Abstract
A case of disseminated infection due to Nocardia otitidiscaviarum is described in a Caucasian man infected with the human immunodeficiency virus. The patient presented with no previous AIDS-defining conditions, a CD4 lymphocyte count of 206 cells/mm3 and enlarging intra-abdominal and chest wall abscesses with bilateral pulmonary infiltrates. Aggressive surgical debridement and antimicrobial therapy with trimethoprim/sulfamethoxazole and amikacin resulted in clinical cure. Long term suppressive therapy was needed to prevent relapse.
Keywords: AIDS, Human immunodeficiency virus, Nocardia otitidiscaviarum
RÉSUMÉ :
Un cas d’infection disséminée à Nocardia otitidiscaviarum est décrit chez un homme de race blanche infecté au virus de l’immunodéficience humaine. Le patient s’est présenté sans maladie antérieure liée au diagnostic du sida, une numération des lymphocytes CD4 à 206 cellules/mm3 et des abcès intra-abdominaux et thoraciques en progression avec infiltrats pulmonaires bilatéraux. Un débridement chirurgical énergique et un traitement antibiotique par triméthoprime/sulfaméthoxazole et amikacine ont amené une guérison clinique. Le traitement suppressif prolongé a été nécessaire pour prévenir une rechute.
Disseminated and pulmonary nocardiosis is increasingly reported as an opportunistic infection in patients with advanced human immunodeficiency virus (HIV) disease, but it is not considered an AIDS-defining condition. Often presenting as an indolent chronic infection, diagnosis may be delayed or missed. Most case reports and reviews discuss Nocardia asteroides or Nocardia brasiliensis as the etiological agent in nocardiosis in patients with advanced HIV infection (1–3). This report describes a patient with disseminated infection due to Nocardia otitidiscaviarum.
CASE PRESENTATION
A 59-year-old Caucasian man, diagnosed as HIV-1 positive two years prior, previously asymptomatic and antiretroviral naive, presented in July 1995 with a three-week history of weight loss, fever, night sweats, dyspnea, productive cough and an enlarging mass in his left upper abdominal quadrant and left chest wall. The patient’s chest had sustained no local penetrating trauma. He had returned from the west coast of Africa, where he had resided since 1989, because of his illness.
Past medical history included smoking, past malaria, remote alcohol abuse and shingles in 1990. There was no history of tuberculosis, positive tubercullin skin tests, syphilis, gonorrhea, chlamydia, malignancies or hepatitis. His HIV risk factors included heterosexual contacts in Africa.
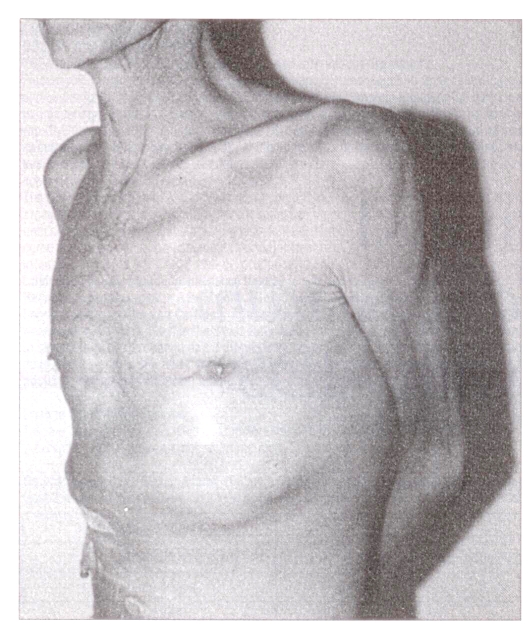
Physical examination revealed a thin cachectic man, hemodynamically stable with a low grade fever. Pertinent findings included oral thrush and a large protuberant mass over his left upper abdominal quadrant and left chest wall which was tender to palpation and fluctuant (Figure 1). Chest auscultation revealed coarse crepitations bilaterally at the bases, with dullness to percussion of the left base. Large bilateral nontender mobile axillary lymph nodes were palpable. Neurological examination was normal.
Figure 1.
Clinical photograph of the patient at the time of presentation demonstrating a large mass in the left uppper abdominal quadrant and chest wall
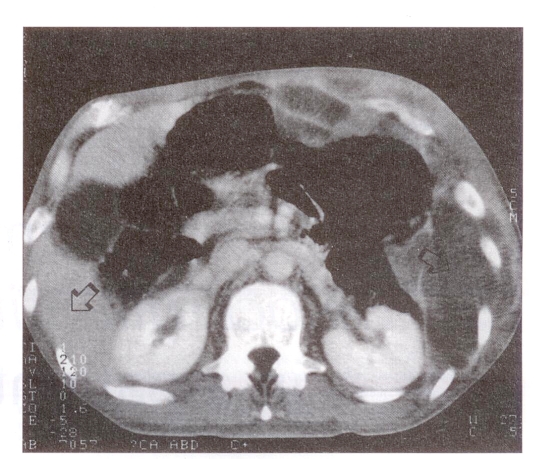
Investigations revealed a white blood cell count of 11.3×109/L, hemoglobin 101 g/L, platelet count 641×109/L and CD4 lymphocyte count 206 cells/mm3. Chest radiograph revealed ill-defined masses in the left lung and a consolidation in the left costophrenic angle and pleura. Faint nodules were also evident in the right lung. Augmented computed tomography of the chest and abdomen revealed a large septated extraperitoneal mass crossing both inside and outside the left thoracic and abdominal wall, and extending down to the iliac crest (Figure 2).
Figure 2.
Augmented computed tomography revealing a large septated intra-abdominal mass
Percutaneous aspiration of the mass produced thick green fluid revealing abundant beaded branching Gram-positive bacilli on Gram stain; these bacteria were also detected in sputum samples. Further identification revealed N otitidiscaviarum.
N otitidiscaviarum was initially identified by Gordon techniques as outlined by Mishra et al (4) and Lechevalier (5). Direct microscopy for nocardial filamentation was done on four-day-old cultures grown in slide cultures on Sabouraud peptone glucose agar and pyruvate-yeast extract agar (6,7), a specialized medium promoting aerial filamentation in aerobic actinomycetous organisms. In addition, cellular fatty acid constituents were assayed with the MIDI gas-liquid chromatography system (Microbial ID Inc, Delaware).
The organism produced acid from arabinose, glucose, glycerol, inositol and galactose, but not adonitol, cellobiose, erythritol, lactose, maltose, mannitol, melezitose, alpha-methyl-D-glucoside, raffinose, rhamnose, sorbitol, trehalose, dulcitol, sucrose, melibiose and inulin. It cleared suspended xanthine solids but not casein, tyrosine or adenine. It was urease positive. Visible orange carotenoid pigments were seen in cultures. Bromersol purple-milk solids-glucose agar was alkalinized without clearing of milk solids. In chromatography of fatty acid methyl esters, the organism possessed the signature tuberculostearic acid distinguishing nocardioforms from Streptomyces species and many other comparable filamentous aerobic actinomycetes (5). The MIDI databases available at the time (August 1995, TSBA Revision 3.80, CLIN Revision 3.80) did not find a match for the fatty acid profile. Species identification was based on Gordon characters, which were unambiguously characteristic of N otitidiscaviarum.
Susceptibility testing was done by the modified Kirby-Bauer disk diffusion methods of Wallace et al (8) and Wallace and Steele (9). Standardized inoculum was prepared by grinding colonies scraped from the surface of Sabouraud peptone-glucose agar plates to a fine slurry in a small amount of sterile distilled water in a glass tissue grinder with sintered pestle and tube interior. The homogenized inoculum was then diluted with reference to a 0.5 McFarland barium sulfate standard and streaked evenly on Mueller-Hinton agar. Control isolate ATCC 19247 was used as recommended by Wallace and Steele (9). Only tests where the control’s inhibition zone size was within the specified range were read for the test organism. Results showed that the organism gave susceptible values for amikacin and trimethoprim/sulfamethoxazole (TMP/SMX), although the zone size for the latter was near the borderline. Resistance was shown to cefotaxime, tobramycin, gentamicin, minocycline and erythromycin. Certain drugs without published breakpoints for nocardiae were tested for taxonomic reasons or to supply a rough estimate of relative responsiveness in case of extreme clinical urgency. The organism was completely resistant to cefamandole and streptomycin (no zone) and had a moderately wide zone size for imipenem (39 mm).
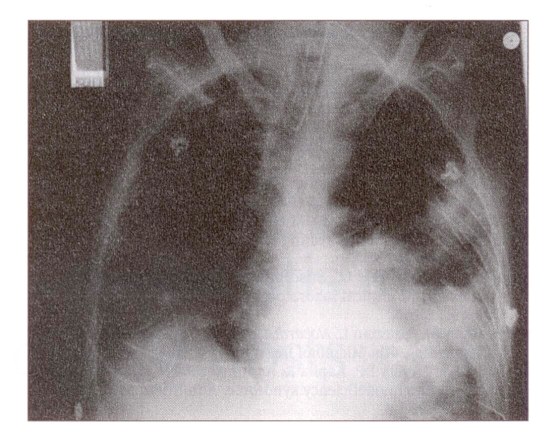
Following surgical drainage and debridement of the abscess, the patient deteriorated with rapid widespread pulmonary involvement (Figure 3) requiring intubation and ventilation in the intensive care unit. Initial medical therapy consisted of TMP/SMX (160 mg TMP, 800 mg SMX) parenterally every 6 h and amikacin 500 mg parenterally every 12 h, for six weeks. Initial treatment also included parenteral cefotaxime. He responded well to treatment and eventually was discharged from the hospital and was initially maitained on TMP/SMX (160 mg TMP, 800 mg SMX) daily. He remained stable, with weight gain, no recurrence of the nocardiosis and a CD4 lymphocyte count of 257 cells/mm3; he was maintained on combination antiretroviral therapy with zidovudine and zalcitabine.
Figure 3.
Chest radiograph of the patient postoperatively demonstrating extensive bilateral pulmonary involvement
DISCUSSION
Nocardiosis in patients with HIV infection is probably under-recognized and not as rare as previously suspected (10). In previous reports of nocardiosis due to N asteroides in AIDS patients, the incidence rate was 1.8% (1), higher than the rate of 0.2% to 0.3% previously reported by the Centers for Disease Control and Prevention, Atlanta, Georgia (11). The majority of pulmonary and extrapulmonary cases of nocardia in AIDS patients have involved N asteroides (1,2,12–15), N brasiliensis (3) or Nocardia farcinica (14,15), and most frequently involved the lung (51.7%) and brain (11.7%) in one series (10). There are six forms of disease recognized in humans: pulmonary; systemic or disseminated; central nervous system; extrapulmonar; cutaneous or lymphocutaneous; and actinomycetomas (10). Large abscesses enlarge by progressive extension of filaments into the tissue and may behave like a bacterial abscess (10). The mortality rate due to nocardiosis was 63% in one series and largely attributed to disseminated infection, relapse of infection and severe immunosuppression (1). N otitidiscaviarum, however, is rarely reported as a cause of infection in patients with AIDS, described in only scattered case reports (14,16,17).
N otitidiscaviarum is a soil saphrophyte with worldwide distribution and an infrequent cause of mycetomas in humans (10). Disseminated infection in humans has been reported in the pre-AIDS era in patients immunocompromised by underlying malignancy or corticosteroids (18).
This report describes a case of disseminated infection due to N otitidiscaviarum according to previously defined criteria (10). Despite the high morbidity and mortality reported in previous series (1), the patient responded well to aggressive surgical drainage and parenteral antibiotics with long term suppressive therapy with TMP/SMX. The patient described had an initial CD4 lymphocyte count of 206 cells/mm3 at presentation, higher than the mean CD4 lymphocyte count of 109 cells/mm3 in the previous series (1). Antimicrobial susceptibility testing of Nocardia species has been problematic because of a lack of standardization in methodology. However, optimal drug selection will depend on antimicrobial susceptibility because of drug resistance patterns and often the need for long term therapy (19). The N otitidiscaviarum isolate in this case was susceptible to amikacin and TMP/SMX.
The optimal therapy for nocardiosis has yet to be determined; however, the use of TMP/SMX is a mainstay of therapy with approximately 80% of patients initially responding in one series (1). The patient described remains on long term suppressive therapy with TMP/SMX, which appears to be necessary to prevent relapse in patients with advanced HIV infection (1,2), and likely protects patients from primary infection with nocardia. Nocardia are opportunistic organisms causing serious infections in HIV-infected patients and are associated with a high mortality. Nocardiosis is likely under-recognized, and consideration should be given to adding this infection to AIDS-defining conditions in adults.
Acknowledgments
Myrna Decastro, Carol Headley and Sergio Dalla Rosa.
REFERENCES
- 1.Uttamchandani RB, Daikos GL, Reyes RR, et al. Nocardiosis in 30 patients with advanced human immunodeficiency virus infection: Clinical features and outcome. Clin Infect Dis. 1994;18:348–53. doi: 10.1093/clinids/18.3.348. [DOI] [PubMed] [Google Scholar]
- 2.Coker RJ, Bignardi G, Horner P, et al. Nocardia infection in AIDS: A clinical and microbiological challenge. J Clin Pathol. 1992;45:821–2. doi: 10.1136/jcp.45.9.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sieratzki HJ. Nocardia brasiliensis infection in patients with AIDS. Clin Infect Dis. 1992;14:977–8. doi: 10.1093/clinids/14.4.977-b. (Lett) [DOI] [PubMed] [Google Scholar]
- 4.Mishra SK, Gordon RE, Barnett DA. Identification of nocardiae and streptomycetes of medical importance. J Clin Microbiol. 1980;11:728–36. doi: 10.1128/jcm.11.6.728-736.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechevalier HA. Nocardioform actinomycetes In: Bergey’s Manual of Systematic Bacteriology. Vol. 4. Baltimore: Williams & Wilkins; 1989. pp. 2384–404. [Google Scholar]
- 6.Bannantyne RM, Small WL, Summerbell RC. Disseminated Nocardia farcinica infection. Clin Microbiol Newsl. 1993;15:70–2. [Google Scholar]
- 7.Saksun JM, Kane J, Schachter RK. Mycetoma caused by Nocardia madurae. Can Med Assoc J. 1978;119:911–4. [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace RJ, Jr, Septimus EJ, Musher DM, Martin RR. Disk diffusion susceptibility testing of Nocardia species. J Infect Dis. 1977;135:568–76. doi: 10.1093/infdis/135.4.568. [DOI] [PubMed] [Google Scholar]
- 9.Wallace RJ, Jr, Steele LC. Susceptibility testing of Nocardia species for the clinical laboratory. Diagn Microbiol Infect Dis. 1988;9:155–66. doi: 10.1016/0732-8893(88)90025-9. [DOI] [PubMed] [Google Scholar]
- 10.Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7:213–64. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holtz HA, Lavery DP, Kapila R. Actinomycetales infection in the acquired immunodeficiency syndrome. Ann Intern Med. 1985;102:203–5. doi: 10.7326/0003-4819-102-2-203. [DOI] [PubMed] [Google Scholar]
- 12.Khorrami P, Heffeman EJ. Pneumonia and meningitis due to Nocardia asteroides in a patient with AIDS. Clin Infect Dis. 1993;17:1084–5. doi: 10.1093/clinids/17.6.1084. (Lett) [DOI] [PubMed] [Google Scholar]
- 13.Garcia del Palacio JI, Perez IM. Response of pulmonary nocardiosis to ceftriaxone in a patient with AIDS. Chest. 1993;103:1925–6. doi: 10.1378/chest.103.6.1925. (Lett) [DOI] [PubMed] [Google Scholar]
- 14.Poonwan N, Kusum M, Mikami Y, et al. Pathogenic nocardia isolated from clinical specimens including those of AIDS patients in Thailand. Eur J Epidemiol. 1995;11:507–12. doi: 10.1007/BF01719301. [DOI] [PubMed] [Google Scholar]
- 15.Long PF. A retrospective study of nocardia infections associated with the acquired immune deficiency syndrome (AIDS) Infection. 1994;22:362–4. doi: 10.1007/BF01715551. (Lett) [DOI] [PubMed] [Google Scholar]
- 16.Castelli L, Zlotnik H, Ponti R, Vidotto V. First reported Nocardia otitidiscaviarum infection in an AIDS patient in Italy. Mycopathologia. 1994;126:131–6. doi: 10.1007/BF01103766. [DOI] [PubMed] [Google Scholar]
- 17.Boiron P, Provost F, Chevrier G, Dupont B. Review of nocardial infections in France 1987 to 1990. Eur J Clin Microbiol Infect Dis. 1992;11:709–13. doi: 10.1007/BF01989975. [DOI] [PubMed] [Google Scholar]
- 18.Arroyo JC, Nichols S, Carroll GF. Disseminated Nocardia caviae infection. Am J Med. 1977;62:409–12. doi: 10.1016/0002-9343(77)90839-7. [DOI] [PubMed] [Google Scholar]
- 19.Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891–905. doi: 10.1093/clinids/22.6.891. [DOI] [PubMed] [Google Scholar]