Abstract
Background
Smoking and stress, pancreatic cancer (PanCa) risk factors, stimulate nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and catecholamines production respectively. NNK and catecholamine bind the β-adrenoceptors and induce PanCa cell proliferation; and we have previously suggested that β-adrenergic antagonists may suppress proliferation and invasion and stimulate apoptosis in PanCa. To clarify the mechanism of apoptosis induced by β2-adrenergic antagonist, we hypothesize that blockage of the β2-adrenoceptor could induce G1/S phase arrest and apoptosis and Ras may be a key player in PanCa cells.
Results
The β1 and β2-adrenoceptor proteins were detected on the cell surface of PanCa cells from pancreatic carcinoma specimen samples by immunohistochemistry. The β2-adrenergic antagonist ICI118,551 significantly induced G1/S phase arrest and apoptosis compared with the β1-adrenergic antagonist metoprolol, which was determined by the flow cytometry assay. β2-adrenergic antagonist therapy significantly suppressed the expression of extracellular signal-regulated kinase, Akt, Bcl-2, cyclin D1, and cyclin E and induced the activation of caspase-3, caspase-9 and Bax by Western blotting. Additionally, the β2-adrenergic antagonist reduced the activation of NFκB in vitro cultured PanCa cells.
Conclusions
The blockage of β2-adrenoceptor markedly induced PanCa cells to arrest at G1/S phase and consequently resulted in cell death, which is possibly due to that the blockage of β2-adrenoceptor inhibited NFκB, extracellular signal-regulated kinase, and Akt pathways. Therefore, their upstream molecule Ras may be a key factor in the β2-adrenoceptor antagonist induced G1/S phase arrest and apoptosis in PanCa cells. The new pathway discovered in this study may provide an effective therapeutic strategy for PanCa.
Keywords: β-adrenergic antagonists, G1/S phase arrest, apoptosis, Ras
Introduction
Pancreatic cancer (PanCa) remains a lethal disease [1]. There is increasing evidence suggesting that many factors such as smoking, stress, chronic depression and a high-fat diet, with cardiovascular disease and stress patients may contribute to PanCa genesis and development but the underlying mechanisms are not clear [2-4]. Previous studies indicate that the enhanced tumour progression by smoking-stimulated nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) production and stress-stimulated autonomic activation of nervous system [5,6]. The autonomic activation of nerve system results in the release of catecholamines from the adrenal gland and sympathetic nerve terminals. Further studies suggest that both NNK and constantly high level of catecholamines modulate the activity of multiple components of the tumour microenvironment and consequently promote tumour-cell growth via β-adrenoceptors [4,7-9].
β-adrenoceptors are members of the superfamily of G protein-coupled adrenergic receptors, which mediate actions of the endogenous catecholamines in a variety of target cells [10,11]. β1- and β2-adrenoceptors have been found to be expressed in the BxPC-3, MIA PaCa-2, and Panc-1 cell lines [12-14]. NNK functions as a β-adrenergic agonist and it has been shown that the binding of NNK or catecholamines to the β-adrenoceptors induce PanCa cell proliferation by activating the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) pathways in PanCa cells [15]. The consequence of PKA signalling leads to the transcriptional activation of proteins involved in proliferation via cAMP response element binding protein (CREB), activator protein 1 (AP-1) or NF-κB [15-17]. Recent reports have shown that the agonists of the β2-adrenoceptor stimulate the activation of Ras and Src tyrosine kinases via the mitogen-activated protein kinase (MAPK) pathway in cancer cells and fibroblasts [18-20]. Ras activates several signalling pathways that lead to transcriptional activation of genes related to cell proliferation and antiapoptotic signalling cascades, including the Raf/MEK/extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K)/ATP-dependent tyrosine kinases (Akt)/Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) pathways and NF-κB [17,21].
Previous studies suggested that β-adrenergic antagonists may suppress cell proliferation and invasion and induce apoptosis in PanCa [14,22], and also β2-adrenergic agonist can stimulate the production of cAMP and activation of G-protein effectors Gs [23]. However, the mechanism of PanCa cell death induced by β2-adrenergic antagonist is not clear. In this study, we determined the effects of β2-adrenergic antagonist ICI118,551 on PanCa tumor growth and the underlying mechanism in vitro and in vivo.
Materials and methods
Tumor tissues, Cell lines and cell culture
Forty-eight pancreatic carcinoma specimens were obtained from the Department of Hepatobiliary and Pancreas Surgery, the First Affiliated Hospital of Xi'an Jiaotong University. The protocol was approved by The Human Research Review Committee at the University Hospital. The human ductal pancreatic adenocarcinoma cell lines, MIA PaCa-2 and the BxPC-3 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The MIA PaCa-2 cell line harbours an activating point mutation in codon 12 of the k-ras gene, whereas BxPC-3 does not have that k-ras mutation [24]. The cells were cultured in DMEM (Gibco, Grand Island, NY, USA) with 10% (v/v) heat-inactivated FBS (Gibco, Grand Island, NY, USA), penicillin (100 U/ml) and streptomycin (100 mg/ml)at 37°C with 5% CO2 and 95% relative humidity. Before each experiment, cells were seeded at a density of 5 × 104 cells/cm2.
Immunohistochemistry staining
Immunohistochemical staining for β1-adrenoceptor and β2-adrenoceptor were performed using the SABC kit according to the manufacturer's instruction (Abfrontier, Korea). The sections were incubated with primary antibodies (Abcam, Cambridge, MA, USA) overnight at 4°C and then incubated with the appropriate biotinylated secondary antibody (Abcam, Cambridge, MA, USA) for 30 min at room temperature. Sections were then incubated with avidin/biotin complexes for 30 min at room temperature. Following washing in PBS, immunoreactivity was visualized by using DAB reaction followed by hematoxylin counter staining. For evaluation of protein expression, the staining intensity was graded as 0 (negative), 1 (weak), 2 (medium) or 3 (strong). The slides were graded by two investigators. The densitometry analysis of immunohistochemical staining was performed using the Image-Pro Plus 4.5 software.
Cell cycle analysis
The cells after treatment were stained with propidium iodide (PI) and then analysed using flow cytometry for cell cycle according to the manufacturer's protocol. After stimulation for 24 h with ICI118,551 (100 μM) [13,14,25] (Sigma Chemical, St. Louis, MO, USA), MIA PaCa-2 and BxPC-3 cells were fixed in ice-cold 70% ethanol at 4°C. On the day of analysis, the cells were then centrifuged (400 × g for 5 min) and washed once in staining buffer. After centrifugation, the cells were resuspended in 100 μL of staining buffer. The cells were then treated with RNase A and incubated for 30 min at 37°C. After RNase A digestion, the cells were stained for 30 min at room temperature (protected from light) with 10 μg of PI in a final volume of 0.5 mL of staining buffer. The samples were then analysed by flow cytometry. The percentages of cells in G0-G1, S, and G2-M were calculated using the ModFit computer program.
Animal studies and subrenal capsular assay
BALB/c athymic nude mice, 4 to 6 weeks old (Shanghai Experimental Animal Center (Chinese Academy of Sciences, China) were used for the subrenal capsule assay. PanCa xenografts were generated by implantation of MIA PaCa-2 and BxPC-3 cells into one flank of athymic nude mice. Each group contains n ≥ 8 male athymic nude mice (10 mice each group). On the day of the experiment, mice were anesthetized, and the left kidney was exposed. The same number of cells (1 × 108) in 25 μl was then injected into each mouse under the left renal capsule. After three days, a 5 mg/kg/dose of ICI118,551 was intraperitoneally injected into the mice every day. After inoculation with the tumor cells, mice were observed and sacrificed on day ten, when their left kidneys were excised. The left renal capsule were fixed in 10% neutral buffered formalin and processed in paraffin. Sections were cut on a microtome and mounted on glass slides. For histopathology, routine H&E staining was carried out. Animal care and experiments were carried out in accordance with the guidelines of the Xi'an Jiaotong University.
Electron microscopy
To evaluate the morphological features of cell death, the cells in the control group or cells treated with or without ICI118,551 (100 μM) were processed for transmission electron microscopy (TEM). Briefly, cells were pelleted by centrifugation and fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4 for 1 h at 4°C. After rinsing with cacodylate buffer, the specimens were fixed in 1% cacodylate-buffered osmium tetroxide for 2 h at room temperature, dehydrated in a graded series of ethanol, briefly transferred to propylene oxide and embedded in Epon-Araldite. Ultrathin sections (60-80 nm thick) were cut with a diamond knife, placed on formvar-carbon coated copper grids (200 mesh), stained with uranyl acetate and lead citrate and observed with a Jeol 100 SX TEM.
Determination of cell apoptosis by hoechst 33342 fluorescent staining
The cell death was assessed using Hoechst staining. Briefly, cells were seeded in 24-well plates at 5 × 104 cells/well and treated with ICI118,551 (100 μM) for 24 h. After treatment, cells were stained with 10 μg/ml Hoechst 33342 dye (Sigma Chemical, St. Louis, MO, USA). Fixed cells were incubated overnight at 4°C and visualized under a 365 nm UV light microscope. Quantitative analysis was performed by counting the blue fluorescent (apoptosis positive) cells from three independent fields at 400× magnification. Values were expressed as the percentage of apoptotic cells relative to the total number of cells per field.
Analysis of the apoptosis rate by Annexin V-FITC/PI
The apoptosis rate was measured using Annexin V-FITC/PI double staining followed by flow cytometry (FCM) according to the instructions (BD Biosciences, San Jose, CA, USA). After treatment with metoprolol (25-200 μM) and ICI118,551 (25-200 μM) for 24 h, MIA PaCa-2 and BxPC-3 cells were washed once with ice-cold PBS and resuspended in binding buffer at a concentration of 1 × 106 cells/ml. A total of 5 μL of Annexin V-FITC and 10 μL of 20 mg/mL PI were added to the cells, and the mixture was incubated for 15 min in the dark prior to the addition of 400 μl of PBS. Quantitative analysis of the apoptotic level was performed using a flow cytometer (BD Biosystems, CA, USA).
Electrophoretic mobility shift assays (EMSA)
The activity of NF-κB in PanCa cells was determined using An EMSA. Binding reaction mixtures (20 μl) containing 3 μl of nuclear extract protein (2-5 μg/μl), 1 μl of poly (dI-dC) in 10 mM Tris and 1 mM EDTA (1 μg/μl), 1 μl of MgCl2 (100 mM), 2 μl of 25 mM DTT/2.5% Tween-20, 1 μl of 1% NP-40, 2 μl of 10 × Binding Buffer (100 mM Tris, 500 mM KCl, 10 mM DTT, pH 7.5), 1 μl of IRDye™ end labelled oligo probe (50 nM) (LI-COR Biosciences, Lincoln, NE, USA) and 9 μl of Ultra Pure water were incubated for 30 minutes at room temperature. Proteins were separated by electrophoresis in a native 5% polyacrylamide gel at 4°C in running buffer (12.5 mM Tris borate, 0.25 mM EDTA; pH 8.0), then imaged using the LI-COR Bioscience Odyssey Imaging System according to the manufacturer's instructions. The sequence of the NF-κB-specific probe was 5'-AGT TGA GGG GAC TTT CCC AGG C-3'.
Western blotting analysis
The MIA PaCa-2 and BxPC-3 cells were treated with metoprolol (100 μM) and ICI118,551 (100 μM) for 24 h. The proteins were then extracted from the treated cells. The supernatant was collected and the total protein concentration was measured using the BCA assay kit (Pierce, Rockford, IL, USA) according to the manufacturer's instructions. The total proteins were used for Western blotting analysis using specific antibodies against caspase-3, caspase-9, Bcl-2, Bax, cyclin D1, cyclin E (Abcam, Cambridge, MA, USA), pERK, ERK, pAkt, and Akt (Cell Signal Technology, Beverly, MA, USA). Thirty μg of total protein each sample was separated by 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and the protein was electrically transferred onto a nitrocellulose membrane (Millipore, Bedford, MA, USA). The membrane was then blocked with TBST (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Tween-20) containing 5% w/v non-fat dry milk and incubated with the primary antibody in TBST at 4°C overnight. The membrane was washed three times and was then incubated with the secondary antibody for 2 h at room temperature. After washing three times for 10 min each with 15 ml of TBST, signallings were visualised by enhanced chemiluminescence (Pierce, Rockford, IL, USA) associated fluorography.
Statistical analysis
The data were analysed by one-way ANOVA, and by the nonparametric Kruskal-Wallis test. The relationship between β-AR expression and clinical-pathologic character were analysed by Chi-square test. Values were expressed as the mean ± SEM and the differences were considered statistically significant at P < 0.05.
Results
Expression of β-adrenoceptors in PanCa cell lines and clinical samples
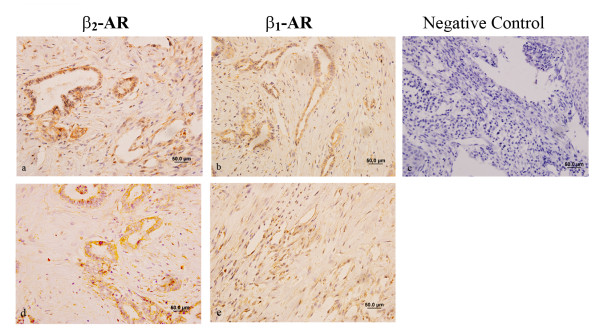
Our previous study showed that β1- and β2-adrenergic receptors were expressed in PanCa cells by Real-time RT-PCR and Western blotting [14]. In the current study, we further determined the expression of β1- and β2-adrenergic receptors in pancreatic carcinoma specimens using immunohistochemical staining. As shown in Figure 1, PanCa cells in biopsy specimens express bothβ1-AR and β2-AR on the membrane in a large number of epithelial cells and the β-ARs are localized in the plasma membrane of pancreatic cancer cells, and the representative images are shown in Figure 1. The 48 pancreatic carcinoma specimen samples from 30 males and 18 females include 18 poorly differentiated pancreatic carcinoma samples, 23 moderately differentiated pancreatic carcinoma samples, and 7 well-differentiated pancreatic carcinoma samples. The relationship between β-AR expression and clinical-pathologic character is showed in the Table 1.
Figure 1.
Expression levels of β1 and β2-adrenoceptor in the human ductal pancreatic adenocarcinoma cells. Two representative immunohistochemical staining of PanCa cells presented from 48 pancreatic carcinoma specimen samples (expressions of β1-adrenergic receptor [b and e], the expressions of β2-adrenergic receptor [a and d] and the negative control [c] in).
Table 1.
The relationship between β-AR expression and clinical-pathologic character
| Clinical and pathologic character | β1-AR expression score | β2-AR expression score |
|---|---|---|
| Pathology | ||
| Well-differentiated (7) | 3.2 | 2.5 |
| Moderately differentiated (23) | 4.9 | 4.3 |
| Poorly differentiated (18) | 5.4 | 5.3 |
| TNM stage | ||
| I phase (7) | 1.6 | 1.5 |
| II phase (9) | 3.9 | 3.4 |
| III phase (19) | 5.5* | 5.9* |
| IV phase (13) | 7.8* | 7.9* |
* P < 0.05 Analysed by Chi-square test.
Cell cycle analysis after β-adrenergic antagonist treatment
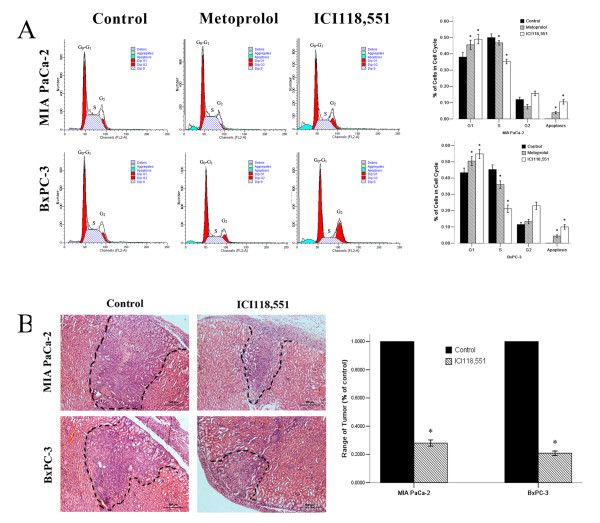
Our early published study showed the optimal concentrations of ICI118,551 and metoprolol for PanCa cell inhibition [14]. In brief, MIA PaCa-2 and BxPC-3 cells were treated with various concentrations from 25 to 200 μM of ICI118,551 and metoprolol for 24 h, the maximal response was obtained with a range of 100 - 200 μM ICI118,551 and metoprolol [14]. In this study, we used 100 μM of β2-adrenoceptor antagonist to examine the effect on the cell cycle phase distribution of MIA PaCa-2 and BxPC-3 cells. Based on the data of cell cycle analysis, we found a prolonged and prominent delay in progression from G0-G1 phase after the cells treated with β-adrenoceptor antagonists (Figure 2A) (G0-G1 phase: Control < Metoprolol < ICI118,551, P < 0.05), a decrease at the S phase (S phase: Control > Metoprolol > ICI118,551, P < 0.05). Moreover, sub-G1 apoptotic compartment was also observed in MIA PaCa-2 and BxPC-3 cells (sub-G1 apoptotic compartment: Control < Metoprolol < ICI118,551, P < 0.05). The percentages of G0-G1 phase increased, and the percentages of S phase decreased significantly in the cells exposed to ICI118,551 compared with metoprolol treatment groups (P < 0.05). The effect of the ICI118,551 on G1/S phase arrest was greater in BxPC-3 cells than in MIA PaCa-2 cells (P < 0.05), suggesting a lower sensitivity to β2-adrenoceptor antagonist in the cells containing an activating k-ras mutation (Figure 2A).
Figure 2.
β-adrenoceptor antagonists regulate cell cycle distribution in vitro cultured PanCa cells and limited prevented tumour progression in vivo. The cell cycle distribution of PanCa cells in response to β-adrenoceptor antagonists (A), MIA PaCa-2 and BxPC-3 cells were untreated (control) or treated with β-adrenoceptor antagonists: 100 μM metoprolol and 100 μM ICI118,551 for 24 h. β-adrenoceptor antagonists limited tumor development in BALB/c athymic nude mice (B), the black dashed line was used to label the tumor range. H&E staining statistical graph of tumor range showed that the proliferation of PanCa cells was strongly suppressd in renal capsule xenografts after ICI118,551 treatment compared to non-treated cells (P < 0.05). *P < 0.05 when compared with controls.
Subrenal capsular assay
In the subrenal capsular assay, PanCa cells maintained a solid structure and 3-dimensional growth under the kidney capsule. The implanted tumors could invade the kidney parenchyma, as demonstrated by detection of the tumor cells surrounding the renal glomeruli and tubules. As shown in Figure 2B, the implanted tumors are characterized by malignant growth in vivo, including frequent mitosis, neovascularization and invasion into normal tissues. H&E staining results showed that the proliferation of PanCa cells was strongly suppressed in the renal capsule xenografts in mice after ICI118,551 treatment, the range and the depth of invasion was significantly inhibited compared to the non-treated cells (Figure 2B, P < 0.05).
Cell apoptosis analysis after β-adrenergic antagonist treatment
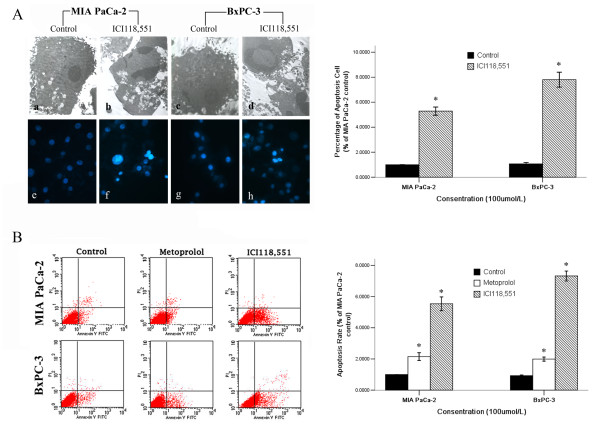
To investigate the morphological features of cellular death induced by β-adrenergic antagonists, MIA PaCa-2 and BxPC-3 cells were analysed by TEM (Figure 3A). Untreated MIA PaCa-2 and BxPC-3 cells exhibited typical ultrastructure that was characterised by a well-preserved plasma membrane, a nucleus with finely granular and uniformly dispersed chromatin, and a cytoplasm containing randomly distributed organelles and electron-dense granules (a and c). When cells were exposed to ICI118,551 for 24 h, unequivocal signs of apoptosis were detected. The morphological changes observed in these cells included a progressive margination and condensation of the chromatin abutting the inner nuclear envelope, nuclear fragmentation and cytoplasm shrinkage (b and d).
Figure 3.
β-adrenoceptor antagonists induce PanCa cell death. The morphological features of cell death (necrosis and/or apoptosis) were analyzed by using TEM (A [a-d]×5000). MIA PaCa-2 and BxPC-3 cell induced cell death by ICI118,551 (100 μM) was further confirmed by Hoechst 33342 fluorescent staining (A [e-h]×400), BxPC-3 cells were more sensitive to apoptosis induction than MIA PaCa-2 cells (P < 0.05). The apoptotic MIA PaCa-2 and BxPC-3 cells were detected by flow cytometry (B). MIA PaCa-2 and BxPC-3 cells were treated with β-adrenoceptor antagonists (100 μM). Exposure to the β2-adrenergic antagonist ICI118,551 significantly increased cell apoptosis compared to the metoprolol group (P < 0.05), BxPC-3 cells were more sensitive to apoptosis induction than MIA PaCa-2 cells(P < 0.05) (B). *P < 0.05 when compared with controls.
In this study, the effect of ICI118,551 was examined in PanCa cells by Hoechst staining (Figure 3A). Figure 3A shows the characteristic appearance of the apoptotic PanCa cells after treatment with ICI118,551 for 24 h. The ICI118,551 treatment resulted in a significant increase in the number of apoptotic cells (f and h in Figure 3A), compared with basal apoptosis levels in untreated controls (e and g in Figure 3A). The percentage of apoptotic cells in the ICI118,551-treated group was statistically different from the untreated control cells (P < 0.05).
The percentages of early apoptotic cells increased significantly after treatment with metoprolol (100 μM) and ICI118,551 (100 μM) in MIA PaCa-2 and BxPC-3 cells (Figure 3B). Additionally, exposure to the β2-adrenergic antagonist ICI118,551 significantly increased cell apoptosis when compared to the group treated with the β1-adrenergic antagonist metoprolol. BxPC-3 cells, which contain the wild-type k-ras gene, were more sensitive to apoptosis induction than MIA PaCa-2 cells, which harbour an activation point mutation in codon 12 of the k-ras gene (P < 0.05).
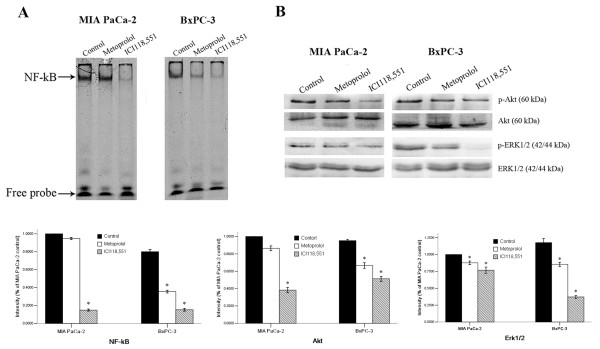
Detection of ERK1/2 and Akt phosphorylation and NF-κB activation
We next investigated whether the effect of ICI118,551 on MIA PaCa-2 and BxPC-3 cells is associated with the inhibition of NF-κB activation and the induction of ERK, and Akt dephosphorylation. The EMSA and WB results showed that ICI118,551 completely suppressed NF-κB activation and significantly inhibited, ERK1/2 and Akt phosphorylation in MIA PaCa-2 and BxPC-3 cells after 24 h of treatment (Figure 4). We also found that the β2-adrenergic antagonists inhibited NF-κB activation, ERK1/2 and Akt phosphorylation more significantly than metoprolol in MIA PaCa-2 and BxPC-3 cells (Figure 4).
Figure 4.
β-adrenoceptor antagonists limited the activation of NF-κB, ERK, and Akt. The NF-κB activity in PanCa cells in response to β2-adrenoceptor antagonists (A) and the phosphorylation state of ERK and Akt in PanCa cells in response to β2-adrenoceptor antagonists (B) *P < 0.05 when compared with controls.
The relationship of β-adrenoceptor antagonist treatment with the expression of caspase-3, caspase-9, Bcl-2, Bax, cyclin D1, and cyclin E
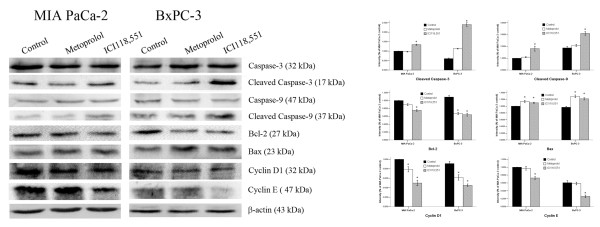
The caspase family proteases, caspase-3, caspase-9, Bcl-2, and Bax, have been reported to be involved in apoptosis. In this current study, caspase-3, caspase-9, Bcl-2, and Bax were detected by Western blotting in MIA PaCa-2 and BxPC-3 cells after treatment with metoprolol (100 μM) and ICI118,551 (100 μM) for 24 h. As shown in Figure 5, the cleavage of caspase-3, caspase-9, and Bax increased after treatment with β-adrenergic antagonists compared to the controls; ICI118,551 yielded more prominent bands than metoprolol. However, Bcl-2 decreased after treatment with β-adrenergic antagonists. These results suggested that the β-adrenergic antagonists affect the expression levels of Bax, caspase-3, and caspase-9 in MIA PaCa-2 and BxPC-3 cells.
Figure 5.
Expression of caspase-3, caspase-9, Bcl-2, Bax, cyclin D1 and cyclin E in MIA PaCa-2 and BxPC-3 cells in response to β-adrenoceptor antagonists. A negative control group is also included. The expression of the respective proteins in the whole cell lysates was examined by Western blotting. β-adrenoceptor antagonists increased the level of caspase-3, caspase-9, and Bax, while they negatively affected Bcl-2, cyclin D1, and cyclin E. *P < 0.05 when compared with controls.
We also examined the effects of ICI118,551 on the regulatory proteins responsible for cell cycle progression. The protein levels of cyclin D1 and cyclin E were decreased in the two cell lines treated with β-adrenergic antagonists. These results indicate that β2-adrenergic antagonists have an effect on some of the cell cycle regulatory proteins responsible for the G1/S phase transition and on cell cycle progression (Figure 5).
Discussion
In this study, we have demonstrated that β-adrenoceptors are expressed in PanCa cells. Our data suggested that the β-adrenergic antagonists were associated with the inhibition of PanCa cell proliferation and the induction of apoptosis and G1/S phase arrest. In addition, these effects mediated by the β2-adrenergic antagonist were associated with the decreased expression of pERK1/2, pAkt, and the activation of NF-κB. Furthermore, we have found that the β2-adrenergic antagonist ICI118,551 significantly increased PanCa cell apoptosis and G1/S phase arrest. The effects of ICI118,551 on the rate of apoptosis and on G1/S phase arrest were stronger than the β1-adrenoceptor antagonist metoprolol.
The G1/S phase transition is a particularly important cell cycle control point. The cyclin D1 is a critical cell cycle regulatory protein and required for the progression through G1 to S phase [17,26]. The growth stimulatory signals in the form of increasing levels of cyclin D1 are received by the cells in the early stage of G1, which are then followed by increasing levels of cyclin E at the later stage [26]. ICI118,551 could remarkably down-regulate cyclin D1 and cyclin E expression, indicating that the negatively regulatory effect of the ERK and Akt pathway on the PanCa cell might be associated with the inhibition of NF-κB activation, a decrease in cyclin D1 expression and a delay in the G1/S transition, and cell cycle process. Our data has demonstrated that ICI118,551 could disengage the prevention of apoptosis in PanCa cells via suppression of the ERK and Akt pathways and NF-κB activation, and consequently limited the activation of Bax, caspase-9, and caspase-3. The ERK and Akt pathway can phosphorylate Bad, which allows Bcl-2 to form homodimers that result in the generation of an antiapoptotic response. This phosphorylation allows Bcl-2, Bcl-XL, and Mcl-1 to bind Bax and prevent its activation [27-29].
The Ras pathway is commonly altered in PanCa. Activating point mutations in the K-ras gene are common in ductal pancreatic carcinomas. NNK induces activating point mutations in the ras gene, so it is conceivable that the binding of NNK to β2- adrenoceptors may stimulate this pathway via further activation of the Src tyrosine kinase and Ras. The effects of the β2-adrenergic antagonist ICI118,551 on the G1/S phase arrest and apoptosis are stronger in the BxPC-3 cells (expressing wild-type k-ras) than in the MIA PaCa-2 cells containing a k-ras mutation. Therefore, it suggests that k-ras may be a key molecule involved in the blockage of the β-adrenoceptor resulting in G1/S arrest and apoptosis in PanCa cells. Previous study suggested that β2-adrenergic receptor antagonist induces cell death by inhibiting the Ras/Raf/Erk1/2 and PI3K/Akt pathways and mitochondrial death pathway [30]. Our results demonstrated that β2-adrenergic receptor antagonist induced cell death in MIA PaCa-2 and BxPC-3 cells is independent of the mitochondrial Apaf-1/caspase-9 pathway.
Strategies to block β-adrenoceptors might be used to prevent cancer in smokers and stress patients, and for the prevention of cancer relapse and metastasis after conventional chemotherapy or surgical resection [4,15]. Considering that the β2-adrenergic antagonists could be developed as therapeutic and preventive agents, we suggest that understanding the relationship between β2-adrenoceptor signalling and G1/S phase arrest and apoptosis may provide a new strategic therapy for pancreatic cancer.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DZ, ZW, MZ, and KG carried out the experimental work, FW provided data analysis, QM provided tumor samples, clinical information, and histopathological analysis, DZ, ZW, QM, FW, and EW designed the study and participated in writing the paper. All authors read and approved the manuscript.
Contributor Information
Dong Zhang, Email: pavilion1021@hotmail.com.
Qingyong Ma, Email: qyma56@mail.xjtu.edu.cn.
Zheng Wang, Email: zheng.wang11@mail.xjtu.edu.cn.
Min Zhang, Email: sun.1978@stu.xjtu.edu.cn.
Kun Guo, Email: guokun@stu.xjtu.edu.cn.
Fengfei Wang, Email: fengfei.wang@ndsu.edu.
Erxi Wu, Email: Erxi.Wu@ndsu.edu.
Acknowledgements
The authors acknowledge the staff of the Institution of Genetic Disease Research and Dr. Yue Wu of the Institution of Cardiovascular Disease Research, Xi'an Jiaotong University for their technical assistance. This work was supported by grants from the National Natural Science Foundation of China (No. 81172360), a 13115 major project of Shaanxi province (#2010ZDKG-49) (QM), ND EPSCoR (EW) and Pilot Project Grant (EW) from the Centers of Biomedical Research Excellence (COBRE) grant NIH P20 RR020151 from the National Center for Research Resources (NCRR). NCRR is a component of the National Institutes of Health (NIH). The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH or NCRR).
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Wadei HA, Schuller HM. Nicotinic receptor-associated modulation of stimulatory and inhibitory neurotransmitters in NNK-induced adenocarcinoma of the lungs and pancreas. J Pathol. 2009;218:437–445. doi: 10.1002/path.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R, Rizwani W, Banerjee S, Kovacs M, Haura E, Coppola D, Chellappan S. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PLoS One. 2009;4:e7524. doi: 10.1371/journal.pone.0007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Pena GA, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra Vi, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;2:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WK, Wong HP, Luo SW, Chan K, Huang FY, Hui MK, Lam EK, Shin VY, Ye YN, Yang YH, Cho CH. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone from cigarette smoke stimulates colon cancer growth via beta-adrenoceptors. Cancer Res. 2005;65:5272–5277. doi: 10.1158/0008-5472.CAN-05-0205. [DOI] [PubMed] [Google Scholar]
- Schuller HM. Neurotransmission and cancer: implications for prevention and therapy. Anticancer Drugs. 2008;19:655–671. doi: 10.1097/CAD.0b013e3283025b58. [DOI] [PubMed] [Google Scholar]
- McGraw DW, Liggett SB. Molecular mechanisms of β2-adrenergic receptor function and regulation. Proc Am Thorac Soc. 2005;2:292–296. doi: 10.1513/pats.200504-027SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller HM, Al-Wadei HA. Neurotransmitter receptors as central regulators of pancreatic cancer. Future Oncol. 2010;6:221–228. doi: 10.2217/fon.09.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weddle DL, Tithoff P, Williams M, Schuller HM. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473–479. doi: 10.1093/carcin/22.3.473. [DOI] [PubMed] [Google Scholar]
- Zhang D, Ma QY, Hu HT, Zhang M. Beta2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NF-kappaB and AP-1. Cancer Biol Ther. 2010;10:19–29. doi: 10.4161/cbt.10.1.11944. [DOI] [PubMed] [Google Scholar]
- Schuller HM. Mechanisms of smoking-related lung and pancreatic adenocarcinoma development. Nat Rev Cancer. 2002;2:455–463. doi: 10.1038/nrc824. [DOI] [PubMed] [Google Scholar]
- Askari MD, Tsao MS, Schuller HM. The tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through beta-adrenergic transactivation of EGF receptors. J Cancer Res Clin Oncol. 2005;131:639–648. doi: 10.1007/s00432-005-0002-7. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, David TM, Michele M, Agostino T, Franca S, Massimo L, Jorg B, Camilla E, Alberto MM, Richard AF. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi M, Al-Wadei HA, Takahashi T, Schuller HM. Nongenomic beta estrogen receptors enhance beta1 adrenergic signaling induced by the nicotine-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human small airway epithelial cells. Cancer Res. 2007;67:6863–6871. doi: 10.1158/0008-5472.CAN-07-0483. [DOI] [PubMed] [Google Scholar]
- Vargiu P, De Abajo R, Garcia-Ranea JA, Valencia A, Santisteban P, Crespo P, Bernal J. The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors. Oncogene. 2004;23:559–568. doi: 10.1038/sj.onc.1207161. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin FT, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. β-Arrestin-dependent formation of β2 adrenergic receptor-src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Ciraolo E, Ghigo A, Costa C. Taming the PI3K team to hold inflammation and cancer at bay. Pharmacol Therapeut. 2008;118:192–205. doi: 10.1016/j.pharmthera.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Zhang D, Ma Q, Shen S, Hu H. Inhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction: the study of beta-adrenoceptor antagonist's anticancer effect in pancreatic cancer cell. Pancreas. 2009;38:94–100. doi: 10.1097/MPA.0b013e318184f50c. [DOI] [PubMed] [Google Scholar]
- Hu HT, Ma QY, Zhang D, Shen SG, Han L, Ma YD, Li RF, Xie KP. HIF-1alpha links beta-adrenoceptor agonists and pancreatic cancer cells under normoxic condition. Acta Pharmacol Sin. 2010;31:102–110. doi: 10.1038/aps.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Thorpe J, Ito H, Ashley SW, Whang EE. Differential display of expressed genes in pancreatic cancer cells. Biochem Biophys Res Commun. 2002;293:391–395. doi: 10.1016/S0006-291X(02)00237-1. [DOI] [PubMed] [Google Scholar]
- Chen ZB, Liu C, Chen FQ, Li SY, Liang Q, Liu LY. Effects of tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) on the activation of ERK1/2 MAP kinases and the proliferation of human mammary epithelial cells. Environ Toxicol Pharmacol. 2006;22:283–291. doi: 10.1016/j.etap.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Foster I. Cancer: A cell cycle defect. Radiography. 2008;14:144–149. doi: 10.1016/j.radi.2006.12.001. [DOI] [Google Scholar]
- Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, Guri T. Raf kinases: Function, regulation and role in human cancer. Biochim Biophys Acta. 2007;1773:1196–1212. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyden L, Adams DJ. The Ras-association domain family (RASSF) members and their role in human tumourigenesis. Biochim Biophys Acta. 2007;1776:58–85. doi: 10.1016/j.bbcan.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard JC, Lefloch R, Pouysségur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773:1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–2614. doi: 10.1182/blood.V98.9.2603. [DOI] [PubMed] [Google Scholar]