Abstract
BACKGROUND
Poor neurodevelopmental outcomes and recurrences of cutaneous lesions remain unacceptably frequent among survivors of neonatal herpes simplex virus (HSV) disease.
METHODS
We enrolled neonates with HSV disease in two parallel, identical, double-blind, placebo-controlled studies. Neonates with central nervous system (CNS) involvement were enrolled in one study, and neonates with skin, eye, and mouth involvement only were enrolled in the other. After completing a regimen of 14 to 21 days of parenteral acyclovir, the infants were randomly assigned to immediate acyclovir suppression (300 mg per square meter of body-surface area per dose orally, three times daily for 6 months) or placebo. Cutaneous recurrences were treated with open-label episodic therapy.
RESULTS
A total of 74 neonates were enrolled — 45 with CNS involvement and 29 with skin, eye, and mouth disease. The Mental Development Index of the Bayley Scales of Infant Development (in which scores range from 50 to 150, with a mean of 100 and with higher scores indicating better neurodevelopmental outcomes) was assessed in 28 of the 45 infants with CNS involvement (62%) at 12 months of age. After adjustment for covariates, infants with CNS involvement who had been randomly assigned to acyclovir suppression had significantly higher mean Bayley mental-development scores at 12 months than did infants randomly assigned to placebo (88.24 vs. 68.12, P = 0.046). Overall, there was a trend toward more neutropenia in the acyclovir group than in the placebo group (P = 0.09).
CONCLUSIONS
Infants surviving neonatal HSV disease with CNS involvement had improved neurodevelopmental outcomes when they received suppressive therapy with oral acyclovir for 6 months. (Funded by the National Institute of Allergy and Infectious Diseases; CASG 103 and CASG 104 ClinicalTrials.gov numbers, NCT00031460 and NCT00031447, respectively.)
The outcomes of neonatal herpes simplex virus (HSV) disease are dependent on the extent of the disease.1 Approximately 30% of babies with disseminated disease die, but only 20% of survivors have neurologic sequelae.2 In contrast, only 6% of babies with central nervous system (CNS) disease die, but approximately 70% have permanent neurologic impairment.2 Skin, eye, and mouth disease is not associated with death, and neurologic impairment is rare with this manifestation of neonatal herpes.3
HSV establishes latency in sensory ganglia, with periodic reactivation and recurrence of localized disease.4,5 Whether the virus subclinically reactivates in the brain after neonatal HSV disease is not known. If reactivation in the brain does occur, it could contribute to the serious neurologic sequelae associated with neonatal HSV disease with CNS involvement, as has been suggested.6
Antiviral suppressive therapy prevents the recurrence of localized disease in persons with genital7–10 or orolabial11,12 HSV infection. The National Institute of Allergy and Infectious Diseases (NIAID) Collaborative Antiviral Study Group (CASG) conducted parallel, identical, phase 3, placebo-controlled studies of oral acyclovir suppressive therapy after neonatal HSV disease to determine the efficacy and safety of long-term antiviral administration during infancy.
METHODS
STUDY DESIGN AND OVERSIGHT
From August 1997 through April 2008, we enrolled infants with HSV disease in two trials: infants with CNS involvement (stratified according to CNS disease alone or disseminated disease with CNS involvement) were enrolled in the CASG 103 trial, and infants with skin, eye, and mouth disease only were enrolled in the CASG 104 trial. The institutional review board at each participating institution approved the studies. The protocols, including the statistical analysis plans, are available with the full text of this article at NEJM.org. All authors vouch for the completeness and accuracy of the data presented, as well as the fidelity of the studies to the protocols. The NIAID convened a data and safety monitoring board, which oversaw the studies. Oral acyclovir and matching placebo were supplied by GlaxoSmithKline, Alpharma USPD, and Pharm Ops. None of these companies had any role in the design or conduct of the trials, the collection or analysis of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication. Written informed consent was obtained from a parent or guardian of each study subject before study activities proceeded.
STUDY POPULATION
Inclusion criteria for both studies were HSV infection, as confirmed by culture or, in the case of infants in the CASG 103 study who did not have skin lesions, detection of HSV DNA in cerebrospinal fluid by polymerase-chain-reaction (PCR) assay; disease onset within the first 28 days of age; and a body weight of at least 800 g at the time of enrollment. Infants in the CASG 103 study had to have documentation of abnormal cerebrospinal fluid indexes or abnormal findings on neuroimaging studies or electroencephalograms or had to have cerebrospinal fluid that was positive for HSV DNA, as detected by PCR assay, whereas subjects in the CASG 104 study had normal results. In order for infants in the CASG 103 study to be eligible to undergo randomization after the initial regimen of parenteral acyclovir, they had to have cerebrospinal fluid that was negative for HSV DNA, as detected by PCR assay, after completion of that regimen.
The primary end point of both studies was neurodevelopmental status at 12 months of age, as assessed with the use of the Bayley Scales of Infant Development, second edition. Secondary end points were two or fewer recurrences of cutaneous lesions during the first 12 months of age and detection by PCR assay of HSV DNA in the cerebrospinal fluid during or after suppressive therapy. The tertiary end point was toxic effects of grade 2 or higher, as assessed with the use of the World Health Organization (WHO) Toxicity Grading Scale.13
All the infants completed a 14-day course (in the case of the infants with skin, eye, and mouth disease) or a 21-day course (in the case of infants with CNS involvement) of parenteral acyclovir, as is the standard of care.14 Infants in both studies were then randomly assigned, in a 1:1 ratio, to oral acyclovir or placebo, in a double-blind fashion. The study drug was administered at a dose of 300 mg per square meter of body-surface area, administered three times daily for 6 months.15 Per protocol, after an infant had a second cutaneous recurrence (i.e., exceeded the secondary end point), the randomly assigned oral acyclovir or placebo was discontinued, and open-label oral acyclovir suppression was allowed.
CLINICAL AND SAFETY ASSESSMENTS
Assessments were performed at 2 weeks and 4 weeks after the first administration of the study drug and then monthly throughout the 6-month period during which the infants received suppressive treatment with the study drug or an open-label drug. The infants were then seen at 1 year of age for an assessment of end points. The neurodevelopmental pediatricians and psychologists performing the Bayley Scales of Infant Development testing were not aware of the treatment assignments. Laboratory testing to assess safety was performed at each visit during the period in which the infants were receiving suppressive treatment.
STATISTICAL ANALYSIS
Demographic and baseline clinical characteristics and results of the grading of laboratory-test abnormalities after intravenous acyclovir therapy were compared with the use of the Kruskal–Wallis test for continuous variables and Fisher’s exact test for categorical variables. For the primary efficacy analyses, a two-sample t-test was used to determine whether assignment to suppressive therapy with oral acyclovir was associated with significantly improved neurodevelopmental outcomes at 12 months of age. An analysis-of-covariance model was also used to adjust for baseline clinical factors that were unbalanced between the two treatment groups (P≤0.10). A Kaplan–Meier analysis with a log-rank test was used to determine whether suppressive therapy with oral acyclovir reduced the time to the second recurrence of skin lesions and whether it prevented the appearance of HSV DNA in the cerebrospinal fluid, as assessed by PCR, during the 12-month period after completion of intravenous acyclovir therapy. Fisher’s exact test was used to compare toxic effects between the two groups. A repeated-measures analysis was used to compare the two groups with respect to abnormalities in laboratory-test results during the period from baseline to month 6.
RESULTS
DEMOGRAPHIC AND BASELINE CHARACTERISTICS OF THE SUBJECTS
A total of 45 infants underwent randomization at 19 institutions as part of the CASG 103 study — 37 with CNS disease and 8 with disseminated disease and CNS involvement; 29 infants with skin, eye, and mouth disease underwent randomization at 12 institutions as part of the CASG 104 study. As shown in Table 1, the two groups in each study were balanced with respect to the extent of neurologic involvement, viral type, and gestational age. Infants with CNS disease who were randomly assigned to acyclovir, as compared with those assigned to placebo, had a lower median birth weight (P = 0.01), median weight at study enrollment (P = 0.01), and median head circumference at birth (P = 0.03). Infants with skin, eye, and mouth disease who were randomly assigned to acyclovir, as compared with those assigned to placebo, had lower median weight at enrollment (P = 0.03) (Table 1 in the Supplementary Appendix, available at NEJM.org).
Table 1.
Baseline Demographic and Clinical Characteristics, According to Study-Drug Assignment.*
| Characteristic | CASG 103 | CASG 104 | ||||
|---|---|---|---|---|---|---|
| CNS Disease | Disseminated Disease with CNS Involvement |
Skin, Eye, and Mouth Disease | ||||
| acyclovir (N = 21) |
placebo (N = 16) |
acyclovir (N = 3) |
placebo (N = 5) |
acyclovir (N = 15) |
placebo (N = 14) |
|
| Gestational age — wk | ||||||
| Median | 37 | 38 | 40 | 39 | 38 | 39 |
| Range | 25–41 | 35–40 | 40–41 | 38–40 | 27–40 | 27–41 |
| HSV type — no./total no. (%)† | ||||||
| I | 2/14 (14) | 3/11 (27) | 1/2 (50) | 1/3 (33) | 5/12 (42) | 5/11 (45) |
| II | 12/14 (86) | 8/11 (73) | 1/2 (50) | 2/3 (67) | 7/12 (58) | 6/11 (55) |
| White-cell count in cerebrospinal fluid at presentation — cells/mm3 | ||||||
| Median | 91.5 | 109.0 | 3.0 | 2.0 | 4.0 | 6.0 |
| Range | 10–1216 | 5–58,080 | 1–18 | 0–13 | 0–20 | 0–33 |
| HSV DNA in cerebrospinal fluid at presentation — no./total no. (%)‡ | ||||||
| Positive | 17/21 (81) | 12/16 (75) | 2/3 (67) | 3/5 (60) | 0 | 0 |
| Negative | 4/21 (19) | 4/16 (25) | 1/3 (33) | 2/5 (40) | 14/14 (100) | 13/13 (100) |
| Evidence of HSV disease on MRI — no./total no. (%) | 10/16 (62) | 9/14 (64) | 1/3 (33) | 0 | 0 | 0 |
| Abnormal EEG — no./total no. (%) | 9/13 (69) | 10/14 (71) | 1/1 (100) | 0 | NA | NA |
None of the characteristics differed significantly between the groups (P>0.05 for all comparisons). EEG denotes electroencephalogram, HSV herpes simplex virus, MRI magnetic resonance imaging, and NA not applicable.
The viral type was unknown in the case of some infants.
HSV DNA in cerebrospinal fluid was detected with the use of a polymerase-chain-reaction assay.
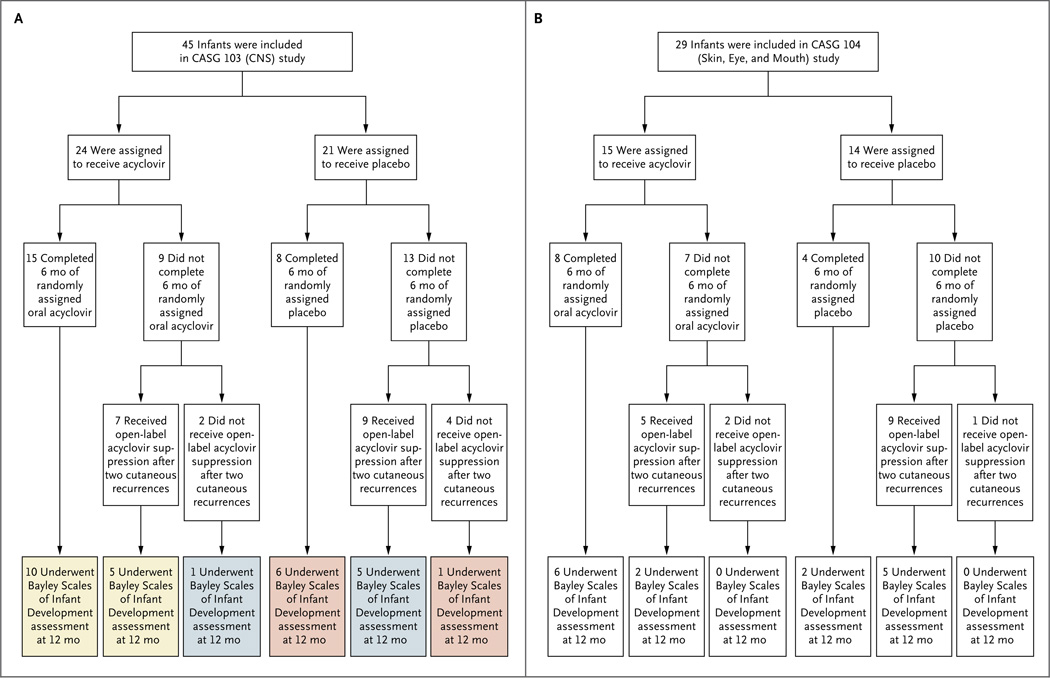
Of the 45 infants in the CASG 103 (CNS) study, 39 (87%) either completed 6 months of therapy (23 subjects) or had two recurrences of cutaneous lesions (16); the other 6 infants did not complete the full 6 months of therapy for the following reasons: loss to follow-up (1 infant), nonadherence to the protocol (2), withdrawn consent (2), and death (1 in the placebo group) (Fig. 1A). The baseline characteristics of the 6 infants who did not complete 6 months of treatment did not differ significantly from those of the infants who did.
Figure 1. Enrollment, Randomization, and Follow-up in the Two Parallel Studies.
The CASG 103 study (Panel A) included neonates who had HSV disease with central nervous system (CNS) involvement. The yellow boxes represent the 15 infants with a Bayley Scales of Infant Development mental-development assessment at 12 months who received active suppression for 6 months, either with the randomly assigned oral acyclovir for the entire 6-month period (10 infants) or with the randomly assigned oral acyclovir for part of the time and open-label oral acyclovir for part of the time (5 infants); the blue boxes represent the 6 infants with a mental-development assessment at 12 months who received acyclovir suppression for only part of the 6-month treatment period; and the pink boxes represent the 7 infants with a mental-development assessment at 12 months who received no acyclovir suppression at any time during the study. The CASG 104 study (Panel B) included neonates who had HSV disease with skin, eye, and mouth involvement.
Of the 29 infants in the CASG 104 (skin, eye, and mouth disease) study, 26 (90%) either completed 6 months of therapy (12) or reached the study end point after two cutaneous recurrences (14); the other 3 infants did not complete the full 6 months of therapy because of loss to follow-up (1) and nonadherence to the protocol (2) (Fig. 1B). The baseline characteristics of the 3 infants who did not complete 6 months of treatment did not differ significantly from those of the infants who did.
CUTANEOUS RECURRENCES
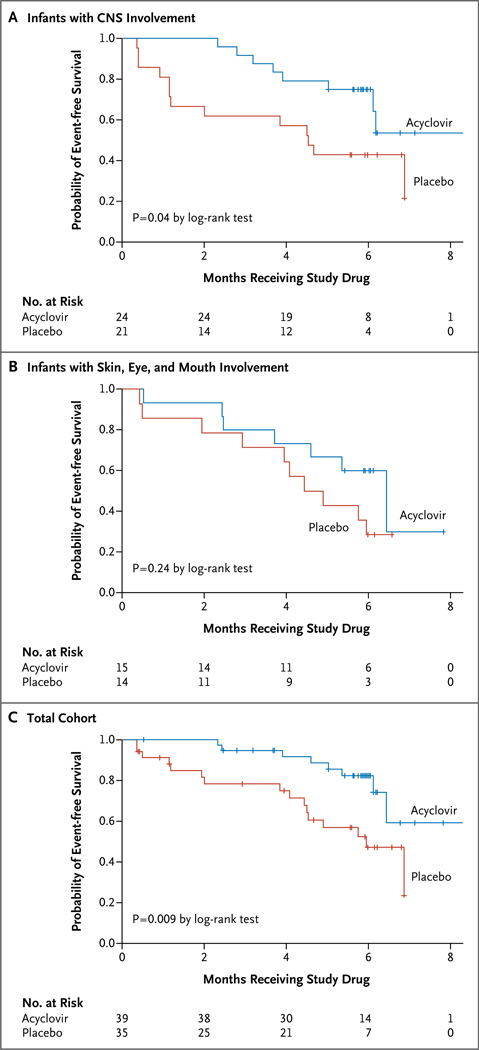
The likelihood of skin recurrences after neonatal HSV disease is not affected by disease classification.3 In an analysis that included data from the 74 infants in the two studies combined and that evaluated the time to discontinuation of the study medication because the infant had two cutaneous recurrences, the median time the infants received the study drug was 2.5 months longer among infants assigned to acyclovir than among those assigned to placebo (P = 0.009) (Fig. 2C). In the CASG 103 (CNS) study, the median time the infants received the study drug was 4.1 months longer among infants assigned to acyclovir than to those assigned to placebo (P = 0.04) (Fig. 2A). In the CASG 104 (skin, eye, and mouth disease) study, the median time the infants received the study drug was 1.7 months longer among infants assigned to acyclovir than to those assigned to placebo (P = 0.24) (Fig. 2B). In a regression analysis of the two thirds of infants for whom the viral type was known (Table 1), the likelihood of cutaneous recurrences was not affected by viral type.
Figure 2. Time to Discontinuation of Study Drug.
Per protocol, the study drug was discontinued if an infant had two cutaneous recurrences. The time to discontinuation of the study drug is shown among subjects in the CASG 103 study (Panel A), which included neonates with HSV disease with central nervous system (CNS) involvement, among subjects in the CASG 104 study (Panel B), which included neonates with HSV disease with skin, eye, and mouth involvement only, and among the subjects in the two groups combined (Panel C).
RECURRENCES OF CNS DISEASE
Three infants in the CASG 103 study had a recurrence of CNS disease during the 12 months after enrollment in the study. Two had been assigned to placebo (both term babies), and one had been assigned to acyclovir (a 28-week premature infant) (P = 0.59). All three had cerebrospinal fluid that was positive for HSV DNA, as detected by PCR assay, at the time of the recurrence of CNS disease and had an adequate therapeutic and virologic response to another course of parenteral acyclovir, suggesting that acyclovir resistance was unlikely. During the same period, no infant in the CASG 104 study had a recognized occurrence of CNS disease.
NEURODEVELOPMENTAL OUTCOMES
A total of 28 of the 45 infants (62%) enrolled in the CASG 103 (CNS) study had a Bayley Scales of Infant Development assessment at 12 months (Fig. 1A). Baseline demographic and clinical characteristics, including assessments of the extent of neurologic involvement, were similar between the 28 subjects who had a Bayley Scales of Infant Development assessment at 12 months of age and the 17 who did not (Table 2, and Table 2 in the Supplementary Appendix). For subjects with CNS disease, an analysis-of-covariance model was used to adjust for the covariates at baseline that were unbalanced between the groups. The covariates that were examined included age at enrollment, birth weight, weight at enrollment, head circumference at birth, head circumference at enrollment, Apgar scores at 1 and 5 minutes, gestational age, the presence of cutaneous lesions during the initial illness, and viral type (HSV-1 or HSV-2). After adjustment for covariates that had P values of 0.10 or less (head circumference at birth, birth weight, and enrollment weight), infants assigned to receive acyclovir had significantly higher mean Bayley mental-development scores at 1 year (88.24 vs. 68.12, P = 0.046). Among subjects in the CASG 103 (CNS) study assigned to suppressive therapy with oral acyclovir, 69% had normal neurologic outcomes, 6% had mild impairment, 6% had moderate impairment, and 19% had severe impairment; the corresponding proportions among subjects assigned to placebo were 33%, 8%, 25%, and 33%. Among subjects in the CASG 104 (skin, eye, and mouth) study, no significant differences in Bayley mental-development scores were noted. In a regression analysis of data from the two thirds of subjects for whom the viral type was known (Table 1), neurodevelopmental outcomes did not differ significantly according to viral type in either study.
Table 2.
Demographic and Clinical Characteristics of Infants with and without 12-Month Bayley Scales of Infant Development Assessments.*
| Characteristic | CASG 103 (CNS) Study | CASG 104 (Skin, Eye, and Mouth) Study | ||||
|---|---|---|---|---|---|---|
| Bayley Assessment (N = 28) |
No Bayley Assessment (N = 17) |
P Value | Bayley Assessment (N = 15) |
No Bayley Assessment (N = 14) |
P Value | |
| Gestational age — wk | 0.17 | 0.69 | ||||
| Median | 39.5 | 38 | 38 | 39 | ||
| Range | 28–41 | 25–40 | 27–41 | 27–40 | ||
| HSV type — no./total no. (%)† | 0.03 | 0.003 | ||||
| I | 7/19 (37) | 0/11 | 2/13 (15) | 8/10 (80) | ||
| II | 12/19 (63) | 11/11 (100) | 11/13 (85) | 2/10 (20) | ||
| White-cell count in cerebrospinal fluid at presentation — cells/mm3 | 0.98 | 0.48 | ||||
| Median | 65 | 71 | 6 | 5 | ||
| Range | 1–58,080 | 0–1216 | 0–20 | 2–33 | ||
| HSV DNA in cerebrospinal fluid at presentation — no./total no. (%)‡ | 0.16 | 0.22 | ||||
| Positive | 19/28 (68) | 15/17 (88) | 0/15 | 0/12 | ||
| Negative | 9/28 (32) | 2/17 (12) | 15/15 (100) | 12/12 (100) | ||
| Evidence of HSV disease on MRI — no./total no. (%) | 13/23 (57) | 7/13 (54) | 1.00 | 0 | 0 | — |
| Abnormal EEG — no./total no. (%) | 14/19 (74) | 6/12 (50) | 0.26 | NA | NA | |
EEG denotes electroencephalogram, HSV herpes simplex virus, MRI magnetic resonance imaging, and NA not applicable.
The viral type was unknown in the case of some infants.
HSV DNA in cerebrospinal fluid was detected with the use of a polymerase-chain-reaction assay.
A total of 15 infants in the CASG 103 (CNS) study received suppressive therapy with oral acyclovir for the entire 6-month study-drug period (Fig. 1A, yellow boxes), whereas 6 infants received acyclovir suppression for part of the 6-month treatment period (Fig. 1A, light blue boxes) and 7 subjects received no active suppression throughout the study (Fig. 1A, pink boxes). The 12-month Bayley mental-development scores were incrementally higher as infants were receiving acyclovir suppression for longer periods of time: among infants receiving suppressive therapy for 6 months, the mean (±SE) scores were 85±5 (median, 91); among infants receiving suppressive therapy for less than 6 months, the mean scores were 80±8 (median, 70); and among infants receiving no suppressive therapy, the mean scores were 73±10 (median, 58). Bayley motor-development scores did not differ significantly between the groups in either study.
SAFETY ASSESSMENTS
There was no significant difference in absolute neutrophil counts between the infants receiving oral acyclovir and those receiving placebo in either study (Table 3). The time to the first absolute neutrophil count of 500 cells per cubic millimeter or less in the combined studies did not differ significantly between the infants assigned to acyclovir and those assigned to placebo, although a trend for neutropenia was noted among those who received acyclovir (P = 0.09). In total, an absolute neutrophil count of 500 cells per cubic millimeter or less developed in 25% of the subjects in the CASG 103 (CNS) study and in 20% of those in the CASG 104 (skin, eye, and mouth) study who were assigned to acyclovir, as compared with 5% and 7%, respectively, of those who were assigned to placebo. Neutropenia resolved in all the affected infants, in some cases with and in other cases without cessation of suppressive therapy, and none had associated complications.
Table 3.
Maximum Change in Absolute Neutrophil Count during Receipt of Antiviral Suppressive Therapy.*
| Change | CASG 103 (CNS) Study | CASG 104 (Skin, Eye, and Mouth) Study | ||
|---|---|---|---|---|
| Acyclovir (N = 24) |
Placebo (N = 21) |
Acyclovir (N = 15) |
Placebo (N = 14) |
|
| no. of infants/total no. (%) | ||||
| Worsening by ≥2 grades | 5/22 (23) | 3/18 (17) | 2/13 (15) | 0/13 |
| Worsening by 1 grade | 3/22 (14) | 2/18 (11) | 2/13 (15) | 5/13 (38) |
| No change | 13/22 (59) | 13/18 (72) | 4/13 (31) | 6/13 (46) |
| Improvement by ≥1 grade | 1/22 (5) | 0/18 | 5/13 (38) | 2/13 (15) |
Grading was performed with the use of the World Health Organization Toxicity Grading Scale.13 The absolute neutrophil count was not measured in some of the infants. P=0.94 and P=0.24 for the comparison of acyclovir with placebo in the CASG 103 study and the CASG 104 study, respectively.
No significant differences between the groups were seen with respect to other hematologic or chemical laboratory tests or with respect to adverse events or serious adverse events (Table 3 in the Supplementary Appendix). There were no adverse events that led to discontinuation of the study drug.
DISCUSSION
Therapeutic studies of rare diseases such as neonatal herpes present unique challenges. Each of the previous major investigations of the management of neonatal HSV conducted by the CASG has spanned the course of a decade.2,3,16,17 The current studies were no exception, requiring 11 years to enroll 74 infants. Many clinicians assumed that oral acyclovir would be efficacious, on the basis of the results of small, uncontrolled studies,18,19 further challenging the conduct of these randomized, controlled trials. The long-term follow-up required to assess neurodevelopmental outcomes also affected the ability to follow all the enrolled infants for the primary protocol end point, with only 43 of the 74 infants (58%) having a Bayley Scales of Infant Development assessment at 12 months (Fig. 1A and 1B).
Interpretation of the results of the neurodevelopmental assessment should be tempered by the fact that Bayley Scales of Infant Development assessments were not performed in 38% of subjects in the CASG 103 (CNS) study. This substantial attrition renders the primary protocol end point less interpretable. For the 62% of infants in the CASG 103 study with a neurologic evaluation at 12 months, our finding of improved neurodevelopmental outcomes among subjects who started oral acyclovir suppression at the end of the 21-day course of intravenous therapy provides the first controlled data that suggest that ongoing neurologic injury occurs in infants who survive neonatal HSV disease and that it can be decreased by longer-term antiviral suppression. This finding had previously been implied in small, uncontrolled case series.6,19
The distribution of neurologic outcomes among infants in the CASG 103 (CNS) study who were randomly assigned to placebo (normal, 33%; mild impairment, 8%; moderate impairment, 25%; severe impairment, 33%) was similar to that among infants in an earlier trial (in whom the neurologic outcomes were measured by a different tool) who received high-dose parenteral acyclovir for the treatment of acute disease but no subsequent suppression (normal, 31%; mild impairment, 15%; moderate impairment, 15%; severe impairment, 39%).2 Similarly, the distribution of outcomes among infants in the CASG 103 (CNS) study who were randomly assigned to oral acyclovir was virtually identical to that from a small, uncontrolled case series (involving 16 infants) of oral acyclovir suppression after neonatal HSV CNS disease (69% with normal outcomes in both studies).19 The finding of incrementally higher neurodevelopmental scores among infants treated with oral antiviral suppressive therapy for the full 6 months as compared with those treated for only part of the 6 months and as compared with those who received no antiviral suppression (median 12-month Bayley mental-development score of 91 vs. 70 and 58, respectively) also supports the conclusion that oral acyclovir suppressive therapy after treatment of acute neonatal HSV disease confers a benefit. If the infants who were randomly assigned to placebo had not been allowed to switch to active suppression after two cutaneous recurrences, it is possible that the difference between the acyclovir and placebo groups would have been more pronounced. In addition, the subjects with CNS disease who were randomly assigned to acyclovir were smaller in size than were those assigned to placebo, as reflected in the lower birth weights and smaller head circumferences. However, any bias introduced by this difference should be in favor of the null hypothesis, since prematurity or small-for-gestational-age status would be more likely to be associated with worse developmental outcomes.
Not surprisingly,7–12 these studies provided evidence that suppressive therapy with oral acyclovir decreases the number of recurrences of cutaneous lesions after neonatal HSV disease, as has been suggested previously.15 Since skin lesions occur in approximately 70% of all babies with neonatal HSV,1 the positive socioeconomic effect of decreased recurrences should not be underestimated. For example, prevention of skin recurrences can translate to the need for fewer medical evaluations and to more days of child care attendance, which in turn results in fewer days of work missed by the parent.
Previous uncontrolled studies have suggested that acyclovir therapy may be associated with neutropenia.2,15,20–24 In the current placebo-controlled studies, neutropenia was not more likely to develop in infants receiving acyclovir than in infants receiving placebo, although the P values approached significance. It is possible that there is indeed an association that our studies were underpowered to detect; thus, we believe that neutropenia should continue to be considered as a possible toxic effect of longer-term oral acyclovir therapy.15
These data support the use of suppressive therapy with 300 mg of oral acyclovir per square meter per dose administered three times daily for 6 months after initial treatment of neonatal HSV disease. Babies with skin, eye, and mouth disease can benefit because this therapy helps to prevent skin recurrences, whereas babies with CNS disease may have additional benefit with respect to neurodevelopmental outcomes. There are no controlled data that suggest that suppressive therapy administered longer than 6 months or with the use of higher doses of oral acyclovir is beneficial. An extemporaneously compounded oral solution of valacyclovir has not been sufficiently studied in neonates and young infants to warrant its use instead of oral acyclovir for antiviral suppression.25
Supplementary Material
Acknowledgments
Supported by contracts (N01-AI-30025, N01-AI-65306, N01-AI-15113, and N01-AI-62554) with the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases (NIAID). Oral acyclovir and matching placebo were supplied by GlaxoSmithKline, Alpharma USPD, and Pharm Ops.
Footnotes
Presented in part at the 48th Annual Meeting of the Infectious Diseases Society of America, Vancouver, BC, Canada, October 22, 2010; abstract 2977.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Kimberlin DW, Lin CY, Jacobs RF, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108:223–229. doi: 10.1542/peds.108.2.223. [DOI] [PubMed] [Google Scholar]
- 2.Kimberlin DW, Lin CY, Jacobs RF, et al. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics. 2001;108:230–238. doi: 10.1542/peds.108.2.230. [DOI] [PubMed] [Google Scholar]
- 3.Whitley R, Arvin A, Prober C, et al. A controlled trial comparing vidarabine with acyclovir in neonatal herpes simplex virus infection. N Engl J Med. 1991;324:444–449. doi: 10.1056/NEJM199102143240703. [DOI] [PubMed] [Google Scholar]
- 4.Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:541–553. doi: 10.1086/514600. [DOI] [PubMed] [Google Scholar]
- 5.Kimberlin DW, Rouse DJ. Genital herpes. N Engl J Med. 2004;350:1970–1977. doi: 10.1056/NEJMcp023065. [DOI] [PubMed] [Google Scholar]
- 6.Gutman LT, Wilfert CM, Eppes S. Herpes simplex virus encephalitis in children: analysis of cerebrospinal fluid and progressive neurodevelopmental deterioration. J Infect Dis. 1986;154:415–421. doi: 10.1093/infdis/154.3.415. [DOI] [PubMed] [Google Scholar]
- 7.Douglas JM, Critchlow C, Benedetti J, et al. A double-blind study of oral acyclovir for suppression of recurrences of genital herpes simplex virus infection. N Engl J Med. 1984;310:1551–1556. doi: 10.1056/NEJM198406143102402. [DOI] [PubMed] [Google Scholar]
- 8.Patel R, Bodsworth NJ, Woolley P, et al. Valaciclovir for the suppression of recurrent genital HSV infection: a placebo controlled study of once daily therapy. Genitourin Med. 1997;73:105–109. doi: 10.1136/sti.73.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reitano M, Tyring S, Lang W, et al. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. J Infect Dis. 1998;178:603–610. doi: 10.1086/515385. [DOI] [PubMed] [Google Scholar]
- 10.Mertz GJ, Loveless MO, Levin MJ, et al. Oral famciclovir for suppression of recurrent genital herpes simplex virus infection in women: a multicenter, double-blind, placebo-controlled trial. Arch Intern Med. 1997;157:343–349. [PubMed] [Google Scholar]
- 11.Spruance SL, Hamill ML, Hoge WS, Davis LG, Mills J. Acyclovir prevents reactivation of herpes simplex labialis in skiers. JAMA. 1988;260:1597–1599. [PubMed] [Google Scholar]
- 12.Gold D, Corey L. Acyclovir prophylaxis for herpes simplex virus infection. Antimicrob Agents Chemother. 1987;31:361–367. doi: 10.1128/aac.31.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Durham, NC: International Clinical Sciences Support Center; 2003. WHO Toxicity Grading Scale. http://www.icssc.org/Documents/Resources/AEManual2003AppendicesFebruary_06_2003%20final.pdf. [Google Scholar]
- 14.Pickering LK, Baker CJ, Long SS, Kimberlin DW, editors. Red book: 2009 report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2009. Herpes simplex; pp. 363–373. [Google Scholar]
- 15.Kimberlin D, Powell D, Gruber W, et al. Administration of oral acyclovir suppressive therapy after neonatal herpes simplex virus disease limited to the skin, eyes and mouth: results of a phase I/II trial. Pediatr Infect Dis J. 1996;15:247–254. doi: 10.1097/00006454-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Whitley RJ, Nahmias AJ, Soong SJ, Galasso GG, Fleming CL, Alford CA. Vidarabine therapy of neonatal herpes simplex virus infection. Pediatrics. 1980;66:495–501. [PubMed] [Google Scholar]
- 17.Whitley RJ, Yeager A, Kartus P, et al. Neonatal herpes simplex virus infection: follow-up evaluation of vidarabine therapy. Pediatrics. 1983;72:778–785. [PubMed] [Google Scholar]
- 18.Rudd C, Rivadeneira ED, Gutman LT. Dosing considerations for oral acyclovir following neonatal herpes disease. Acta Paediatr. 1994;83:1237–1243. doi: 10.1111/j.1651-2227.1994.tb13004.x. [DOI] [PubMed] [Google Scholar]
- 19.Tiffany KF, Benjamin DK, Jr, Palasanthiran P, O’Donnell K, Gutman LT. Improved neurodevelopmental outcomes following long-term high-dose oral acyclovir therapy in infants with central nervous system and disseminated herpes simplex disease. J Perinatol. 2005;25:156–161. doi: 10.1038/sj.jp.7211247. [DOI] [PubMed] [Google Scholar]
- 20.Feder HM, Jr, Goyal RK, Krause PJ. Acyclovir-induced neutropenia in an infant with herpes simplex encephalitis: case report. Clin Infect Dis. 1995;20:1557–1559. doi: 10.1093/clinids/20.6.1557. [DOI] [PubMed] [Google Scholar]
- 21.Wade JC, Hintz M, McGuffin R, Springmeyer SC, Connor JD, Meyers JD. Treatment of cytomegalovirus pneumonia with high-dose acyclovir. Am J Med. 1982;73:249–256. doi: 10.1016/0002-9343(82)90100-0. [DOI] [PubMed] [Google Scholar]
- 22.Wade JC, McGuffin RW, Springmeyer SC, Newton B, Singer JW, Meyers JD. Treatment of cytomegaloviral pneumonia with high-dose acyclovir and human leukocyte interferon. J Infect Dis. 1983;148:557–562. doi: 10.1093/infdis/148.3.557. [DOI] [PubMed] [Google Scholar]
- 23.Bean B, Fletcher C. Neutropenia in immunocompromised patients receiving intravenous acyclovir. Program and abstracts of the 25th Interscience Conference on Antimicrobial Agents and Chemotherapy; Minneapolis. [September 29–October 2, 1985]. p. 786. abstract. [Google Scholar]
- 24.McPherson ML, Plaisance KI. Neutropenia associated with oral acyclovir and multiple antibacterial agents in a patient with acquired immunodeficiency syndrome. Clin Pharm. 1988;7:398–401. [PubMed] [Google Scholar]
- 25.Kimberlin DW, Jacobs RF, Weller S, et al. Pharmacokinetics and safety of extemporaneously compounded valacyclovir oral suspension in pediatric patients from 1 month through 11 years of age. Clin Infect Dis. 2010;50:221–228. doi: 10.1086/649212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.