Abstract
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) improves motor function including gait and stability in people with PD, but differences in DBS contact locations within the STN may contribute to variability in the degree of improvement. Based on anatomic connectivity, dorsal STN may be preferentially involved in motor function and ventral STN in cognitive function. To determine whether dorsal DBS affects gait and balance more than ventral DBS, we conducted a double-blind evaluation of 23 PD patients with bilateral STN DBS. Each participant underwent gait analysis and balance testing off Parkinson medication in three DBS conditions (unilateral DBS in dorsal STN region, unilateral DBS in ventral STN region, and both stimulators off) on one day. For UPDRS-III scores and velocity for Fast and Pref gait, as well as stride length for Fast and Pref gait, dorsal and ventral stimulation improved gait, compared to the off condition (post hoc tests, p<0.05). However, there were no differences with dorsal compared to ventral stimulation. Balance, assessed using a multi-item clinical balance test (mini-BESTest), was similar across conditions. Absence of differences in gait and balance between dorsal and ventral conditions suggests motor connections involved in gait and balance may be more diffusely distributed in STN than previously thought, as opposed to neural connections involved in cognitive processes, such as response inhibition, which are more affected by ventral stimulation.
Keywords: Parkinson Disease, Gait, Balance, Deep Brain Stimulation, Subthalamic Nucleus
INTRODUCTION
Gait abnormalities and postural stability deficits are commonly reported in Parkinson disease (PD). Pharmacological treatments and surgical interventions may ameliorate some aspects of gait and balance impairments, but surgical interventions may provide additional benefit as the disease advances. In particular, bilateral subthalamic nucleus (STN) deep brain stimulation (DBS) at clinically optimal settings improves motor function including gait and stability in PD patients. Unilateral STN DBS produces similar, but smaller magnitude improvements in UPDRS-III scores and gait velocity compared to bilateral stimulation.[1–3] Unilateral STN DBS improved motor function on the side contralateral to stimulation,[4–6] and also had lower magnitude effects on the side ipsilateral to stimulation.[1,2,4,7–9]
Despite these apparent benefits, substantial variability across studies and from person to person within studies has been noted for STN DBS-induced improvements in gait and balance in PD.[10–11] Differences in the location of DBS contacts within the STN may contribute to this variability. Surgical placement of each DBS electrode, with four equally spaced contacts, follows a dorsolateral to ventromedial trajectory through the STN region. Segregation of anatomic connections of STN suggests it may be divided into three distinct regions: a dorsal sensorimotor region, a ventral associative region, and a medial limbic region.[12] Motor function may preferentially involve the dorsal STN, based on its anatomic connections with motor areas of the brain, including the primary motor cortex, premotor cortex, and supplementary motor cortex.[13–14] Thus, stimulation of the dorsal portion of the STN would be expected to produce greater motor and gait benefits than stimulation of ventral regions. In support of this notion, ventral stimulation preferentially affects response inhibition to a greater extent than dorsal stimulation.[15]
More precise understanding of anatomic relationships underlying motor benefits from STN DBS may provide a foundation to optimize this treatment. This study aimed to determine whether DBS in the dorsal versus ventral STN regions differentially affected quantitative measures of gait and balance. To maximally distinguish the differential effects of dorsal versus ventral stimulation, uniform suboptimal DBS stimulation variables were used for each individual, rather than using higher voltage clinically identified DBS settings. By using the same DBS stimulation settings across participants, we were able to eliminate the within group variability in stimulation parameters present in many studies that use each individual’s clinical stimulator settings. Further, using these uniform suboptimal settings, we were able to minimize potential spatial overlap of current spread between conditions. We hypothesized that DBS of either dorsal or ventral STN regions would improve gait and balance but that dorsal DBS would provide greater benefit.
MATERIALS AND METHODS
Participants
We conducted a double-blind evaluation of 23 PD patients (age 62±9 yrs, mean±SD, 19 males, PD duration 15±6 yrs) after at least 12-hour withdrawal from Parkinson medication. Thirty participants were enrolled, but six did not complete gait and balance tasks for all three conditions because they could not stand or walk independently (n=3) or ended the study early due to discomfort (n=3). One participant was excluded due to a DBS programming error. Data from these seven participants were excluded from all analyses. Participants met specific inclusion criteria, including diagnosis of idiopathic PD according to standard criteria,[16] previously implanted bilateral STN stimulating electrodes, no serious medical problems, other neurological diseases, confounding medications (i.e. dopamine blockers, depleters, or drugs that may impair balance), or injuries that could impair gait or balance. All had DBS for at least 3 months prior to participation, to confirm they responded adequately to DBS therapy and allow for resolution of most microsubthalamotomy effects. Participants were recruited from Washington University in Saint Louis and the University of Cincinnati. This study was approved by the Washington University Human Research Protection Office, and all participants provided informed consent.
Contact Localization
Participants were pre-screened to ensure they had at least one dorsal and one ventral STN DBS contact tip contralateral to the side of the body with worse motor signs, based on lateralized UPDRS-III administered off medication and off stimulation after DBS surgery.[15] Contact localization was performed using images from pre-operative magnetic resonance imaging (MRI) scans and post-operative computed tomography (CT) scans, a validated method previously described.[17]
All participants had quadripolar deep brain stimulators implanted (electrode model 3389, Medtronic Activa System, Medtronic Inc.). Each contact was 1.27 mm in diameter and extended 1.5 mm. Contacts were separated from adjacent contacts by 0.5 mm gaps. DBS contacts are numbered 0–3 along the electrode (ventromedially to dorsolaterally). Standardized suboptimal DBS settings were used in an effort to control for current spread and to minimize adverse effects. The DBS settings used were: monopolar stimulation (with pulse generator case as cathode, 185 Hz, 60 μs, 2.5 V. The frequency and pulse width settings were selected to match the clinical settings used for most participants. The chosen voltage setting was less than the clinically prescribed voltage for 20 of the 23 participants, and thus was expected to be a tolerable setting for most participants, even when applied via suboptimal contacts. The DBS current likely activates neurons and axons within a 2 mm radius sphere around the active contact tip, based on previous research [18–19], as well as electrode characteristics and DBS variables used in this study. To select the DBS contact tips within the dorsal STN and ventral STN regions, 2 mm spheres were drawn around the contact tip and contacts were selected if they intersected with either the dorsolateral or ventromedial STN. When possible, dorsal and ventral contacts were selected on either side of an unused contact to minimize overlap of current spread.
Experimental Design
Gait and balance assessments were conducted in three DBS conditions on one day: unilateral DBS of the dorsal STN region, unilateral DBS of the ventral STN region, and both stimulators off. The order of DBS conditions was counterbalanced across participants, and all evaluations were double-blind. Thirteen individuals had right and ten had left unilateral DBS during stimulation conditions. Testing sessions began at least 42 minutes after DBS settings were changed, as it has been shown that 90% of changes in motor performance (UPDRS-III) occur within 45 minutes of DBS being turned off, and changes after DBS is turned on occur more quickly, with 90% of changes in motor performance occurring in 15–30 minutes.[20] Within our own laboratory, we have found that 85% of changes in motor performance occur by 42 minutes.
A 4.8m GAITRite walkway (CIR Systems, Havertown, PA) was used to calculate gait velocity in six walking trials: three at preferred pace [Pref gait], three as fast as possible [Fast gait]. We anticipated changes in gait variables would be more pronounced in Fast gait since the task was more challenging. Our primary gait variable of interest was gait velocity, as it is improved with STN DBS.[21–22] In each stimulation condition, postural stability was assessed using the mini-Balance Evaluation Systems Test of dynamic balance (mini-BESTest). The mini-BESTest is a fourteen item clinical balance test (with 32 points possible) that includes commonly used items, such as rising from a chair and one-leg stance.[23] UPDRS-III was administered by a validated rater at the beginning of each stimulation condition to assess overall motor impairment.
Statistical Analysis and Power
The primary variables of interest were gait velocity and total mini-BESTest score. Stride length was also calculated from GAITRite trials and examined as a secondary gait variable. In each condition, the gait variables for each participant’s 3 Fast gait trials and 3 Pref gait trials were averaged separately. Separate one-way RM ANOVAs (repeated measure: DBS condition) were run for Fast and Pref gait velocity and stride length, as well as for total mini-BESTest score and UPDRS-III score. Bonferroni corrections on ANOVA p values were used because of multiple gait comparisons. The Greenhouse-Geisser epsilon adjustment of the degrees of freedom estimate was used to correct for violations of sphericity. Post hoc pairwise comparisons, with Bonferroni corrections for multiple comparisons, were used to clarify differences between all conditions (dorsal DBS, ventral DBS, and off) when a significant effect of stimulation condition was seen in the RM ANOVA. For all statistical tests, significance was defined as p≤0.05. A priori power analyses suggest 15 participants would be necessary to detect a difference between stimulation conditions with a small effect size of 0.2 using a two-tailed repeated measures ANOVA (0.82 correlation among repeated measures), an alpha level of 0.05, and power (1-beta) of 0.8.[24]
RESULTS
An attempt was made to separate dorsal and ventral DBS contacts by one (n=18) or two (n=2) unused contacts when possible, but 3 participants were tested with adjacent dorsal and ventral contacts. Figure 1 shows the average DBS contact locations across participants. Six participants could not tolerate the standardized DBS settings due to adverse side effects. Side effects noted were eliminated with use of lower voltages listed in parentheses. One participant had jaw dystonia (2.3 V), one had paresthesia and nausea (2.3 V), one could not tolerate higher voltages in clinical programming (2.2 V), one had paresthesia and dystonia (2.0 V), and two had dyskinesias (1.8 V). Table 1 shows the DBS settings used for this study, as well as the normal clinical settings, for each participant.
Figure 1.
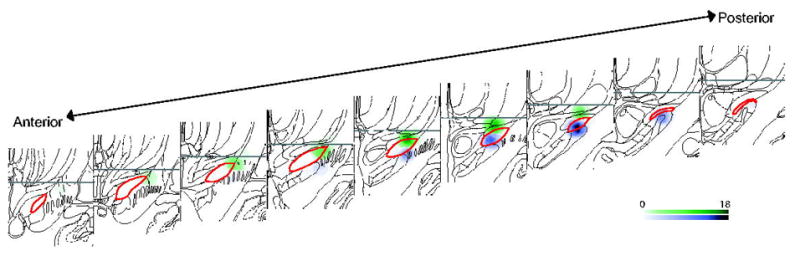
Contact locations for included participants. Coronal sections containing the STN (red outline) are shown anterior to posterior. All left side contacts used have been mirrored on the right side for ease of display. Spheres represent estimated 2 mm current spread around the dorsal (green) and ventral (purple) contact tip across subjects. Scale bar indicates the number of participants with overlapping spheres for that voxel.
Table 1.
Clinical DBS settings and DBS settings used for this study for each participant. DBS settings include DBS contacts used (C+ indicates pulse generator case as cathode), volts (V), pulse width (μs), frequency (Hz).
| Clinical Settings | Study Settings | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | L Contacts | L Volts | L Pulse | L Freq | R Contacts | R Volts | R Pulse | R Freq | Study Side | D Contact | D Volts | D Pulse | D Freq | V Contact | V Volts | V Pulse | V Freq |
| 1 | 1−, 2−, C+ | 3.0 | 90 | 185 | 1−, 2−, C+ | 3.0 | 90 | 185 | R | 2−, C+ | 2.5 | 60 | 185 | 0−, C+ | 2.5 | 60 | 185 |
| 2 | 2−, C+ | 3.2 | 60 | 185 | 2−, C+ | 3.2 | 60 | 185 | R | 2−, C+ | 2.5 | 60 | 185 | 0−, C+ | 2.5 | 60 | 185 |
| 3 | 2−, C+ | 3.6 | 60 | 185 | 2−, C+ | 3.6 | 60 | 185 | L | 3−, C+ | 2.5 | 60 | 185 | 1−, C+ | 2.5 | 60 | 185 |
| 4 | 3−, C+ | 2.4 | 60 | 185 | 3−, C+ | 3.5 | 60 | 185 | R | 2−, C+ | 2.0 | 60 | 185 | 0−, C+ | 2.0 | 60 | 185 |
| 5 | 2−, C+ | 3.8 | 90 | 185 | 2−, C+ | 3.8 | 90 | 185 | R | 3−, C+ | 2.5 | 60 | 185 | 0−, C+ | 2.5 | 60 | 185 |
| 6 | 2−, C+ | 2.8 | 60 | 185 | 1−, C+ | 2.8 | 60 | 185 | R | 2−, C+ | 2.5 | 60 | 185 | 0−, C+ | 2.5 | 60 | 185 |
| 7 | 3−, C+ | 3.3 | 60 | 185 | 1−, C+ | 3.0 | 60 | 185 | R | 3−, C+ | 2.5 | 60 | 185 | 1−, C+ | 2.5 | 60 | 185 |
| 8 | 2−, C+ | 3.6 | 60 | 185 | 2−, C+ | 3.6 | 60 | 185 | R | 3−, C+ | 2.5 | 60 | 185 | 1−, C+ | 2.5 | 60 | 185 |
| 9 | 2−, C+ | 3.0 | 60 | 185 | 1−, C+ | 3.2 | 60 | 185 | L | 2−, C+ | 2.5 | 60 | 185 | 0−, C+ | 2.5 | 60 | 185 |
| 10 | 0−, C+ | 3.0 | 60 | 185 | 0−, C+ | 1.6 | 60 | 185 | L | 3−, C+ | 2.5 | 60 | 185 | 0−, C+ | 2.5 | 60 | 185 |
| 11 | 2−, C+ | 3.9 | 60 | 185 | 2−, C+ | 3.8 | 60 | 185 | L | 3−, C+ | 2.5 | 60 | 185 | 1−, C+ | 2.5 | 60 | 185 |
| 12 | 2−, C+ | 3.5 | 60 | 185 | 1−, C+ | 3.1 | 60 | 185 | R | 3−, C+ | 2.5 | 60 | 185 | 1−, C+ | 2.5 | 60 | 185 |
| 13 | 1−, 2+ | 3.5 | 60 | 185 | 1−, C+ | 3.0 | 60 | 185 | L | 2−, C+ | 2.5 | 60 | 185 | 1−, C+ | 2.5 | 60 | 185 |
| 14 | 3−, 2−, C+ | 3.6 | 60 | 185 | 2−, 1+ | 4.0 | 60 | 185 | R | 1−, C+ | 2.5 | 60 | 185 | 0−, C+ | 2.5 | 60 | 185 |
| 15 | 2−, C+ | 2.9 | 60 | 185 | 2−, C+ | 3.2 | 60 | 185 | L | 2−, C+ | 2.5 | 60 | 185 | 0−, C+ | 2.5 | 60 | 185 |
| 16 | 2−, C+ | 2.8 | 60 | 185 | 2−, C+ | 2.8 | 60 | 185 | R | 3−, C+ | 1.8 | 60 | 185 | 1−, C+ | 1.8 | 60 | 185 |
| 17 | 0−, 1+ | 3.9 | 60 | 185 | 0−, 1+ | 3.9 | 60 | 185 | L | 2−, C+ | 2.5 | 60 | 185 | 0−, C+ | 2.5 | 60 | 185 |
| 18 | 2−, C+ | 2.9 | 60 | 185 | 2−, C+ | 2.3 | 60 | 185 | L | 2−, C+ | 2.5 | 60 | 185 | 0−, C+ | 2.5 | 60 | 185 |
| 19 | 1−, C+ | 1.8 | 60 | 185 | 1−, C+ | 2.0 | 60 | 185 | L | 3−, C+ | 2.5 | 60 | 185 | 1−, C+ | 2.3 | 60 | 185 |
| 20 | 1−, C+ | 2.2 | 60 | 185 | 1−, 3+ | 2.2 | 60 | 185 | R | 2−, C+ | 2.2 | 60 | 185 | 0−, C+ | 2.2 | 60 | 185 |
| 21 | 1−, C+ | 3.5 | 60 | 185 | 1−, C+ | 3.3 | 60 | 185 | R | 3−, C+ | 2.5 | 60 | 185 | 1−, C+ | 2.5 | 60 | 185 |
| 22 | 2−, C+ | 2.5 | 60 | 185 | 2−, C+ | 2.3 | 60 | 185 | L | 1−, C+ | 1.8 | 60 | 185 | 0−, C+ | 1.8 | 60 | 185 |
| 23 | 2−, C+ | 2.8 | 60 | 185 | 2−, C+ | 2.9 | 60 | 185 | R | 2−, C+ | 2.3 | 60 | 185 | 0−, C+ | 2.3 | 60 | 185 |
Gait
Mean gait velocity and mean stride length for Pref and Fast gait are shown in Figure 2a and 2b, respectively. Mean Fast and Pref gait velocity differed between the dorsal DBS, ventral DBS, and DBS off conditions (F(2,44)=10.66, p<0.001 and (F(1.38.30.28)=6.28, p=0.044, respectively), as did Fast and Pref stride length (F(1.47,32.35)=16.79, p<0.001 and F(1.21,26.63)=11.09, p=0.008, respectively), suggesting gait was improved with DBS (Figure 2).
Figure 2.
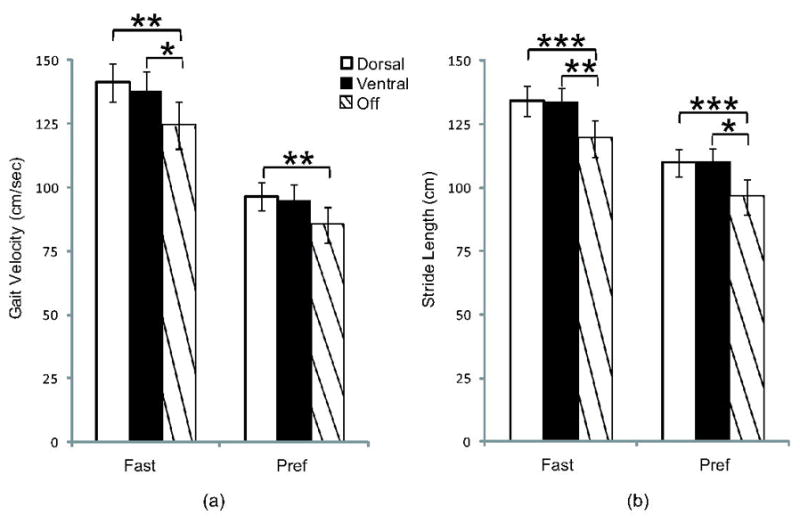
Mean velocity and stride length for Fast and Pref gait for each stimulation condition. Fast velocity and Fast and Pref stride length were significantly higher with dorsal and ventral DBS, compared to off. Velocity for Pref gait was significantly different between dorsal DBS and off. There were no differences between dorsal DBS and ventral DBS. Mean values ± SEM. *p≤0.05, **p≤0.01, ***p≤0.001.
Post-hoc pairwise comparisons showed that during Fast gait, participants walked faster with dorsal stimulation (M=141.2±36.0 cm/sec, mean±SD) and ventral stimulation (M=137.9±37.1 cm/sec) compared to DBS off (M=124.3±44.2 cm/sec, p=0.002, and p=0.015, respectively). In Pref gait, participants walked faster with dorsal stimulation (M=96.5±25.8 cm/sec, mean±SD) compared to DBS off (M=85.1±33.7 cm/sec, p=0.002) but not ventral stimulation (M=94.8±29.4 cm/sec), compared to DBS off (p=0.127). Velocity did not differ between dorsal and ventral conditions in Fast gait (p=0.750, 95% CI (−3.94,10.55)) or Pref gait (p=1.0, 95% CI (−5.41,8.88)).
Participants walked with longer strides in Fast gait during dorsal (M=134.3±28.3 cm) and ventral (M=133.3±30.0 cm) stimulation conditions, compared to DBS off (M=119.3±35.7 cm, p<0.001 and p=0.002, respectively). They also used longer strides in Pref gait with dorsal (M=109.8±26.0 cm) and ventral (M=109.8±27.9 cm) stimulation, compared to DBS off (M=96.4±32.8 cm, p=0.001, and p=0.017, respectively). Stride length did not differ between dorsal and ventral stimulation conditions for Fast or Pref gait (p=1.0, 95% CI (−3.86,5.89), and p=1.0, 95% CI (−5.21,5.34) respectively).
Postural Stability and Overall Motor Performance
No significant differences were found in total mini-BESTest scores between dorsal (M=20.7±4.9), ventral (M=20.7±4.9), and DBS off (M=19.1±5.8) conditions (F(1.51,33.31)=2.73, p=0.093), suggesting postural stability did not change measurably across the three conditions (Figure 3a). Overall, the UPDRS-III postural stability item (Item 3.12) was correlated with mini-BESTest Score. (ρ(67)= −0.44, P<0.001). Examining individual mini-BESTest scores, some participants exhibited improved balance with either dorsal or ventral DBS, while others performed worse, compared to DBS off. The location of the DBS contact within or outside the STN border was assessed in participants who did and those who did not show balance improvements with STN stimulation. Among those whose balance improved with dorsal STN stimulation (n=13), compared to DBS off, 38% had STN DBS within the STN, while 62% had the dorsal contact outside the STN. Among those whose balance worsened or did not change with dorsal STN stimulation (n=10), compared to DBS off, 50% had the dorsal contact within, and 50% had the dorsal contact outside the STN. Among those whose balance improved with ventral STN stimulation (n=13), compared to DBS off, 46% had the ventral contract within the STN, while 54% had the ventral contact outside the STN. Among those whose balance did not improve with ventral STN stimulation (n=10), compared to DBS off, 60% had the ventral contact within the STN, while 40% had the ventral contact outside the STN.
Figure 3.
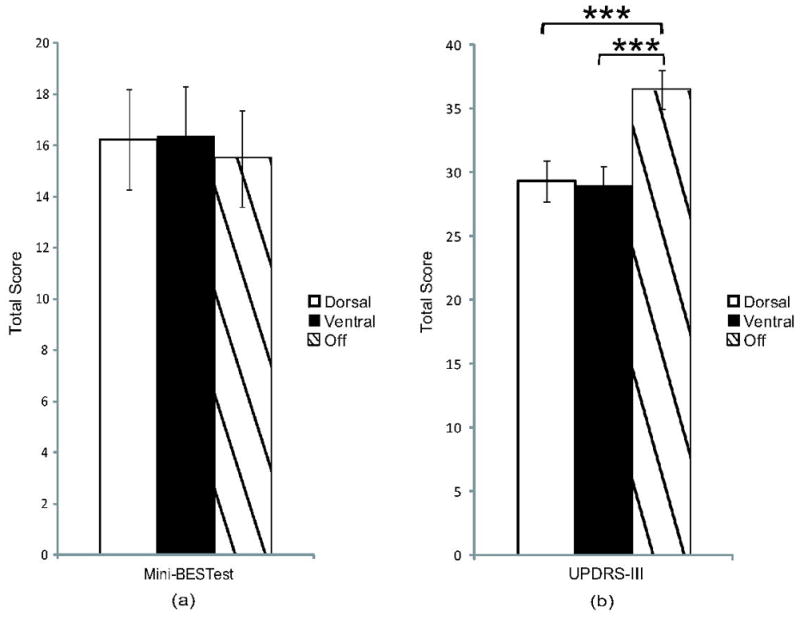
Total a) mini-BESTest and b) UPDRS-III scores were compared across conditions. Balance did not differ between conditions, but total UPDRS-III scores were significantly improved with dorsal and ventral DBS, compared to off. There were no differences between dorsal DBS and ventral DBS. Mean values ± SEM. ***p≤0.001.
RM ANOVA indicated a difference (F(2,44)=24.75, p<0.001) in total UPDRS-III scores between stimulation conditions (Figure 3b). Similar to gait results, post hoc tests on total UPDRS-III scores showed differences between dorsal (M=29.3±7.8) and off (M=36.5±7.3, p<0.001) and ventral (M=28.9±7.5) and off (p<0.001), but not between dorsal and ventral (p=1.0, 95% CI (−2.50, 3.33)) DBS conditions.
DISCUSSION
Based on the differences in anatomic connectivity between the dorsal and ventral STN,[12] it has been suggested that DBS should target the dorsal STN, which has anatomic connections with sensorimotor brain regions.[25] We hypothesized that targeted stimulation in the dorsal STN region would improve gait and balance more than stimulation in the ventral STN region in PD patients. Although we found improvements in gait with DBS similar to those reported with unilateral[2] or bilateral[21] STN DBS using the most therapeutic contacts at clinically-optimal DBS settings, the results of our current study indicate unilateral dorsal DBS does not improve gait and balance more than unilateral ventral STN region stimulation at suboptimal voltages. Improvement in gait with STN DBS, regardless of contact location, supports the possibility that motor connections may be more diffusely distributed throughout the STN. These regions of STN may not be sharply divided, but instead the motor connections may exist throughout STN, potentially allowing for integration across the functional regions.[26]
Although there were no significant balance improvements with either unilateral dorsal or ventral DBS, compared to DBS off, the mini-BESTest data show a similar pattern to that seen with the gait data. Our results suggest that unilateral STN DBS at suboptimal DBS settings does not have a substantial clinically or functionally meaningful effect on balance. In contrast, previous studies have demonstrated that bilateral STN DBS with optimal clinical settings improved balance and postural stability as measured by the Berg Balance Scale (BBS) and the postural stability item of the UPDRS-III.[27] Further, balance as measured by assessing center of mass displacement following postural perturbations improves with bilateral STN DBS in some participants, and worsens in others.[11] Similarly, some participants in our study showed considerable improvements in mini-BESTest score in both unilateral dorsal and ventral DBS conditions, while others performed worse, regardless of the order of the stimulation conditions. Based on the percentages of those with the DBS contact within versus outside the STN, it does not appear that the position of the contact within or outside the STN border predominantly dictated whether balance improved with DBS in the dorsal or ventral stimulation conditions. The worsening of balance in some participants with unilateral dorsal or ventral stimulation, compared to off DBS, may be due to stimulation of certain pathways or structures near the STN, such as the internal capsule, zona incerta (dorsally), or the substantia nigra (ventrally).
We expected dorsal STN DBS would improve overall motor performance more than ventral STN DBS. In contrast, UPDRS-III scores improved with either unilateral dorsal or ventral DBS, compared to DBS off, and there were no differences between motor benefits seen with unilateral dorsal and ventral stimulation. Our results concur with previous reports of improvements in UPDRS-III scores with unilateral STN DBS,[1,2,4] compared to DBS off. Further, unilateral dorsal or ventral STN region stimulation produce similar improvements in contralateral side UPDRS-III scores. This differs from the greater effect of ventral STN stimulation compared to dorsal STN stimulation on changes in response inhibition.[15] Uniform improvements in motor performance regardless of DBS contact location also supports a model of STN organization where motor connections may be more diffusely distributed, rather than primarily restricted to the dorsal region.
Unilateral DBS of dorsal and ventral STN regions produced similar improvements in gait and overall motor performance in PD. Balance was more variably affected. Though our results do not support the proposed dorsal-ventral model of STN motor organization, some prior evidence does support this model. One study examining locations of clinically active contacts in those with PD found that DBS of the dorsal STN region produced significantly greater increases in lateralized UPDRS-III scores, step length, step velocity, and balance (percentage of time with both feet on the ground during gait).[28] However, the design of that study was not ideal because each participant’s left and right active contacts were not necessarily placed in similar positions within the STN on both sides. Further, each participant’s left and right side contacts were considered separately, measuring the effects of activation of each contact (during bilateral stimulation) on contralateral motor performance. Results were potentially confounded since STN DBS has both ipsilateral and contralateral effects.[1,2,4,7–9] Additionally, stimulator settings were not standardized across participants in the previous study.
Our study was unique in that we measured changes in gait and balance with unilateral DBS in the dorsal versus ventral STN regions in the same individuals with standardized DBS settings. We were able to detect significant differences between DBS off and DBS on conditions despite use of suboptimal DBS settings. Utilizing the lower voltage settings was important to minimize the overlap in stimulation area between the dorsal and ventral contacts. The contact localization and targeted dorsal and ventral stimulation methods used in our study have been successfully employed to assess the effects of dorsal versus ventral stimulation on response inhibition in individuals with PD.[15] In this recent study, differences were detected in cognitive performance between dorsal and ventral stimulation conditions,[15] supporting our ability to differentially stimulate dorsal and ventral STN regions.
Conclusions
The exact mechanism by which STN DBS provides motor benefits in PD remains unclear. STN DBS may normalize irregular or pathological STN output seen in PD.[29–30] It is also not known which axons or neurons in or near the STN should be stimulated to produce optimal improvements in gait and balance. The neuroanatomic basis of gait and balance deficits in PD is also not clear, although the pedunculopontine nucleus (PPN) may play an important role in these gait impairments. Since the PPN has reciprocal connections with the basal ganglia, and specifically the STN,[31–32] stimulation of the STN might alleviate potential aberrant suppression of PPN activity and/or compensate for neuron loss in the PPN, improving gait impairments in PD.[31] These STN-PPN motor connections may be more diffusely distributed throughout the STN, rather than located primarily in the dorsal STN region. At this time, DBS contact location in the dorsal/ventral orientation of the STN need not be a major consideration for surgical targeting or programming purposes with respect to gait and balance. However, attention to dorsal/ventral location may be critical with respect to cognitive function [15]. Future research is necessary to improve our understanding of STN organization with the ultimate goal of optimizing the target for DBS within the STN to maximize motor benefit while also minimizing cognitive side effects.
Acknowledgments
We thank John Michael Rotello, Ryan Duncan, Phillip Lintzenich, and Abigail Leddy for data collection assistance, and Vanessa Heil-Chapdelaine for data analysis assistance.
Funding: Support was provided by NIH grants RO1NS41509, CO6 RR020092, and RR024992 (Washington University Institute of Clinical and Translational Sciences – Brain, Behavioral and Performance Unit), the American Parkinson Disease Association (APDA) Advanced Center for PD Research at Washington University, the Greater St. Louis Chapter of the APDA, and the Barnes Jewish Hospital Foundation (Stein Family Fund and the Jack Buck Fund for PD Research).
Footnotes
Competing Interests: None to report.
Licensing Statement: “The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in the Journal of Neurology, Neurosurgery & Psychiatry editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence.”
References
- 1.Kumar R, Lozano AM, Sime E, Halket E, Lang AE. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology. 1999;53:561–6. doi: 10.1212/wnl.53.3.561. [DOI] [PubMed] [Google Scholar]
- 2.Tabbal SD, Ushe M, Mink JW, Revilla FJ, Wernle AR, Hong M, Karimi M, Perlmutter JS. Unilateral subthalamic nucleus stimulation has a measurable ipsilateral effect on rigidity and bradykinesia in Parkinson disease. Exp Neurol. 2008;211:234–42. doi: 10.1016/j.expneurol.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, Suelter M, Jacobson CE, IV, Wang X, Gordon CW, II, Zeilman P, Romrell J, Martin P, Ward H, Rodriguez RL, Foote KD. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009;65:586–95. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slowinski JL, Putzke JD, Uitti RJ, Lucas JA, Turk MF, Kall BA, Wharen RE. Unilateral deep brain stimulation of the subthalamic nucleus for Parkinson disease. J Neurosurg. 2007;106:626–632. doi: 10.3171/jns.2007.106.4.626. [DOI] [PubMed] [Google Scholar]
- 5.Agostino R, Dinapoli L, Modugno N, Iezzi E, Romanelli P, Berardelli A. Effects of unilateral subthalamic deep brain stimulation on contralateral arm sequential movements in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79:76–78. doi: 10.1136/jnnp.2007.122010. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Paek SH, Kim JY, Lee JY, Lim YH, Kim DG, Jeon BS. Two-year follow-up on the effect of unilateral subthalamic deep brain stimulation in highly asymmetric Parkinson’s disease. Mov Disord. 2009;24:329–35. doi: 10.1002/mds.22211. [DOI] [PubMed] [Google Scholar]
- 7.Linazasoro G, Van Blercom N, Lasa A. Unilateral subthalamic deep brain stimulation in advanced Parkinson’s disease. Mov Disord. 2003;18:713–16. doi: 10.1002/mds.10407. [DOI] [PubMed] [Google Scholar]
- 8.Germano IM, Gracies JM, Weisz DJ, Tse W, Koller WC, Olanow CW. Unilateral stimulation of the subthalamic nucleus in Parkinson disease: a double-blind 12-month evaluation study. J Neurosurg. 2004;101:36–42. doi: 10.3171/jns.2004.101.1.0036. [DOI] [PubMed] [Google Scholar]
- 9.Walker HC, Watts RL, Guthrie S, Wang D, Guthrie BL. Bilateral effects of unilateral subthalamic deep brain stimulation on Parkinson’s disease at 1 year. Neurosurgery. 2009;65:302–10. doi: 10.1227/01.NEU.0000349764.34211.74. [DOI] [PubMed] [Google Scholar]
- 10.Kelly VE, Israel SM, Samii A, Slimp JC, Goodkin R, Shumway-Cook A. Assessing the effects of subthalamic nucleus stimulation on gait and mobility in people with Parkinson disease. Disabil Rehabil. 2010;32:929–36. doi: 10.3109/09638280903374139. [DOI] [PubMed] [Google Scholar]
- 11.Visser JE, Allum JH, Carpenter MG, Esselink RA, Speelman JD, Borm GF, Bloem BR. Subthalamic nucleus stimulation and postural instability in Parkinson’s disease. J Neurol. 2008;255:205–210. doi: 10.1007/s00415-008-0636-x. [DOI] [PubMed] [Google Scholar]
- 12.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 13.Benarroch EE. Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology. 2008;70:1991–5. doi: 10.1212/01.wnl.0000313022.39329.65. [DOI] [PubMed] [Google Scholar]
- 14.Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 15.Hershey T, Campbell MC, Videen TO, Lugar HM, Weaver PM, Hartlein J, Karimi M, Tabbal SD, Perlmutter JS. Mapping Go–No-Go performance within the subthalamic nucleus region. Brain. 2010 September 20; doi: 10.1093/brain/awq256. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes AJ, Daniels SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 caes. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Videen TO, Campbell MC, Tabbal SD, Karimi M, Hershey T, Perlmutter JS. Validation of a fiducial-based atlas localization method for deep brain stimulation contacts in the area of the subthalamic nucleus. J Neurosci Methods. 2008;168:275–81. doi: 10.1016/j.jneumeth.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butson CR, McIntyre CC. Tissue and electrode capacitance reduce neural activation volumes during deep brain stimulation. Clin Neurophysiol. 2005;116:2490–500. doi: 10.1016/j.clinph.2005.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butson CR, McIntyre CC. Role of electrode design on the volume of tissue activated during deep brain stimulation. J Neural Eng. 2006;3:1–8. doi: 10.1088/1741-2560/3/1/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- 21.Johnsen EL, Mogensen PH, Sunde NA, Østergaard K. Improved asymmetry of gait in Parkinson’s disease with DBS: gait and postural instability in Parkinson’s disease treated with bilateral deep brain stimulation in the subthalamic nucleus. Mov Disord. 2009;24:590–97. doi: 10.1002/mds.22419. [DOI] [PubMed] [Google Scholar]
- 22.Hausdorff JM, Gruendlinger L, Scollins L, O’Herron S, Tarsy D. Deep brain stimulation effects on gait variability in Parkinson’s disease. Mov Disord. 2009;24:1688–92. doi: 10.1002/mds.22554. [DOI] [PubMed] [Google Scholar]
- 23.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the balance evaluation systems test: The mini-BESTest. J Rehabil Med. 2010;42:323–331. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. A power primer. Psychol Bull. 1992;112:155–59. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 25.Aravamuthan BR, Muthusamy KA, Stein JF, Aziz TZ, Johansen-Berg H. Topography of cortical and subcortical connections of the human pedunculopontine and subthalamic nuclei. Neuroimage. 2007;37:694–705. doi: 10.1016/j.neuroimage.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 26.Mallet L, Schupbach M, N’diaye K, Remy P, Bardinet E, Czernecki V, Welter ML, Pelissolo A, Ruberg M, Agid Y, Yelnik J. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc Natl Acad Sci. 2007;104:10661–6. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson MH, Törnqvist AL, Rehncrona S. Deep-brain stimulation in the subthalamic nuclei improves balance performance in patients with Parkinson’s disease, when tested without anti-parkinsonian medication. Acta Neurol Scand. 2005;111:301–8. doi: 10.1111/j.1600-0404.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnsen EL, Sunde N, Mogensen PH, Ostergaard K. MRI verified STN stimulation site - gait improvement and clinical outcome. Eur J Neurol. 2010;17:746–753. doi: 10.1111/j.1468-1331.2010.02962.x. [DOI] [PubMed] [Google Scholar]
- 29.Vitek JL. Mechanisms of deep brain stimulation: Excitation or inhibition. Mov Disord. 2002;17:S69–S72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- 30.Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain. 2000;123:1767–83. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- 32.Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–8. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]


