Abstract
Since its identification in April 2009 an A(H1N1) virus containing a unique combination of gene segments from both North American and Eurasian swine lineages has continued to circulate in humans. The 2009 A(H1N1) virus is distantly related to its nearest relatives, indicating that its gene segments have been circulating undetected for an extended period. Low genetic diversity among the viruses suggests the introduction into humans was a single event or multiple events of similar viruses. Molecular markers predicted for adaptation to humans are not currently present in 2009 A(H1N1) viruses, suggesting previously unrecognized molecular determinants could be responsible for the transmission among humans. Antigenically the viruses are homogeneous and similar to North American swine A(H1N1) viruses but distinct from seasonal human A(H1N1).
Influenza pandemics occur when an influenza virus with a hemagglutinin (HA), against which there is little or no existing immunity, emerges in the human population and efficiently transmits from human-to-human. The genomes of the last three pandemic influenza viruses (1918 H1N1, 1957 H2N2, and 1968 H3N2) all originated in whole or in part from non-human reservoirs and the HA genes of all of the pandemic viruses ultimately originated from avian influenza viruses.
A(H1N1) influenza viruses were first isolated from swine in 1930 (1) and have been shown to be highly similar antigenically to a recently reconstructed human 1918 A(H1N1) virus (2), and likely share a common ancestor (3, 4). From 1930 to the late 1990s these “classical swine influenza” viruses circulated in swine and remained relatively antigenically stable (5, 6).
In, or just before, 1998 the classical swine influenza viruses reassorted with a contemporary human A(H3N2) influenza virus and an American lineage avian influenza virus of an unknown subtype resulting in the emergence of a triple reassortant H3N2 (rH3N2) swine virus in swine populations throughout North America (7-9). Shortly after the initial detection of the rH3N2 virus, subsequent reassortment between the rH3N2 virus and classical H1N1 swine virus is believed to have resulted in the generation of further triple reassortant swine A(H1N1) and A(H1N2) viruses (6). In addition to the detection of these triple reassortants in North American swine populations since the late 1990s, triple reassortant swine viruses of the North American lineage have also recently been detected in Asian swine populations (10-12). Since 1999 there has been antigenic divergence within the various triple reassortant H1 viruses, with as much as 16-fold difference in hemagglutination inhibition assay (HI) titer from the pre-reassortment strains when measured with swine antisera (6) (which if it were seen in human viruses would be sufficient antigenic change to require an update of the human seasonal influenza vaccine strain).
A(H1N1) viruses circulated in humans from 1918 until the A(H2N2) influenza pandemic of 1957. During this period there was substantial antigenic drift of A(H1N1) viruses in humans away from the 1918 virus (2, 13). A(H1N1) influenza viruses from the early 1950s re-emerged in humans in 1977 (14). From 1977 to 2009, there was substantial further antigenic evolution of the human A(H1N1) viruses that was sufficient to warrant eight updates of the H1 component of the influenza virus vaccine (15).
The relative antigenic stasis of classical H1N1 influenza viruses in swine until 1998 during the time when significant antigenic drift of H1 in humans was observed, has created a substantial antigenic gap between classical swine H1 and human seasonal H1 viruses. Thus, swine have become a reservoir of H1 viruses with the potential to cause significant respiratory outbreaks or even a possible pandemic in humans.
In recent decades, both classical swine influenza and triple reassortant swine influenza viruses have occasionally been isolated from humans (14-18). Although these infections cause clinical disease, and occasionally hospitalizations and deaths, only limited human-to-human transmission has previously been documented.
In April 2009 a previously undescribed A(H1N1) influenza virus was isolated from humans in Mexico and the USA (19). As of May 18th 2009, there have been 8829 laboratory confirmed cases in 40 countries, resulting in 74 deaths (20-23). We have sequenced full or partial genomes of 17 2009 A(H1N1) viruses isolated in Mexico, and 59 2009 A(H1N1) viruses from 12 states in the USA (Table S1).
This 2009 A(H1N1) virus contains a combination of gene segments that previously has not been reported in swine or human influenza viruses in the USA or elsewhere. The NA and M gene segments are in the Eurasian swine genetic lineage (Fig. S1F and G). Viruses with NA and M gene segments in this lineage, were originally derived from a wholly avian influenza virus and thought to have entered the Eurasian swine population in 1979 (24), continue to circulate throughout Eurasia (25), and have not been previously reported outside Eurasia. The HA, NP, and NS gene segments are in the classical swine lineage (Fig. S1D, E and H). Viruses that seeded this lineage are thought to have entered pigs around 1918 (1) and subsequently circulated in classical swine viruses and triple reassortant swine viruses (26). The PB2 and PA gene segments are in the swine triple reassortant lineage (Fig. S1A and C). Viruses that seeded this lineage, originally of avian origin, entered pigs in North America around 1998 (9). Finally, the PB1 gene segment is in the swine triple reassortant lineage (Fig. S1B). This lineage of PB1 was seeded in swine from humans at the time of the North American swine triple reassortment events (9), and was itself seeded from birds around 1968 (27). Fig. 1 summarizes these host and lineage origins for the gene segments of the 2009 A(H1N1) virus.
Fig. 1.
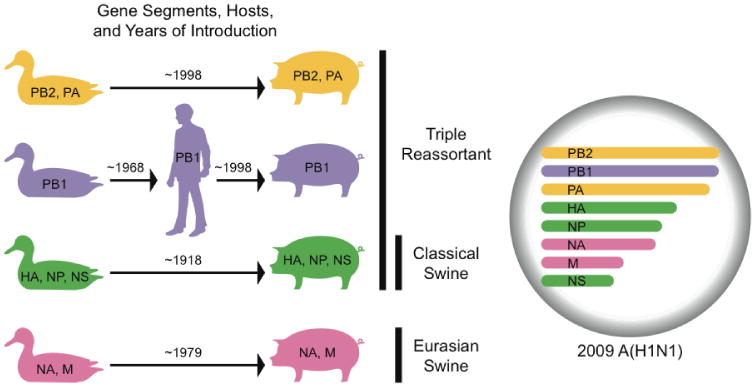
Host and lineage origins for the gene segments of the 2009 A(H1N1) virus: PB2 polymerase basic 2, PB1 polymerase basic 1, PA polymerase acidic, HA hemagglutinin, NP nucleoprotein, NA neuraminidase, M matrix gene, NS nonstructural gene. Color of gene segment in circle indicates host. Determination of lineage explained in main text.
The most closely related M gene segment to the 2009 A(H1N1) viruses is from A/Hong Kong/1774/1999 (H3N2) which was isolated from a human case of swine influenza (28). A further human case of swine influenza, A/Thailand/271/2005, contains genes from both North American and Eurasian swine influenza lineages (29) indicating previous reassortment between these two swine virus lineages.
Given the history of reassortment events of swine influenza, it is likely that additional reassortant viruses have emerged but have not been sampled. The poor surveillance for swine influenza viruses and the observation that the closest ancestral gene for each of the eight gene segments is of swine origin suggests that this virus might have been circulating undetected among swine herds somewhere in the world. Several scenarios exist, including reassortment in Asia or the Americas, for the events that have led to the genesis of the 2009 A(H1N1) virus. Where the reassortment event(s) most likely happened is currently unclear.
BLAST searches on GenBank (blastn using default settings) for each gene segment of the 2009 influenza A(H1N1) outbreak viruses showed that viruses with genes of highest nucleotide sequence identity were isolated, on average, 10 years ago (range 1992-2004) and top BLAST results for each gene segment had a sequence identity of 94 to 97% to the 2009 influenza A(H1N1) outbreak strains. This substantial divergence from previously sequenced strains is also shown by the long branch lengths to the current outbreak strains in the phylogenetic tree for each gene segment (Fig. 2 and Fig. S1) (30). Though long, these branch lengths are not unusual for swine viruses, there are 52 other similar or longer branch lengths in the swine phylogenetic trees (Fig. S2), further substantiating the gaps in swine influenza virus surveillance.
Fig. 2.
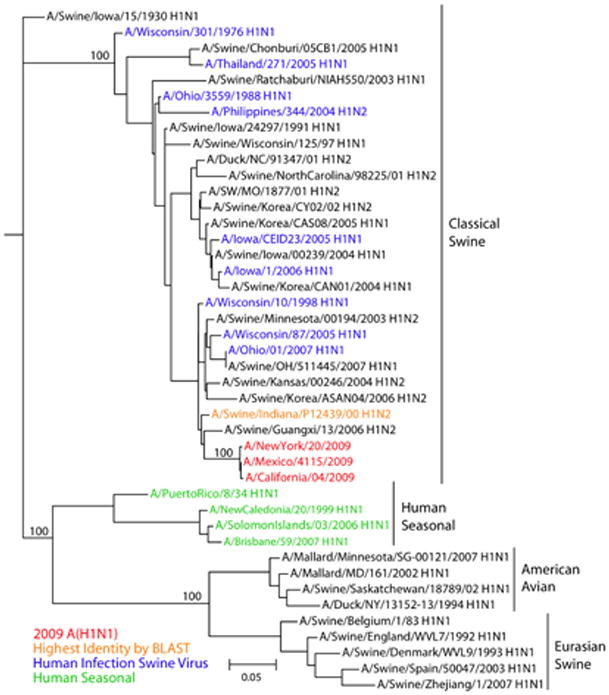
A maximum likelihood phylogenetic tree for nucleotide sequences of the HA gene of influenza viruses selected to be representative of HA gene segments in relevant hosts. Phylogenetic trees of a larger number of representative HA gene segments, and of all H1 HA swine gene segments are shown in Figs. S1D and S2D respectively. Tree was inferred using PAUP* (version 4.0b10) (39), using GTR+I+Γ4 (the general time-reversible model with the proportion of invariant sites and the gamma distribution of among-site rate variation with four categories estimated from the empirical data) as determined by ModelTest (40). Global optimization of the tree topology was performed by tree bisection-reconnection branch swapping. The robustness of individual nodes of the tree was assessed using a bootstrap resampling analysis (1000 replicates, with topologies inferred using the neighbor-joining method under the GTR+I+Γ4 substitution model).
Within each gene segment, there is high (99.9%) identity among the outbreak viruses sequenced to date, suggesting the cross-species introduction into humans was a single event or multiple events of genetically very similar viruses. Analysis across the genomes of the 2009 A(H1N1) viruses from Mexico and the USA to date, found five minor genome variants: (i) the consensus sequence, (ii) T373I mutation in the NP paired with M582L mutation in the PA, (iii) amino acid substitutions of V106I and N247D in the NA (N2 numbering) paired with V100I in the NP and (iv) amino acid substitutions of S206T in the HA1 (H3 numbering) clustering with both V106I and N247D in the NA (N2 numbering), V100I in the NP and I123V in the NS1, and (v) amino acid substitutions of S91P, V323I (H3 numbering) together with S224P in the PA (Table S2). The inclusion of isolates from Mexico or border states among all five genome variants reflects the likelihood that these early genome variants represent initial independent introductions into the USA from Mexico. Due to the short time interval since the 2009 A(H1N1) virus was first detected, it is not clear what effect, if any, these genome variations may have on viral characteristics such as transmissibility or pathogenesis.
Sequence analysis of the US and Mexico isolates of the 2009 A(H1N1) viruses to date has not identified molecular features previously shown to confer increased transmissibility or virulence in studies of other influenza A viruses. The known receptor binding sites of the H1 hemagglutinin protein are typical of many other classical swine H1N1 viruses recently isolated in North America. Although there are some mutations detected in the HA of the 2009 A(H1N1) viruses that differ from the classical swine consensus sequence, none of these were identified in known functionally significant receptor binding sites. As expected, many of the 2009 A(H1N1) viruses contain amino acid substitutions at putative antigenic sites in comparison to seasonal H1 HA; the effect of these substitutions is examined in the antigenic analysis below.
The 2009 A(H1N1) influenza viruses have been shown to have the genetic marker (S31N in M2) for resistance to the adamantane antivirals, and be sensitive to oseltamivir and zanamivir in functional assays (22, 31). Adamantane resistance is a characteristic marker of the Eurasian swine lineage. Like the M gene segment, the closest available ancestor for the NA is also from a Eurasian swine virus. All further viruses tested to date (102 in total from Mexico and 23 states of the USA) have the same pattern of resistance and sensitivity. Additionally, no genetic markers have been found in the NA that are known to decrease neuraminidase inhibitor sensitivities.
Many of the molecular markers predicted to be associated with adaptation to a human host or to the generation of a pandemic virus, such as in 1918 H1N1 or highly pathogenic H5N1, are not present in the 2009 A(H1N1) viruses characterized here. All 2009 A(H1N1) viruses to date have an E at position 627 in the PB2 protein, which is unexpected as all known human influenza viruses have a K at this position, while 627E is typical for avian influenza viruses. The PB1-F2 protein has previously been associated with the increased pathogenicity of the 1918 virus and highly pathogenic H5N1 virus (32-34). However, the PB1-F2 protein of the 2009 A(H1N1) viruses to date are truncated by the presence of a stop codon at position 12. The NS1 protein is also truncated, by a stop codon at position 220, which creates a deletion of the PDZ ligand domain, a protein-protein recognition domain involved in a variety of cell-signaling pathways that have been implicated in the pathogenicity of 1918 H1N1 and highly pathogenic H5N1 viruses (35). Together these data suggest that other previously unrecognized molecular determinants are responsible for the ability of the 2009 A(H1N1) virus to replicate and transmit in humans.
Antibodies against the surface glycoprotein HA are of major importance for protection against infection, and the HA is the primary component of the currently licensed influenza virus vaccines. To determine the antigenic properties of the 2009 A(H1N1) viruses, 18 viruses isolated in Mexico, and 38 isolated in the United States were characterized in HI assays using post-infection ferret antisera raised against a selection of swine H1 viruses, swine H1 viruses that have previously infected humans, 2009 A(H1N1) viruses, and representative viruses of the currently circulating seasonal human H1 and H3 viruses (Table 1, Table S3 and Table S4; Fig. 3).
Table 1. Hemagglutination Inhibition Reactions of Influenza H1N1 Swine Like Viruses(05/0709).
HI table of representative previous swine, and current outbreak, H1 influenza viruses. Complete HI tables of all outbreak strains tested to date are shown as Table S3 and Table S4. Swine viruses previously isolated from humans and sera raised to those viruses are shown in blue. 2009 A(H1N1) viruses and sera raised to them are shown in red.
| REFERENCE FERRET ANTISERA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| STRAIN DESIGNATION | SW/IA/30 | NJ/8/76 | WI/10 | SW/MN | OH/2 | IL/9 | CA/04 | CA/05 | DATE COLLECTED | PASSAGE |
| A/SWINE/IOWA/1930 | 320 | 40 | 5 | 20 | 5 | 5 | 5 | 5 | UNK | XEXE6 |
| A/NEWJERSEY/8/1976 | 80 | 160 | 10 | 40 | 5 | 5 | 5 | 5 | UNK | SpfE6 |
| A/WISCONSIN/10/1998 | 5 | 2560 | 1280 | 640 | 5120 | 2560 | 640 | 2560 | 01/01/03 | C3/C3E2 |
| A/SWINE/MINNESOTA/2002 | 40 | 640 | 640 | 2560 | 5120 | 2560 | 1280 | 5120 | UNK | SIVCX/C3 |
| A/OHIO/2/2007 | 80 | 1280 | 640 | 2560 | 5120 | 5120 | 2560 | 5120 | 08/18/11 | C1 |
| A/ILLINOIS/9/2007 | 80 | 1280 | 1280 | 2560 | 5120 | 5120 | 2560 | 5120 | 09/02/11 | C1 |
| A/CALIFORNIA/04/2009 | 10 | 320 | 320 | 640 | 5120 | 640 | 1280 | 2560 | 04/02/13 | C2 |
| A/CALIFORNIA/05/2009 | 40 | 320 | 320 | 1280 | 5120 | 1280 | 1280 | 5120 | 03/31/13 | C2 |
|
| ||||||||||
| TEST ANTIGENS | ||||||||||
|
| ||||||||||
| A/California/06/2009 | 80 | 640 | 640 | 1280 | 5120 | 2560 | 1280 | 5120 | 04/17/13 | M1/C1 |
| A/California/07/2009 | 320 | 1280 | 1280 | 2560 | 5120 | 5120 | 2560 | 5120 | 04/10/13 | C1 |
| A/California/07/2009 | 80 | 640 | 320 | 1280 | 5120 | 1280 | 2560 | 5120 | 04/10/13 | E2 |
| A/California/08/2009 | 160 | 1280 | 640 | 2560 | 5120 | 2560 | 2560 | 5120 | 04/10/13 | C1 |
| A/California/08/2009 | 160 | 320 | 320 | 1280 | 5120 | 1280 | 2560 | 5120 | 04/10/13 | E2 |
| A/Kansas/2/2009 | 160 | 640 | 640 | 1280 | 5120 | 2560 | 2560 | 5120 | 04/25/13 | C1 |
| A/Kansas/3/2009 | 40 | 320 | 320 | 640 | 2560 | 640 | 1280 | 5120 | 04/25/13 | C1 |
| A/Ohio/07/2009 | 80 | 640 | 640 | 640 | 2560 | 1280 | 1280 | 5120 | 04/25/13 | E2 |
| A/Ohio/07/2009 | 80 | 640 | 320 | 640 | 5120 | 1280 | 1280 | 5120 | 04/25/13 | C1 |
| A/New York/18/2009 | 160 | 1280 | 640 | 2560 | 2560 | 2560 | 2560 | 5120 | UNK | E2 |
| A/New York/20/2009 | 160 | 640 | 640 | 1280 | 5120 | 2560 | 5120 | 5120 | UNK | E2 |
| A/New York/23/2009 | 160 | 640 | 320 | 1280 | 2560 | 1280 | 1280 | 5120 | 04/25/13 | C1 |
| A/New York/23/2009 | 80 | 640 | 640 | 1280 | 5120 | 1280 | 640 | 5120 | 04/25/13 | E2 |
| A/Texas/04/2009 | 20 | 160 | 160 | 640 | 1280 | 640 | 640 | 1280 | 04/15/13 | X/C1 |
| A/Texas/05/2009 | 320 | 1280 | 640 | 2560 | 5120 | 2560 | 2560 | 5120 | 04/16/13 | X/C1 |
| A/Texas/08/2009 | 40 | 320 | 320 | 640 | 5120 | 1280 | 1280 | 2560 | 04/25/13 | E2 |
| A/Texas/08/2009 | 40 | 320 | 320 | 640 | 2560 | 1280 | 1280 | 2560 | 04/25/13 | C1 |
| A/Indiana/9/2009 | 40 | 640 | 320 | 640 | 5120 | 1280 | 1280 | 2560 | 04/23/13 | C1 |
| A/Minnesota/02/2009 | 80 | 640 | 640 | 1280 | 5120 | 2560 | 2560 | 5120 | UNK | C1 |
| A/Georgia/01/2009 | 80 | 640 | 320 | 640 | 5120 | 1280 | 1280 | 2560 | 04/28/13 | E1 |
| A/South Carolina/09/2009 | 80 | 640 | 640 | 1280 | 5120 | 2560 | 2560 | 5120 | 04/27/13 | C1 |
| A/Nebraska/02/2009 | 320 | 1280 | 1280 | 1280 | 5120 | 2560 | 2560 | 5120 | UNK | C1 |
| A/Colorado/03/2009 | 320 | 640 | 640 | 1280 | 5120 | 2560 | 2560 | 5120 | 04/28/13 | C1 |
| A/Arizona/02/2009 | 160 | 640 | 640 | 1280 | 5120 | 1280 | 2560 | 2560 | 04/27/13 | C1 |
| A/Delaware/02/2009 | 160 | 640 | 640 | 1280 | 5120 | 1280 | 2560 | 5120 | 04/29/13 | E1 |
| A/Delaware/03/2009 | 320 | 1280 | 1280 | 2560 | 5120 | 2560 | 5120 | 5120 | 04/29/13 | E1 |
| A/Mexico/4486/2009 | 160 | 1280 | 1280 | 1280 | 5120 | 2560 | 2560 | 5120 | 04/15/13 | C1 |
| A/Mexico/4486/2009 | 160 | 1280 | 640 | 1280 | 2560 | 1280 | 2560 | 5120 | 04/15/13 | E2 |
| A/Mexico/4108/2009 | 320 | 1280 | 1280 | 2560 | 5120 | 2560 | 2560 | 5120 | 04/04/13 | C1 |
| A/Mexico/4108/2009 | 80 | 320 | 320 | 1280 | 5120 | 1280 | 1280 | 2560 | 04/04/13 | E1 |
| A/Mexico/3955/2009 | 160 | 640 | 640 | 2560 | 5120 | 2560 | 2560 | 5120 | 04/04/13 | E2 |
| A/Mexico/4486/2009 | 160 | 640 | 640 | 1280 | 5120 | 1280 | 1280 | 2560 | 04/15/13 | C1/C1 |
| A/Mexico/4516/2009 | 40 | 640 | 320 | 1280 | 2560 | 1280 | 2560 | 5120 | 04/17/13 | C1/C1 |
| A/Mexico/4603/2009 | 80 | 320 | 320 | 640 | 2560 | 1280 | 1280 | 2560 | 04/20/13 | E2 |
| A/Mexico/4603/2009 | 160 | 640 | 640 | 1280 | 5120 | 1280 | 1280 | 5120 | 04/20/13 | C1/C1 |
| A/Mexico/4627/2009 | 80 | 640 | 640 | 1280 | 2560 | 640 | 1280 | 5120 | 04/21/13 | C1/C1 |
| A/Mexico/4635/2009 | 160 | 640 | 640 | 1280 | 5120 | 2560 | 2560 | 5120 | 04/21/13 | C1/C1 |
| A/Mexico/4646/2009 | 160 | 640 | 320 | 1280 | 5120 | 1280 | 1280 | 5120 | 04/21/13 | C1/C1 |
Fig. 3.
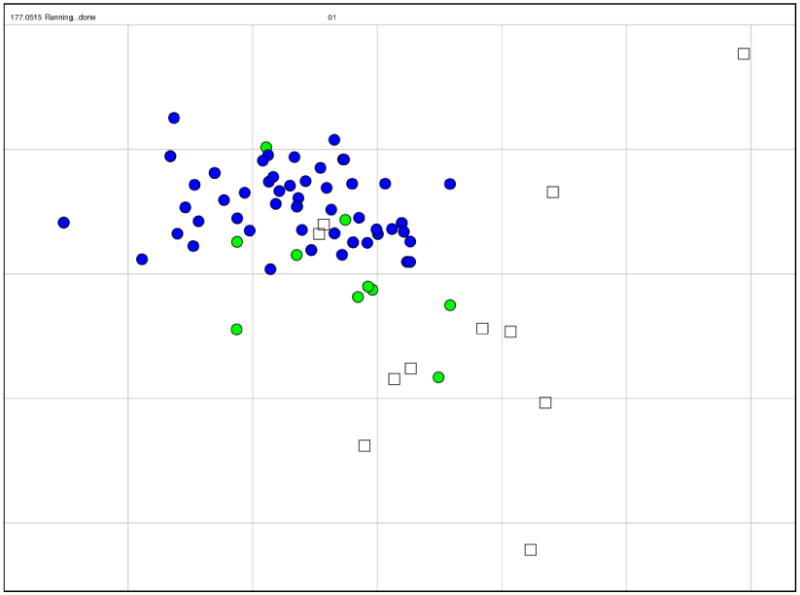
Antigenic map of 71 early swine-origin 2009 A(H1N1) influenza viruses and 11 antisera. An antigenic map is a geometric representation of binding assay data, in this case the HI assay data in Tables S3 and S4. In such a map, the relative positions of strains (colored circles) and antisera (uncolored squares) are adjusted such that the distances between strains and antisera in the map represent the corresponding HI measurements with the least error. Distance in the map thus represents antigenic distance and the closer antigens are to each other in the map the more similar they are antigenically (37). The color of a circle in the map indicates whether the strain is a 2009 A(H1N1) influenza virus (blue) or an A(H1) swine influenza virus isolated between 1998 and 2007 from either a swine or a human infected with a swine influenza virus (green). The vertical and horizontal axes both represent antigenic distance, and because only the relative positions of antigens and antisera can be determined, the orientation of the map within these axes is free (thus an antigenic map can be rotated in the same way that a geographic map can be rotated). The spacing between grid lines is one unit of antigenic distance--corresponding to a 2-fold dilution of antiserum in the HI assay. Two units correspond to 4-fold dilution, three units to 8- fold dilution, etc. A difference higher than 4-fold in HI titer is usually considered to be sufficient to necessitate an update of the human seasonal influenza virus vaccine. Antigenic clusters of human seasonal influenza viruses typically have a radius of 2 antigenic units (4-fold in HI) (37). (see Fig. S3 for a zoomable PDF that includes the names of each strain and antiserum).
Antigenically the 2009 A(H1N1) viruses are homogeneous, and among historical viruses, are antigenically similar to classical swine A(H1N1) viruses, as well as to North American lineage triple reassortant A(H1N1) viruses that have circulated in swine over the last 10 years in the US, and that have occasionally infected humans during the same period (18). There have been only a few amino acid substitutions in the HA among the 2009 H1N1 viruses analyzed to date (Table S5), and none of these amino acid changes appear to have an antigenic effect. The antigenic variation among the 2009 A(H1N1) viruses is currently less than that seen during a typical influenza season in humans (36, 37).
Ferret post-infection antisera raised against the currently circulating seasonal human A(H1N1) viruses did not react with the 2009 A(H1N1) strains (Table 1). This lack of cross-reactivity does not however directly equate to a lack of cross-protection in humans between seasonal A(H1N1) and 2009 A(H1N1) viruses as humans have a more complex immune profile than the single-infection used in ferrets for antigenic characterization. Tumpey et al. (2) showed a small boost of cross-reactive antibodies (measured by HI assay) to A/Swine/Iowa/1930 A(H1N1) in a proportion of human sera post vaccination with A/New Caledonia/20/1999 A(H1N1). Whether this boost would be protective, and the magnitude of the boost against the 2009 A(H1N1) viruses after vaccination with the current H1 component of the influenza virus vaccine, remains to be determined.
Circulation of an influenza A(H1N1) swine-origin virus in humans with an antigenically and genetically divergent HA and a previously unrecognized genetic composition is of concern to public health officials around the world. That this virus appears readily transmissible between humans is further cause for alarm. The evolutionary distances between the gene segments of this virus and its closest relatives indicate a lack of surveillance in swine populations that may harbour influenza viruses with pandemic potential. Worldwide monitoring of the antigenic and genetic properties of the 2009 A(H1N1) viruses continues for, among other reasons, detecting any changes and thus any necessity for selecting further vaccine candidates or changes in antiviral recommendations. Ongoing full genome sequencing will monitor for the possibility of future reassortment events (38).
Supplementary Material
Acknowledgments
We thank the many individuals at the local, state and national levels for their enormous contributions to the surveillance of the 2009 A(H1N1) virus; the entire CDC Influenza Division staff and emergency staff; the maintainers of the GISAID EPIFLUDB and NCBI GenBank/IVR; and the members of the WHO Global Influenza Surveillance Network. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. R.A.M.F. was supported by National Institute of Allergy and Infectious Diseases – NIH contract, HHSN266200700010C. ES was supported in part by the IFPMA through grant number RG51953. CAR was supported in part by a Research Fellowship from Clare College, Cambridge. DJS, CAR, ES, and DFB were supported by an NIH Director's Pioneer Award, part of the NIH roadmap for medical research, through grant number DP1-OD000490-01; an FP7 grant, 223498 EMPERIE, from the European Union; and program grant RG P0050/2008 from the Human Frontier Science Program.
Footnotes
This manuscript has been accepted for publication in Science. This version has not http://www.sciencemag.org/content/325/5937/197.short/
References
- 1.Shope RE. J Exp Med. 1931;54:373. doi: 10.1084/jem.54.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tumpey TM, et al. Proc Natl Acad Sci U S A. 2004;101:3166. doi: 10.1073/pnas.0308391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorman OT, et al. J Virol. 1991;65:3704. doi: 10.1128/jvi.65.7.3704-3714.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid AH, Taubenberger JK. J Gen Virol. 2003;84:2285. doi: 10.1099/vir.0.19302-0. [DOI] [PubMed] [Google Scholar]
- 5.Sheerar MG, Easterday BC, Hinshaw VS. J Gen Virol. 1989;70(Pt 12):3297. doi: 10.1099/0022-1317-70-12-3297. [DOI] [PubMed] [Google Scholar]
- 6.Vincent AL, et al. Vet Microbiol. 2006;118:212. doi: 10.1016/j.vetmic.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Karasin AI, et al. Virus Res. 2000;68:71. doi: 10.1016/s0168-1702(00)00154-4. [DOI] [PubMed] [Google Scholar]
- 8.Webby RJ, et al. J Virol. 2000;74:8243. doi: 10.1128/jvi.74.18.8243-8251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou NN, et al. J Virol. 1999;73:8851. doi: 10.1128/jvi.73.10.8851-8856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, et al. Wei Sheng Wu Xue Bao. 2008;48:466. [PubMed] [Google Scholar]
- 11.Lee CS, et al. Virus Genes. 2008;37:168. doi: 10.1007/s11262-008-0251-z. [DOI] [PubMed] [Google Scholar]
- 12.Song DS, et al. Virus Res. 2007;125:98. doi: 10.1016/j.virusres.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Kilbourne ED, et al. Proc Natl Acad Sci U S A. 2002;99:10748. doi: 10.1073/pnas.162366899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Microbiol Rev. 1992;56:152. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hay AJ, Gregory V, Douglas AR, Lin YP. Philos Trans R Soc Lond B Biol Sci. 2001;356:1861. doi: 10.1098/rstb.2001.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kendal AP, Goldfield M, Noble GR, Dowdle WR. J Infect Dis. 1977;136(Suppl):S381. doi: 10.1093/infdis/136.supplement_3.s381. [DOI] [PubMed] [Google Scholar]
- 17.Myers KP, Olsen CW, Gray GC. Clin Infect Dis. 2007;44:1084. doi: 10.1086/512813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinde V, et al. New England Journal of Medicine. 2009;361 [Google Scholar]
- 19.MMWR Morb Mortal Wkly Rep. 2009;58:400. [PubMed] [Google Scholar]
- 20.MMWR Morb Mortal Wkly Rep. 2009;58:453. [PubMed] [Google Scholar]
- 21.CDC. [accessed 18 May 2009];H1N1 Flu (Swine Flu) http://www.cdc.gov/h1n1flu/
- 22.Dawood New England Journal of Medicine. 2009;361 [Google Scholar]
- 23.WHO. [accessed 18 May 2009];Influenza A(H1N1) http://www.who.int/csr/disease/swineflu/en/index.html.
- 24.Pensaert M, Ottis K, Vandeputte J, Kaplan MM, Bachmann PA. Bull World Health Organ. 1981;59:75. [PMC free article] [PubMed] [Google Scholar]
- 25.Maldonado J, et al. Vet J. 2006;172:377. doi: 10.1016/j.tvjl.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Olsen CW. Virus Res. 2002;85:199. doi: 10.1016/s0168-1702(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 27.Kawaoka Y, Krauss S, Webster RG. J Virol. 1989;63:4603. doi: 10.1128/jvi.63.11.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregory V, et al. J Gen Virol. 2001;82:1397. doi: 10.1099/0022-1317-82-6-1397. [DOI] [PubMed] [Google Scholar]
- 29.Komadina N, et al. Virus Genes. 2007;35:161. doi: 10.1007/s11262-007-0097-9. [DOI] [PubMed] [Google Scholar]
- 30.Materials and methods are available as supporting material on Science online.
- 31.MMWR Morb Mortal Wkly Rep. 2009;58:433. [PubMed] [Google Scholar]
- 32.Conenello GM, Zamarin D, Perrone LA, Tumpey T, Palese P. PLoS Pathog. 2007 Oct 5;3:1414. doi: 10.1371/journal.ppat.0030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steel J, Lowen AC, Mubareka S, Palese P. PLoS Pathog. 2009;5:e1000252. doi: 10.1371/journal.ppat.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zamarin D, Ortigoza MB, Palese P. J Virol. 2006;80:7976. doi: 10.1128/JVI.00415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson D, Hossain MJ, Hickman D, Perez DR, Lamb RA. Proc Natl Acad Sci U S A. 2008;105:4381. doi: 10.1073/pnas.0800482105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell CA, et al. Science. 2008;320:340. doi: 10.1126/science.1154137. [DOI] [PubMed] [Google Scholar]
- 37.Smith DJ, et al. Science. 2004;305:371. [Google Scholar]
- 38.Sequences will continue to be uploaded to the sequence databases (NCBI (http://www.ncbi.nlm.nih.gov/genomes/FLU/) GISAID (http://gisaid.org)) as they are generated. See Table S1 for a list of GenBank accession numbers. Antigenic data will be available at ACORG (http://antigenic-cartography.org/).
- 39.Swofford DL. PAUP* Sinauer Associates; Sunderland: 2003. [Google Scholar]
- 40.Posada D, Crandall KA. Bioinformatics. 1998;14:817. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


