Abstract
Purpose
Greater chronic disease burden may decrease quality of life (QOL) of breast-cancer survivors. Our objective was to investigate the association between chronic disease burden and QOL in breast-cancer survivors at one year post-diagnosis.
Methods
We analyzed cross-sectional data collected one year post-diagnosis from a sample of female breast-cancer survivors identified from the Missouri cancer registry. We used eight RAND-36 subscales to assess physical, emotional and social functioning QOL domains. Using Katz’s measure of comorbidity, we computed chronic disease burden (0, 1, 2+). Multivariable general linear models for each QOL subscale were used to examine associations between chronic disease burden and QOL after controlling for potential covariates: socio-demographic, clinical, psychosocial, behavioral risk factors, and access to medical care.
Results
Participants (n=1089) were 58 years old on average (range 27-96) and mostly White (92%), married (68%), had at least a high school education (95%), and had health insurance (97%). Sixty-six percent of survivors had a chronic disease burden score of 0, 17% had 1, and 17% had 2+. Chronic disease burden was significantly associated with each QOL subscale in crude models (p<0.001). In fully adjusted models, chronic disease burden was still significantly correlated with six subscales, but not with the emotional well-being and role limitations due to emotional problems subscales.
Conclusions
One year post-diagnosis, breast-cancer survivors with higher chronic disease burden had lower physical and social functioning than survivors without additional health conditions. These differences were not fully explained by relevant covariates. Identifying modifiable targets for intervention will be critical for improving QOL outcomes among survivors who have other chronic health conditions.
Keywords: breast-cancer, survivors, comorbidity, quality of life
Introduction
The availability of an effective screening test, more effective and less harmful treatments, and better overall access to cancer care has resulted in better outcomes and improved survival after breast cancer diagnosis for many women. In 2007, there were an estimated 2.7 million female breast cancer survivors in the US [1]. As this number continues to grow, attention has expanded beyond the study of factors associated with recurrence and survival to include a focus on understanding the “continuing care” phase of the cancer experience and quality of life (QOL) outcomes of cancer survivors [2].
QOL is a multidimensional construct and includes the subjective evaluation of several important aspects of a person’s life including physical functioning, emotional well being, role functioning, and social functioning [3, 4]. QOL after a cancer diagnosis can vary dramatically over time with physical and treatment-related problems occurring most acutely immediately following treatment [5]. A number of studies have shown that breast cancer survivors have lower QOL than non-cancer controls [6-11]. Although these differences appear to attenuate over time following completion of treatment [12-14], other effects of breast cancer diagnosis and treatment such as fatigue, breast symptoms, lymphedema, functional limitations, and psychosocial concerns, can persist years after completion of treatment [12, 15-17]. Numerous studies have identified patient characteristics associated with QOL among female breast cancer survivors at different points during diagnosis and treatment, including age, ethnicity, socioeconomic status, type of treatment, comorbidity, functional deficits, breast symptoms, and fatigue [13, 14, 16, 18-20].
Less attention has focused on the independent association of chronic disease burden with QOL among breast cancer survivors despite the high prevalence of chronic conditions among older adults and the impact of existing chronic conditions on type of treatment received [21], development of disability [15], prognosis [21, 22], and survival [23]. QOL following breast cancer diagnosis and treatment may be affected by comorbid conditions in addition to the cancer itself. Chronic health conditions may impact QOL domains individually due to unique physiologic aspects of the specific health condition, but chronic disease burden (having a greater number and/or severity of conditions) may itself be an important influence on QOL among breast cancer survivors because greater chronic disease burden may have already led to decrements in QOL, may exacerbate breast cancer symptoms leading up to or following diagnosis, may affect type of cancer treatments received or effects from treatment, and may impact recovery over time.
The objective of this study was to examine how QOL varies by chronic disease burden across eight QOL domains in a sample of female breast cancer survivors interviewed at one year post-diagnosis. A better understanding of the effects of chronic disease burden on QOL post-diagnosis is needed to improve processes of care and maximize QOL among breast cancer survivors at all points during the cancer survivorship continuum.
Methods
Study sample
Missouri women age 25 or older diagnosed with first primary breast cancer during June, 2006 through June 2008 were identified from the statewide Missouri Cancer Registry. Women were initially recruited by mail. Participants provided informed consent and completed computer-assisted telephone interviews one year after diagnosis. Women who scored more than 10 on the Orientation-Memory-Concentration (OMC) test were excluded [24].
During the study period, 4020 women with first primary breast cancer were eligible to participate, 675 of whom we were unable to contact after seven attempts. Of the remaining women, 1166 women completed the telephone interview for a participation rate of 34.9 percent. Available cancer registry data of all eligible women showed that nonparticipants were statistically more likely than participants to be older, African American, and to live in the northwest part of Missouri. There was no difference in stage at diagnosis among study participants and those who did not participate. Of the 1166 women who completed the interview, 65 women were excluded because of high scores on the OMC test, and an additional 12 women were excluded because of missing data on one or more covariates of interest. This left a total of 1089 women available for the current analysis.
Measures
QOL
The RAND 36-Item Health Survey 1.0 was used to measure quality of life. It contains eight subscales that represent three general areas of health-related QOL: physical, emotional, and social well-being [25, 26]. The subscales include: general health status (5 items), physical functioning (10 items), role limitations due to physical problems (4 items), energy-fatigue (4 items), bodily pain (2 items), emotional well-being (5 items), role limitations due to emotional problems (3 items), and social functioning (2 items). Scores for each subscale were standardized, ranging from 0 to 100, with higher scores indicating better functioning within that subscale [25].
Chronic disease burden
Katz’s validated self-report adaptation of the Charlson comorbidity index was used to measure the presence and/or history of several chronic health conditions [27]. Conditions assessed in this measure included: myocardial infarction, diabetes, congestive heart failure, peripheral vascular disease, stroke, hemiplegia, chronic obstructive pulmonary disease, ulcer disease, renal disease, connective tissue disease, and other conditions such as dementia, liver cirrhosis, and other cancers. We did not include questions about HIV/AIDS as in the original measure. A weighted score is derived for each individual that reflects the presence and severity of the conditions assessed [27].
Covariates associated with QOL
We examined factors, previously associated with QOL among cancer survivors, as covariates in a potential association between CDB and the QOL subscales, including: 1) socio-demographic factors, 2) cancer-related clinical factors, 3) access to medical care characteristics, 4) psychosocial factors, and 5) cancer-related behavioral risk factors [28]. Factors included in the analysis were patterned after other studies among cancer survivors [29-32].
Socio-demographic factors included race (white, African American, multiple, other), age at interview, educational attainment (less than high school, high school, more than high school), employment (employed or not), marital status (married or not), and income adequacy. Income adequacy was measured by asking participants whether they felt their household income was comfortable, enough to make ends meet, or not enough to make ends meet.
Cancer-related clinical factors included stage at diagnosis, a surgical side effects index, and types of treatment received. Stage at diagnosis was obtained from the Missouri Cancer Registry. Based on the literature [33] and surgeons’ anecdotal reports of patients’ complaints after surgery, we developed a five-item measure of breast-surgery-associated side effects with higher scores indicating more severe side effects (alpha=0.74) [34]. The five items included in this side effects measure were: limited arm mobility/frozen shoulder, tightness/tenderness in chest wall, tightness/tenderness/discomfort in breast, arm weakness, and lymphedema of the arm. Treatment received consisted of type of surgery (mastectomy, lumpectomy, both, neither), axillary lymph node removal (yes, no), receipt of chemotherapy (yes, no), receipt of radiotherapy (yes, no), and taking hormonal therapy (Tamoxifen, Raloxifene, or aromatase inhibitors) at the time of the interview (yes, no). Self-reported treatment has been shown to be accurate relative to medical record review [35].
Access to medical care variables included having health care insurance at the time of the interview (yes, no), being unable to see a doctor during the 12 months prior to the interview because of cost (yes, no), having a place to go when sick or needing advice about health issues (yes, no), and lacking transportation (yes, no). These items were adapted questions routinely used by the Behavioral Risk Factor Surveillance System (BRFSS).
Psychosocial factors consisted of social support, perceived stress, and depressive symptoms. Four items from the Cohen perceived stress scale were used (alpha=0.82) [36]. Perceived availability of social support was measured using the Medical Outcomes Study Social Support Survey [37]. Depressive symptoms were measured using the 11-item version of the Center for Epidemiologic Studies Depression (CES-D) scale [38]. This version accurately reproduces the results from the original 20-item CES-D and functions well in community-dwelling subjects [38]. A score of 9 or greater on this version is equivalent to the usual clinically relevant levels of depressive symptoms (CRLDS) criterion of 16 or greater on the 20-item scale [38].
Cancer-related behavioral risk factors included current smoking status (current, former, never), participation in physical activity in the past month (yes vs. no), body mass index (BMI) (calculated from self-reported height and weight and categorized into the following categories: normal (<25.0), overweight (25.0-29.9), and obese (>=30.0), and alcohol use during the past month (<=1 drink per day vs. > 1 drink per day) [39]. These items were adapted from questions routinely used by the BRFSS.
Statistical Analysis
Standard statistical methods were used to examine potential differences in covariates by chronic disease burden group, including chi-squared tests for categorical variables and one-way analysis of variance (ANOVA) for continuous variables. Next, general linear modeling was used to model the relationship between chronic disease burden group and each of the eight QOL subscales. Crude mean scores and 95% Confidence Intervals (CI) for each disease burden group were generated using ANOVA. Multivariable general linear models were used to determine the relationship between chronic disease burden and each QOL subscale as well as calculate estimated mean scores for each QOL subscale after controlling for potential confounders and covariates of the outcome. Adjusted mean scores (estimated marginal means) and 95% CI were generated for these multivariable models and reflect the mean QOL subscale score for each chronic disease burden group adjusted for all other variables included in the model. Data were analyzed using SPSS 16.0. P-values < 0.05 indicate statistical significance.
Results
Demographic characteristics and chronic disease burden of the study sample are provided in Table 1. Participants were 58.1 years of age on average (range 27-96), mostly White (92.0%), married (68.3%), had at least a high school education (95.4%), and had health insurance (97.2%). Chronic disease burden scores ranged from 0 to 8. Chronic disease burden was categorized for subsequent analyses into three categories: 0, 1, 2 or more (2+) based on the distribution of scores in the study population as well as for comparison to other studies of comorbidity and QOL among cancer survivors [6]. Sixty-six percent of survivors had a disease burden score of 0, 17% had a score of 1, and 17% had a score of 2+.
Table 1.
Socio-demographic Characteristics and Chronic Disease Burden Score of Female Breast Cancer Survivors One Year Post-Diagnosis (2006-2008).
| n (%) | |
|---|---|
| Age (mean/std dev) | 58.1 (11.6) |
| Race | |
| White | 1002 (92.0) |
| African-American | 65 (6.0) |
| Other | 22(2.0) |
| Marital status | |
| not married | 345 (31.7) |
| married | 744 (68.3) |
| Education | |
| less than HS | 50 (4.6) |
| HS grad | 326 (29.9) |
| more than HS | 713 (65.5) |
| Employment | |
| employed | 592 (54.4) |
| unemployed | 33 (3.0) |
| homemaker | 108 (9.9) |
| student | 1 (0.1) |
| retired | 299 (27.5) |
| unable to work | 56 (5.1) |
| Adequacy of Income | |
| comfortable | 659 (60.5) |
| just enough to make ends meet | 304 (27.9) |
| not enough to make ends meet | 119 (10.9) |
| don’t know/not sure | 4 (0.4) |
| refused | 3 (0.3) |
| Health Insurance | |
| yes | 1058 (97.2) |
| no | 31 (2.8) |
| Chronic Disease Burden | |
| 0 | 720 (66.1) |
| 1 | 185 (17.0) |
| 2+ | 184 (16.9) |
Unadjusted mean scores for each QOL subscale for the total study sample at one year post-diagnosis are shown in Figure 1. Mean scores for the physical subscales were generally lower than mean scores for the emotional or social functioning subscales.
Figure 1.
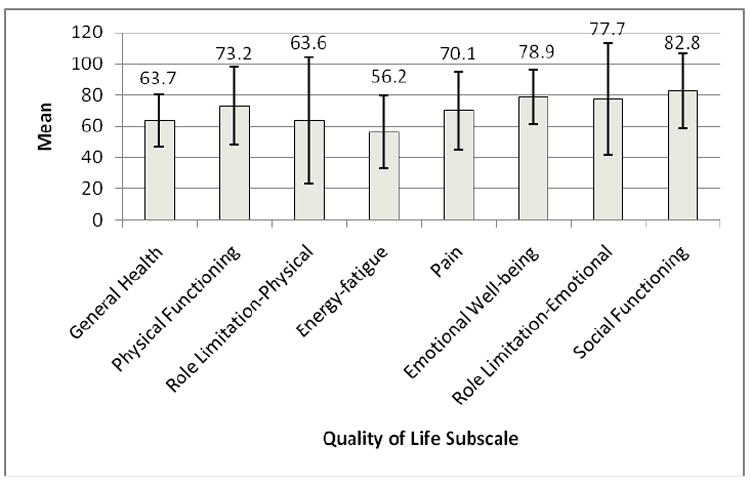
Unadjusted RAND-36 Subscale Mean Scores, Female Breast Cancer Survivors, One Year Post-Diagnosis.
As shown in Table 2, there were significant differences in socio-demographics, clinical factors, access to medical care, psychosocial factors, and cancer-related behavioral risk factors across disease burden categories. Women with higher chronic disease burden were more likely to be non-White, older, unmarried, less educated, unemployed, have less adequate income, and have reduced access to medical care based on characteristics measured. Additionally, women with higher chronic disease burden had more depressive symptoms, higher levels of perceived stress, lower levels of social support, a greater burden of surgery side effects, and were less likely to receive chemotherapy and radiation treatments. Higher chronic disease burden was also associated with no physical activity in the past month and higher BMI but with lower alcohol use per day.
Table 2.
Characteristics of Breast Cancer Survivors by Chronic Disease Burden Score at One Year Post-Diagnosis (2006-2008).
| Chronic Disease Burden Score | ||||
|---|---|---|---|---|
| 0 (N=720) | 1 (N=185) | 2+ (N=184) | p-value | |
| Socio-demographic Characteristics | n(%) | n(%) | n(%) | |
| Race | <.001 | |||
| White | 683 (94.9) | 159 (85.9) | 160 (87.0) | |
| African-American | 26 (3.6) | 21 (11.4) | 18 (9.8) | |
| Other | 11 (1.5) | 5 (2.7) | 6 (3.2) | |
| Age (years) mean/std dev | 56.4 (11.1) | 58.8 (11.8) | 63.6 (11.5) | <.001 |
| Marital status | <.001 | |||
| not married | 199 (27.6) | 71 (38.4) | 75 (40.8) | |
| married | 521 (72.4) | 114 (61.6) | 109 (59.2) | |
| Education | <.001 | |||
| less than HS | 21 (2.9) | 12 (6.5) | 17 (9.2) | |
| HS grad | 208 (28.9) | 50 (27.0) | 68 (37.0) | |
| more than HS | 491 (68.2) | 123 (66.5) | 99 (53.8) | |
| Employment | <.001 | |||
| employed | 446 (61.9) | 84 (45.4) | 62 (33.7) | |
| unemployed | 12 (1.7) | 14 (7.6) | 7 (3.8) | |
| homemaker | 73 (10.1) | 21 (11.4) | 14 (7.6) | |
| student | 0 (0.0) | 1 (0.5) | 0 (0.0) | |
| retired | 169 (23.5) | 53 (28.6) | 77 (41.8) | |
| unable to work | 20 (2.8) | 12 (6.5) | 24 (13.0) | |
| Adequacy of income | <.001 | |||
| comfortable | 468 (65.0) | 99 (53.5) | 92 (50.0) | |
| just enough to make ends meet | 200 (27.8) | 46 (24.9) | 58 (31.5) | |
| not enough to make ends meet | 49 (6.8) | 39 (21.1) | 31 (16.8) | |
| don’t know/not sure | 2 (0.3) | 0 (0.0) | 2 (1.1) | |
| refused | 1 (0.1) | 1 (0.5) | 1 (0.5) | |
| Health insurance | 0.811 | |||
| yes | 699 (97.1) | 179 (96.8) | 180 (97.8) | |
| no | 21 (2.9) | 6 (3.2) | 4 (2.2) | |
| Psychosocial Characteristics | ||||
| CESD cutoff | <.001 | |||
| less than 9 | 595 (82.6) | 139 (75.1) | 115 (62.5) | |
| 9 or greater | 125 (17.4) | 46 (24.9) | 69 (37.5) | |
| Cohen Stress Scale (CSTR) mean/std dev | 7.1 (2.9) | 7.9 (3.1) | 8.5 (3.8) | <.001 |
| MOS Social Support Scale mean/std dev | 4.4 (0.6) | 4.3 (0.8) | 4.2 (0.8) | <.001 |
| Chronic Disease Burden Score | ||||
| 0 | 1 | 2+ | Chi-sq | |
| N (%) | N (%) | N (%) | p-value | |
| Cancer-Related Clinical Factors | ||||
| Stage at diagnosis | 0.154 | |||
| in situ | 133 (18.5) | 37 (20.0) | 31 (16.8) | |
| localized | 397 (55.1) | 100 (54.1) | 104 (56.5) | |
| regional | 177 (24.6) | 45 (24.3) | 41 (22.3) | |
| distant | 8 (1.1) | 2 (1.1) | 8 (4.3) | |
| unstaged | 5 (0.7) | 1 (0.5) | 0 (0.0) | |
| Type of surgery received | 0.467 | |||
| mastectomy | 227 (31.5) | 67 (36.2) | 62 (33.7) | |
| lumpectomy | 423 (58.8) | 98 (53.0) | 98 (53.3) | |
| both | 52 (7.2) | 13 (7.0) | 15(8.2) | |
| neither | 18 (2.5) | 7 (3.8) | 9 (4.9) | |
| Chemotherapy received | 0.05 | |||
| yes | 342 (47.5) | 82 (44.3) | 69 (37.5) | |
| no | 378 (52.5) | 103 (55.7) | 132 (63.5) | |
| Radiation therapy received | 0.04 | |||
| yes | 525 (72.9) | 123 (66.5) | 119 (64.7) | |
| no | 195 (27.1) | 62 (33.5) | 65 (35.3) | |
| Hormonal therapy received | 0.538 | |||
| yes | 472 (65.6) | 130 (70.3) | 115 (62.5) | |
| no | 232 (32.2) | 51 (27.6) | 66 (35.9) | |
| don’t know/not sure | 16 (2.2) | 4 (2.2) | 3 (1.6) | |
| Lymph node removal | 0.852 | |||
| Yes | 564 (78.3) | 148 (80.0) | 139 (75.5) | |
| No | 151 (21.0) | 36 (19.5) | 43 (23.4) | |
| Don’t know/not sure | 5 (0.7) | 1 (0.5) | 2 (1.1) | |
| Surgical Side Effects Scale (mean/median) | 7.9 (3.1) | 8.6 (3.6) | 9.2 (4.2) | <0.001 |
| Access to Medical Care Factors | ||||
| Unable to afford doctor past 12 months | 0.002 | |||
| yes | 21 (2.9) | 13 (7.0) | 15 (8.2) | |
| no | 699 (97.1) | 172 (93.0) | 169 (91.8) | |
| Unable to get prescription past 12 months | <0.001 | |||
| yes | 44 (6.1) | 29 (15.7) | 25 (13.6) | |
| no | 674 (93.6) | 156 (84.3) | 159 (86.4) | |
| did not need a prescription | 2 (0.3) | 0 (0.0) | 0 (0.0) | |
| 0 | 1 | 2+ | Chi-sq | |
| N (%) | N (%) | N (%) | p-value | |
| Access to Care Characteristics, continued | ||||
| Unable to afford breast cancer treatment | 0.082 | |||
| yes | 10 (1.4) | 5 (2.7) | 8 (4.3) | |
| no | 709 (98.5) | 179 (96.8) | 176 (95.7) | |
| don’t know/not sure | 1 (0.1) | 1 (0.5) | 0 (0.0) | |
| Lack of transportation | <0.001 | |||
| yes | 8 (1.1) | 10 (5.4) | 12 (6.5) | |
| no | 712 (98.9) | 175 (94.6) | 172 (93.5) | |
| Has a Primary care physician/clinic | 0.62 | |||
| yes, one place | 602 (83.6) | 159 (85.9) | 153 (83.2) | |
| yes, more than one place | 101 (14.0) | 25 (13.5) | 27 (14.7) | |
| no | 17 (2.4) | 1 (0.5) | 4 (2.2) | |
| Cancer-related Behavioral Risk Factors | ||||
| Physical activity in the past month | <0.001 | |||
| yes | 568 (78.9) | 125 (67.6) | 103 (56.0) | |
| no | 152 (21.1) | 60 (32.4) | 81 (44.0) | |
| Body Mass Index (BMI) | <0.001 | |||
| <25.0 | 251 (34.9) | 39 (21.1) | 41 (22.3) | |
| 25.0-29.9 | 257 (35.7) | 62 (33.5) | 58 (31.5) | |
| 30.0-34.9 | 137 (19.0) | 38 (20.5) | 40 (21.7) | |
| 35+ | 75 (10.4) | 46 (24.9) | 45 (24.5) | |
| Smoking status | 0.025 | |||
| current smoker | 68 (9.4) | 25 (13.5) | 22 (12.0) | |
| former smoker | 246 (34.2) | 63 (34.1) | 81 (44.0) | |
| never smoked | 406 (56.4) | 97 (52.4) | 81 (44.0) | |
| Alcohol use | 0.001 | |||
| 1 or fewer drinks/day (includes non-drinkers) | 566 (78.6) | 157 (84.9) | 165 (89.7) | |
| 2 or more drinks/day | 154 (21.4) | 27 (14.6) | 19 (10.3) | |
| Don’t know/not sure | 0 (0.0) | 1 (0.5) | 0 (0.0) | |
Using general linear regression models, we calculated crude and adjusted means for each RAND-36 QOL subscale by chronic disease burden group (Table 3). For each QOL subscale, higher disease burden was significantly associated with lower mean QOL score in unadjusted analyses (p<0.001). Survivors with a chronic disease burden score of 2+ had significantly lower unadjusted mean subscale scores than survivors with a score of 0 for general health status, physical functioning, role limitations due to physical problems, energy-fatigue, pain, emotional well-being, role limitations due to emotional problems, and social functioning. After adjustment for potential confounders, chronic disease burden was no longer significantly associated with emotional well-being (p=0.158) or role limitations due to emotional problems (p=0.267). However, CDB was still significantly associated with each of the physical and social functioning subscales even after adjustment for all covariates, although differences in the adjusted mean scores were reduced.
Table 3.
Crude and Adjusted Mean Scores for Eight RAND-36 Quality of Life Subscales for Female Breast Cancer Survivors in Missouri, One Year Post-Diagnosis.
| Chronic Disease Burden Alone Crude Means (95% CI) | p-value | Chronic Disease Burden + Covariates* Adjusted Means (95% CI) | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2+ | 0 | 1 | 2+ | |||
| General Health | 67.7 (66.6-68.8) | 60.1 (57.9-62.3) | 51.4 (49.2-53.7) | <0.001 | 65.7 (64.7-66.6) | 62.4 (60.5-64.3) | 57.1 (55.1-59.1) | <0.001 |
| Physical Functioning | 79.5 (77.8-81.2) | 67.5 (64.1-70.8) | 54.7 (51.3-58.0) | <0.001 | 75.4 (74.1-76.8) | 71.4 (68.8-74.1) | 66.5 (63.8-69.3) | <0.001 |
| Role limitations-Physical | 70.3 (67.4-73.2) | 57.7 (52.1-63.4) | 43.2 (37.5-48.9) | <0.001 | 65.9 (63.4-68.4) | 61.9 (57.0-66.8) | 56.3 (51.1-61.4) | 0.005 |
| Energy-Fatigue | 60.4 (58.7-62.0) | 52.0 (48.7-55.2) | 44.3 (41.1-47.6) | <0.001 | 57.3 (56.0-58.7) | 55.1 (52.5-57.7) | 53.1 (50.4-55.9) | 0.025 |
| Pain | 74.7 (72.9-76.4) | 65.2 (61.8-68.7) | 57.3 (53.8-60.8) | <0.001 | 71.6 (70.1-73.2) | 68.6 (65.6-71.6) | 65.8 (62.7-68.9) | 0.004 |
| Emotional Well-Being | 80.6 (79.3-81.8) | 76.7 (74.2-79.2) | 74.5 (72.0-77.0) | <0.001 | 78.6 (77.8-79.5) | 78.3 (76.7-80.0) | 80.4 (78.7-82.1) | 0.158 |
| Role limitations-Emotional | 81.9 (79.3-84.4) | 72.1 (67.0-77.1) | 66.8 (61.8-71.9) | <0.001 | 78.6 (76.5-80.7) | 74.7 (70.6-78.8) | 77.0 (72.7-81.3) | 0.267 |
| Social Functioning | 86.9 (85.2-88.6) | 79.0 (75.7-82.3) | 70.2 (66.9-73.6) | <0.001 | 84.0 (82.7-85.2) | 82.1 (79.6-84.6) | 78.7 (76.1-81.3) | 0.003 |
Covariates included in the multivariable model are socio-demographic variables (race, marital status, education, employment status, age, adequacy of income), cancer-related clinical variables (stage, type of surgery, receipt of chemotherapy, receipt of radiation therapy, currently receiving hormonal therapy, surgical side effects scale score, lymph node removal), access to care variables (insurance, having a usual source of primary care, unable to afford doctor in past 12 months, unable to afford medications in past 12 months, lack of transportation), psychosocial variables (depressive symptoms, social support, perceived stress), and cancer-related health behaviors (BMI, smoking, physical activity, alcohol use).
Discussion
At one year post-diagnosis, greater chronic disease burden was significantly associated with lower QOL mean scores for all eight domains in unadjusted analyses. Even after adjusting for a large number of relevant covariates, six of the eight QOL domains remained significantly associated with greater chronic disease burden. These findings highlight the impact of a breast cancer survivor’s underlying health on physical and social domains of QOL post-diagnosis and suggest the need to consider chronic health conditions as an important contextual characteristic of the patient during survivorship.
Our findings are largely consistent with previous research linking chronic disease to aspects of QOL among cancer survivors [6, 15, 40, 41]. However, a few of these studies examining the influence of comorbidity on QOL among cancer survivors were limited by their focus on older adults, use of different methods for assessing and “counting” comorbidity, not sufficiently controlling for potential confounders, and/or use of diverse measures of QOL (some including activities of daily living, instrumental activities of daily living, or performance status). Other studies focused on global or summary component measures of QOL or included a variety of cancer types together in their analyses, which might mask important associations between chronic disease burden and particular domains of QOL [6, 15, 41]. Perhaps to date, the most specific examination of the influence of comorbidity on QOL among cancer survivors was reported by Smith and colleagues [6]. Using the large sample of cancer survivors from the Surveillance, Epidemiology, and End Results-Medicare Health Outcomes Study (SEER-MHOS) data linkage, they reported on PCS and MCS scores by cancer site (for breast, prostate, colorectal and lung cancers) by time since diagnosis (0-1 year, 1-5 years, and 5+ years). Twelve common chronic health conditions were assessed as part of the MHOS. Approximately 85% of their overall sample had one or more of these chronic health conditions and the average age was over 75 years. Their analysis showed that among breast cancer survivors 65 years of age and older within one year of diagnosis, women who had 2 or more comorbidities had lower physical and mental component summary scores on the SF-36. Unfortunately, they had a limited number of confounding variables for which they were able to control, and by focusing on component summary scores of the SF-36, they potentially missed important differences in QOL domains that might lead to important clinical and behavioral intervention points for breast cancer patients at one year post-diagnosis. Our findings extend findings by Smith and colleagues by examining QOL in a sample that included younger, generally healthier, breast cancer survivors. We examined chronic disease burden using a validated measure that incorporates both presence of disease as well as severity rather than a simple enumeration of chronic conditions. We also add to the literature on comorbidity and QOL among breast cancer survivors by examining QOL domains separately. We found that chronic disease burden primarily impacts physical and social functioning domains of QOL compared with emotional domains, even after controlling for a broad range of other explanatory variables. Identifying which QOL domains are affected by existing health conditions is important for designing interventions to improve QOL for cancer survivors. Additionally, most differences in adjusted mean scores are greater than minimally important differences (2-5 points) that have been previously reported [42]. Only the differences in energy-fatigue by chronic disease burden group did not fall above the minimally important difference range of 2-5 points. This indicates that differences in QOL scores by chronic disease burden group are likely to be clinically meaningful suggesting that cancer survivors with chronic conditions may require ongoing surveillance and follow up care to prevent deficits in physical and social functioning even after treatment ends.
The finding that greater chronic disease burden is associated with deficits in physical and social functioning domains, but is not highly correlated with emotional domains is interesting. With the emotional subscales unadjusted differences in scores between disease burden groups were eliminated after controlling for clinical and psychosocial factors indicating that factors such as breast symptoms, depression, mental distress, or anxiety can explain the observed relationship between disease burden and emotional QOL domains [43]. On the other hand, with the physical and social domains differences in QOL that remained after controlling for the wide range of covariates that we assessed might indicate that precancer functioning or functioning at diagnosis might be important to functioning and QOL at one year post-diagnosis, that effects of treatment in the first year combined with higher chronic disease burden have significant impact on physical and social functioning, or that the physiologic impact of chronic disease burden on QOL plays a significant role in predicting QOL above and beyond other socio-demographic, clinical, behavioral, and psychosocial factors. It will be important to determine how these different mechanisms might be related to specific QOL subscales such as physical functioning, role limitations due to physical problems, and general health.
Our study has a few limitations worth noting. The response rate to the overall survey was low at 35.2%. We found that nonparticipants were more likely to be older, African American, and to live in the northwest part of Missouri than women who participated in the study. We might also speculate that nonparticipants were sicker overall either due to their cancer or other health conditions. Since breast cancer survivors who are older and African American may be more likely to have high chronic disease burden, we may have underestimated the effect of chronic health conditions on QOL among breast cancer survivors. We also did not have information about QOL prior to cancer diagnosis or at the time of diagnosis and were unable to separate out the effects of pre-existing deficits in functioning from those induced by breast cancer treatments. Also, although comorbidity measures, such as the Katz self-report instrument, are increasingly used in survey studies and have been shown to be accurate assessments of chronic health conditions [27], several common conditions that may impact QOL domains are not currently included in this co-morbidity index. Common chronic conditions such as osteoarthritis, back pain, and hypertension are less directly related to mortality, but would greatly impact QOL, particularly physical functioning. Additionally, self-reported comorbidity measures typically ask about the presence and severity of a condition (based on treatments or complications) but do not capture the patient’s perception of the burden of these comorbidities on their functioning or QOL nor allow us to identify physiologic aspects of the comorbidities that would directly impact physical functioning. This might be a particularly important distinction when considering QOL outcomes [44] and should be considered in future studies. Finally, we observed that there was a great deal of variability around the unadjusted and adjusted mean QOL scores for cancer survivors with chronic health conditions (1 or 2+) which may be due to the variability in perceptions of the impact of these health conditions on QOL or variability in treatments received for these conditions.
Despite these limitations, we have provided new evidence that chronic disease burden is an important correlate of QOL at one year post-diagnosis for female breast cancer survivors. Our results support the continued examination of chronic health conditions as an important contextual characteristic of cancer survivors. Additionally, we’ve shown that chronic disease burden is strongly related to physical components of QOL but less so to emotional components of QOL. These findings have significant clinical implications for the care and recovery of breast cancer patients. Processes of care for cancer survivors must include the assessment and evaluation of chronic health conditions not only at the time of cancer diagnosis and treatment, but also as part of ongoing medical surveillance of cancer patients to identify progression of existing conditions as well as the development of new ones. Whether the decrements in the physical aspects of QOL that we observed improve over time or are further impacted by additional newly diagnosed conditions are important questions that still need to be addressed in long term cancer survivorship studies. Additionally, the impact of low physical and social functioning on breast cancer survivors’ risk of recurrence or survival has yet to be studied. Improved planning and coordination of health care between the oncologist/surgeon and primary care physician will address existing and emerging health issues of survivors. Additionally, cancer researchers must continue to study the impact of chronic health burden on QOL particularly in defining those chronic health conditions that are predictive of QOL outcomes at different points on the survivorship continuum and the impact of those conditions over time so that researchers and physicians can identify modifiable targets for intervention at different phases of cancer recovery resulting in better short and long-term physical and mental health outcomes for all breast cancer survivors.
Acknowledgments
This research was supported in part by grants from the National Cancer Institute (CA112159, CA91842). The funders did not have any role in the design of the study; the analysis, and interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript. We thank Dr. Jeannette Jackson-Thompson and the staff of the Missouri Cancer Registry at the University of Missouri-Columbia for data collection; the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri, for the use of the Health Behavior, Communication, and Outreach Core; and Drs. Amy McQueen and Sandi Pruitt for review and comments on early versions of this manuscript.
All funding sources supporting the work and all institutional or corporate affiliations of the authors are acknowledged at the end of the text.
Footnotes
Conflict of Interest Statement
All authors of this manuscript have no commercial associations that might pose a conflict of interest in connection with the submitted article.
References
- 1.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK. SEER Cancer Statistics Review, 1975-2007. National Cancer Institute; 2010. [July 15, 2010]. http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 2.Ramsey S, Andersen M, Etzioni R, Moinpour C, Peacock S, Potosky AL. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88(6):1294–1303. [PubMed] [Google Scholar]
- 3.Cella DF, Cherin EA. Quality of life during and after cancer treatment. Compr Ther. 1988;14:69–75. [PubMed] [Google Scholar]
- 4.Ganz PA, Hirji K, Sim MS, Coscarellis Schag CA, Fred C, Polinsky ML. Predicting psychosocial risk in patients with breast cancer. Med Care. 1993;31:419–431. doi: 10.1097/00005650-199305000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Shimozuma K, Ganz PA, Petersen L, Hirji K. Quality of life in the first year after breast cancer surgery: rehabilitation needs and patterns of recovery. Breast Cancer Res Treat. 1999;56:45–57. doi: 10.1023/a:1006214830854. [DOI] [PubMed] [Google Scholar]
- 6.Smith AW, Reeve BB, Bellizzi KM, Harlan LC, Klabunde CN, Amsellem M, Bierman AS, Hays RD. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev. 2008;29(4):41–56. [PMC free article] [PubMed] [Google Scholar]
- 7.Reeve BB, Potosky AL, Smith AW, Han PK, Hays RD, Davis WW, Arora NK, Haffer SC, Clauser SB. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101(12):860–868. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazovich D, Robien K, Cutler G, Virnig B, Sweeney C. Quality of life in a prospective cohort of elderly women with and without cancer. Cancer. 2009;115(18 Suppl):4283–4297. doi: 10.1002/cncr.24580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 Suppl):2577–2592. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michael YL, Kawachi I, Berkman LF, Holmes MD, Colditz GA. The persistent impact of breast carcinoma on functional health status. Cancer. 2000;89(11):2176–2186. doi: 10.1002/1097-0142(20001201)89:11<2176::aid-cncr5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Baker F, Haffer SC, Denniston M. Health-related quality of life of cancer and noncancer patients in Medicare managed care. Cancer. 2003;97(3):674–681. doi: 10.1002/cncr.11085. [DOI] [PubMed] [Google Scholar]
- 12.Ganz PA, Guadagnoli E, Landrum MB, Lash TL, Rakowski W, Silliman RA. Breast cancer in older women: quality of life and psychosocial adjustment in the 15 months after diagnosis. J Clin Oncol. 2003;21(21):4027–4033. doi: 10.1200/JCO.2003.08.097. [DOI] [PubMed] [Google Scholar]
- 13.Engel J, Kerr J, Schlesinger-Raab A, Eckel R, Sauer H, Holzel D. Comparison of breast and rectal cancer patients’ quality of life: results of a four year prospective field study. Eur J Cancer Care (Engl) 2003;12(3):215–223. doi: 10.1046/j.1365-2354.2003.00414.x. [DOI] [PubMed] [Google Scholar]
- 14.Vacek PM, Winstead-Fry P, Secker-Walker RH, Hooper GJ, Plante DA. Factors influencing quality of life in breast cancer survivors. Qual Life Res. 2003;12(5):527–537. doi: 10.1023/a:1025098108717. [DOI] [PubMed] [Google Scholar]
- 15.Garman KS, Pieper CF, Seo P, Cohen HJ. Function in elderly cancer survivors depends on comorbidities. J Gerontol A Biol Sci Med Sci. 2003;58(11):1119–1124. doi: 10.1093/gerona/58.12.m1119. [DOI] [PubMed] [Google Scholar]
- 16.Janz NK, Mujahid M, Chung LK, Lantz PM, Hawley ST, Morrow M, Schwartz K, Katz SJ. Symptom experience and quality of life of women following breast cancer treatment. J Womens Health. 2007;16(9):1348–1361. doi: 10.1089/jwh.2006.0255. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed RL, Prizment DL, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: The Iowa Women’s Health Study. J Clin Oncol. 2008;26(35):5689–5696. doi: 10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowen D, Alfano C, McGregor B, Kuniyuki A, Bernstein L, Meeske K, Baumgartner K, Fetherolf J, Reeve B, Smith A, Ganz P, McTiernan A, Barbash R. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106:85–95. doi: 10.1007/s10549-006-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashing-Giwa KT, Lim JW. Examining the impact of socioeconomic status and socioecologic stress on physical and mental health quality of life among breast cancer survivors. Oncol Nurs Forum. 2009;36(1):79–88. doi: 10.1188/09.ONF.79-88. [DOI] [PubMed] [Google Scholar]
- 20.Wedding U, Röhrig B, Klippstein A, Pientka L, Höffken K. Age, severe comorbidity and functional impairment independently contribute to poor survival in cancer patients. J Cancer Res Clin Oncol. 2007;133(12):945–950. doi: 10.1007/s00432-007-0233-x. [DOI] [PubMed] [Google Scholar]
- 21.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25(14):1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 22.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 23.Yancik R, Wesley MN, Ries LAG, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 24.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration test of cognitive impairment. Am J Psychiatry. 1983;140(6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 25.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36) Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 27.Katz JN, Chang LC, Sangha O, Fosse lAH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Zheng Y, Zheng W, Gu K, Chen Z, Lu W, Shu XO. The effect of regular exercise on quality of life among breast cancer survivors. Am J Epidemiol. 2009;170(7):854–862. doi: 10.1093/aje/kwp209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med. 2005;40(6):702–711. doi: 10.1016/j.ypmed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Ness KK, Wall MM, Oakes JM, Robison LL, Gurney JG. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol. 2006;16(3):197–205. doi: 10.1016/j.annepidem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional limitations in elderly female cancer survivors. J Natl Cancer Inst. 2006;98(8):521–529. doi: 10.1093/jnci/djj130. [DOI] [PubMed] [Google Scholar]
- 32.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol. 2003;58(1):82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 33.Schag CA, Ganz PA, Polinsky ML, Fred C, Hirji K, Petersen L. Characteristics of women at risk for psychosocial distress in the year after breast cancer. J Clin Oncol. 1993;11:783–793. doi: 10.1200/JCO.1993.11.4.783. [DOI] [PubMed] [Google Scholar]
- 34.Collins KK, Liu Y, Schootman M, Aft R, Yan Y, Dean G, Eilers M, Jeffe DB. Effects of breast cancer surgery and surgical side effects on body image over time. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1077-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schootman M, Jeffe D, West M, Aft RL. Comparison of self-reported breast cancer treatment and medical records among older women. J Clin Epidemiol. 2005;58:1316–1319. doi: 10.1016/j.jclinepi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 37.Sherbourne C, Stewart A. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 38.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5(2):179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 39.Castellino SM, Casillas J, Hudson MM, Mertens AC, Whitton J, Brooks SL, Zeltzer LK, Ablin A, Castleberry R, Hobbie W, Kaste S, Robison LL, Oeffinger KC. Minority adult survivors of childhood cancer: a comparison of long-term outcomes, health care utilization, and health-related behaviors from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23(27):6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 40.Kurtz ME, Kurtz JC, Stommel M, Given CW, Given B. Predictors of physical functioning among geriatric patients with small cell or non-small cell lung cancer 3 months after diagnosis. Support Care Cancer. 1999;7:328–331. doi: 10.1007/s005200050270. [DOI] [PubMed] [Google Scholar]
- 41.Wedding U, Röhrig B, Klippstein A, Brix C, Pientka L, Höffken K. Co-morbidity and functional deficits independently contribute to quality of life before chemotherapy in elderly cancer patients. Support Care Cancer. 2007;15(9):1097–1104. doi: 10.1007/s00520-007-0228-9. [DOI] [PubMed] [Google Scholar]
- 42.Hays RD, Farivar SS, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD. 2004;2(1):63–67. doi: 10.1081/copd-200050663. [DOI] [PubMed] [Google Scholar]
- 43.Grov EK, Fosså SD, Dahl AA. Is somatic comorbidity associated with more somatic symptoms, mental distress, or unhealthy lifestyle in elderly cancer survivors? J Cancer Surviv. 2009;3:109–116. doi: 10.1007/s11764-009-0081-6. [DOI] [PubMed] [Google Scholar]
- 44.Bayliss EA, Ellis JL, Steiner JF. Subjective assessments of comorbidity correlate with quality of life health outcomes: initial validation of a comorbidity assessment instrument. Health Qual Life Outcomes. 2005;3:51. doi: 10.1186/1477-7525-3-51. [DOI] [PMC free article] [PubMed] [Google Scholar]


