Abstract
OBJECTIVE:
To compare three sampling methods and to pretest methods for the determination of fecal coliform (FC) counts and Toxocara species from sand in the day care outdoor environment.
DESIGN:
The sand samples were obtained from the play area and the sandbox of a day care centre and examined for the presence of FC and Toxocara species, the common roundworm of dogs and cats. The sampling methods included random selection and two types of judgement methods. The latter included one method where domestic animals were judged to be likely to defecate and the other where children would be likely to be playing. In addition, to obtain a global estimate of contamination, the entire areas of both the sandbox and the play area were sampled on the last day.
SETTING:
Outdoor day care environment.
MAIN RESULTS:
The most representative levels of bacterial contamination and Toxocara species originated from the combined sample of the entire surface areas rather than from any separate random or judgement method of sampling. FCs were found in all sampled areas of the sandbox (median 910 FCs/g of sand) and of the play area (median 350 FCs/g of sand). Toxocara species were recovered from a number of areas in both the sandbox and the play area.
CONCLUSIONS:
Research on environmental microbial contamination of outdoor day care settings would benefit from the application of standardized and validated sampling and laboratory methods.
Keywords: Contamination, Day care centre, Environment, Fecal coliforms, Methodology, Toxocara species
Abstract
OBJECTIF :
Comparer trois méthodes d’échantillonnage et prétester des méthodes pour déterminer le nombre de coliformes fécaux et rechercher les espèces de Toxocara dans le sable d’une cour de garderie.
MODÈLE :
Les échantillons de sable ont été prélevés dans le bac à sable et sur le terrain de jeu d’une garderie et analysés pour une recherche de coliformes fécaux (CF) et des espèces de Toxocara, le nématode commun des chiens et des chats. Les méthodes d’échantillonnage comprenaient une sélection au hasard et deux types d’échantillonnage au jugé, dont une estimant l’endroit où les animaux domestiques iraient probablement déféquer, et l’autre estimant l’endroit où les enfants iraient probablement jouer. De plus, pour obtenir une estimation globale de la contamination, des échantillons ont été prélevés dans tout le bac à sable et dans toutes les aires de jeu le dernier jour.
CONTEXTE :
Terrain de jeu d’une garderie.
PRINCIPAUX RÉSULTATS :
Les niveaux les plus représentatifs de contamination bactérienne et de contamination par les espèces de Toxocara provenaient de l’échantillon combiné prélevé sur les surfaces entières plutôt que d’une quelconque méthode d’échantillonnage distincte par sélection au hasard ou au jugé. On a décelé des CF dans toute les aires échantillonnées du bac à sable (médiane de 910 CF/g de sable) et des aires de jeux (médiane de 350 CF/g de sable). On a prélevé les espèces de Toxocara dans plusieurs endroits du bac à sable et du terrain de jeu.
CONCLUSIONS:
La recherche sur la contamination microbienne environnementale touchant les terrains de jeu et les installations extérieures des garderies bénéficierait de l’application de méthodes d’échantillonnage et de laboratoire validées et normalisées.
It is recognized that children who attend day care centres (DCCs) have a higher incidence of infectious diseases than children who do not attend DCCs (1–4). In particular, toddlers are considered to be the group at most elevated risk because their hygiene skills are not yet fully developed, they are in the ‘oral’ stage of their development and they are increasingly mobile (4–6). Black et al (7) have shown that children under three years of age put their hands or other objects into their mouths every 2 to 3 mins. This normal behaviour sometimes results in exposure to environmental contamination. It has been shown that fecal coliform (FC) contamination present in the indoor day care environment accounts for almost one-third of diarrhea in toddlers (5). These observations highlight the need to understand where and when microbial contamination is highest in the environment surrounding young children in order to initiate appropriate measures of prevention and control. Unfortunately, much remains unknown about the sources of microbial contamination, especially in the outdoor environment.
To date, only two studies have assessed sand- or soil-associated microorganism contamination in the outdoor environment of DCCs (ie, sandboxes and play areas). These studies, carried out in Canada (8) and in France (9), used the presence of Toxocara species as an indicator of domestic animal fecal contamination because they are a zoonosis and, therefore, of public health concern, and because they specifically represent contamination from domestic animal sources (dogs and cats). Both studies showed that this parasite is present in outdoor DCC play areas. Seasonality of contamination may occur, but in sandboxes of three nursery schools in Marseille (France), Toxocara species ova were recovered throughout the year (9). Animal feces can also contain viral (eg, rotavirus) and bacterial microorganisms (eg, Escherichia coli) (10). These microorganisms can remain viable in the environment for some time, especially in fecal matter (11). FC contamination has been reported in sandboxes in parks of the Angers region of France (12) and in lawns and sandboxes of parks in Poland (13). Birds may also be a potential source of contamination because they shed microorganisms in their droppings that can be infectious to humans (10). In Canada, one-third of seagulls in the Montreal area were shown to carry Salmonella species, Listeria monocytogenes and Campylobacter species in their cloacae (14). Transmission of these microorganisms to young children via the outdoor environment is thus possible, but the magnitude of risk remains unknown.
Guidelines regarding the prevention and control of contamination of sand and toys in outdoor DCC playgrounds have been established by public health authorities in Quebec (15), in Canada (16) and in the United States (17). However, these guidelines vary from one authority to another. Moreover, their efficacy and effectiveness have not been evaluated.
Numerous studies have assessed the presence of Toxocara species in sandpits, sandboxes and soil in public parks, kindergardens, schools, gardens and backyards. A comprehensive list of the results of these studies is shown in Table 1. The great variation in results is immediately apparent and highlights several issues. First, identification of Toxocara species from the outdoor environment is recognized internationally as an important indicator of potential pathogenic contamination. Second, there is a lack of documentation from day care centres, settings that may previously have been thought to present little risk of exposure. Third, sampling methodology differs greatly from one study to the next. Lastly, there is a large amount of information missing from the published reports.
TABLE 1.
Reported prevalence of Toxocara species in outdoor environments by country
| Reference | Year | Country | Number of sites and type | Number of samples per site (total) | Prevalence number (%) | |
|---|---|---|---|---|---|---|
| Per site | Per sample | |||||
| 18 | 1984 | Australia | 6 parks | ? (?) | 0 | 0 |
| 19 | 1990 | Australia | 41 sandpits in 30 kindergardens | 2–3 (?) | 0 | 0 |
| 20 | 1994 | Brazil | 39 parks | 5 (195) | 9 (23) | ? |
| 21 | 1976 | Canada | 10 parks | 1–5 | 6 (60) | 14 (33) |
| 33 sandboxes in 10 parks | 7 (18) | |||||
| 22 | 1986 | Canada | 21 playgrounds in parks | ? (510) | 11 parks (5) | 8 (2) |
| 8 | 1994 | Canada | 10 play areas in 10 DCCs | 10 (100) | 2 (20) | ? |
| 23 | 1980 | France | 17 parks | ? | 11 (65) | ? |
| 24 | 1982 | France | 15 sandboxes in 8 parks | 4–11 (58) | 2 (13) in 1 park | 4 (7) |
| 9 | 1986 | France | 13 sandboxes: 10 parks, 3 DCCs | ? | 8 (62):2 DCCs | ? |
| 25 | 1994 | France | 5 sandboxes: 3 parks, 2 kinder-gardens | 10 (50) | 4 (80) | 17 (34) |
| 26 | 1984 | Germany | 31 sandpits: ? | 4–10 (562) | 27 (87) | ? |
| 27 | 1987 | Germany | 18 sandboxes | ? (86) | 4 (22) | 4 (5) |
| 28 | 1990 | Germany | 52 sandpits in playground | 4 (208) | 29 (56) | 51 (25) |
| 29 | 1991 | Ireland | 26 gardens | ? | 10 (38) | ? |
| 17 parks | 2–6 (53) | 2 (12) | 3 (6) | |||
| 30 | 1994 | Ireland | 9 playgrounds | 12–40 (228) | 8 (89) | 35 (15) |
| 31 | 1993 | Japan | 24 sandpits in parks | 5 (120) | 21 (80) | ? |
| 22 sandpits in kindergardens | 5 (110) | 8 (36) | ? | |||
| 32 | 1993 | Japan | 13 sandpits in parks | 5–8 (?) | 12 (92) | ? |
| 33 | 1989 | Jordan | ? schools | ? (86) | ? | 5 (6) |
| ? public places | ? (94) | ? | 7 (8) | |||
| 34 | 1986 | La Réunion* | 13 playgrounds: park and school | 1 | 6 (46) | 6 (46) |
| 35 | 1993 | Netherlands | 27 parks | 6 (162) | ? | 13 (8) |
| ? sandboxes | 2 (61) | ? | 15 (25) | |||
| 36 | 1980 | Scotland | ? parks | ? (234) | ? | 17 (7) |
| 37 | 1989 | Spain | 132 urban park, street | 1 (132) | 6 (5) | 6 (5) |
| 310 rural play areas | 1 (310) | 28 (9) | 28 (9) | |||
| 38 | 1973 | United Kingdom | 10 parks | 40 (400) | 10 (100) | 93 (23) |
| 39 | 1987 | United Kingdom | 5 play areas in 5 parks | Vary (226) | 5 (100) | 147 (65) |
| 5 parks | Vary (277) | 5 (100) | 169 (61) | |||
| 40 | 1991 | United Kingdom | 8 parks | 8–229 (521) | 7 (88) | 33 (6) |
| 41 | 1975 | United States | 2 parks | 42 and 48 (90) | 2 (100) | 26 (29) |
| 42 | 1979 | United States | 23 swing areas in 10 parks | 1 (23) | 4 (17) | 4 (17) |
| 23 sandboxes in 10 parks | 1 (23) | 9 (39) | 9 (39) | |||
| 43 | 1980 | United States | 32 play areas in parks | Vary (285) | 1 (3) | 1 (0.4) |
| 44 | 1984 | United States | 20 parks | ? (1529) | 4 (20) | 6 (0.4) |
| 45 | 1985 | United States | 146 backyards | 3 (438) | 16 (11) | ? |
| 46 | 1988 | United States | 23 play areas in parks | Vary (135) | 11 (48) | 22 (16) |
| 47 | 1989 | United States | 3 parks | 13–53 (114) | 2 (67) | 22 (19) |
| 48 | 1983 | Yugoslavia | 10 parks | 10 (100) | 8 (80) | 27 (27) |
La Réunion is an Overseas French Department (France).
? Indicates that this information is not provided in the publication. DCC Day care centre
The details of the various sampling and laboratory methodologies used in previous studies are shown in Table 2. The types of sampling most frequently used were random, systematic and two types of judgement: one, where children would play, and two, where domestic animals would be expected to defecate (eg, shaded areas, near walls). When reported, the depth and surface from which the sand or soil specimens were sampled and the weight of sample varied extensively. A similar observation was found with respect to laboratory methods used. Missing information combined with the great variation in methods provide insufficient evidence for an accurate assessment of the occurrence and/or intensity of microbial contamination reported in this literature.
TABLE 2.
Methodologies used in the recovery of Toxocara species ova from sand and soil
| Reference | Sampling methods | Laboratory method | |||||
|---|---|---|---|---|---|---|---|
| Type* | Depth (cm) | Surface | Weight (g) | Pretreatment | Flotation | Sieving | |
| 18 | ? | ? | ? | ? | ? | ? | ? |
| 19 | Children | 10 | ? | 250 | ? | ? | ? |
| 20 | ? | 5 | ? | ? | ? | MgSO4 + KI | No |
| 21 | Random | Various | 100 cm2 | 200 | NaCl + water | Brine | No |
| 22 | Children | 1 | 15 cm2 | ? | ? | ZnSO4 + NaOH | No |
| 8 | ? | 12 | ? | 75 | Water | ZnSO4 | No |
| 23 | Animal | ? | ? | 450–2350 | Water | NA | Yes |
| 24 | Random | 8–10 | 3– 4 cm† | 500–600 | Water | NA | Yes |
| 9 | ? | 40 | 3.5 cm† | 250–300 | Water | NaCl | No |
| 25 | Children | 15 | ? | 1000 | Water | KIHg | No |
| 26 | Systematic | 10 | ? | 250–300 | Water + mesh | Saline | No |
| 27 | Animal | 10 | ? | 250 | Hypochlorite sodium | NaCl | No |
| 28 | Systematic | Surface | ? | 1000 | Tween 80 | Sugar | Yes |
| 29 | Children | 2 | 130 cm2 | 250 | None | NaNO3 | No |
| 30 | Random | 1 | 1 m2 | 450 | Tween 80 | NaNO3 | No |
| 31 | Systematic | Upper | 1000 cm2 | ? | Water + mesh | NaNO3 | No |
| 32 | Systematic | 3 | 6 cm† | 100–150 | ? | Sucrose | No |
| 33 | ? | 10 | ? | 250–300 | None | ZnSO4 | No |
| 34 | ? | Surface | ? | 10 | ? | ZnSO4 | No |
| 35 | ? | 5 | ? | 10 | Teepol + sieve | ZnSO4 | Yes |
| 36 | ? | 3 | ? | ? | Tween 80 | MgSO4 + KI | No |
| 37 | ? | 3 | 100 cm2 | ? | Tween 60 | MgSO4 + KI | No |
| 38 | Systematic | ? | ? | 250 | Water | ZnSO4 | No |
| 39 | Systematic | 3 | ? | 200 | Tween 60 | ZnSO4 | No |
| 40 | ? | ? | ? | 50 | Tween 80 | MgSO4 | No |
| 41 | ? | 0.5 | 15×15 cm2 | ? | Tween 60 | NaNO3 | No |
| 42 | Systematic | 0.5–1 | 930 cm2 | 250 | NaOH | ZnSO4 | No |
| 43 | ? | Upper | ? | ? | ? | ZnSO4 | No |
| 44 | Children | ? | ? | ? | Tween 40 | NaNO3 | No |
| 45 | Animal | 1 | ? | 250 | Tween 60 | ZnSO4 | No |
| 46 | Systematic | ? | ? | 50 | Tween 40 | NaNO3 | No |
| 47 | Systematic | 1–2 | ? | 40 | Tween 40 | NaNO3 | No |
| 48 | 0.5–1 | 500 cm2 | 250 | N NaOH | ZnSO4 | No | |
Method by which the sample was taken: children – from areas where children play; animals – from areas where animals are expected to defecate; systematic; random.
Diameter of sample.
? Indicates that this information is not provided in the publication. KI Potassium Iodide; KI Potassium Iodine; KIHg Mercury potassium iodide; MgSO4 Magnesium sulphate; NaNO3 Sodium nitrate; NaOH Sodium hydroxide; ZnSO4 Zinc sulphate
Based on the above considerations, we designed a study with two objectives: to compare three of the most commonly used types of sampling methods (one random and two types of judgement sampling), and to pretest field and laboratory methods for the determination of Toxocara species and FC counts from sand.
METHODS
Selection of the study DCC:
The sampling frame consisted of 10 DCCs located in the Montreal and Laval regions of Quebec. Participating DCCs had at least one outdoor sandbox and play area. One 100 g sample of sand from each DCC was examined for the presence of FCs. Of the 10 DCCs, six were found to have no FCs (or a coliform level below that detectable at the screening dilution). Contamination levels found at the other four centres were 1 FCs/g, 40 FCs/g, 660 FCs/g and 1600 FCs/g, respectively. The DCC having the highest number of FCs was selected for this study.
Sampling methods:
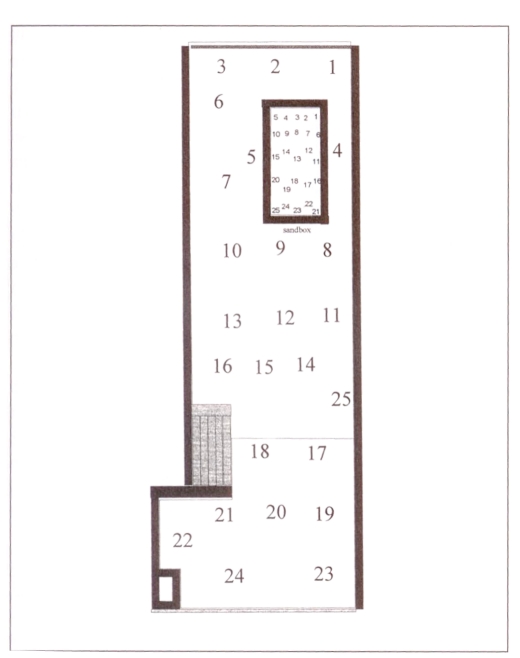
Surfaces of both the sandbox and the play area were measured and divided into 25 areas of approximately equal size (Figure 1). The grid coordinates for the areas were indicated on the sides of the sandbox and the play area with a black marker. A total of 25 areas were, therefore, identified and numbered from 1 to 25.
Figure 1.
Division of sandbox and play area into 25 areas for sampling
On each day over a nine-day period, five areas from the sandbox and five areas from the play area were sampled for a total of 90 sand samples. The areas sampled varied from day to day according to the method used, random or by judgement (two types). The methods are described below.
Random method (R): Five numbers from 1 to 25 were selected at random using a table of random numbers. The areas corresponding to the selected numbers were sampled. The numbers selected for the sandbox differed from the ones selected for the play area.
Judgement 1 method (J1): In order to assess soil contamination by sand or soil-associated microorganisms, the World Health Organization (WHO) recommends that sand be sampled in shaded areas and near trees (49). Therefore, this judgement method focused on covered areas, places where traces of cats were visible, areas near walls and shaded areas.
Judgement 2 method (J2): Areas where children were the most likely to play were sampled in the sandbox and in the play area. These areas were identified by observing children at play.
The nine-day period was divided into three blocks of three days each. The three methods were each used once in each block. For each three-day block, the order of the sampling method used each day was chosen at random in order to avoid an order effect. The order of the sampling methods is described in Table 3.
TABLE 3.
Order in which areas at day care centre where sampled
| Block | 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Method | J2 | R | J1 | J1 | R | J2 | R | J1 | J2 |
J1 Judgement 1 method; J2 Judgement 2 method; R Random method
All 25 areas of the sandbox and all 25 areas of the play area were sampled on day 10.
Sand sampling for laboratory analysis:
The sampling took place every morning before the arrival of the children at the DCC. In each selected area, 100 g of sand were obtained, to a depth of 10 cm, with a 4 cm diameter sterile container for bacteriological analysis. The container was placed at 4°C until it was transported to the laboratory, where it was immediately processed (within 1 h of sampling). Another 100 g of sand, similarly obtained, was placed in a container filled with sodium-acetic acid-formalin (SAF) for the parasitological analysis.
Method of quantification of the FCs:
Bacteriological analyses were performed at the Centre de recherche en virologie, Institut Armand-Frappier, location. A membrane filtration method for the identification of FCs was used (50). First, the sand was shaken to homogenize the sample. Then, 10 g was weighed and placed in a solution of 100 mL phosphate-buffered saline 10×. The samples were left at 4°C for 24 h. The sample was filtered, placed on m-FC medium and incubated at 44°C for 24 h. Blue colonies with metallic sheen were counted.
Recovery methods for Toxocara species:
Parasitological analyses were performed at the Centre for Tropical Diseases at the Montreal General Hospital, Montreal, Quebec. Recovery of Toxocara species ova from sand included a pretreatment stage to homogenize the sand and to ‘unstick’ the ova from the sand particles. A flotation-centrifugation method was used to separate and collect the ova from the sand sediment.
One millilitre of Tween 80 solution (Anachema, Quebec) was added to the sand sample (100 g) diluted in SAF to obtain a 0.1% solution and then shaken for 1 min. This solution was poured into 15 mL centrifuge tubes and centrifuged for 2 mins at 700 g. The supernatant was discarded. The sediment was then suspended in a solution of zinc sulphate (specific gravity 1.2) (51) and centrifuged for 2 mins at ×700 g. A small amount of the supernatant was pipetted and placed on a microscope slide. The slide was examined promptly at 40× magnification.
Statistical analyses:
The results are described by sampling method and by block. A logarithmic transformation (log10) was used because the data were not normally distributed. Ninety-five per cent confidence intervals were calculated for the difference in FC counts in the play area and the sandbox. SAS software (Statistical Analysis Systems Institute Inc, North Carolina) was used to obtain summary statistics (quartiles, 95% CI, median, ranges).
RESULTS
FCs:
FC counts by method and by block for the sandbox and the play area are shown in Table 4. The FC counts varied extensively from area to area and from day to day. All three methods almost constantly underestimated the overall contamination found on the last day of sampling. Only in one instance did the J2 method (where children play) provide a FC count higher than the count on day 10. However, because of the extreme variation in the FC levels both within the same day and between days, there was insufficient power to conduct any meaningful parametric or nonparametric test. Therefore, it was not possible to identify a method that was superior to the other methods.
TABLE 4.
Fecal coliforms (FCs) recovered from the study sandbox and play area
| Method* | Number of FCs (number of colonies/g sand) from sandbox Block | Number of FCs (number of colonies/g sand) from play area Block | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Random | 200 | 200 | 260 | 460 | 250 | 96 |
| 1020 | 2800 | 115 | 1 | 2100 | 750 | |
| 100 | 10,500 | 690 | 2 | 24 | 0 | |
| 16 | 18 | 149 | 34 | 10 | 70 | |
| 87 | 18 | 4640 | 152 | 24,800 | 130 | |
| Average | 285 | 2707 | 1171 | 130 | 5437 | 1046 |
| Judgement 1 ‘animal’ | 21 | 140,000 | 1050 | 240 | 25,000 | 1090 |
| 7 | 17 | 5 | 50 | 620 | 610 | |
| 270 | 380 | 1680 | 3840 | 530 | 230 | |
| 40 | 30 | 7200 | 0 | 1 | 4110 | |
| 4 | 7 | 54 | 4 | 22 | 80 | |
| Average | 68 | 28,087 | 1998 | 827 | 5235 | 779 |
| Judgement 2 ‘children’ | 800 | 430 | 1440 | 48 | 28 | 215 |
| 4360 | 370 | 460 | 260 | 610 | 2480 | |
| 2810 | 1770 | 900 | 570 | 230 | 3870 | |
| 790 | 210 | 680 | 330 | 4110 | 210 | |
| 170 | 68 | 1000 | 12 | 8 | 13 | |
| Average | 2186 | 570 | 896 | 244 | 997 | 1358 |
See text for explanation of methods used
On the last day of sampling (day 10), the average counts were 3036±7700 FCs/g and 915±930 FCs/g of sand for the play area and the sandbox, respectively. These counts were not normally distributed. The median counts of FC were 910 FCs/g (interquartile range [IQR]=1050) and 350 FCs/g (IQR=1160 of sand for the sandbox and the play area, respectively). The difference in the log transformed FC counts between the play area and sandbox was 0.0093 with a 95% CI of −0.5589 to 0.5776.
Toxocara species:
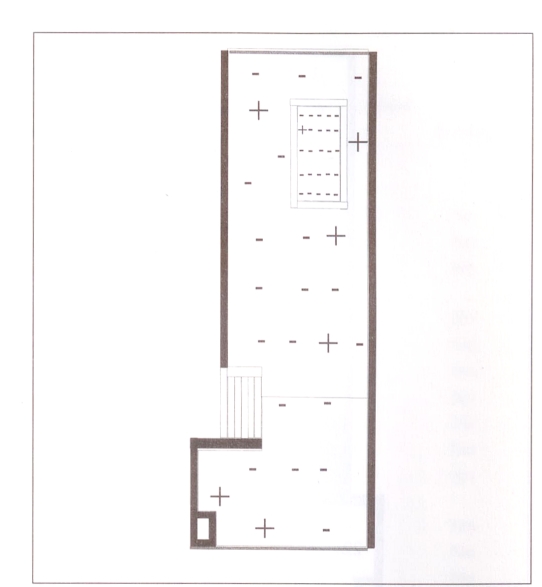
The areas where Toxocara species ova were found using the different sampling methods for the three blocks are shown in Table 5. Because very few ova were recovered in each sample, a qualitative measure was used to describe the presence or absence of Toxocara species. No differentiation was made between Toxocara canis and Toxocara cati. The recovery varied considerably from one sampling area and day to another. Figure 2 shows the areas in which toxocara ova were recovered on the last day of sampling. Toxocara ova were recovered in one area of the sandbox and in six areas of the play area. The presence of toxocara ova was not associated with any particular region of the play area.
TABLE 5.
Presence of Toxocara species in the study sandbox and play area
| Sandbox Block | Play area Block | |||||
|---|---|---|---|---|---|---|
| Method | 1 | 2 | 3 | 1 | 2 | 3 |
| Random | – | – | – | – | – | – |
| – | – | – | – | – | – | |
| – | – | – | – | + | – | |
| – | – | – | – | – | – | |
| – | – | – | – | – | – | |
| Judgement 1 ‘animal’ | – | + | – | – | – | – |
| – | – | – | – | – | – | |
| – | – | + | – | – | – | |
| – | – | – | + | – | – | |
| – | + | – | – | – | – | |
| Judgement 2 ‘children’ | – | – | + | – | – | + |
| – | – | – | – | – | – | |
| – | – | – | – | – | – | |
| – | – | – | – | + | – | |
| – | – | – | – | – | – | |
– No Toxocara species present in sample;
+ Toxocara species present in sample
Figure 2.
Spatial distribution of the presence of Toxocara species (+) on the last day of sampling. – No Toxocara species present
DISCUSSION
The number of FCs and the presence of Toxocara species recovered from the play area and the sandbox of the study day care centre varied extensively, both in time and space. Environmental factors, such as temperature and humidity, and physical factors, such as the shifting of sand by children or animals, and the presence of domestic and small wild animals or birds defecating in different areas of the playground on different days may partially explain the observed variation.
In Quebec, the presence of animals in DCCs is prohibited (52). In addition, outdoor playgrounds of DCCs must be surrounded by a fence of at least 1.2 m in height (52). However, no mention is made of the spacing between the ground and the lower perimeter of the fence or fence maintenance. Small animals such as dogs, cats and raccoons consequently may have access to the playgrounds. In a 1994 study of 10 DCCs from three different geographical regions of Quebec (Quebec City, Trois-Rivière and Montreal), the presence of dogs, cats, raccoons, pigeons and mice during the night was reported by four DCC directors (8).
Due to the magnitude of the random variation in bacterial and parasite contamination observed, a statistical comparison among the three sampling methods was not possible. To best represent the overall level of contamination in the outdoor DCC environment (sandbox and play area), results from the sampling of all squares (as observed from the results obtained on day 10) were determined to be the most useful.
To evaluate the importance of the level of contamination found, we obtained the microbiological standards (for total and FC contamination) established by Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec for the interpretation of the results of food analyses (53). For example, 30 FCs/g is the upper ‘acceptable’ limit of contamination in molluscs prepared for human consumption; a norm of 1000 total coliforms is the upper limit in ice milk. The proportion of FCs to total coliforms varies considerably from one medium to another (personal communication) but to our knowledge, no standards have been established for sand. Standards used by the Ministère de l’Environnement du Québec in assessing beach water contamination are 200 FCs/100 mL (55). The results we obtained (medians of 350 and 910 FCs/g of sand from the sandbox and play area, respectively) are clearly higher than these standards. Indoor FC contamination from surfaces, toys, and from children’s and staff’s hands has been reported at (median) levels between 0 and 39.8 FCs (5). Although our results from sand cannot be directly compared with results from food, water, indoor surfaces or hands, they indicate significant fecal contamination.
Based on data obtained over a two-week period, 65 children age one to four years were reported to have ingested a median of 40 mg of soil per day in a DCC setting in the United States (55). One child in this study had ingested 5 to 8 g of soil per day. Using our data, it is possible, therefore, that children playing in a play area contaminated with an average of 1000 FCs/g of sand, could ingest a median of 40 FCs per day.
Our study confirms previous reports documenting the presence of Toxocara species ova in the outdoor DCC environment. This result may have been missed if the sampling method had been limited to one method only. It is impossible to know whether this is due to a poor recovery rate due to the method itself, because the sand is moved by children and animals each day or because the samples were not taken exactly at the same place from day to day. The percentage recovery of toxocara ova in experimental studies is reported to range from 0% to 70%, but this can vary with the number of grams processed, the level of contamination, the pretreatment techniques, the type of flotation solution used and the type of sand/soil examined (36,56–60). Consequently, the prevalence of toxocara observed can only be an underestimate of the true level of contamination.
CONCLUSION
The contamination levels found in this study indicate a risk of potentially pathogenic bacterial and parasite contamination in the outdoor day care environment. The most representative levels of bacterial contamination were found in a combined sample of the total surface area rather than from a random or judgement sampling method. Research on environmental microbial contamination of outdoor day care settings would benefit from the application of standardized and validated sampling and laboratory methods.
Acknowledgments
This work was supported by the Animal Branch of Pfizer Canada and, in part, by the National Health Research and Development Program (NHRDP) through a National Health Research Scholar Award to Theresa W Gyorkos; by NHRDP and the Fonds pour la formation de Chercheurs et l’Aide à la Recherche (FCAR) through a doctoral fellowship to Hélène Carabin; and by the Fonds de la Recherche en Santé du Québec (FRSQ) through a Chercheur boursier to Lawrence Joseph. Denis Minville provided expert assistance with the bacteriological analyses. The authors gratefully acknowledge all the day care centres that participated in this study, particularly the day care centre in which the complete series of sampling methods were studied.
REFERENCES
- 1.Bartlett A, Moore M, Gary G, Starko K, Erben J, Meredith B. Diarrheal illness among infants and toddlers in child care centers. II. Comparison with day care homes and households. J Pediatr. 1985;107:503–9. doi: 10.1016/s0022-3476(85)80005-6. [DOI] [PubMed] [Google Scholar]
- 2.Collet JP, Burtin P, Kramer MS, et al. Type of day care setting and risk of recurrent infection. Respiration. 1994;61(Suppl 1):16–9. doi: 10.1159/000196375. [DOI] [PubMed] [Google Scholar]
- 3.Fleming DW, Cochi SL, Hightower AW, Broome CV. Childhood upper respiratory tract infections: To what degree is incidence affected by day-care attendance? Pediatrics. 1987;79:55–60. [PubMed] [Google Scholar]
- 4.Thompson SC. Infectious diarrhoea in children: Controlling transmission in the child care setting. J Pediatr. 1994;30:210–9. doi: 10.1111/j.1440-1754.1994.tb00621.x. [DOI] [PubMed] [Google Scholar]
- 5.Laborde D, Weigle C, Weber D, Kotch J. Effect of fecal contamination on diarrheal illness rates in day-care centers. Am J Epidemiol. 1993;138:243–55. doi: 10.1093/oxfordjournals.aje.a116853. [DOI] [PubMed] [Google Scholar]
- 6.Soto JC. Infectious disease control in daycare centres: a Canadian experience. Can J Pediatr. 1993;5:330–6. [Google Scholar]
- 7.Black R, Merson M, Huq I, Alim A, Yunus M. Incidence and severity of rotavirus and Escherichia coli diarrhea in rural Bangladesh: implications for vaccine development. Lancet. 1981;i:141–3. doi: 10.1016/s0140-6736(81)90719-4. [DOI] [PubMed] [Google Scholar]
- 8.Gyorkos TW, Kokoskin-Nelson E, MacLean JD, Soto JC. Parasite contamination of sand and soil from daycare sandboxes and play areas. Can J Infect Dis. 1994;5:17–20. doi: 10.1155/1994/786090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gasquet M, Julien J, Delmas F, Andrac A, Timon-David P. Étude parasitologique des bacs à sable et sables de plages de la région de Marseille. Rev Fr Santé Publ. 1986;35:33–43. [Google Scholar]
- 10.Acha N, Szyfres B. Zoonoses et maladies transmissibles communes à l’homme et aux animaux. Paris: Office international des épizooties; 1989. [Google Scholar]
- 11.Keswick B, Pickering L, Dupont H, Woodward W. Survival and detection of rotaviruses on environmental surfaces in day care centers. Appl Environ Microbiol. 1983;46:813–7. doi: 10.1128/aem.46.4.813-816.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chabasse D, Bouchara JP, Rivet M. Étude parasitologique et mycologique des bacs à sable des aires de jeux de l’agglomération angevine. Médecine Maladies Infectieuses. 1983;13:436–42. [Google Scholar]
- 13.Zurawska-Olszewska J, Misiak G. Preliminary evaluation of biological-sanitary contamination of grass lawns and children playgrounds in Warsaw in 1991. Medycyna Doswiadczalna i Mikrobiologia. 1994;46:103–6. [PubMed] [Google Scholar]
- 14.Quessy S, Messier S. Prevalence of Salmonella spp, Campylobacter spp and Listeria spp in ring-billed gulls (Larus delawarensis) J Wildl Dis. 1992;28:526–31. doi: 10.7589/0090-3558-28.4.526. [DOI] [PubMed] [Google Scholar]
- 15.Comité provincial des maladies infectieuses en garderie Le comité provincial des maladies infectieuses en service de garde répond à vos questions. In: Comité organisateur d’événements pour les services de garde à l’enfance, ed. Actes du Colloque québecois sur les services de garde à l’enfance. Actes du Colloque québécois sur les services de garde à l’enfance. Montréal. 1991:147–50. [Google Scholar]
- 16.Canadian Paediatric Society . Well Beings: A Guide to Promote the Physical Health, Safety and Emotional Well-being of Children in Child Care Centers and Family Day Care Homes. Ottawa: Canadian Paediatric Society; 1992. pp. 98–9. [Google Scholar]
- 17.American Public Health Association and American Academy of Pediatrics . National health and safety performance standards: Guidelines for out-of-home child care program. In: Chang A, editor. Caring for Our Children. Elk Grove: American Academy of Pediatrics; 1992. p. 363. [Google Scholar]
- 18.Dunsmore JD, Thompson RCA, Bates IA. Prevalence and survival of Toxocara canis in the urban environment of Perth, Australia. Vet Parasitol. 1984;16:303–11. doi: 10.1016/0304-4017(84)90048-7. [DOI] [PubMed] [Google Scholar]
- 19.Winkel KD, Saw TH, Prociv P. Risk of parasitic infections from sandpits. Med J Aust. 1990;153:503. doi: 10.5694/j.1326-5377.1990.tb126177.x. (Lett) [DOI] [PubMed] [Google Scholar]
- 20.Costa-Cruz JM, Nunes RS, Buso AG. Presença de ovos de Toxocara spp em praças públicas da cidade de Uberlândia. Minas Gerais, Brasil Rev Inst Med Trop Säo Paulo. 1994;36:39–42. [PubMed] [Google Scholar]
- 21.Ghadirain E, Viens P, Dubreuil F. Epidemiology of toxocariasis in the Montreal area. Prevalence of Toxocara and other helminth ova in dogs and soil. Can J Public Health. 1976;67:495–8. [PubMed] [Google Scholar]
- 22.Gualazzi DA, Embil JA, Pereira LH. Prevalence of helminth ova in recreational areas of peninsular Halifax, Nova Scotia. Can J Public Health. 1986;77:147–51. [PubMed] [Google Scholar]
- 23.Laborde C, Bussieras J, Chermette R. Recherche des oeufs de Toxocara spp. dans le sol des jardins de Paris. Prophylaxie des infestations humaines. Rec Med Vet Ec Alfort. 1980;156:733–8. [Google Scholar]
- 24.Chabasse D, Rivet M. Recherche des oeufs de Toxocara species dans les bacs à sable des aires de jeux de l’agglomération angevine. Ouest Med. 1982;35:807–10. [Google Scholar]
- 25.Doucet M. Étude de la contamination des bacs à sable de la ville deLyon par Toxocara canis. Thèse de D.M.V. Lyon: École nationale vétérinaire de Lyon, 1994.
- 26.Duwell D. The prevalence of toxocara eggs in the sand in children’s playgrounds in Frankfurt/M. Ann Trop Med Parasitol. 1984;78:633–6. [PubMed] [Google Scholar]
- 27.Knaus VB-U, Lange U, Volcsik R. Larva migrans visceralis – Occurrence of ascarid eggs in sand-pits in the GDR district town of Cottbus. Angewandte Parasitologie. 1987;28:81–3. [PubMed] [Google Scholar]
- 28.Horn K, Schieder T, Stoye M. Contamination of public children playgrounds with helminth eggs in Hannover (German) Dtsch Tierärztl Wschr. 1990;97:122–5. [PubMed] [Google Scholar]
- 29.Holland C, O’Connor P, Taylor MR, Hughes G, Girdwood RWA, Smith H. Families, parks, gardens and toxocariasis. Scand J Infect Dis. 1991;23:225–31. doi: 10.3109/00365549109023405. [DOI] [PubMed] [Google Scholar]
- 30.O’Lorcain P. Prevalence of Toxocara canis ova in public playgrounds in the Dublin area of Ireland. J Helminthol. 1994;68:237–41. doi: 10.1017/s0022149x00014401. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu T. Prevalence of toxocara eggs in sandpits in Tokushima city and its outskirts. J Vet Med Sci. 1993;55:807–11. doi: 10.1292/jvms.55.807. [DOI] [PubMed] [Google Scholar]
- 32.Uga S. Prevalence of toxocara eggs and number of fecal deposits from dogs and cats in sandpits of public parks in Japan. J Helminthol. 1993;67:78–82. doi: 10.1017/s0022149x0001289x. [DOI] [PubMed] [Google Scholar]
- 33.Abo-Shehada MN. Prevalence of Toxocara ova in some schools and public grounds in northern and central Jordan. Ann Trop Med Parasitol. 1989;83:73–5. doi: 10.1080/00034983.1989.11812313. [DOI] [PubMed] [Google Scholar]
- 34.Thompson DE, Bundy DAP, Cooper ES, Schantz PM. Epidemiological characteristics of Toxocara canis zoonotic infection of children in a Carribean community. Bull WHO. 1986;64:283–90. [PMC free article] [PubMed] [Google Scholar]
- 35.Jansen J, van Knappen F, Schreurs M, van Wijngaarden Th. Toxocara eieren in parken en zandbakken in de stad Utrecht. Tijdschr Diergeneeskd. 1993;118:611–4. [PubMed] [Google Scholar]
- 36.Quinn R, Smith HV, Bruce RG, Girdwood RWA. Studies on the incidence of Toxocara and Toxascaris spp ova in the environment. 1. A comparison of flotation procedures for recovering Toxocara spp ova from soil. J Hyg (Lond) 1980;84:83–9. doi: 10.1017/s0022172400026553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conde Garcia L, Muro Alvarez A, Simon Martin F. Epidemiological studies on toxocariasis and visceral larva migrans in a zone of Western Spain. Ann Trop Med Parasitol. 1989;83:615–20. doi: 10.1080/00034983.1989.11812395. [DOI] [PubMed] [Google Scholar]
- 38.Borg OA, Woodruff AW. Prevalence of infective ova of Toxocara species in public places. Br Med J. 1973;4:470–2. doi: 10.1136/bmj.4.5890.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snow KR, Ball SJ, Bewick JA. Prevalence of Toxocara species eggs in the soil of five east London parks. Vet Rec. 1987;120:66–7. doi: 10.1136/vr.120.3.66. [DOI] [PubMed] [Google Scholar]
- 40.Gillepsie SH, Ramsay A. The prevalence of Toxocara canis ova in soil samples from parks and gardens in the London area. Public Health. 1991;105:335–9. doi: 10.1016/s0033-3506(05)80219-7. [DOI] [PubMed] [Google Scholar]
- 41.Dubin S, Segall S, Martindale J. Contamination of soil in two city parks with canine nematode ova including Toxocara canis: a preliminary study. Am J Public Health. 1975;65:1242–5. doi: 10.2105/ajph.65.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dada BJO, Lindquist WD. Studies on flotation techniques for the recovery of helminth eggs from soil and the prevalence of eggs of Toxocara spp in some Kansas public places. J Am Vet Med Assoc. 1979;174:1208–10. [PubMed] [Google Scholar]
- 43.Surgan MH, Colgan KB, Kennett BS, Paffman JV. A survey of canine toxocariasis and toxocaral soil contamination in Essex County, New Jersey. Am J Public Health. 1980;760:1207–8. doi: 10.2105/ajph.70.11.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith RE, Hagstad H, A’Beard GB. Visceral larva migrans: a risk assessment in Baton Rouge, Louisiana. Int J Zoonoses. 1984;11:189–94. [PubMed] [Google Scholar]
- 45.Childs JE. The prevalence of Toxocara species ova in backyards and gardens of Baltimore, Maryland. Am J Public Health. 1985;75:1092–4. doi: 10.2105/ajph.75.9.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul AJ, Tood KS, Dipietro JA. Environmental contamination by eggs of Toxocara species. Vet Parasitol. 1988;26:339–42. doi: 10.1016/0304-4017(88)90102-1. [DOI] [PubMed] [Google Scholar]
- 47.Ludlam KE, Platt TR. The relationship of park maintenance and accessibility to dogs to the presence of Toxocara spp ova in the soil. Am J Public Health. 1989;79:633–4. doi: 10.2105/ajph.79.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rapic D. Kontaminiranost javih povrsina jajascima helminata. Vet Arch. 1983;53:233–8. [Google Scholar]
- 49.World Health Organization (WHO) Expert Committee on the Control of Ascariasis Control of Ascariasis report of a WHO expert committee World Health Organization Technical Report Series no 379. Geneva: WHO; 1967. pp. 14–22. [Google Scholar]
- 50.American Public Health Association . Standard Methods for the Examination of Water and Wastewater. 18th edn. Washington: American Public Health Association; 1992. [Google Scholar]
- 51.Ash LR, Orinel TC. Parasites: A Guide to Laboratory Procedures and Identification. Chicago: American Society of Clinical Pathology Press; 1987. [Google Scholar]
- 52.Editeur officiel du Québec . Québec: Gouvernement du Québec; 1994. Règlement sur les services de garde en garderie. [S-4.1,r.2]: Article #43. [Google Scholar]
- 53.Comité sur l’uniformisation des méthodes d’analyses et l’interprétation des résultats analytiques. Critères microbiologiques pour l’interprétation des résultats en analyse alimentaire. Gouvernement du Québec, ed. Québec: Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec, 1993
- 54.Thibault M, Blaney S, Lévesque B. Québec: Centre de santé publique de Québec, Équipe Santé et environnement; 1995. Étude de la contamination microbiologique du fleuve Saint-Laurent et ses tributaires et impacts possibles sur la santé humaine. [Google Scholar]
- 55.Calabrese EJ, Barnes R, Stanek EJ, et al. How much soil do young children ingest: an epidemiologic study. Reg Toxicol Pharmacol. 1989;10:123–37. doi: 10.1016/0273-2300(89)90019-6. [DOI] [PubMed] [Google Scholar]
- 56.Dada BJO. A new technique for the recovery of toxocara eggs from soil. J Helminthol. 1979;53:141–4. doi: 10.1017/s0022149x00005873. [DOI] [PubMed] [Google Scholar]
- 57.Delmas F, Gasquet J, Julien J, Timon-David P. Intérêt de la standardisation des méthodes de recherche parasitologiques dans les bacs à sable. Rev Fr Santé Publ. 1988;42:13–7. [Google Scholar]
- 58.Horn K, Schieder T, Stoye M. Quantitative comparison of various methods for detecting eggs of Toxocara canis in samples of sand. J Vet Med (Ser B) 1990;37:241–50. [PubMed] [Google Scholar]
- 59.Kazacos KR. Improved method for recovering ascarid and other helminth eggs from soil associated with epizootics and during survey studies. Am J Vet Res. 1983;44:896–900. [PubMed] [Google Scholar]
- 60.Nunes CM, Sinhorini IL, Ogassawara S. Influence of soil in the recovery of Toxocara canis eggs by a flotation method. Vet Parasitol. 1994;54:269–74. doi: 10.1016/0304-4017(94)90190-2. [DOI] [PubMed] [Google Scholar]