Abstract
Laboratory studies have demonstrated that vitamin D has a number of chemopreventive properties, and that these properties may be mediated or modified by other molecules in the vitamin D pathway, such as parathyroid hormone (PTH) or calcium. However, there is little epidemiologic data exploring the effects of vitamin D on breast cancer risk in the context of these other molecules. We examined a panel of molecules in the vitamin D pathway in relation to mammographic breast density, a marker of breast cancer risk, in the Wisconsin Breast Density Study. A total of 238 postmenopausal women (ages 55-70, with no history of postmenopausal hormone use) were enrolled from mammography clinics in Madison, Wisconsin. Subjects provided blood samples that were analyzed for levels of 25-hydroxy vitamin D [25(OH)D], PTH, insulin-like growth factor-1 (IGF1), IGF-binding protein 3 (IGFBP3), retinol, and calcium. Percent breast density was measured using Cumulus software. In age-adjusted analyses there was a positive association between 25(OH)D and percent breast density (P=0.05; mean percent density=11.3% vs. 15.6% for 1st vs. 4th quartile of 25(OH)D). Breast density was inversely associated with PTH (P=0.05; 16.0% vs. 11.4% for Q1 vs. Q4) and positively associated with the IGF-1:IGFBP-3 molar ratio (P=0.02; 11.9% vs. 15.6% for Q1 vs. Q4). However, these associations were all null after further adjustment for body mass index (BMI; P>0.25). The independent relation between 25(OH)D and breast density remained null among subgroups defined by BMI and serum levels of retinol, calcium, and estradiol. These results suggest no strong independent associations between the circulating molecules of the vitamin D pathway and mammographic breast density in postmenopausal women. While it remains possible that vitamin D could influence breast cancer risk, our results suggest that such an effect would be mediated through pathways other than breast density.
Keywords: mammographic breast density, vitamin D, calcium, parathyroid hormone, breast cancer
INTRODUCTION
Despite abundant laboratory evidence that vitamin D has chemopreventive properties in relation to breast carcinogenesis [1], the impact of circulating levels of vitamin D on breast tissue and breast cancer risk in humans is unclear. In the Nurses Health Study [2] and the French 3N Cohort [3], women with elevated circulating levels of vitamin D had an approximately 25% reduced risk of developing breast cancer. However, no associations between vitamin D and breast cancer risk were observed in other large cohorts [4, 5] or in the Women’s Health Initiative randomized trial of 400 IU vitamin D/1000 mg calcium supplementation [6].
Inconsistency among animal and human studies could potentially be caused by the complex interactions of vitamin D with other circulating molecules involved in its regulatory pathway (Figure 1) [7]. Some of these molecules, such as calcium, parathyroid hormone, and insulin-like growth factor-1, may also have direct effects on breast cancer cell proliferation and breast cancer risk [8-10]. Other molecules, such as retinol (vitamin A), may antagonize vitamin D’s actions through competing signaling pathways within the cell [11].
FIGURE 1.
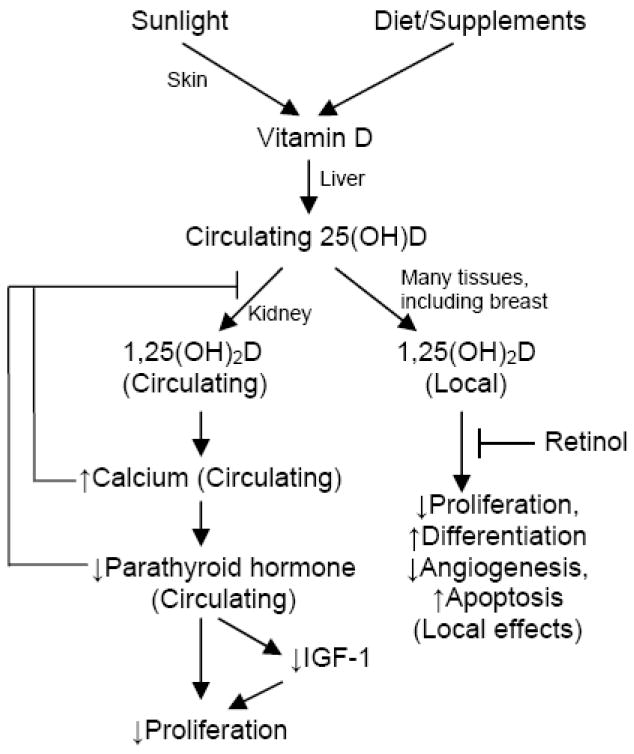
Hypothesized pathways by which Vitamin D may influence breast carcinogenesis.
Mammographic density refers to the radiologic appearance of breast tissue on a mammogram, and is determined by the relative amounts of radiodense epithelial/stromal tissue and radiolucent fat tissue. Given the strong association of breast density with breast cancer risk and the fact that breast density is sensitive to known breast cancer risk factors [12], breast density is an attractive marker for evaluating the influence of vitamin D on breast tissue. Few previous studies have examined the relation between circulating vitamin D and breast density [13-17]. None of these studies evaluated vitamin D in the context of the interacting molecules which may modify vitamin D’s effects on breast tissue.
We have examined the relation between vitamin D and mammographic breast density in the Wisconsin Breast Density Study, a cross-sectional study of postmenopausal women aged 55-70 years receiving a screening mammogram. We also evaluated the association between other molecules in the vitamin D pathway and breast density, and examined the potential modifying effect of these molecules on the relation between vitamin D and breast density.
MATERIALS AND METHODS
Study population
This study was approved by the University of Wisconsin Health Sciences Institutional Review Board. All subjects provided written informed consent. Details of the study population and recruitment methods have been previously described [18]. Eligibility was limited to postmenopausal women, aged 55-70 years, receiving a screening mammogram at the UW Health West Clinic or UW Health Breast Center in Madison, Wisconsin. Women who reported having no menstrual cycles within the past 12 months were considered postmenopausal. Exclusion criteria included a previous diagnosis of breast cancer, breast implants, or having ever used postmenopausal hormone therapy. Over the course of one year (June 2008 – July 2009), 268 subjects were recruited into the study.
Data collection
All subjects completed a questionnaire, provided a blood sample, and permitted access to their screening mammogram for the assessment of mammographic breast density.
Questionnaire
The questionnaire included items known or suspected to be associated with breast cancer or mammographic density, including age, height, weight, age at menarche, age at first pregnancy, parity, age at menopause, first degree family history of breast cancer, alcohol consumption, physical activity, smoking, and education level. All subjects were asked if they were currently taking any vitamins or supplements, and if so, to name them.
Blood analyses
A single blood draw was obtained from each subject at the time of her mammogram. Serum was aliquoted in 2 mL vials and immediately frozen at -70° C. Quantification of 25(OH)D, IGF-1, and IGFBP-3 was performed at the Reproductive Endocrine Research Laboratory at the University of Southern California, using previously described techniques [19, 20]. 25(OH)D was measured using a commercial 125I-based radioimmunoassay (RIA) kit (DiaSorin, Stillwater, MN), with a preliminary organic solvent extraction step. Previous studies using the DiaSorin kit have reported excellent intra-assay and inter-assay coefficients of variation (CV) of <10% [13, 14]. Additionally, the 125I-based RIA for measuring 25(OH)D has been validated against gold standard methods based on high performance liquid chromatography (HPLC; r2 = 0.98) [21] and liquid chromatography tandem mass spectroscopy (LC-MS/MS; r2 = 0.74 to 0.96) [22, 23]. IGF-1 and IGFBP-3 were quantified by direct chemiluminescent immunoassays using the Immulite analyzer (Siemens Medical Solutions Diagnostics, Malvern, PA). Previous use of this assay by the Reproductive Endocrine Research Laboratory demonstrated inter-assay CVs for IGF-1 and IGFBP-3 of 4.7% and 8.8%, respectively [19].
PTH, calcium, and retinol were analyzed at the University of Wisconsin Carbone Cancer Center’s Analytical Instrumentation Laboratory for Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Intact PTH was measured by a two-site Enzyme-Linked Immunosorbent Assay (ELISA; MD Biosciences, St. Paul, MN), which has previously been shown to have intra-assay and inter-assay CVs of less than 4% [24, 25]. Calcium was measured by quantitative colorimetric determination using the QuantiChrom calcium assay kit (Bioassay Systems, Hayward, CA). Retinol was quantified by HPLC, using the validated method described by Taibi and Nicotra [26], who reported intra-assay and inter-assay CVs of less than 3.5%.
Since the effect of vitamin D on breast tissue may also depend on sex hormone levels, we also examined potential effect modification by serum estradiol. As previously described [18], estradiol was measured at the Reproductive Endocrine Research Laboratory at the University of Southern California using a validated RIA [27]. Previous use of this assay by the laboratory has demonstrated a CV of 8.5% [28].
Mammographic density
As previously described [18], all participants underwent their normally scheduled screening mammogram on either a Senographe 2000D (GE Medical Systems) or a Clearview CSm2 CR (Fujifilm Corporation) machine. Full resolution lossless digital images of the craniocaudal view of the left breast were obtained for the quantitative analysis of mammographic breast density using Cumulus software [29]. Total breast area and dense area were recorded to permit the calculation of percent breast density. All density measurements were performed with high reliability [18] by the same reader (EJAB), who was blinded in respect to other study data.
For both types of mammography machines, the pixel intensity values in the raw mammogram images are automatically processed using a proprietary algorithm to highlight contrast for more sensitive evaluation by the radiologist. This processing can introduce non-linear relations between breast tissue density and pixel brightness. However, the raw digital images are not directly readable in Cumulus software. For mammograms obtained on GE machines, software developed by Drs. Martin Yaffe and Chris Peressotti (University of Toronto) was used to convert the pixel values of the raw digital image to those expected if the same patient was imaged with a film screened mammography unit and the film image was digitized. This algorithm makes the raw image suitable for analysis in Cumulus while preserving the linear relation between density and pixel brightness (Martin Yaffe, University of Toronto, personal communication, 2008).
The correlation between density measurements on processed and raw GE mammogram images was assessed by a small sub-study. Percent density was measured on 28 processed GE images and compared to the values obtained using the converted raw GE image. The intraclass correlation coefficients were 0.872, 0.997, and 0.854 for percent density, total breast area, and dense area, respectively.
No algorithm was available to convert raw Fuji images to a form appropriate for density reading by Cumulus software. Thus, the processed Fuji images were directly evaluated in Cumulus. The high correlation between breast density as assessed using the raw and processed GE images provides reassurance that the processed Fuji images are suitable for Cumulus analysis.
At the mammography clinics, women were assigned to a particular machine for imaging based primarily on machine availability. However, for the comfort of the subjects, women with larger breasts are imaged on the Fuji machine when possible. As breast density is inversely related to body mass index, the density values were on average lower for Fuji images (mean percent density = 12.4%) compared to GE images (mean percent density = 18.8%). After adjustment for body mass index the difference in percent density between mammogram machines was greatly attenuated and not statistically significant (15.0% for Fuji vs. 15.8% for GE; p = 0.67).
Statistical analyses
Of the 268 women recruited, 20 women refused to consent to future analyses of their serum and 2 women had insufficient serum for vitamin D analyses. In addition, eight (all under 60 years old) had estradiol levels greater than 35 pg/mL, suggesting that they were not truly postmenopausal. Exclusion of these thirty women left a total of 238 samples available for analysis. Quantification of PTH, retinol, and calcium were missing for three women and certain covariate data were missing for a small fraction of subjects (See Table 1). Multiple imputation was used to impute missing PTH, retinol, calcium and covariate data. Ten imputations were conducted using the Markov Chain Monte Carlo method [30]. The imputation model contained percent breast density and all variables listed in Tables 1 and 2. For statistical analyses, each model was fit separately to the ten imputed datasets and the results combined for statistical inferences using the methods of Rubin [31].
Table 1.
Characteristics of study participants (N=238), Wisconsin Breast Density Study, 2008-2009.
| N | % | 25(OH)D (ng/mL) Mean (SD) | |
|---|---|---|---|
| Age | |||
| 55-59 | 126 | 52.9 | 34.2 (9.6) |
| 60-64 | 64 | 26.9 | 34.5 (9.5) |
| 65-70 | 48 | 20.2 | 34.0 (12.4) |
| Ptrend = 0.95 | |||
| Body mass index (kg/m2) | |||
| <18.5 | 2 | 0.8 | 33.6 (3.4) |
| 18.5-24.9 | 72 | 30.3 | 38.5 (9.5) |
| 25.0-29.9 | 76 | 31.9 | 34.0 (9.2) |
| ≥30.0 | 86 | 36.1 | 31.0 (10.2) |
| Missing | 2 | 0.8 | - |
| Ptrend < 0.0001 | |||
| First degree family history of breast cancer | |||
| No | 182 | 76.5 | 34.0 (9.8) |
| Yes | 56 | 23.5 | 35.0 (11.3) |
| Ptrend = 0.52 | |||
| Age at menarche (years) | |||
| ≤11 | 58 | 24.4 | 34.1 (10.8) |
| 12-13 | 137 | 57.6 | 34.0 (10.1) |
| ≥14 | 43 | 18.1 | 35.4 (9.7) |
| Ptrend = 0.55 | |||
| Parity | |||
| 0 | 59 | 24.8 | 35.7 (10.1) |
| 1 | 30 | 12.6 | 31.6 (9.7) |
| 2 | 84 | 35.3 | 34.2 (9.9) |
| ≥3 | 65 | 27.3 | 34.4 (10.8) |
| Ptrend = 0.65 | |||
| Age at first birth (years)* | |||
| <24 | 69 | 38.6 | 33.4 (10.7) |
| 24-29 | 67 | 37.4 | 34.3 (10.5) |
| ≥30 | 43 | 24.0 | 33.6 (9.1) |
| Ptrend = 0.84 | |||
| Age at menopause (years) | |||
| <50 | 64 | 26.9 | 33.6 (10.2) |
| 50-54 | 127 | 53.4 | 34.2 (10.0) |
| ≥55 | 42 | 17.7 | 34.7 (10.3) |
| Missing | 5 | 2.1 | - |
| Ptrend = 0.58 | |||
| Education | |||
| High school | 44 | 18.5 | 33.4 (10.7) |
| Some college | 54 | 22.7 | 31.7 (9.7) |
| College diploma | 71 | 29.8 | 35.1 (9.7) |
| Advanced degree | 69 | 29.0 | 36.0 (10.4) |
| Ptrend = 0.05 | |||
| Alcohol consumption (drinks/wk)† | |||
| None | 85 | 35.7 | 34.4 (10.2) |
| <5 per week | 112 | 47.1 | 33.7 (10.5) |
| ≥5 per week | 30 | 12.6 | 35.4 (8.7) |
| Missing | 11 | 4.6 | - |
| Ptrend = 0.88 | |||
| Vigorous physical activity (hours per week)‡ | |||
| 0-1.0 | 71 | 29.8 | 33.2 (9.6) |
| 1.1-4.0 | 82 | 34.5 | 34.4 (10.5) |
| >4.0 | 85 | 35.7 | 35.0 (10.4) |
| Ptrend = 0.29 | |||
| Smoking history (pack-years) | |||
| None | 144 | 60.5 | 34.6 (10.3) |
| 1-15 | 45 | 18.9 | 34.3 (9.6) |
| >15 | 40 | 16.8 | 34.2 (11.2) |
| Missing | 9 | 3.8 | - |
| Ptrend = 0.79 | |||
| Vitamin D supplement use | |||
| No | 58 | 24.4 | 27.7 (8.5) |
| Yes | 180 | 75.6 | 36.4 (9.8) |
| Ptrend < 0.0001 |
SD, standard deviation.
Among parous women only.
Includes beer, wine, and hard liquor.
Physically vigorous activities that cause large increases in heart rate or breathing, such as sports activities, climbing stairs, heavy gardening, or lifting/carrying heavy objects.
Table 2.
Distribution of circulating molecules in study participants (N=238), Wisconsin Breast Density Study, 2008-2009.
| Mean | Standard Deviation | Q1 | Median | Q3 | Range | Correlation* with 25(OH)D (P-Value) | Correlation* with BMI (P-Value) | |
|---|---|---|---|---|---|---|---|---|
| 25(OH)D (ng/mL) | 34.3 | 10.2 | 27.4 | 33.3 | 39.9 | 6.7, 67.8 | -0.31 (<0.0001) | |
| PTH (pg/mL)† | 41.7 | 21.1 | 27.3 | 37.1 | 51.3 | 7.0, 182.4 | -0.22 (0.001) | 0.12 (0.06) |
| Calcium (mg/dL)† | 10.5 | 2.3 | 9.2 | 10.3 | 11.3 | 4.8, 19.5 | 0.09 (0.19) | -0.11 (0.10) |
| IGF-1 (ng/mL) | 137 | 46 | 102 | 134 | 161 | 37.6, 287.0 | 0.20 (0.002) | -0.18 (0.005) |
| IGFBP-3 (μg/mL) | 4.28 | 0.95 | 3.70 | 4.24 | 4.92 | 2.00, 7.75 | 0.04 (0.57) | 0.05 (0.45) |
| IGF-1/IGFBP-3 molar ratio | 0.11 | 0.03 | 0.09 | 0.11 | 0.13 | 0.06, 0.20 | 0.23 (<0.001) | -0.29 (<0.0001) |
| Retinol (ng/mL)† | 0.75 | 0.24 | 0.59 | 0.72 | 0.88 | 0.31, 2.16 | 0.13 (0.04) | 0.03 (0.62) |
Q1, first quartile; Q3, third quartile; BMI, body mass index.
Spearman correlation coefficient.
Values were missing for 3 subjects.
Linear regression was used to determine the univariate association between various questionnaire items of interest and serum vitamin D levels. Spearman correlation coefficients were computed to describe the association between serum vitamin D and levels of the other measured molecules.
Multivariable linear regression was used to assess the association between the circulating molecules and percent breast density, while sequentially adjusting for 1) age and season of blood draw; 2) body mass index; and 3) other variables which have previously been shown to be associated with density in this study population: parity, family history of breast cancer, vigorous physical activity, and pack-years of smoking [18]. Percent density was square root transformed to improve the normality of the data. An ordinal term was included in separate regression models to test for trends across increasing hormone quartile groups. Adjusted least-squares means of square root density were calculated according to quartiles of each molecule level and reverse-transformed for display purposes. Tests for effect modification of the relation between 25(OH)D and percent breast density by other circulating molecules and BMI were conducted by including continuous cross-product interaction terms in the regression models. Interactions were considered statistically significant if p-values associated with cross-product interaction terms were less than 0.05. All analyses were repeated using the square root of dense area (rather than percent density) as the outcome of interest. All statistical analyses were performed using SAS Statistical Software (Version 9; SAS Institute, Inc., Cary, North Carolina).
RESULTS
Characteristics of the study population are described in Table 1. The mean age of participants was 60.7 (standard deviation, 4.3). Over two-thirds were either overweight (31.9%) or obese (36.1%). In general, the study population was highly educated (81.5% had attended college), and reported low smoking rates (60.5% smoked fewer than 100 cigarettes in their life) and high use of vitamin D supplements (75.6%). In univariate analyses, circulating 25(OH)D levels were inversely associated with BMI and positively associated with education and supplement use (Table 1). Established predictors of breast density, such as age, body mass index, and parity, were inversely associated with percent breast density in this study as expected (data not shown; previously described in [18]).
The distributions of the circulating molecules of interest are described in Table 2. Of the 238 subjects, 15 (6.3%) had 25(OH)D levels considered deficient (<20 ng/mL), 66 (27.7%) had insufficient levels (between 20-29 ng/mL), and 157 (66.0%) had sufficient levels (≥30 ng/mL). There was a negative correlation between 25(OH)D and PTH levels (r = -0.22), and positive correlations between 25(OH)D and IGF-1 (r = 0.20), the IGF-1/IGFBP-3 molar ratio (r = 0.23), and retinol (r = 0.13). There was little correlation between 25(OH)D and calcium or IGFBP-3 levels. Vitamin D and IGF-1 were both inversely associated with body mass index (P<0.05). PTH and calcium exhibited weak correlations (positive and negative, respectively) with BMI that were of borderline statistical significance.
Mean percent breast density in the study population was 15.3% (standard deviation, 12.5; range 0.4-71.2). After adjusting for age and season of blood draw, 25(OH)D was positively associated with percent breast density (P = 0.05; Table 3). Mean percent density rose from 11.3% among women in the first quartile of 25(OH)D to 15.6% among women in the fourth quartile. There was an inverse association between PTH and percent density (P = 0.05), with mean percent density declining from 16.0% among women in the first quartile to 11.4% in the fourth quartile. IGF-1 and IFGBP3 were not individually associated with percent density, yet the molar ratio between the two was positively associated with percent density (P = 0.02), which rose from 11.9% among women in the first quartile to 15.6% among women in the fourth quartile of the IGF-1:IGFBP-3 molar ratio. Calcium and retinol were not associated with percent density (P>0.9).
Table 3.
The association between circulating molecules and mammographic breast density (N=238), Wisconsin Breast Density Study, 2008-2009.
| Hormone | Linear regression of square root percent breast density on molecule levels | Mean percent density by molecule quartile (95% Confidence Interval)* | Ptrend | |||||
|---|---|---|---|---|---|---|---|---|
| β coefficient | SE (β) | P | Q1 | Q2 | Q3 | Q4 | ||
| 25(OH)D (ng/mL) | ||||||||
| Age and season adjusted | 0.018 | 0.009 | 0.05 | 11.3 (9.0, 13.9) | 13.5 (10.9, 16.3) | 12.2 (9.7, 15.0) | 15.6 (12.7, 18.8) | 0.07 |
| Age, season, and BMI adjusted | -0.004 | 0.009 | 0.65 | 13.7 (11.3, 16.4) | 14.0 (11.6, 16.6) | 11.2 (9.1, 13.6) | 13.4 (11.1, 16.1) | 0.52 |
| Multivariable-adjusted† | -0.004 | 0.009 | 0.68 | 13.6 (11.3, 16.2) | 14.3 (11.9, 16.9) | 11.2 (9.1, 13.5) | 13.3 (11.0, 15.9) | 0.49 |
| PTH (pg/mL) | ||||||||
| Age and season adjusted | -0.010 | 0.005 | 0.05 | 16.0 (13.1, 19.2) | 12.2 (9.7, 15.0) | 12.8 (10.2, 15.6) | 11.4 (8.9, 14.2) | 0.05 |
| Age, season, and BMI adjusted | -0.005 | 0.004 | 0.27 | 14.9 (12.4, 17.7) | 12.8 (10.5, 15.4) | 12.0 (9.8, 14.5) | 12.5 (10.2, 15.2) | 0.18 |
| Multivariable-adjusted† | -0.003 | 0.004 | 0.48 | 14.9 (12.5, 17.6) | 12.3 (10.0, 14.7) | 12.3 (10.1, 14.7) | 12.8 (10.5, 15.4) | 0.27 |
| Calcium (mg/dL) | ||||||||
| Age and season adjusted | 0.004 | 0.045 | 0.93 | 11.7 (9.2, 14.5) | 13.1 (10.4, 16.0) | 13.6 (10.9, 16.6) | 14.0 (11.2, 17.0) | 0.25 |
| Age, season, and BMI adjusted | -0.020 | 0.041 | 0.62 | 12.8 (10.5, 15.5) | 12.6 (10.3, 15.2) | 13.0 (10.6, 15.5) | 13.8 (11.4, 16.5) | 0.57 |
| Multivariable-adjusted† | -0.016 | 0.039 | 0.69 | 12.7 (10.4, 15.3) | 12.5 (10.3, 15.1) | 13.2 (10.9, 15.8) | 13.7 (11.3, 16.3) | 0.53 |
| IGF-1 (ng/mL) | ||||||||
| Age and season adjusted | 0.003 | 0.002 | 0.15 | 11.9 (9.4, 14.7) | 13.3 (10.8, 16.1) | 11.9 (9.4, 14.7) | 15.1 (12.4, 18.2) | 0.11 |
| Age, season, and BMI adjusted | 0.000 | 0.002 | 0.87 | 13.6 (11.1, 16.2) | 13.2 (11.0, 15.7) | 11.6 (9.4, 14.1) | 13.8 (11.5, 16.4) | 0.90 |
| Multivariable-adjusted† | -0.001 | 0.002 | 0.67 | 14.2 (11.8, 16.9) | 12.6 (10.4, 15.0) | 11.8 (9.6, 14.2) | 13.7 (11.4, 16.2) | 0.74 |
| IGFBP-3 (μg/mL) | ||||||||
| Age and season adjusted | -0.041 | 0.102 | 0.69 | 13.7 (11.0, 16.7) | 11.7 (9.3, 14.4) | 14.4 (11.7, 17.5) | 12.5 (10.0, 15.3) | 0.90 |
| Age, season, and BMI adjusted | -0.042 | 0.090 | 0.64 | 14.2 (11.8, 16.8) | 11.5 (9.4, 13.9) | 13.3 (11.0, 15.9) | 13.3 (11.0, 15.9) | 0.94 |
| Multivariable-adjusted† | -0.063 | 0.088 | 0.48 | 14.3 (11.9, 16.9) | 11.6 (9.5, 13.9) | 13.5 (11.2, 16.1) | 13.0 (10.8, 15.4) | 0.77 |
| IGF-1/IGFBP-3 molar ratio | ||||||||
| Age and season adjusted | 7.5 | 3.3 | 0.02 | 11.9 (9.5, 14.7) | 11.3 (8.9, 13.9) | 13.7 (11.0, 16.6) | 15.6 (12.7, 18.7) | 0.04 |
| Age, season, and BMI adjusted | 0.5 | 3.1 | 0.86 | 14.1 (11.7, 16.8) | 11.4 (9.2, 13.7) | 13.1 (10.7, 15.6) | 13.9 (11.5, 16.5) | 0.83 |
| Multivariable-adjusted† | -0.1 | 3.0 | 0.97 | 14.4 (12.0, 17.0) | 11.6 (9.5, 13.9) | 12.5 (10.2, 14.9) | 13.9 (11.5, 16.5) | 0.96 |
| Retinol (ng/mL) | ||||||||
| Age and season adjusted | 0.000 | 0.430 | 0.99 | 15.0 (12.2, 18.2) | 11.0 (8.6, 13.6) | 14.5 (11.8, 17.5) | 11.9 (9.4, 14.8) | 0.44 |
| Age, season, and BMI adjusted | 0.049 | 0.382 | 0.90 | 15.3 (12.8, 18.0) | 10.7 (8.6, 13.0) | 13.5 (11.1, 16.1) | 13.0 (10.6, 15.7) | 0.55 |
| Multivariable-adjusted† | 0.070 | 0.373 | 0.85 | 15.8 (13.3, 18.5) | 10.4 (8.5, 12.6) | 12.9 (10.7, 15.4) | 13.4 (11.0, 16.1) | 0.49 |
SE, standard error; BMI, body mass index.
Mean percent density displayed is reverse transformed from model of square root density; quartile cut-points are described in Table 2.
Adjusted for age, body mass index, parity, family history of breast cancer, vigorous physical activity, and smoking.
Further adjustment for BMI essentially eliminated any associations between these circulating molecules and percent breast density (Table 3). Additional adjustment for parity, physical activity, pack-years of smoking, and family history of breast cancer had little influence on the estimates of association.
The association between 25(OH)D and percent density in the fully adjusted models is shown for selected subgroups in Table 4. No statistically significant interactions were detected between 25(OH)D and any of the other molecules in Table 3 in their association with percent breast density in the fully adjusted models (all Pinteraction > 0.25). While there was a negative association between 25(OH)D and percent density among women with high retinol levels, this relation did not reach statistical significance. There was a significant interaction between BMI and 25(OH)D (P=0.05); the association between 25(OH)D and percent density became more strongly negative as BMI increased. However, the relation between 25(OH)D and percent breast density did not reach statistical significance in any particular subgroup defined by BMI. Since BMI among postmenopausal women is closely associated with circulating estrogen levels, we also examined the interaction between 25(OH)D and serum estradiol levels. There was a negative association between 25(OH)D and percent breast density among women with high (above the median) estradiol levels, but neither this association nor the test for interaction was statistically significant.
Table 4.
The association between vitamin D and mammographic breast density among various subgroups, Wisconsin Breast Density Study, 2008-2009.
| Subgroup | N | Linear regression of square root percent breast density on 25(OH)D levels† | Mean Percent Density by 25(OH)D Quartile (95% Confidence Interval)*† | Ptrend | |||||
|---|---|---|---|---|---|---|---|---|---|
| β coefficient | SE (β) | P | Q1 | Q2 | Q3 | Q4 | |||
| All women | 238 | -0.004 | 0.009 | 0.68 | 13.6 (11.3, 16.2) | 14.3 (11.9, 16.9) | 11.2 (9.1, 13.5) | 13.3 (11.0, 15.9) | 0.49 |
| Retinol ≤ 0.72 ng/mL | 119 | 0.008 | 0.012 | 0.52 | 11.4 (8.7, 14.6) | 15.6 (12.4, 19.2) | 9.3 (6.7, 12.4) | 14.5 (11.0, 18.5) | 0.72 |
| Retinol > 0.72 ng/mL | 119 | -0.017 | 0.013 | 0.21 | 16.1 (11.8, 21.1) | 13.2 (9.7, 17.2) | 13.7 (10.3, 17.6) | 12.2 (9.1, 15.7) | 0.22 |
| Pinteraction = 0.36 | |||||||||
| Calcium ≤ 10.3 mg/dL | 119 | -0.005 | 0.012 | 0.69 | 12.3 (9.6, 15.3) | 17.3 (13.6, 21.5) | 7.7 (5.4, 10.5) | 12.2 (9.2, 15.5) | 0.27 |
| Calcium > 10.3 mg/dL | 119 | -0.003 | 0.014 | 0.82 | 15.1 (11.0, 20.0) | 12.7 (9.6, 16.2) | 14.3 (11.0, 18.1) | 14.3 (10.8, 18.4) | 0.99 |
| Pinteraction = 0.87 | |||||||||
| BMI < 25 kg/m2 | 74 | 0.006 | 0.021 | 0.79 | 18.7 (9.1, 31.8) | 26.8 (19.4, 35.3) | 15.1 (10.5, 20.6) | 23.5 (18.2, 29.5) | 0.99 |
| 25 kg/m2 ≤ BMI < 30 kg/m2 | 76 | 0.006 | 0.014 | 0.67 | 10.1 (7.3, 13.5) | 14.3 (10.6, 18.5) | 10.4 (7.7, 13.5) | 11.7 (8.2, 15.9) | 0.89 |
| BMI ≥ 30 kg/m2 | 86 | -0.016 | 0.013 | 0.21 | 10.8 (8.4, 13.5) | 6.9 (4.7, 9.6) | 10.2 (6.6, 14.4) | 7.0 (4.3, 10.4) | 0.14 |
| Pinteraction = 0.05 | |||||||||
| Estradiol ≤ 8.8 pg/mL | 119 | 0.002 | 0.013 | 0.87 | 15.6 (11.6, 20.2) | 17.5 (13.7, 21.8) | 12.8 (9.5, 16.6) | 16.7 (13.2, 20.5) | 0.93 |
| Estradiol > 8.8 pg/mL | 119 | -0.017 | 0.012 | 0.15 | 11.7 (9.0, 14.7) | 11.8 (8.9, 15.1) | 10.1 (7.4, 13.2) | 8.8 (6.0, 12.1) | 0.14 |
| Pinteraction = 0.29 | |||||||||
SE, standard error; BMI, body mass index.
Mean percent density displayed is reverse transformed from model of square root density.
Adjusted for age, body mass index, family history of breast cancer, parity, vigorous physical activity, and smoking.
No statistically significant associations were observed between the absolute measure of dense area and any of the molecules of interest in either the age and season adjusted models or the fully adjusted models (all P > 0.10).
DISCUSSION
The results of this study indicate that there is a cross-sectional relation between serum vitamin D and percent breast density in postmenopausal women, yet this association is eliminated by adjustment for BMI. We found no evidence that any of the examined molecules of the vitamin D pathway are independently associated with percent breast density after adjustment for BMI, and little evidence that they modify the relation between vitamin D and percent breast density.
These findings add to growing evidence suggesting no strong relation between vitamin D and mammographic breast density in postmenopausal women. A handful of studies have examined dietary intake of vitamin D in relation to breast density. With a couple of exceptions [32, 33], the majority of these studies reported null associations in postmenopausal women [34-38]. In contrast, an inverse association between dietary intake of vitamin D and breast density has been more consistently observed among premenopausal women [32, 34, 37, 39]. To our knowledge, only three previous studies have examined breast density in relation to circulating vitamin D levels in postmenopausal women, each of which reported null associations [13, 15, 16]. Inverse [14] and null [17] findings have been reported in studies of premenopausal women.
To our knowledge, this is the first study to examine circulating PTH in relation to breast density. PTH can stimulate the proliferation of quiescent MCF7 breast cancer cells in vitro [40], and a high percentage of human mammary cancers and hyperplastic mammary epithelial cells express the PTH receptor [9]. A number of medical record linkage studies have reported that hyperparathyroidism is a risk factor for breast cancer [41-43]. However, we found that there was no independent association between PTH and percent breast density in our study.
Previous studies have observed a positive association between circulating IGF-1 levels and breast cancer risk, though this has largely been limited to premenopausal women (reviewed in [10]). We observed no independent associations between breast density and IGF-1, IGFBP-3, or the IGF-1:IGFBP-3 molar ratio. These results are consistent with previous studies which observed null relations between breast density and IGF-1, IGFBP-3, and their molar ratio in postmenopausal women [44-46]. In contrast, significant associations have been observed in premenopausal women [44-47].
We found no evidence for an influence of either calcium or retinol on the relation between vitamin D and breast density. We had also hypothesized that the effect of vitamin D on breast tissue may vary according to sex hormone levels. For instance, the influence of vitamin D may be limited in women with high estrogen levels, such as those who are obese. We observed some suggestion that the relation between vitamin D and percent breast density varied according to BMI (Pinteraction = 0.05); the association was positive among women with low BMI and negative among women with high BMI. However, this association did not reach statistical significance in any specific BMI category. Direct evaluation of effect modification by serum estradiol did not reveal a strong interaction, though there was the suggestion of an inverse association between vitamin D and breast density in women with estradiol levels above the median.
Our results provide further evidence against a strong relation between the molecules of the vitamin D pathway and breast density in postmenopausal women. Interpretation of these results should be balanced by consideration of the study’s limitations. Measurements of serum molecules and breast density were made at one single point in time in this cross-sectional study. The half-life of 25(OH)D in the blood is only 2-4 weeks [48, 49], thus variation in past exposure levels are not captured by a single measurement. The resulting misclassification may attenuate any association between vitamin D and breast density, and we cannot evaluate the temporality of the relation. However, blood levels of vitamin D generally remain consistent over time in individuals. In the Nurses Health Study an intraclass correlation coefficient of 0.72 was observed for 25(OH)D measures taken two to three years apart among postmenopausal women [50]. In the general population, moderate correlation (r = 0.5) has been observed at up to 14 years between measurements [51]. Additionally, very little intra-individual diurnal variation in 25(OH)D levels has been observed [48, 52].
Breast density was assessed from digital mammograms using Cumulus software. Cumulus was designed for use on film images that have been digitized. Use of digital images may have introduced measurement error which could obscure a relation between breast density and vitamin D or other circulating molecules. However, we were able to detect the expected associations between breast density and established risk factors, such as age, body mass index, and parity, suggesting that any measurement error for breast density was within reasonable limits.
Multiple imputation was used to impute the small numbers of missing laboratory and covariate data. Sensitivity analyses in which subjects with missing laboratory values were excluded revealed a negligible impact upon the results.
While the study was sufficiently powered to detected clinically relevant differences in breast density, the relatively small sample size of the study limited the power to detect smaller differences in breast density, and we are unable to rule out potential interactions between circulating molecules. Since all women were recruited from UW Health Clinics in Madison, Wisconsin, the generalizability of the results is somewhat limited. The limited geographic range of participants likely restricted the observed variation in vitamin D exposure. Ethnic diversity in our sample was minimal, as ~97% of subjects reported white race (reflecting the clinic patient demographics). Additionally, eligibility was restricted to postmenopausal women, aged 55-70, who had never used postmenopausal hormones. While this provided an optimal opportunity to detect an effect of vitamin D on density (i.e. in a low endogenous hormone background), the impact of vitamin D in premenopausal women or users of postmenopausal hormones could not be evaluated.
In summary, we found little evidence to support an independent association between molecules of the vitamin D pathway and breast density in postmenopausal Caucasian women. While it remains possible that vitamin D could influence breast cancer risk, our results suggest that such an effect would be mediated through pathways other than breast density. Continued investigation into the influence of vitamin D on breast tissue will be needed to understand the potential mechanisms by which this molecule may reduce breast cancer risk.
Acknowledgments
This work was supported by the National Cancer Institute (CA139548, CA014520), the Department of Defense (BC062649), and the Susan G. Komen Foundation (FAS0703857). Dr. Sprague is supported by a fellowship from the American Society of Preventive Oncology sponsored by the Prevent Cancer Foundation and the Susan G. Komen Foundation. The authors would like to thank Kristi Klein and the staff of UW Health Clinics, Dr. Walter Peppler, Eva Baird, and Lori Wollett and staff of the UW OCT for their assistance in subject recruitment and data collection; Dr. Marty Kanarek, John Hampton, Tammy LeCaire, Tanya Watson, Matt Walsh, Jane Maney, and Cecilia Bellcross for study-related advice; Dr. Martin Yaffe and Chris Peressotti for assistance in breast density measurements; Dr. Karen Cruickshanks, Carla Schubert, and Scott Nash for assistance in sample storage; and Julie McGregor, Kathy Peck, and Dawn Fitzgibbons for study support.
Footnotes
CONFLICT OF INTEREST The authors report no conflicts of interest.
References
- 1.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 2.Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, Hankinson SE. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1991–1997. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 3.Engel P, Fagherazzi G, Boutten A, Dupre T, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Serum 25(OH) vitamin D and risk of breast cancer: a nested case-control study from the French E3N cohort. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2341–2350. doi: 10.1158/1055-9965.EPI-10-0264. [DOI] [PubMed] [Google Scholar]
- 4.Freedman DM, Chang SC, Falk RT, Purdue MP, Huang WY, McCarty CA, Hollis BW, Graubard BI, Berg CD, Ziegler RG. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2008;17(4):889–894. doi: 10.1158/1055-9965.EPI-07-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, Horst RL, Gapstur SM, Thun MJ, Calle EE. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res. 2009;11(4):R64. doi: 10.1186/bcr2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Rossouw J, Lane D, O’Sullivan MJ, Yasmeen S, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100(22):1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16(2):83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 8.Almquist M, Manjer J, Bondeson L, Bondeson AG. Serum calcium and breast cancer risk: results from a prospective cohort study of 7,847 women. Cancer Causes Control. 2007;18(6):595–602. doi: 10.1007/s10552-007-9001-0. [DOI] [PubMed] [Google Scholar]
- 9.Birch MA, Carron JA, Scott M, Fraser WD, Gallagher JA. Parathyroid hormone (PTH)/PTH-related protein (PTHrP) receptor expression and mitogenic responses in human breast cancer cell lines. Br J Cancer. 1995;72(1):90–95. doi: 10.1038/bjc.1995.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 11.Johansson S, Melhus H. Vitamin A antagonizes calcium response to vitamin D in man. J Bone Miner Res. 2001;16(10):1899–1905. doi: 10.1359/jbmr.2001.16.10.1899. [DOI] [PubMed] [Google Scholar]
- 12.Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010;102(16):1224–1237. doi: 10.1093/jnci/djq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight JA, Vachon CM, Vierkant RA, Vieth R, Cerhan JR, Sellers TA. No association between 25-hydroxyvitamin D and mammographic density. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1988–1992. doi: 10.1158/1055-9965.EPI-06-0241. [DOI] [PubMed] [Google Scholar]
- 14.Brisson J, Berube S, Diorio C, Sinotte M, Pollak M, Masse B. Synchronized seasonal variations of mammographic breast density and plasma 25-hydroxyvitamin d. Cancer Epidemiol Biomarkers Prev. 2007;16(5):929–933. doi: 10.1158/1055-9965.EPI-06-0746. [DOI] [PubMed] [Google Scholar]
- 15.Green AK, Hankinson SE, Bertone-Johnson ER, Tamimi RM. Mammographic density, plasma vitamin D levels and risk of breast cancer in postmenopausal women. Int J Cancer. 2010;127(3):667–674. doi: 10.1002/ijc.25075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuhouser ML, Bernstein L, Hollis BW, Xiao L, Ambs A, Baumgartner K, Baumgartner R, McTiernan A, Ballard-Barbash R. Serum vitamin D and breast density in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2010;19(2):412–417. doi: 10.1158/1055-9965.EPI-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chai W, Maskarinec G, Cooney RV. Serum 25-hydroxyvitamin D levels and mammographic density among premenopausal women in a multiethnic population. Eur J Clin Nutr. 2010;64(6):652–654. doi: 10.1038/ejcn.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprague BL, Trentham-Dietz A, Gangnon RE, Buist DSM, Burnside ES, Aiello Bowles EJ, Stanczyk FZ, Sisney GS. Circulating sex hormones and mammographic breast density among postmenopausal women. Hormones and Cancer. 2011;2(1):62–72. doi: 10.1007/s12672-010-0056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chia VM, Quraishi SM, Graubard BI, Rubertone MV, Erickson RL, Stanczyk FZ, McGlynn KA. Insulin-like Growth Factor 1, Insulin-like Growth Factor-Binding Protein 3, and Testicular Germ-Cell Tumor Risk. Am J Epidemiol. 2008;167(12):1438–1445. doi: 10.1093/aje/kwn080. [DOI] [PubMed] [Google Scholar]
- 20.Troisi R, Lagiou P, Trichopoulos D, Xu B, Chie L, Stanczyk FZ, Potischman N, Adami HO, Hoover RN, Hsieh CC. Cord serum estrogens, androgens, insulin-like growth factor-I, and insulin-like growth factor binding protein-3 in Chinese and U.S. Caucasian neonates. Cancer Epidemiol Biomarkers Prev. 2008;17(1):224–231. doi: 10.1158/1055-9965.EPI-07-0536. [DOI] [PubMed] [Google Scholar]
- 21.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–186. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 22.Tsugawa N, Suhara Y, Kamao M, Okano T. Determination of 25-hydroxyvitamin D in human plasma using high-performance liquid chromatography--tandem mass spectrometry. Anal Chem. 2005;77(9):3001–3007. doi: 10.1021/ac048249c. [DOI] [PubMed] [Google Scholar]
- 23.Saenger AK, Laha TJ, Bremner DE, Sadrzadeh SM. Quantification of serum 25-hydroxyvitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006;125(6):914–920. doi: 10.1309/J32U-F7GT-QPWN-25AP. [DOI] [PubMed] [Google Scholar]
- 24.Seamans KM, Hill TR, Wallace JM, Horigan G, Lucey AJ, Barnes MS, Taylor N, Bonham MP, Muldowney S, Duffy EM, et al. Cholecalciferol supplementation throughout winter does not affect markers of bone turnover in healthy young and elderly adults. J Nutr. 2010;140(3):454–460. doi: 10.3945/jn.109.113480. [DOI] [PubMed] [Google Scholar]
- 25.Cashman KD, Wallace JM, Horigan G, Hill TR, Barnes MS, Lucey AJ, Bonham MP, Taylor N, Duffy EM, Seamans K, et al. Estimation of the dietary requirement for vitamin D in free-living adults >=64 y of age. Am J Clin Nutr. 2009;89(5):1366–1374. doi: 10.3945/ajcn.2008.27334. [DOI] [PubMed] [Google Scholar]
- 26.Taibi G, Nicotra CM. Development and validation of a fast and sensitive chromatographic assay for all-trans-retinol and tocopherols in human serum and plasma using liquid-liquid extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;780(2):261–267. doi: 10.1016/s1570-0232(02)00529-9. [DOI] [PubMed] [Google Scholar]
- 27.Goebelsmann U, Bernstein GS, Gale JA, Kletzky OA, Nakamura RM, Coulson AH, Korelitz JJ. Serum gonadotropin, testosterone, estradiol and estrone levels prior to and following bilateral vasectomy. In: Lepow IH, Crozier R, editors. Vasectomy: Immunologic and pathophysiologic effects in animals and man. New York: Academic Press; 1979. [Google Scholar]
- 28.Dorgan JF, Stanczyk FZ, Kahle LL, Brinton LA. Prospective case-control study of premenopausal serum estradiol and testosterone levels and breast cancer risk. Breast Cancer Res. 2010;12(6):R98. doi: 10.1186/bcr2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39(10):1629–1638. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- 30.Schafer JL. Analysis of incomplete multivariate data. New York: Chapman and Hall; 1997. [Google Scholar]
- 31.Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 32.Berube S, Diorio C, Verhoek-Oftedahl W, Brisson J. Vitamin D, calcium, and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1466–1472. [PubMed] [Google Scholar]
- 33.Tseng M, Byrne C, Evers KA, Daly MB. Dietary intake and breast density in high-risk women: a cross-sectional study. Breast Cancer Res. 2007;9(5):R72. doi: 10.1186/bcr1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berube S, Diorio C, Masse B, Hebert-Croteau N, Byrne C, Cote G, Pollak M, Yaffe M, Brisson J. Vitamin D and calcium intakes from food or supplements and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1653–1659. doi: 10.1158/1055-9965.EPI-05-0068. [DOI] [PubMed] [Google Scholar]
- 35.Masala G, Ambrogetti D, Assedi M, Giorgi D, Del Turco MR, Palli D. Dietary and lifestyle determinants of mammographic breast density. A longitudinal study in a Mediterranean population. Int J Cancer. 2006;118(7):1782–1789. doi: 10.1002/ijc.21558. [DOI] [PubMed] [Google Scholar]
- 36.Vachon CM, Kushi LH, Cerhan JR, Kuni CC, Sellers TA. Association of diet and mammographic breast density in the Minnesota breast cancer family cohort. Cancer Epidemiol Biomarkers Prev. 2000;9(2):151–160. [PubMed] [Google Scholar]
- 37.Thomson CA, Arendell LA, Bruhn RL, Maskarinec G, Lopez AM, Wright NC, Moll CE, Aickin M, Chen Z. Pilot study of dietary influences on mammographic density in pre- and postmenopausal Hispanic and non-Hispanic white women. Menopause. 2007;14(2):243–250. doi: 10.1097/01.gme.0000235362.72899.7b. [DOI] [PubMed] [Google Scholar]
- 38.Mishra G, McCormack V, Kuh D, Hardy R, Stephen A, dos Santos Silva I. Dietary calcium and vitamin D intakes in childhood and throughout adulthood and mammographic density in a British birth cohort. Br J Cancer. 2008;99(9):1539–1543. doi: 10.1038/sj.bjc.6604697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diorio C, Berube S, Byrne C, Masse B, Hebert-Croteau N, Yaffe M, Cote G, Pollak M, Brisson J. Influence of insulin-like growth factors on the strength of the relation of vitamin D and calcium intakes to mammographic breast density. Cancer Res. 2006;66(1):588–597. doi: 10.1158/0008-5472.CAN-05-1959. [DOI] [PubMed] [Google Scholar]
- 40.Carron JA, Fraser WD, Gallagher JA. PTHrP and the PTH/PTHrP receptor are co-expressed in human breast and colon tumours. Br J Cancer. 1997;76(8):1095–1098. doi: 10.1038/bjc.1997.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer M, Adami HO, Krusemo UB, Ljunghall S. Increased risk of malignant diseases after surgery for primary hyperparathyroidism. A nationwide cohort study. Am J Epidemiol. 1988;127(5):1031–1040. doi: 10.1093/oxfordjournals.aje.a114879. [DOI] [PubMed] [Google Scholar]
- 42.Pickard AL, Gridley G, Mellemkjae L, Johansen C, Kofoed-Enevoldsen A, Cantor KP, Brinton LA. Hyperparathyroidism and subsequent cancer risk in Denmark. Cancer. 2002;95(8):1611–1617. doi: 10.1002/cncr.10846. [DOI] [PubMed] [Google Scholar]
- 43.Michels KB, Xue F, Brandt L, Ekbom A. Hyperparathyroidism and subsequent incidence of breast cancer. Int J Cancer. 2004;110(3):449–451. doi: 10.1002/ijc.20155. [DOI] [PubMed] [Google Scholar]
- 44.Byrne C, Colditz GA, Willett WC, Speizer FE, Pollak M, Hankinson SE. Plasma insulin-like growth factor (IGF) I, IGF-binding protein 3, and mammographic density. Cancer Res. 2000;60(14):3744–3748. [PubMed] [Google Scholar]
- 45.Boyd NF, Stone J, Martin LJ, Jong R, Fishell E, Yaffe M, Hammond G, Minkin S. The association of breast mitogens with mammographic densities. Br J Cancer. 2002;87(8):876–882. doi: 10.1038/sj.bjc.6600537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diorio C, Pollak M, Byrne C, Masse B, Hebert-Croteau N, Yaffe M, Cote G, Berube S, Morin C, Brisson J. Insulin-like growth factor-I, IGF-binding protein-3, and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1065–1073. doi: 10.1158/1055-9965.EPI-04-0706. [DOI] [PubMed] [Google Scholar]
- 47.Maskarinec G, Williams AE, Kaaks R. A cross-sectional investigation of breast density and insulin-like growth factor I. Int J Cancer. 2003;107(6):991–996. doi: 10.1002/ijc.11505. [DOI] [PubMed] [Google Scholar]
- 48.Cugini P, Coen G, Scavo D, Lucia P, Mazzaferro S, Bianchini G, Massimetti C, Donato G. Circannual versus seasonal variations of longitudinally sampled 25-hydroxycholecalciferol serum levels. Biochem Med. 1984;32(1):22–29. doi: 10.1016/0006-2944(84)90004-8. [DOI] [PubMed] [Google Scholar]
- 49.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 50.Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, Mantzoros CS, Ricchiuti V, Willett WC, Hankinson SE, et al. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19(4):938–946. doi: 10.1158/1055-9965.EPI-09-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171(8):903–908. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 52.Tangrea J, Helzlsouer K, Pietinen P, Taylor P, Hollis B, Virtamo J, Albanes D. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control. 1997;8(4):615–625. doi: 10.1023/a:1018450531136. [DOI] [PubMed] [Google Scholar]


