude odds ratio (95% Cl) | P* | Adjusted odds ratio (95% Cl) | P adjusted |
|---|---|---|---|---|---|---|
| Clindamycin | 23 | 2 | 15.74 (3.57–69.46) | <0.0001 | 15.16 (2.93–78.44) | 0.004 |
| Ceftazidime | 23 | 3 | 10.36 (2.97–36.18) | <0.0001 | 26.01 (5.67–119.19) | 0.0001 |
| Cefuroxime | 30 | 17 | 2.22 (1.10–4.48) | 0.037 | 5.17 (1.86–14.36) | 0.005 |
| Vancomycin | 9 | 3 | 3.25 (0.85–12.50) | 0.13 | –† | – |
| Piperacillin | 6 | 8 | 0.73 (0.24–2.21) | 0.78 | – | – |
| Ampicillin | 6 | 4 | 1.54 (0.42–5.68) | 0.75 | 4.04 (0.88–18.62) | 0.07 |
| Aminoglycosides | 20 | 15 | 1.44 (0.68–3.08) | 0.45 | – | – |
| Erythromycin | 12 | 14 | 0.83 (0.36–1.93) | 0.83 | 0.22 (0.06–0.75) | 0.02 |
| Ciprofloxacin | 12 | 5 | 2.65 (0.89–7.90) | 0.12 | 3.81 (1.05–13.79) | 0.04 |
| Antineoplastics | 7 | 4 | 1.82 (0.51–6.49) | 0.53 | – | – |
| Gastrointestinal drugs | 51 | 32 | 2.64 (1.39–5.00) | 0.004 | 3.20 (1.39–7.34) | 0.01 |
Measured by Fisher’s exact method;
Considered for entry but not retained in the stepwise logistic function regression (SLFR) model
All 11 variables were considered for entry into the slfr model; however, the following four variables were removed from the model: vancomycin, piperacillin, aminoglycosides and antineoplastic drugs. Ceftazidime, clindamycin and cefuroxime remained significant risk factors in descending order of significance (Table 1). Ceftazidime had an adjusted odds ratio (aor) differing substantially from the cor. Ceftazidime had increased odds ratio of 26.01 with 95% ci 5.67 to 119.19 in the slfr model. The odds ratio of cefuroxime was also substantially increased (aor=5.17, 95% ci 1.86 to 14.36). In the slfr model, the use of ciprofloxacin was found to be an additional risk factor (aor=3.81, 95% ci 1.05 to 13.79). Use of gastrointestinal drugs (ranitidine, famotidine, cimetidine, omeprazole and sucralfate) also remained a significant risk factor in the slfr model (aor=3.20, 95% ci 1.39 to 7.34). None of the other variables entered in the multivariate logistic regression analysis reached statistical significance.
DISCUSSION
We have previously reported an evolving C difficile outbreak in our institution by tracing strains using numerical analysis of sds-page protein patterns (23). We have also previously identified a toxigenic C difficile clone from the majority of our patients and the environment. The same toxigenic clone has been persistently transmitted for 25 months among the patients on a medical teaching unit, a nonteaching medical unit, a hematological oncology unit and the intensive care unit (24). In the present study, we have identified and quantified specific antimicrobial and nonantimicrobial drug risk factors that may have contributed to the development of C difficile diarrhea during the sustained outbreak.
Antibiotic exposure has been recognized to be of primary importance in the pathogenesis of C difficile infections (1,3–7). In this study, antibiotic exposure was significantly higher among the cases. The case patients received antibiotic combinations more frequently than controls. Other published reports of case-control studies of C difficile outbreaks have also shown a significant risk of C difficile infection associated with multiple antibiotics (11–15). Four antibiotics were found to be specifically associated with C difficile diarrhea in this patient population. The major risk factors were the use of ceftazidime and cefuroxime, in addition to clindamycin, in both the univariate and the slfr model, a finding also reported by others (9,14–16). Among the moderate to high level use antibiotics, use of ciprofloxacin was shown to be an added risk by the regression analysis. The contribution of ciprofloxacin to the development of C difficile diarrhea has not been demonstrated before this study. Although this study involved a considerable proportion of oncology patients, it did not reveal the use of antineoplastic drugs (cytarabine, cyclophosphamide, doxorubicin, vincristine and etoposide) to be a significant risk factor. However, the cases received significantly more gastrointestinal drugs (ranitidine, famotidine, cimetidine, omeprazole and sucralfate) than controls. An earlier report demonstrated the use of ranitidine and cimetidine to be a risk factor for disease (14).
Our study may have certain limitations. We did not reveal the risk associated with numbers of previous admissions or length of stay. Similarly, we did not investigate the risk associated with malignancy and previous gastrointestinal disease or intervention, which has been studied by others (14,15). We have not looked at the overall severity of underlying illness (comorbidity or acute physiology and chronic health evaluation [apache] II score) of cases and controls. The role of these variables and other intrinsic patient characteristics in the development and nosocomial transmission of C difficile disease could not be established in our institution. The study findings about medications contributing to the development of C difficile diarrhea may also be limited by the fact that our institution has a structured antibiotic formulary containing a single antibiotic for each antibiotic class. Thus, the impact of antibiotics, the specific gastrointestinal drugs and antineoplastic agents other than those maintained on the formulary could not be assessed.
CONCLUSIONS
This case-control study revealed that ceftazidime and cefuroxime were highly associated with C difficile diarrhea. As anticipated, clindamycin was a significant risk factor. The slfr model, which considered all variables for entry, indicated that ciprofloxacin in combination with other high risk agents (eg, gastrointestinal drugs) may be linked to C difficile diarrhea in our in-patients. We noted that specific second- and third-generation cephalosporins (cefuroxime and ceftazidime) were more likely associated than other cephalosporins with this infection; these data have not been previously demonstrated (16). In addition, this is the first time that the use of ciprofloxacin has been implicated in the development of C difficile diarrhea. The gastrointestinal drugs (ranitidine, famotidine, cimetidine, omeprazole and sucralfate) were also implicated in the development of C difficile diarrhea.
C difficile diarrhea continues to be a major nosocomial problem. Efforts to contain hospital epidemics of this infection by improving infection control policies have met with variable success. The evaluation and alteration of antibiotic prescribing practices have been proved to be more effective in decreasing the risk of infection (9). Similar studies on larger scales are war-ranted so that the strategies to curtail the use of high risk drugs to reduce nosocomial C difficile diarrhea can be tested. Practices such as mandatory infectious diseases consultation before antibiotic use and structured antibiotic order forms may have an impact on reducing this infection.
Acknowledgments
The authors thank Bernadette Kucera, infection control coordinator, Henderson Hospital, for assistance in collating patient and clinical data.
REFERENCES
- 1.Tedesco FJ. Pseudomembranous colitis: pathogenesis and therapy. Med Clin North Am. 1982;66:655–64. doi: 10.1016/s0025-7125(16)31413-4. [DOI] [PubMed] [Google Scholar]
- 2.Peterson LR, Kelly PJ. The role of the clinical microbiology laboratory in the management of Clostridium difficile-associated diarrhea. Infect Dis Clin North Am. 1993;7:277–94. [PubMed] [Google Scholar]
- 3.Tedesco J, Barton RW, Alpers DH. Clindamycin-associated colitis. Ann Intern Med. 1974;81:429–33. doi: 10.7326/0003-4819-81-4-429. [DOI] [PubMed] [Google Scholar]
- 4.Rifkin GD, Fekety FR, Silva J. Antibiotic-induced colitis: implications of a toxin neutralized by Clostridium sordelli antitoxin. Lancet. 1977;ii:1103. doi: 10.1016/s0140-6736(77)90547-5. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett JG, Onderdonk AB, Cisceros RL, Kasper DL. Clindamycin-associated colitis due to toxin producing species of Clostridium in hamsters. J Infect Dis. 1977;136:701–5. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- 6.Fekety R. Antibiotic-associated colitis. In: Mandell GL, Douglas RG, Bennett JE, editors. Principles and Practice of Infectious Diseases. 3rd edn. New York: Churchill Livingstone; 1990. pp. 863–969. [Google Scholar]
- 7.Lyerly DM, Krivan HC, Wilkins TD. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988;1:1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarland LV, Schwartz CM, Stamm WE. Risk factors for C difficile carriage and C difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis. 1990;162:678–84. doi: 10.1093/infdis/162.3.678. [DOI] [PubMed] [Google Scholar]
- 9.Pear SM, Williamson TH, Bettin KM, Gerding DN, Galgiani JN. Decrease in nosocomial Clostridium difficile-associated diarrhea by restricting clindamycin use. Ann Intern Med. 1994;120:272–7. doi: 10.7326/0003-4819-120-4-199402150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Hall SM, Calver GP, William M. A hospital outbreak of Clostridium difficile? J Hosp Infect. 1985;6:312–22. [PubMed] [Google Scholar]
- 11.Pierce PF, Wilson R, Silva J, et al. Antibiotic-associated pseudomembranous colitis: an epidemiologic investigation of a cluster of cases. J Infect Dis. 1982;145:269–74. doi: 10.1093/infdis/145.2.269. [DOI] [PubMed] [Google Scholar]
- 12.Gerding DN, Olson MM, Peterson LR, et al. Clostridium difficile-associated diarrhea and colitis in adults: a prospective case-controlled epidemiologic study. Arch Intern Med. 1986;146:95–100. [PubMed] [Google Scholar]
- 13.Zimmerman RK. Risk factors for Clostridium difficile cytotoxin-positive diarrhea after control for horizontal transmission. Infect Control Hosp Epidemiol. 1991;12:96–100. doi: 10.1086/646294. [DOI] [PubMed] [Google Scholar]
- 14.Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11:283–90. doi: 10.1086/646173. [DOI] [PubMed] [Google Scholar]
- 15.Thibault A, Millar MA, Gaese C. Risk factors for the development of Clostridium difficile-associated diarrhea during a hospital outbreak. Infect Control Hosp Epidemiol. 1991;12:345–8. doi: 10.1086/646354. [DOI] [PubMed] [Google Scholar]
- 16.Nelson DE, Auerbach SD, Baltch AL, et al. Epidemic Clostridium difficile-associated diarrhea: role of second- and third-generation cephalosporins. Infect Control Hosp Epidemiol. 1994;15:88–94. doi: 10.1086/646867. [DOI] [PubMed] [Google Scholar]
- 17.Wust J, Sullivan NM, Hardegger V, Wilkins TD. Investigation of an outbreak of antibiotic-associated colitis by various typing methods. J Clin Microbiol. 1982;16:1096–101. doi: 10.1128/jcm.16.6.1096-1101.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolan NPM, Kelly CP, Humphreys JFH, et al. An epidemic of pseudomembranous colitis: importance of person-to-person spread. Gut. 1987;28:1467–73. doi: 10.1136/gut.28.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heard SR, O’Farrell S, Holland D, Crook S, Barnett MJ, Tabaqchali S. The epidemiology of Clostridium difficile with use of a typing scheme: nosocomial acquisition and cross-infection among immunocompromised patients. J Infect Dis. 1986;153:159–62. doi: 10.1093/infdis/153.1.159. [DOI] [PubMed] [Google Scholar]
- 20.Savage AM, Alford RH. Nosocomial spread of C difficile. Infect Control. 1983;4:31–3. doi: 10.1017/s0195941700057623. [DOI] [PubMed] [Google Scholar]
- 21.Cudmore HA, Silva J, Fekety R, et al. Clostridium difficile colitis associated with cancer chemotherapy. Arch Intern Med. 1982;142:333–5. [PubMed] [Google Scholar]
- 22.Trynka YM, LaMont JT. Association of Clostridium difficile toxin with symptomatic relapse of chronic inflammatory bowel disease. Gastroenterology. 1981;80:693–6. [PubMed] [Google Scholar]
- 23.Costas M, Holmes B, On SLW, Ganner M, Kelly MC, Nath SK. Investigation of an outbreak of Clostridium difficile infection in a general hospital by numerical analysis of SDS-PAGE protein patterns. J Clin Microbiol. 1994;32:759–65. doi: 10.1128/jcm.32.3.759-765.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nath SK, Thornley JH, Kelly M, et al. A sustained outbreak of Clostridium diffcile in a general hospital: persistance of a toxigenic clone in four units. Infect Control Hosp Epidemiol. 1994;15:382–9. doi: 10.1086/646935. [DOI] [PubMed] [Google Scholar]