Abstract
Objectives
Our objectives were to: 1) determine intra-rater and test-retest reliability of the FTSTS in Parkinson disease (PD), 2) characterize Five Time Sit to Stand (FTSTS) performance in PD at different disease stages, 3) determine predictors of FTSTS performance in PD, and 4) determine utility of the FTSTS for discriminating between fallers and non-fallers with PD, identifying an appropriate cutoff score to delineate between these groups.
Design
Measurement study of community-dwelling individuals with idiopathic PD.
Setting
Participants were examined in a medical school laboratory.
Participants
Eighty-two participants were recruited via population-based sampling. The final sample included eighty participants. Two were excluded per exclusion criteria and unrelated illness, respectively.
Interventions
Not applicable.
Main Outcome Measure(s)
Five Times Sit to Stand Test (FTSTS) time (seconds). Secondary outcome measures included: Mini-Balance Evaluation Systems Test (Mini-BEST), Maximal Voluntary Isometric Contraction – Quadriceps (MVIC), nine hole peg test (9HPT), six minute walk, freezing of gait questionnaire, Activities-specific Balance Confidence Scale, Physical Activity Scale for the Elderly, Parkinson Disease Questionnaire-39, and Movement Disorders Society-Unified Parkinson Disease Rating Scale.
Results
Interrater and test-retest reliability for the FTSTS were high (Intraclass correlation coefficients of 0.99 and 0.76, respectively). Mean FTSTS performance was 20.25 ± 14.12 (seconds). All mobility measures were significantly correlated with FTSTS (p<0.01). The Mini-BEST and 9HPT together explained 53% of the variance in FTSTS. Receiver Operating Characteristic (ROC) analysis determined a cutoff of 16.0 seconds (sensitivity = 0.75, specificity = 0.68) for discriminating between fallers and non-fallers, with an area under the curve (AUC) of 0.77.
Conclusion
The FTSTS is a quick, easily administered measure useful for gross determination of fall risk in individuals with PD.
Keywords: Parkinson disease, falls, sit to stand
Introduction
PD is a progressive neurodegenerative disorder affecting between 1–2% of individuals over 65 years of age and 3–5% of those 85 or older [1]. Characterized by a loss of dopaminergic neurons in the substantia nigra, PD leads to cardinal symptoms of tremor, bradykinesia, rigidity, and postural instability [1]. Weakness has also been proposed as a primary symptom in PD [2]. Lower extremity strength [3], postural instability [4] and freezing of gait [5,6] appear to play major roles in falls which occur in 40%–70% of individuals with PD [7]. Due to the high number of falls and subsequent injuries, establishment of an outcome measure that reflects both lower extremity strength and balance deficiencies in individuals with PD may prove useful.
Repeated performance of sit-to-stand has often been utilized as a measure of lower extremity strength [8,9]. People with PD are known to produce smaller lower extremity forces than controls, especially at the hip, which may contribute to the ability to ascend from a chair [10]. Other studies demonstrated that sit-to-stand performance is also influenced by balance [11,12]. When measured in individuals with and without balance disorders, the positive predictive value of the FTSTS was 61% [13]. The FTSTS has also been shown to have added value in estimating the possibility of recurrent falls in community-dwelling individuals at moderate risk for falls, as those individuals in the “moderate risk” group with an FTSTS score of >15 seconds were found to have twice as many falls as those individuals who performed the FTSTS in less than 15 seconds [14]. Finally, the FTSTS has high test-retest reliability when examined in healthy, older individuals (ICC = 0.89–0.96) [11] as well as those with chronic stroke (ICC range = 0.989–0.999) [15]. While the FTSTS would appear to be very useful for assessing fall risk in individuals with PD, as strength and balance deficiencies are prominent, it has not yet been examined in this population.
In examining individuals with PD, the objectives of this study were to: 1) determine intra-rater and test-retest reliability of the FTSTS in PD, 2) characterize FTSTS performance times in PD at different disease stages, 3) determine the predictors of FTSTS performance in people with PD, and 4) determine the utility of the FTSTS for discriminating between individuals with PD with and without a history of falls and identify an appropriate cutoff score to delineate between these groups.
We hypothesized that: 1) the FTSTS would demonstrate acceptable intra-rater and test-retest reliability when used for people with PD, 2) individuals with more severe PD would perform the FTSTS more slowly than individuals with less severe PD, 3) common features of PD (postural instability, bradykinesia, freezing of gait, weakness, etc.) would be predictive of FTSTS performance, and 4) the FTSTS test would discriminate well between fallers and non-fallers.
Methods
Participants
Participants were recruited using databases from the Washington University School of Medicine’s (WUSM) Movement Disorders Center and the WUSM Volunteers for Health. The population targeted was individuals with a diagnosis of idiopathic PD (H&Y Stages I–IV) who were over the age of forty, community-dwelling and able to give informed consent. Via telephone interview and database records, potential participants were excluded based on the following criteria: 1) atypical parkinsonism, or 2) previous surgical management of PD (e.g. deep brain stimulation).
Outcome Measures
FTSTS Test
Participants began this test sitting in an armless chair that was 43 centimeters from the ground [16]. Each participant was instructed to cross his arms over his chest and sit with his back against the upright back rest of the chair. The rater then demonstrated the correct technique for performance of the test, including coming to a full stand, defined as an upright trunk with the hips and knees extended. Timing began when the rater spoke the word “go” and stopped when the participant’s buttocks reached the seat following the fifth stand.
Hoehn & Yahr Stage of Disease Severity (H&Y)
The H&Y stage [17,18] was used for assessing disease severity in individuals with PD. Stages range from zero (asymptomatic) to five (wheelchair bound/bedridden). To assign an H&Y stage, laterality of symptoms and postural instability are assessed.
Mini-Balance Evaluation Systems Test (Mini-BEST)
The Mini-BEST [19] is a newly developed 14-item balance test that has high inter-rater reliability (ICC ≥ 0.92) and high test-retest reliability (ICC ≥ 0.88) when testing individuals with PD [20]. The instrument examines several postural control systems through performance of dynamic balance tasks. As poor balance is a common manifestation of PD, this test was utilized to determine how balance may affect sit to stand performance.
MVIC -Quadriceps
A MicroFET2 [Hoggan Health Industries, West Jordan, UT] dynamometer was used to measure the maximal voluntary isometric contraction of the quadriceps muscles. Each participant was seated on a table with his back against a wall in a position in which the lower leg was parallel with the support leg of the table. The dynamometer was fixed in place on the tibia five centimeters above the lateral malleolus using a gait belt that was secured around the support leg of the table. Knee and hip flexion were set at ninety degrees and knee angle was confirmed using a goniometer. The participant was then instructed to push out as hard as possible for five seconds as if trying to straighten their knee. A practice trial was given to familiarize the participant with the device and the evaluation process. Following each trial, the peak force value (kg) was recorded and the participant was given a one minute rest period before the next trial. Three trials were recorded for each lower extremity. Quadriceps strength was given a composite value by dividing the mean bilateral strength score (mean bilateral strength score = average of left trials + average of right trials, divided by two) by body weight in kilograms [12,21,22].
Six Minute Walk Test (6MWT)
Distance covered in the 6MWT was utilized as a measure of endurance. This test has high test-retest reliability (ICC = 0.96) in PD [23]. Rationale for employing this test was derived from a previous study showing that mobility impairments (Berg Balance Scale, Timed Up and Go, Freezing of Gait Questionnaire) were related to 6MWT distance in individuals with PD [24]. Therefore, the 6MWT may be related to FTSTS performance. Participants were instructed to walk at a safe, comfortable pace while trying to cover as much ground as possible. A 30.48 meter hallway served as the venue for the 6MWT. Distance covered in six minutes was measured to the nearest meter.
Nine Hole Peg Test (9HPT)
The 9HPT is used in clinical settings to examine upper extremity function. In this study, the 9HPT was used to measure bradykinesia, as a former study demonstrated that bradykinesia was able to explain a significant portion in the variance in 9HPT performance [25]. This would allow determination of whether or not bradykinesia is related to FTSTS performance. Using only one hand, participants were asked to place nine pegs, one at a time, in nine holes as quickly as possible. Once the nine holes were filled, without stopping time, the participant was instructed to remove the pegs individually and set them in the tray. Time was stopped when the last peg was hit the tray. Participants performed two trials with each hand, beginning with the dominant hand. The participant was allowed to use the opposite hand to stabilize the peg board. Scoring for this test was completed by taking the average time of the four trials.
Fall History
Retrospective history of falls was collected from each participant. To determine whether a participant was a faller or non-faller, they were asked how often they fell in the past six months. Response options were: once, 2–10 times, weekly, or daily. If the participant chose one of the final three options, they were considered to be a faller.
Questionnaires
The Freezing of Gait Questionnaire (FOGQ) is a highly reliable (r=0.84) and valid tool that can identify 85.9% of individuals who experience freezing [26]. It includes six questions, which assess walking performance in the participant’s worst state, how gait difficulties affect independence, whether or not one experiences freezing, and characteristics of the freezing episodes. The FOGQ was employed in this study to understand how freezing might relate to FTSTS performance. Balance confidence was assessed using the Activities-specific Balance Confidence Scale (ABC) scale [27], which has high test-retest reliability (ICC = 0.94) in PD [23]. The Physical Activity Scale for the Elderly (PASE)[28], which is reliable and valid, is aimed at assessing activity engagement in older individuals in just the past week [29]. Quality of life was measured using the PD Questionnaire – 39 (PDQ-39), which consists of eight subsections (PDQ-Mobility, PDQ-Stigma, etc.). All of the subsections scored together bring about a total referred to as the Summary Index (PDQ-SI). The PDQ-39 has good internal, construct, and test-retest reliability [30,31]. The summary index and mobility scores were used for this study.
Procedures
All participants gave written informed consent in accordance with the policies and procedures of the Washington University Human Research Protection Office. Demographic information, height, and weight for each participant were obtained and questionnaires, including fall history, were completed. The order of administration for outcome measures was: Mini-BEST, FTSTS, quadriceps dynamometry, 9HPT, and 6MWT. A specially trained physical therapist administered all measures while participants were in the ‘on’ phase of their medication. In order to test inter-rater and test-retest reliability, a smaller sub-sample (n=10) of individuals with PD was tested. Two raters simultaneously timed two trials of the FTSTS for each participant. For test-retest reliability, the same procedure was carried out with seven days between testing periods.
Data Analysis
Intraclass correlation coefficients (ICC) were used to determine inter-rater (ICC 1,1) and test-retest (ICC 2,1) reliability of the FTSTS. A two-sample t-test was used to compare FTSTS performance in males as compared to females. To determine relationships between FTSTS performance and all other measures, Pearson correlations were utilized. Quantitative measures specific to mobility that were most related to FTSTS performance (i.e. correlations with p values < 0.01) were entered into a stepwise multiple regression analysis to establish factors most predictive of FTSTS performance. Also, a cutoff score regarding FTSTS performance in fallers vs. non-fallers was determined using an ROC curve, with the cutoff chosen at the minimum value of: (1-sensitivity)2 + (1-specificity) 2. Data analysis was completed using NCSS software version 7.1.19 [32].
Results
Eighty-two individuals with PD agreed to participate in the study; however, two were eliminated due to exclusion criteria and an unrelated illness, respectively. The final sample included eighty participants (59% men; mean age, 67 ± 9.0, mean H&Y, 2.4±0.6). Individuals in each H&Y stage (I=2, II=2, III=2, and IV=1) were unable to perform the FTSTS as they were unable to arise from a chair without using the upper extremities. As such, these participants were given a score of sixty seconds which was approximately one standard deviation higher than the slowest performance time among those who were able to perform the task.
Reliability of FTSTS in PD
ICCs for inter-rater reliability were 0.99. Test-retest reliability with one week between testing periods had an ICC of 0.76.
FTSTS Performance in PD
The time taken to complete the FTSTS, without regard for disease severity, was 20.25±14.12 (mean ± SD) in individuals with PD. Table 1 displays FTSTS performance as it relates to disease severity. There were no significant differences in FTSTS times for the different H&Y stages nor between men and women. Pearson correlation coefficients reveal the relationships between FTSTS performance and demographics, self-report measures, and mobility measures. All mobility measures were significantly correlated with FTSTS performance (Table 2), most notably Mini-BEST (r =−0.71, p<.001), 6MWT (r = −0.60, p <.001), and 9HPT (r = 0.55, p <.001). All questionnaire scores as well as age were also significantly correlated to FTSTS performance (Table 2).
Table 1.
FTSTS Performance by H & Y Stage.
| Disease Severity | Number of Participants | Mean Time (s) ± SD |
|---|---|---|
| H & Y 1 | 4 | 15.39 ± 5.81 |
| H & Y 2 | 27 | 21.27 ± 14.21 |
| H & Y 2.5 | 31 | 18.87 ± 12.68 |
| H & Y 3 | 12 | 22.70 ± 17.73 |
| H & Y 4 | 6 | 21.15 ± 19.32 |
Table 2.
Correlation coefficients between FTSTS and all variables.
| Variable | Correlation | p Value | |
|---|---|---|---|
| Demographics | Age | 0.37 | 0.001 |
| BMI | −0.10 | 0.40 | |
|
| |||
| Questionnaires | PASE | −0.38 | 0.001 |
| PDQ-Mobility | 0.58 | < 0.001 | |
| FOGQ | 0.44 | < 0.001 | |
| PDQ-SI | 0.38 | 0.001 | |
| ABC | −0.54 | < 0.001 | |
|
| |||
| Mobility Measures | Mini-BEST | −0.71 | < 0.001 |
| Quadriceps MVIC | −0.33 | 0.003 | |
| 9HPT | 0.55 | < 0.001 | |
| 6MWT | −0.60 | < 0.001 | |
Pearson correlations were used for all measures.
Abbreviations: BMI – body mass index, PASE – Physical Activity Scale for the Elderly, PDQ-Mobility – Parkinson Disease Questionnaire – Mobility, FOGQ – Freezing of Gait Questionnaire, PDQ-SI – Parkinson Disease Questionnaire – Summary Index, ABC – Activities Specific Balance Confidence Scale, Mini-BEST – Mini-Balance Evaluations Systems Test, Quadriceps MVIC – Quadriceps Maximal Voluntary Isometric Contraction, 9HPT – Nine Hole Peg Test, 6MWT – Six Minute Walk Test
Predictors of FTSTS Performance in PD
A multiple regression analysis revealed that the Mini-BEST and 9HPT combined explained 53% of the variance in FTSTS performance (Table 3 – Model 1). A second regression model with all measures included was similar, predicting only 55% of the variance in FTSTS performance (Table 3 – Model 2). Cumulative R2 refers to total overall R2 when all variables up to and including the variable in question have been entered into the model.
Table 3.
Regression Analyses
| Cumulative R2 | β | P value | |
|---|---|---|---|
| Model 1 | |||
| Mini-BEST | 0.500 | −0.58 | <0.0001 |
| 9HPT | 0.528 | 0.21 | 0.03 |
|
| |||
| Model 2 | |||
| Mini-BEST | 0.506 | −0.69 | <0.001 |
| 9HPT | 0.535 | 0.22 | 0.05 |
| PDQ-SI | 0.545 | −0.12 | 0.34 |
| Quadriceps MVIC | 0.548 | −0.06 | 0.52 |
| PASE | 0.552 | 0.06 | 0.58 |
| FOGQ | 0.553 | −0.06 | 0.61 |
| 6MWT | 0.554 | −0.06 | 0.73 |
| Age | 0.555 | −0.03 | 0.77 |
| ABC | 0.555 | 0.00 | 0.99 |
Cumulative R2 = total R2 when the variable in question plus all preceding variables have been entered into the model
β= standardized regression coefficients
Abbreviations: Mini-BEST – Mini Balance Evaluations Systems Test, 9HPT – Nine Hole Peg Test, PDQ-SI – Parkinson Disease Questionnaire – Summary Index, Quadriceps MVIC – Quadriceps Maximal Voluntary Isometric Contraction, PASE – Physical Activity Scale for the Elderly, FOGQ – Freezing of Gait Questionnaire, 6MWT – Six Minute Walk Test, ABC – Activities Specific Balance Confidence Scale
Discriminative Ability of FTSTS
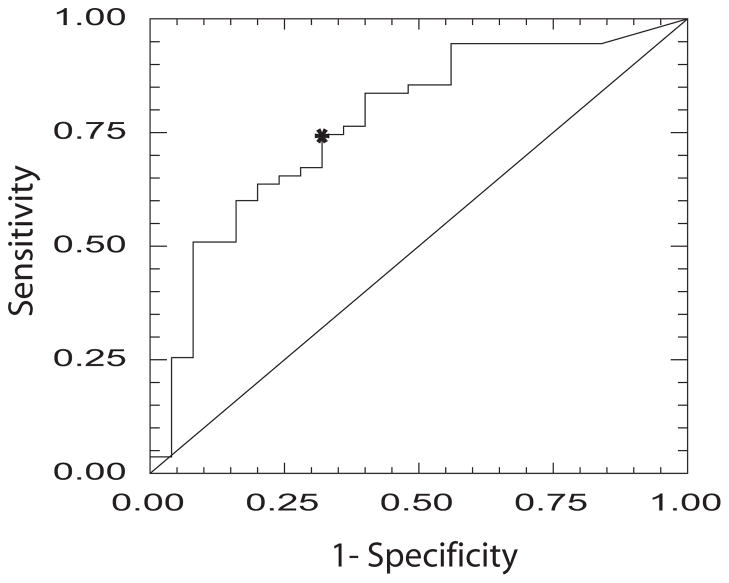
Figure 1 presents the ROC curve for discriminating between fallers and non-fallers utilizing the FTSTS. The cutoff score was 16.0 seconds [AUC = 0.77, sensitivity = 0.75, specificity = 0.68].
Figure 1.
ROC curve for the FTSTS showing sensitivity and specificity for discriminating between individuals with PD with and without a history of falls. Asterisk denotes cutoff score of 16 seconds (sensitivity = .75, specificity = .68).
Discussion
Reliability of FTSTS in PD
To the authors’ knowledge, this is the first study to examine inter-rater and test-retest reliability of the FTSTS in PD. The results of this study demonstrate excellent inter-rater reliability of the FTSTS comparable to that reported in chronic stroke (ICC =0.99)[15]. Test-retest reliability of the FTSTS in PD is also comparable to that of other populations (ICC range 0.64–0.96)[11,33–36].
FTSTS Performance in PD
We sought to examine FTSTS performance as it relates to the H&Y stages of disease severity in PD. In contrast to expectations, there were no differences in FTSTS performance in relation to disease severity, and with respect to individuals (grouped by H&Y stage) unable to complete the test successfully between groups, the number was almost equal. This could be due to specific criteria within the H&Y staging that refer only to the laterality of symptoms and the individual’s ability to stand, ambulate and respond to a postural perturbation without respect for the actual sit-to-stand movement [17]. It is also possible that over time, individuals with PD utilize compensatory strategies in the sit-to-stand movement, which could reduce the expected decline in FTSTS performance as the disease progresses [37].
Measures moderately and significantly correlated with FTSTS performance included age, balance, walking endurance, and bradykinesia (as assessed by the 9HPT). Other studies of healthy older individuals and individuals with stroke have found only weak correlations of age with sit-to-stand performance, but have noted stronger correlations similar to those reported here between balance and sit-to-stand performance [11,12]. Our results suggest that sit-to-stand performance is also correlated with balance confidence (ABC) and mobility-related quality of life (PDQ-Mobility) in PD, with those who take longer to perform the FTSTS having lower balance confidence and poorer quality of life. Those with slower 9HPT performance also had slower FTSTS performance. Overall quality of life (PDQ-SI) and physical activity level (PASE) appear less related to ability to rise from a chair in individuals with PD.
Predictors of FTSTS Performance in PD
Lower extremity strength was initially thought to be the fundamental construct influencing results on the FTSTS test; however in people with PD, balance and bradykinesia seem to be the most important contributing factors per the results of our regression analysis. Overall, the Mini-BEST balance test and 9HPT explained 53% of the variance in FTSTS performance in individuals with PD. Balance has been implicated as a contributing factor in FTSTS performance in other studies [11,12]; however this is the first study to examine a measure of bradykinesia and link it to a timed sit-to-stand test. All other variables, including lower extremity strength, entered into the regression analysis explained only an additional 2% of the variance in sit-to-stand performance. As such, lower extremity strength appears to not be as strongly related to FTSTS performance in PD as it is in other populations.
FTSTS Performance Comparison Between Populations
This is the first study to examine FTSTS performance in individuals with PD. In reviewing previous literature looking at FTSTS performance in other populations, mean performance times (in seconds) are as follows: PD (20.25±14.12), healthy elderly (13.40±2.80)[13], elderly with balance problems (16.40±4.40) [13], and chronic stroke (17.90±9.60) [12]. It is not surprising that the three populations known to have balance problems performed more slowly than the healthy older adults as balance is known to influence sit-to-stand performance [11,12]. It seems that bradykinesia, a symptom unique to PD, may be an underlying factor in the differences between PD, elderly with balance problems, and those with chronic stroke, as bradykinesia was found to be a contributing factor to FTSTS performance in individuals with PD. Regarding healthy older adults, lower extremity strength has been shown to be a strong predictor in sit-to-stand performance [38,11], while the contribution of lower extremity strength to the percentage of explained variance in PD is negligible. This suggests that strength may be a factor that sets apart older individuals with balance problems from individuals with PD when examining FTSTS performance.
Utility of FTSTS as a Fall Risk Assessment in PD
Difficulty rising from a chair has been associated with fall risk for healthy older individuals [39,40]. The ability of the FTSTS to predict the risk of falls in older adults has been previously examined [41]; one objective of the present study was to determine the ability of the FTSTS to distinguish between those who fall and those who do not fall among individuals with PD. The AUC for the FTSTS was 0.77 with a cutoff score of 16 seconds (sensitivity = 0.75, specificity = 0.68). In a study examining older adults, the optimal cutoff score for predicting recurrent fallers was 15 seconds [14]. In contrast, Whitney and colleagues compared FTSTS performance in individuals with and without balance disorders and without controlling for age, the AUC was 0.75 and the cutoff score was 13 seconds [13]. In the same study, when examining only individuals age sixty or older, the AUC was 0.68 [13]. The ability of the FTSTS to discriminate between fallers and non-fallers in the current study is comparable to that of other accepted outcome measures [42,13, 43]. The results of this study support the use of the FTSTS as a quick and objective measure to determine whether or not an individual with PD may be at risk for falling.
Study Limitations and Future Research
One limitation of this study is recording only one trial of the FTSTS test. Taking a mean of two or more trials may have allowed for understanding of FTSTS performance consistency within participants. A second limitation is the small sample size used to examine test-retest reliability. Future research should examine the timed sit-to-stand test in individuals with PD over time to gain a clearer picture of how performance changes over time and how this relates to changes in postural instability and bradykinesia. Finally, our model explained only 55% of the variance in FTSTS times, suggesting that other factors not measured here also influence FTSTS performance. Future studies could incorporate the FTSTS test in a battery of outcome measures to determine if it has additive value in predicting fall risk in individuals with PD and to determine what other factors not measured here may influence FTSTS performance.
Conclusions
Timed sit-to-stand performance does not seem to be related to disease severity in individuals with PD. Performance on the FTSTS test in PD is most related to balance and bradykinesia. The FTSTS test is reliable and easy-to-administer, and maybe useful as a quick means of assessing gross fall risk in individuals with PD.
Acknowledgments
We thank John Michael Rotello for assistance with data collection.
Sources of Support: Davis Phinney Foundation, National Institutes of Health, American Parkinson Disease Association
Funding Sources
The study was funded by the Davis Phinney Foundation and Grant Number UL1 RR024992 and Sub-Award Number TL1 RR024995 from the National Center for Research Resources, a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Additional support came from the Greater St. Louis Chapter of the American PD Association (APDA) and the APDA Center for Advanced PD Research at Washington University.
Abbreviations
- PD
Parkinson Disease
- FTSTS
Five Times Sit to Stand Test
- QOL
Quality of Life
- H&Y
Hoehn & Yahr
- ICC
Intraclass Correlation Coefficient
- Mini-BEST
Mini-Balance Evaluation Systems Test
- MVIC
Maximal Voluntary Isometric Contraction
- 6MWT
Six Minute Walk Test
- 9HPT
Nine Hole Peg Test
- FOGQ
Freezing of Gait Questionnaire
- ABC
Activity Specific Balance Confidence Scale
- PASE
Physical Activity Scale for the Elderly
- PDQ-39
Parkinson Disease Questionnaire - 39
- ROC
Receiver Operating Characteristic
Footnotes
Device Status: The manuscript submitted does not contain information about medical device(s).
Reprints will be available from the authors.
Financial Disclosure and Conflict of Interest: We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, if applicable, we certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript.
References
- 1.Fahn S. Description of Parkinson’s disease as a clinical syndrome. Annals of the New York Academy of Sciences. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 2.Kakinuma S, Nogaki H, Pramanik B, Morimatsu M. Muscle weakness in Parkinson’s disease: isokinetic study of the lower limbs. Eur Neurol. 1998;39:218–222. doi: 10.1159/000007937. [DOI] [PubMed] [Google Scholar]
- 3.Allen NE, Sherrington C, Canning CG, Fung VS. Reduced muscle power is associated with slower walking velocity and falls in people with Parkinson’s disease. Park Rel Disord. 2010;16:261–4. doi: 10.1016/j.parkreldis.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Rudzinska M, Bukowczan S, Banaszkiewicz K, Stozek J, Zajdel K, Szczudlik A. Causes and risk factors of falls in patients with Parkinson’s disease. Neurologia I Neurochirurgia Polska. 2008;42:216–222. [PubMed] [Google Scholar]
- 5.Lamberti P, Armenise S, Castaldo V, de Mari M, Iliceto G, Tronci P, Serlenga L. Freezing gait in Parkinson’s disease. Eur Neurol. 1997;38:297–301. doi: 10.1159/000113398. [DOI] [PubMed] [Google Scholar]
- 6.Giladi N, Treves TA, Simon ES, Shabtai H, Orlov Y, Kandinov B, et al. Freezing of gait in patients with advanced Parkinson’s disease. J Neural Transm. 2001;108:53–61. doi: 10.1007/s007020170096. [DOI] [PubMed] [Google Scholar]
- 7.Pickering RM, Grimbergen YA, Rigney U, Ashburn A, Mazibrada G, Wood B, et al. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov Disord. 2007;22:1892–1900. doi: 10.1002/mds.21598. [DOI] [PubMed] [Google Scholar]
- 8.Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. The American Journal of Medicine. 1985;78:77–81. doi: 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- 9.Bohannon RW. Sit-to-stand test for measuring performance of lower extremity muscles. Percept Mot Skills. 1995;80:163–6. doi: 10.2466/pms.1995.80.1.163. [DOI] [PubMed] [Google Scholar]
- 10.Inkster LM, Eng JJ, MacIntyre DL, Stoessl AJ. Leg muscle strength is reduced in Parkinson’s disease and relates to the ability to rise from a chair. Mov Disord. 2003;18:157–162. doi: 10.1002/mds.10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci. 2002;57:M539–543. doi: 10.1093/gerona/57.8.m539. [DOI] [PubMed] [Google Scholar]
- 12.Ng S. Balance ability, not muscle strength and exercise endurance, determines the performance of hemiparetic subjects on the timed sit-to-stand test. Am J Phys Med Rehab. 2010;89:497–504. doi: 10.1097/PHM.0b013e3181d3e90a. [DOI] [PubMed] [Google Scholar]
- 13.Whitney SL, Wrisley DM, Marchetti GF, Gee MA, Redfern MS, Furman JM. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the five-times-sit-to-stand test. Phys Ther. 2005;85:1034–1045. [PubMed] [Google Scholar]
- 14.Buatois S, Perret-Guillaume C, Gueguen R, Miget P, Vancon G, Perrin P, Benetos A. A simple clinical scale to stratify risk of recurrent falls in community-dwelling adults aged 65 years and older. Phys Ther. 2010;90:550–560. doi: 10.2522/ptj.20090158. [DOI] [PubMed] [Google Scholar]
- 15.Mong Y, Teo TW, Ng SS. 5-repetition sit-to-stand test in subjects with chronic stroke: reliability and validity. Arch Phys Med Rehab. 2010;91:407–413. doi: 10.1016/j.apmr.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 17.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 18.Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. Neurology. 1990;50:1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 19.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42:323–331. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leddy AL, Crowner B, Earhart GM. Utility of the Mini-BESTest, BESTest, and BESTest sections for balance assessments in Parkinson disease. J Neurol Phys Ther. doi: 10.1097/NPT.0b013e31821a620c. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandler J, Duncan P, Studenski S. Choosing the best strength measure in frail older persons: importance of task specificity. Muscle Nerve Suppl. 1997;5:S47–S51. [PubMed] [Google Scholar]
- 22.McCarthy EK, Horvat MA, Holtsberg PA, Wisenbaker JM. Repeated chair stands as a measure of lower limb strength in sexagenarian women. J Gerontol A Biol Sci Med Sci. 2004;59:1207–1212. doi: 10.1093/gerona/59.11.1207. [DOI] [PubMed] [Google Scholar]
- 23.Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88:733–746. doi: 10.2522/ptj.20070214. [DOI] [PubMed] [Google Scholar]
- 24.Falvo MJ, Earhart GM. Six-minute walk distance in persons with Parkinson’s disease: a hierarchical regression model. Arch Phys Med Rehabil. 2009;90:1004–8. doi: 10.1016/j.apmr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Earhart GM. Park Rel Disord. submitted. [Google Scholar]
- 26.Giladi N, Tal J, Azulay T, Rascol O, Brooks DJ, Melamed E, et al. Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov Disord. 2009;24:655–661. doi: 10.1002/mds.21745. [DOI] [PubMed] [Google Scholar]
- 27.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 28.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 29.Shuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol. 1997;50:541–6. doi: 10.1016/s0895-4356(97)00010-3. [DOI] [PubMed] [Google Scholar]
- 30.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res. 1995;4:241–8. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- 31.Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J Neurol. 1998;245 (Suppl 1):10–14. doi: 10.1007/pl00007730. [DOI] [PubMed] [Google Scholar]
- 32.Hintze J. NCSS. NCSS, LLC. Kaysville, Utah: 2009. www.ncss.com. [Google Scholar]
- 33.Ostchega Y, Harris TB, Hirsch R, Parsons VL, Kington R, Katzoff M. Reliability and prevalence of physical performance examination assessing mobility and balance in older persons in the US: data from the Third National Health and Nutrition Examination Survey. J Am Geriatric Soc. 2000;48:1136–1141. doi: 10.1111/j.1532-5415.2000.tb04792.x. [DOI] [PubMed] [Google Scholar]
- 34.Jette AM, Jette DU, Ng J, Plotkin DJ, Bach MA. Are performance-based measures sufficiently reliable for use in multicenter trials? Musculoskeletal Impairment (MSI) Study Group. J Gerontol A Biol Sci Med Sci. 1999;54:M3–6. doi: 10.1093/gerona/54.1.m3. [DOI] [PubMed] [Google Scholar]
- 35.Schaubert KL, Bohannon RW. Reliability and validity of three strength measures obtained from community-dwelling elderly persons. J Strength Cond Res. 2005;19:717–720. doi: 10.1519/R-15954.1. [DOI] [PubMed] [Google Scholar]
- 36.Bohannon RW, Shove ME, Barreca SR, Masters LM, Sigouin CS. Five-repetition sit-to-stand test performance by community-dwelling adults: A preliminary investigation of times, determinants, and relationship with self-reported physical performance. Isokinetics and Exercise Sci. 2007;15:77–81. [Google Scholar]
- 37.Inkster LM, Eng JJ. Postural control during a sit-to-stand task in individuals with mild Parkinson’s disease. Exp Brain Res. 2004;154:33–8. doi: 10.1007/s00221-003-1629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schenkman M, Hughes MA, Samsa G, Studenski S. The relative importance of strength and balance in chair rise by functionally impaired older individuals. J Am Geriatr Soc. 1996;44:1441–1446. doi: 10.1111/j.1532-5415.1996.tb04068.x. [DOI] [PubMed] [Google Scholar]
- 39.Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol. 1989;44:M112–7. doi: 10.1093/geronj/44.4.m112. [DOI] [PubMed] [Google Scholar]
- 40.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. J Am Med Assoc. 1989;261:2663–2668. [PubMed] [Google Scholar]
- 41.Buatois S, Miljkovic D, Manckoundia P, Gueguen R, Miget P, Vancon G, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56:1575–7. doi: 10.1111/j.1532-5415.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 42.Landers MR, Backlund A, Davenport J, Fortune J, Schuerman S, Altenburger P. Postural instability in idiopathic Parkinson’s disease: discriminating fallers from nonfallers based on standardized clinical measures. J Neurol Phys Ther. 2008;32:56–61. doi: 10.1097/NPT.0b013e3181761330. [DOI] [PubMed] [Google Scholar]
- 43.Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75:116–124. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]