Abstract
Ranolazine has been shown to produce atrial-selective depression of sodium channel-dependent parameters and suppress atrial fibrillation (AF) in a variety of experimental models. The present study contrasts the effects of ranolazine and those of a clinically used anti-AF class IC agent, propafenone. Electrophysiological and anti-AF effects of propafenone and ranolazine were compared at clinically relevant concentrations (i.e., 0.3–1.5 and 1–10 μM, respectively) in canine isolated coronary-perfused atrial and ventricular preparations. Transmembrane action potential and pseudo-ECG were recorded. Both ranolazine and propafenone produced atrial-selective prolongation of action potential duration. Propafenone depressed sodium channel-mediated parameters [maximum rate of rise of the action potential upstroke (Vmax), conduction time, and diastolic threshold of excitation] and induced postrepolarization refractoriness to a greater degree than ranolazine, and these effects, unlike those induced by ranolazine, were not or only mildly atrial-selective at normal rates (cycle length 500 ms). At fast pacing rates, however, the effects of propafenone on Vmax and conduction time became atrial-selective, because of the elimination of diastolic interval in atria, but not in ventricles. Propafenone (1.5 μM) and ranolazine (10.0 μM) were effective in preventing the initiation of persistent acetylcholine-mediated AF (6/7 and 9/11 atria, respectively), its termination (8/10 and 8/12 atria, respectively), and subsequent reinduction (8/8 and 7/8 atria, respectively). Thus, propafenone and ranolazine both suppress AF, but ranolazine, unlike propafenone, does it with minimal effects on ventricular myocardium, suggesting a reduced potential for promoting ventricular arrhythmias.
Introduction
A limitation of the use of the currently available anti-atrial fibrillation (AF) agents is the risk of induction of ventricular arrhythmias. This has prompted the development of atrial-specific antiarrhythmic agents. We have shown that ranolazine, an antianginal agent possessing antiarrhythmic properties (Antzelevitch et al., 2004), selectively affects sodium channel-dependent parameters in canine atria versus ventricles and effectively suppresses AF in vitro (Burashnikov et al., 2007). Similar atrial selectivity of ranolazine as well as its anti-AF efficacy have been demonstrated in the porcine heart in vivo (Kumar et al., 2009; Carvas et al., 2010). Consistent with these experimental observations, clinical studies have shown anti-AF efficacy of ranolazine (Scirica et al., 2007; Murdock et al., 2008, 2009, 2010). Propafenone, a potent sodium channel blocker, is used for termination as well as prevention of AF in the clinic (Alboni et al., 2004; Fuster et al., 2011). The aim of the present study was to compare the electrophysiological effects of ranolazine and propafenone in isolated canine coronary-perfused atrial and ventricular preparations and their anti-AF efficacy in an experimental model of AF.
Materials and Methods
This investigation conformed to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (Institute of Laboratory Animal Resources, 1996) and was approved by the animal care and use committee of the Masonic Medical Research Laboratory. Experiments were performed by using isolated arterially perfused canine right atrial preparations and left ventricular arterially perfused wedge preparations (≈2 × 1 × 1 cm). Detailed methods for the isolation and perfusion of these preparations have been described previously (Antzelevitch et al., 2004; Burashnikov et al., 2004). In brief, the preparations were dissected from hearts removed from anesthetized (sodium pentobarbital) adult mongrel dogs (20–25 kg). Unfolded right atria with a rim of the right ventricle was cannulated and perfused through the ostium of the right coronary artery, and the left ventricular wedge was perfused through a branch of the left coronary artery. Unperfused tissue was removed with a razor blade or scissors. The cut ventricular and atrial branches were ligated by using silk thread. After these procedures (performed in cold cardioplegic solution, 4–8°C), the preparations were transferred to a temperature-controlled bath and arterially perfused with Tyrode's solution by use of a roller pump. The composition of the Tyrode's solution was 129 mM NaCl, 4 mM KCl, 0.9 mM NaH2PO4, 20 mM NaHCO3, 1.8 mM CaCl2, 0.5 mM MgSO4 0.5, and 5.5. mM d-glucose, buffered with 95% O2 and 5% CO2 (37 ± 0.5°C; pH 7.35).
Transmembrane action potential (AP) recordings (sampling rate 41 kHz) were obtained by using standard or floating glass microelectrodes (2.7 M KCl; 10–25 MΩ DC resistance). A pseudo-ECG was recorded by using two electrodes consisting of Ag/AgCl half cells placed in the Tyrode's solution, 1.0 to 1.2 cm from opposite ends of the atrial or ventricular coronary-perfused preparations. Conduction time was approximated by measuring the duration of the “P-wave” complex in atria and the “QRS” complex in ventricles on the ECG at a level representing 10 and 90% of P-wave or QRS amplitude. Diastolic threshold of excitation (DTE) was determined by increasing stimulus intensity in 0.01-mA steps. Maximum amplitude of stimulation used for the study was 10 times of the DTE determined in the beginning of each experiment. Effective refractory period (ERP) was measured by delivering premature stimuli after every 10th regular beat at a pacing cycle length (CL) of 500 and 300 ms (with 10-ms resolution; stimulation with a 2× DTE amplitude, determined at each CL). Postrepolarization refractoriness (PRR) was considered to be present when ERP exceeded APD90 in the ventricle and APD75 in atria. Under control conditions, ventricular ERP coincided with APD90, whereas atrial ERP generally coincided with APD75.
Maximum Rate of Rise of the AP Upstroke.
Stable AP recordings and maximum rate of rise of the AP upstroke (Vmax) measurements are difficult to obtain in vigorously contracting perfused preparations. In coronary-perfused atria and ventricles, the effects of propafenone and ranolazine on Vmax were determined by comparing the largest Vmax recorded at any given condition. Because of a substantial interpreparation variability, Vmax values were normalized for each experiment and then averaged.
Experimental Protocols.
The equilibration period for the preparations was 30 to 60 min. The concentrations of ranolazine (1.0, 5.0, and 10.0 μM) and propafenone (0.3 and 1.5 μM) were increased in a stepwise manner, with at least 20 min for ranolazine and 40 min for propafenone at each concentration before starting the collection of the data. This difference reflects the various exposure durations that are required to achieve a steady state in electrophysiological actions of the agents (Delgado et al., 1985; Antzelevitch et al., 2004). The concentrations of the drugs used were within the therapeutic relevant ranges of ranolazine and propafenone achieved when the drugs are prescribed at their recommended doses (Grant, 1996; Antzelevitch et al., 2011). To compare the antiarrhythmic potential of propafenone and ranolazine, we used an acetylcholine (ACh; 0.5–1.0 μM)-dependent AF model in coronary-perfused right atria, where persistent AF is inducible in 100% of preparations (by a single premature beat or rapid pacing) (Burashnikov and Antzelevitch, 2003; Burashnikov et al., 2007). We determined the effect of propafenone and ranolazine to prevent (series 1) the induction of AF as well as, in different preparations, the effect of these drugs to terminate (series 2) persistent AF. In the first series, ACh was added to the perfusate ≥40 min after the start of 1.5 μM propafenone and ≥20 min after the start of 10 μM ranolazine, followed by attempts to induce arrhythmias using programmed electrical stimulation. In the second series, the effect of propafenone or ranolazine to terminate persistent AF was tested by adding these drugs to the coronary perfusate solution after5 to 8 min of persistent AF. After AF termination, reinduction of the arrhythmia was attempted by using programmed electrical stimulation.
Drugs.
Ranolazine (Gilead Sciences, Palo Alto, CA), propafenone (Sigma, St. Louis, MO), and acetylcholine (Sigma) all were dissolved in distilled water, and each was prepared fresh as a stock of 1 to 10 mM before each experiment.
Statistics.
Statistical analysis was performed by using paired or unpaired t tests and one-way repeated-measures or multiple comparison analysis of variance followed by Bonferroni's test, as appropriate. All data are expressed as mean ± S.D.
Results
Propafenone versus Ranolazine: APD90, ERP, PRR, DTE, VMax, and Conduction Time.
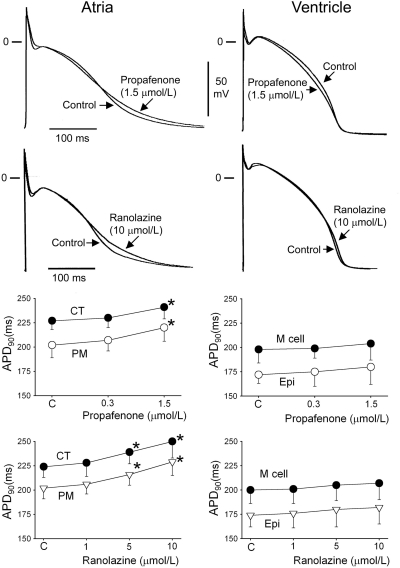
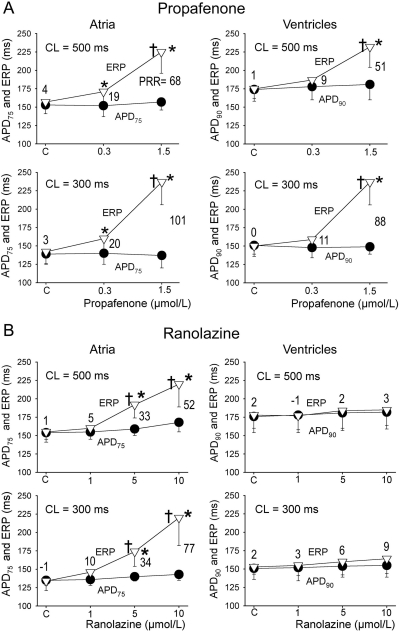
Both propafenone and ranolazine produced atrial, but not ventricular, prolongation of APD90 (Fig. 1). Propafenone rate-dependently lengthened ERP much more than APD75–90 in both atria and ventricles, thereby inducing a significant PRR in both chambers (Fig. 2). Ranolazine induced rate-dependent atrial-selective prolongation of ERP and the development of PRR only in atria (Fig. 2).
Fig. 1.
Effects of propafenone and ranolazine on transmembrane APs from various atrial (left) and ventricular (right) regions. Shown are representative examples of APs and summary data of the effect of propafenone and ranolazine on APD90 in atrial and ventricular preparations stimulated at a cycle of length of 500 ms. CT, crista terminalis; M cell, subendocardial region; Epi, epicardium. *, p < 0.05 versus control. n = 6–18.
Fig. 2.
Effects of propafenone (A) and ranolazine (B) on ERP, APD, and PRR in atrial and ventricular preparations. PRR was measured as the difference between ERP and APD75 in atria and between ERP and APD90 in ventricles (ERP is coincident with APD75 in atria and APD90 in ventricles). Note: whereas APD90 was prolonged by the agents, APD75 was not (see Fig. 1). Ventricular data were obtained from epicardium, and atrial data were from pectinate muscle. *, p < 0.05 versus control. †, p < 0.05 versus APD75 values in atria and APD90 in ventricles. n = 6–18.
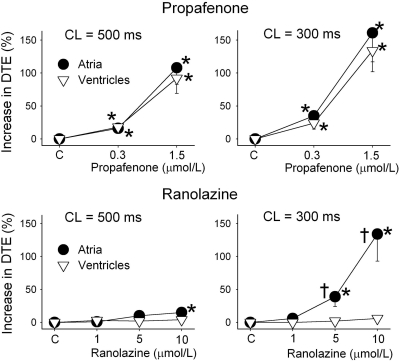
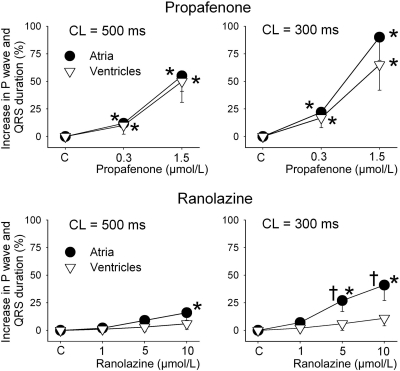
DTE was significantly increased by propafenone in both atria and ventricles at 500 and 300 CLs (Fig. 3). In contrast, ranolazine increased DTE selectively in atria, with a much greater increase at 300 versus 500 ms CL (Fig. 3). Propafenone potently depressed Vmax and increased conduction time similarly in atria and ventricles at a CL of 500 ms, but altered those parameters to a greater extent in atria versus ventricles at a CL of 300 ms (Figs. 4 and 5). Ranolazine produced atrial-selective reduction in Vmax and increase in conduction time, which was more pronounced at faster pacing rates (Figs. 4 and 5). Ranolazine produced a weaker depression of Vmax and conduction velocity compared with propafenone. Figure 4A reveals the mechanisms contributing to the atrial selectivity of propafenone and ranolazine to reduce Vmax at rapid pacing rates. Because of the slow repolarization phase of the atrial action potential and the effect of both propafenone and ranolazine to further slow phase 3, acceleration of rate leads to elimination of the diastolic interval in atria but not in the ventricles. Because much of the recovery from sodium channel block occurs during the diastolic interval, greater accumulation of block occurs in atria versus ventricles at rapid rates of activation.
Fig. 3.
Ranolazine (bottom), but not propafenone (top), causes an atrial-selective, rate-dependent increase in DTE. The increase in DTE caused by propafenone is greater than that of ranolazine. DTE measurements obtained from crista terminalis and pectinate muscle are combined under atria, and those from ventricular epicardium and endocardium are combined under ventricles. *, p < 0.05 versus control. †, p < 0.05 versus values in ventricles. n = 6–10.
Fig. 4.
Rate-dependent effects of propafenone and ranolazine to depress the Vmax in atrial and ventricular preparations. A, representative examples of action potentials and respective Vmax recorded before and upon acceleration of pacing rate from a CL of 500 to 300 ms in atria and ventricles in the absence (control) and presence of propafenone (the AP tracings were taken from the same atrial and ventricular preparations). B and C, graphs summarize the changes in Vmax of atrial and ventricular APs paced at a CL of 500 and 300 ms. Control Vmax values at a CL of 300 ms were normalized to the respective control Vmax value obtained at a CL of 500 ms. Atria includes combined PM and conduction time data. Ventricles includes combined epicardium and M cell data recorded from the ventricular wedge. *, p < 0.05 versus control. †, p < 0.05 versus values in ventricles. n = 6–10.
Fig. 5.
Rate-dependent effects of propafenone (top) and ranolazine (bottom) on conduction times in atrial and ventricular preparations. Atrial and ventricular conduction times were estimated by measuring by the duration of P-wave and QRS complexes of ECG recordings from coronary-perfused atrial and ventricular preparations. *, p < 0.05 versus respective control. †, p < 0.05 versus values in ventricles. n = 6–10.
Propafenone versus Ranolazine: Anti-AF Action.
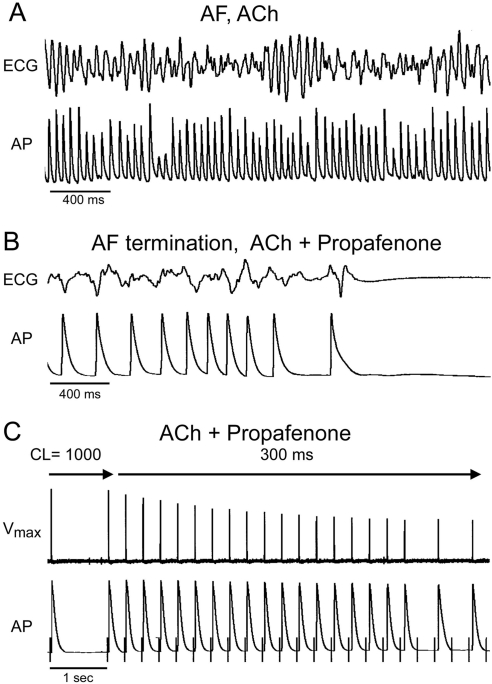
ACh (0.5 μM) alone significantly abbreviated atrial APD90 [from 198 ± 17 to 45 ± 9 ms; p < 0.001; n = 10 for each; pectinate muscle (PM), CL = 500 ms] and ERP (from 149 ± 12 to 51 ± 7 ms; p < 0.001; n = 10 for each; PM, CL = 500 ms), permitting the induction of persistent AF in 100% of atria (10/10 atria). Addition of ACh (0.5 μM) to atrial preparations pretreated with propafenone (1.5 μM) and ranolazine (10 μM) abbreviated APD and ERP to an extent that was less than that observed in the absence of propafenone and ranolazine (Table 1). Under these conditions, both agents were very effective in preventing the induction of persistent ACh-mediated AF, with propafenone being slightly more effective compared with ranolazine (Table 1). In a different set of atrial preparations, we tested the effect of the two drugs to terminate persistent ACh-mediated AF. The addition of propafenone (1.5 μM) or ranolazine (10.0 μM) to the perfusion solution on the fifth to eighth minute of persistent AF terminated the arrhythmia in 8/10 and 8/12 atria, respectively (Fig. 6; Table 1). Average time for AF termination was 7 ± 5 min for propafenone and 14 ± 7 min for ranolazine. Both propafenone and ranolazine effectively prevented the reinduction of persistent AF (Table 1). Brief episodes of nonsustained AF or atrial flutter (<1-min duration) could still be induced in 3/8 and 5/8 atria in the presence of propafenone and ranolazine, respectively. These anti-AF actions of propafenone and ranolazine were associated with rate-dependent depression of excitability, making it impossible for the atria to beat at rapid rates such as those during AF (Fig. 6).
TABLE 1.
Ranolazine (10 μM) vs. propafenone (1.5 μM) to suppress Ach (0.5 μM)-mediated persistent AF in the isolated canine coronary-perfused right atria
APD90 and ERP data presented were obtained from the pectinate muscle region of coronary-perfused atria at a CL of 500 ms. Shortest S1-S1 is the shortest CL permitting 1:1 activation (at a DTE × 2 determined at a CL of 500 ms). n = 6–12.
| APD90 | ERP | Shortest S1-S1 | Induction of Persistent AF | Termination of Persistent AF | Prevention of AF Recurrence | |
|---|---|---|---|---|---|---|
| ms | % | |||||
| ACh | 45 ± 9 | 51 ± 7 | 64 ± 8 | 100 (10/10) | 0 (0/10) | |
| ACh + ranolazine | 69 ± 16* | 112 ± 23*† | 163 ± 46* | 18 (2/11) | 66 (8/12) | 75 (6/8) |
| ACh + propafenone | 83 ± 23* | 142 ± 37*† | 241 ± 69* | 14 (6/7) | 80 (8/10) | 100 (8/8) |
P < 0.05 vs. values at ACh alone.
P < 0.05 vs. APD90.
Fig. 6.
Propafenone suppresses AF and prevents induction/reinduction of the arrhythmia in coronary-perfused right atria. A, persistent AF induced in the presence of Ach (0.5 μM). B, propafenone (1.5 μM) terminates the arrhythmia. C, attempt to reinduce AF fails because of development of use-dependent block of sodium channels (evident from progressive reduction in Vmax), leading to the development of postrepolarization refractoriness and 2:1 activation failure.
Discussion
The main result of the current experimental study is that although both ranolazine and propafenone effectively terminate ACh-mediated AF and prevent the induction of the arrhythmia, ranolazine, in contrast to propafenone, does it without producing significant electrophysiological effects in the ventricles.
Atrial-Selective Sodium Channel Block and AF Suppression.
The risk of induction of ventricular proarrhythmia and/or organ toxicity is a major limitation of currently clinically available anti-AF agents (Fuster et al., 2011). The availability of atrial-specific or atrial-selective agents could obviate this problem. Block of IKur has long been considered to be a promising atrial-selective approach for the management of AF. However, studies indicate that “pure” IKur block is unlikely to be effective in suppressing AF (Burashnikov and Antzelevitch, 2008b,c, 2010; Pandit et al., 2011; Ravens and Wettwer, 2011).
We provided evidence in support of the hypothesis that atrial-selective sodium channel block may effectively suppress AF without inducing ventricular arrhythmias (Burashnikov et al., 2007). This concept stemmed from the finding that certain biophysical properties (e.g., steady-state inactivation) of the sodium channels and action potential morphology in atria differ from those in the ventricles (Burashnikov et al., 2007). Both ranolazine and amiodarone were shown to “take advantage” of these distinctions, producing significant depression of sodium channel-dependent parameters in canine atrial, but not in ventricular, preparations, thus leading to effective suppression of AF at concentrations causing minimal to no effect on ventricular electrophysiology (Burashnikov et al., 2007, 2008; Sicouri et al., 2009; Antzelevitch et al., 2011). The atrial selectivity of ranolazine as well as its anti-AF efficacy have been demonstrated in both in vitro and in vivo animal studies (Burashnikov et al., 2007; Kumar et al., 2009; Carvas et al., 2010; Szél et al., 2011) Atrial-selective depression of sodium channel-mediated parameters in the canine heart has also been reported with exposure to chronic amiodarone and acute tert-butyl (2-{7-[2-(4-cyano-2-fluorophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non3-yl}ethyl)carbamate (AZD1305) (Burashnikov et al., 2008, 2010). In a large clinical study in patients with non-ST segment elevation acute coronary syndrome (Scirica et al., 2007) treatment with ranolazine was associated with the reduced incidence of supraventricular arrhythmias and a 30% reduction in new onset AF. A number of relatively small clinical studies have shown a potent anti-AF efficacy of ranolazine for the termination of paroxysmal AF (Murdock et al., 2008, 2009, 2010). In an exploratory (not placebo-randomized control) clinical study, ranolazine was found to be more effective than amiodarone in preventing postoperative AF (AF incidence was 17.5 versus 26.5%) (Miles et al., 2011). Ranolazine can inhibit early INa, late INa, IKr, and late ICa (Antzelevitch et al., 2004; Burashnikov et al., 2007). Inhibition of early INa by ranolazine is atrial-selective and markedly rate-dependent, which is consistent with the differential effect (atria versus ventricle) of ranolazine on Vmax (Fig. 4) and P-wave and QRS duration (Fig. 5) (Zygmunt et al., 2011). At therapeutically relevant concentrations (1–10 μM) ranolazine possesses antiarrhythmic properties in the ventricles, primarily because of its potent effect to inhibit late INa (Antzelevitch et al., 2004, 2011; Wu et al., 2004), whereas in the atria, this is principally because of its effect to inhibit early INa (Burashnikov et al., 2007). Unlike the block of peak INa, the inhibition of late INa does not directly affect peak INa-mediated parameters such as Vmax, PRR, and DTE.
Propafenone is a well studied and clinically used class IC antiarrhythmic agent, which suppresses AF and prevents its recurrence, largely because of its ability to potently block early INa (Fuster et al., 2011). Within a therapeutic range of concentrations, in addition to INa, propafenone produces relatively mild inhibition of IKr, Ito, ICa, and β-adrenoreceptors (Grant, 1996). We demonstrate an effect of propafenone to potently depress INa-mediated parameters in both atrial and ventricular preparations at moderate to slow pacing rates. This is in sharp contrast to the effects of ranolazine that produces a potent depression of INa-mediated parameters only in atria. However, the anti-AF efficacy of ranolazine was only slightly less than that of propafenone (Table 1).
The electrophysiological effects of both propafenone and ranolazine at clinically relevant concentrations, pacing rates, and temperature are caused largely by the inhibition of peak INa and IKr. The functional potency of these drugs to inhibit peak INa and IKr depends critically on the heart chamber studied as well as pacing rate. Physiologically relevant IC50 values for the comparison of ranolaizne and propafenone are not available at present. Previous studies have reported IC50 values for ranolazine inhibition of peak INa of 285 ± 170 and 286 ± 150 μM for atrial and ventricular cells, respectively; however, the data were obtained at a slow pacing rate, 15°C, and −140 mV holding potential (Zygmunt et al., 2011). Judging from the effects of the drugs on peak INa-mediated parameters (Vmax, PRR, DTE, etc.), the potency of propafenone to block peak INa in the ventricles is much greater than that of ranolazine. Differences in the relative potency of the two agents to suppress peak INa in the atria is less obvious, particularly at a CL of 300 ms. The potency of propafenone and ranolazine to inhibit IKr seems approximately comparable in that both agents produce a similar prolongation of APD90 in atria and no change in the ventricles.
The ranolazine data reported in the current study are very similar to those previously published by our group (Burashnikov et al., 2007) (both studies were conducted in canine coronary-perfused right atrial preparations and used similar experimental protocols). Atrioventricular electrophysiological differences on the effect of propafenone are poorly investigated, but the available data are consistent with the results of the present study. Propafenone reduces Vmax and induces PRR similarly in atrial and ventricular guinea pig superfused preparations (Delgado et al., 1985). 1-[3-(Phenylethyl)-2-benzofuryl]-2-(propylamino)-ethanol hydrochloride (GE-68), an analog of propafenone, selectively prolongs atrial APD, but depresses Vmax to a similar extent in atrial and ventricular guinea pig superfused preparations (Lemmens-Gruber et al., 1997).
Propafenone is used for both conversion of paroxysmal AF and long-term maintenance of sinus rhythm in AF patients with relatively healthy hearts (Alboni et al., 2004; Fuster et al., 2011). One of the clinical uses of propafenone is in the so-called “pill-in-the pocket” approach (Alboni et al., 2004). A disadvantage of propafenone is that its use is contraindicated in patients with structural heart disease (conditions encountered in many patients with AF) because of the risk of induction of life-threatening ventricular arrhythmias. Clinical studies suggest that a single dose of 2000 mg of ranolazine may be effective as a pill-in-the-pocket approach, converting 77% of AF patients, including patients with structural cardiac disease, with no significant adverse reactions (Murdock et al., 2009, 2010). Considering the safety of ranolazine in patients with structural heart diseases (Koren et al., 2007; Wilson et al., 2009), the pill-in-the-pocket approach using ranolazine may prove to have a much wider applicability than previously used class IC antiarrhythmic agents (i.e., propafenone and flecainide).
Atrial-Selective APD Prolongation Potentiates Atrial Selectivity of INa Block.
The primary anti-AF mechanism of propafenone and ranolazine in our study is related to the action of these drugs to block the peak sodium channel current, INa, especially at fast atrial rates. The prolongation of APD90 in atria by both agents probably contributes to the anti-AF effect of these agents both directly and indirectly (i.e., by enhancing the block of early INa; Fig. 4). Atrial-selective prolongation of APD90 by ranolazine and propafenone is likely caused by their effect to block IKr (Grant, 1996; Antzelevitch et al., 2004). Indeed, specific IKr block with N-[4-[1-[2-(6-methylpyridin-2-yl)ethyl]piperidine-4-carbonyl]phenyl] (E-4031) produces atrial-predominant prolongation of APD90 and ERP in canine preparations at a CL of 500 ms (Burashnikov et al., 2008). A similar atrial-predominant effect of IKr blockers to prolong ERP has been demonstrated in vivo in both canine and porcine hearts (Spinelli et al., 1992; Wiesfeld et al., 1996). It is also noteworthy that neither propafenone (Gross and Castle, 1998) nor ranolazine (A. C. Zygmunt and C. Antzelevitch, unpublished work) block IKur and IKur inhibition abbreviates instead of prolonging APD90 in “healthy” atria (Burashnikov et al., 2004; Wettwer et al., 2004).
Atrial-selective prolongation of APD90 induced by propafenone and ranolazine contributes to the abbreviation of the diastolic interval at rapid pacing rates in atria but not ventricles (Fig. 4). Because much of the recovery of the sodium channels from block occurs during the diastolic interval (Whalley et al., 1995), the atrial-selective prolongation of APD90 enhances the effect of both drugs to depress INa and INa-mediated parameters in atria at rapid rates, thus potentiating their effects to suppress AF.
Atrial Selectivity of INa Blockers: Rapid versus Slow Dissociation Kinetics?
Both propafenone and ranolazine are predominantly open-state sodium channel blockers (Whalley et al., 1995; Wang et al., 2008; Zygmunt et al., 2011). Amiodarone and AZD1305 are also atrial-selective sodium channel blockers (Burashnikov et al., 2008, 2010). Although amiodarone is primarily an inactivated-state sodium channel blockers (Whalley et al., 1995), AZD1305 is a potent tonic blocker (i.e., inhibits sodium channel at the resting state) (Burashnikov et al., 2010). Ranolazine produces little to no tonic block at normal resting membrane potential (Zygmunt et al., 2011). Thus, the available data suggest that preferential binding to a given state of the channel (i.e., open, inactivated, or resting) does not necessarily determine atrial selectivity of INa blockers. The unbinding kinetics of propafenone and ranolazine seem to play a determining role. Propafenone dissociates slowly (τ ≥8 s; Whalley et al., 1995), whereas ranolazine dissociation is relatively rapid (τ = 1.6 s; Burashnikov et al., 2007). Consistent with this hypothesis, amiodarone, which has been shown to be an atrial-selective sodium channel blocker (Burashnikov et al., 2008), unbinds rapidly from the sodium channel (Whalley et al., 1995). Other factors that contribute to atrial-selective inhibition of INa include a more negative voltage dependence of steady-state inactivation of the sodium channels, a more positive resting membrane potential, and a much slower phase 3 of the action potential in atria versus ventricles (for detailed discussion see Burashnikov and Antzelevitch, 2008a, 2009, 2010).
Study Limitations.
Extrapolation of our results obtained from in vitro to in vivo animal models or the clinic should be done with great caution. The absence of autonomic and hormonal influences, which can significantly modulate cardiac electrophysiology and thereby the pharmacological response to drugs, are among the limitations of in vitro preparations. In addition, our experiments were carried out using “healthy” atria and ventricles, whereas AF normally occurs in electrically and structurally remodeled atria. Atrial remodeling can significantly affect pharmacological responses.
Acknowledgments
We thank Judy Hefferon and Robert Goodrow for expert technical assistance.
This study was supported by Gilead Sciences, Inc. (C.A.); the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL-47687] (to C.A.); and the New York State and Florida Free and Accepted Masons.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- AF
- atrial fibrillation
- ACh
- acetylcholine
- AP
- action potential
- APD
- AP duration
- CL
- cycle length
- DTE
- diastolic threshold of excitation
- ERP
- effective refractory period
- PM
- pectinate muscle
- PRR
- postrepolarization refractoriness
- Vmax
- maximum rate of rise of the AP upstroke
- AZD1305
- tert-butyl (2-{7-[2-(4-cyano-2-fluorophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non3-yl}ethyl)carbamate
- E-4031
- N-[4-[1-[2-(6-methylpyridin-2-yl)ethyl]piperidine-4-carbonyl]phenyl]
- GE-68
- 1-[3-(phenylethyl)-2-benzofuryl]-2-(propylamino)-ethanol hydrochloride.
Authorship Contributions
Participated in research design: Burashnikov and Antzelevitch.
Conducted experiments: Burashnikov.
Contributed new reagents or analytic tools: Burashnikov, Belardinelli, and Antzelevitch.
Performed data analysis: Burashnikov and Antzelevitch.
Wrote or contributed to the writing of the manuscript: Burashnikov, Belardinelli, and Antzelevitch.
References
- Alboni P, Botto GL, Baldi N, Luzi M, Russo V, Gianfranchi L, Marchi P, Calzolari M, Solano A, Baroffio R, et al. (2004) Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med 351:2384–2391 [DOI] [PubMed] [Google Scholar]
- Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. (2004) Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation 110:904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Burashnikov A, Sicouri S, Belardinelli L. (2011) Electrophysiologic basis for the antiarrhythmic actions of ranolazine. Heart Rhythm 8:1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. (2003) Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation 107:2355–2360 [DOI] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. (2008a) Atrial-selective sodium channel blockers: do they exist? J Cardiovasc Pharmacol 52:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. (2008b) Can inhibition of IKur promote atrial fibrillation? Heart Rhythm 5:1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. (2008c) How do atrial-selective drugs differ from antiarrhythmic drugs currently used in the treatment of atrial fibrillation? J Atr Fibrillation 1:98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. (2009) Atrial-selective sodium channel block for the treatment of atrial fibrillation. Expert Opin Emerg Drugs 14:233–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. (2010) New developments in atrial antiarrhythmic drug therapy. Nat Rev Cardiol 7:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Di Diego JM, Sicouri S, Ferreiro M, Carlsson L, Antzelevitch C. (2008) Atrial-selective effects of chronic amiodarone in the management of atrial fibrillation. Heart Rhythm 5:1735–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. (2007) Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation 116:1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Mannava S, Antzelevitch C. (2004) Transmembrane action potential heterogeneity in the canine isolated arterially perfused atrium: effect of IKr and Ito/IKur block. Am J Physiol Heart Circ Physiol 286:H2393–H2400 [DOI] [PubMed] [Google Scholar]
- Burashnikov A, Zygmunt AC, Di Diego JM, Linhardt G, Carlsson L, Antzelevitch C. (2010) AZD1305 exerts atrial predominant electrophysiological actions and is effective in suppressing atrial fibrillation and preventing its reinduction in the dog. J Cardiovasc Pharmacol 56:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvas M, Nascimento BC, Acar M, Nearing BD, Belardinelli L, Verrier RL. (2010) Intrapericardial ranolazine prolongs atrial refractory period and markedly reduces atrial fibrillation inducibility in the intact porcine heart. J Cardiovasc Pharmacol 55:286–291 [DOI] [PubMed] [Google Scholar]
- Delgado C, Tamargo J, Tejerina T. (1985) Electrophysiological effects of propafenone in untreated and propafenone-pretreated guinea-pig atrial and ventricular muscle fibres. Br J Pharmacol 86:765–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, et al. (2011) 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol 57:e101–e198 [DOI] [PubMed] [Google Scholar]
- Grant AO. (1996) Propafenone: an effective agent for the management of supraventricular arrhythmias. J Cardiovasc Electrophysiol 7:353–364 [DOI] [PubMed] [Google Scholar]
- Gross GJ, Castle NA. (1998) Propafenone inhibition of human atrial myocyte repolarizing currents. J Mol Cell Cardiol 30:783–793 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Koren MJ, Crager MR, Sweeney M. (2007) Long-term safety of a novel antianginal agent in patients with severe chronic stable angina: the Ranolazine Open Label Experience (ROLE). J Am Coll Cardiol 49:1027–1034 [DOI] [PubMed] [Google Scholar]
- Kumar K, Nearing BD, Carvas M, Nascimento BC, Acar M, Belardinelli L, Verrier RL. (2009) Ranolazine exerts potent effects on atrial electrical properties and abbreviates atrial fibrillation duration in the intact porcine heart. J Cardiovasc Electrophysiol 20:796–802 [DOI] [PubMed] [Google Scholar]
- Lemmens-Gruber R, Marei H, Heistracher P. (1997) Electrophysiological properties of the propafenone-analogue GE 68 (1-[3-(phenylethyl)-2-benzofuryl]-2-(propylamino)-ethanol) in isolated preparations and ventricular myocytes of guinea-pig hearts. Naunyn Schmiedebergs Arch Pharmacol 355:230–238 [DOI] [PubMed] [Google Scholar]
- Miles RH, Passman R, Murdock DK. (2011) Comparison of effectiveness and safety of ranolazine versus amiodarone for preventing atrial fibrillation after coronary artery bypass grafting. Am J Cardiol 108:673–676 [DOI] [PubMed] [Google Scholar]
- Murdock DK, Kersten M, Kaliebe J, Larrain G. (2009) The use of oral ranolazine to convert new or paroxysmal atrial fibrillation: a review of experience with implications for possible “pill in the pocket” approach to atrial fibrillation. Indian Pacing Electrophysiol J 9:260–267 [PMC free article] [PubMed] [Google Scholar]
- Murdock DK, Overton N, Kersten M, Kaliebe J, Devecchi F. (2008) The effect of ranolazine on maintaining sinus rhythm in patients with resistant atrial fibrillation. Indian Pacing Electrophysiol J 8:175–181 [PMC free article] [PubMed] [Google Scholar]
- Murdock DK, Reiffel JA, Kaliebe JW, Larrian G. (2010) The conversion of paroxysmal of initial onset of atrial fibrillation with oral ranolazine: implications for “pill in the pocket” approach in structural heart disease. J Am Coll Cardiol 55:A6.E58 [Google Scholar]
- Pandit SV, Zlochiver S, Filgueiras-Rama D, Mironov S, Yamazaki M, Ennis SR, Noujaim SF, Workman AJ, Berenfeld O, Kalifa J, et al. (2011) Targeting atrioventricular differences in ion channel properties for terminating acute atrial fibrillation in pigs. Cardiovasc Res 89:843–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravens U, Wettwer E. (2011) Ultra-rapid delayed rectifier channels: molecular basis and therapeutic implications. Cardiovasc Res 89:776–785 [DOI] [PubMed] [Google Scholar]
- Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, Molhoek P, Verheugt FW, Gersh BJ, McCabe CH, et al. (2007) Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation 116:1647–1652 [DOI] [PubMed] [Google Scholar]
- Sicouri S, Belardinelli L, Carlsson L, Antzelevitch C. (2009) Potent antiarrhythmic effects of chronic amiodarone in canine pulmonary vein sleeve preparations. J Cardiovasc Electrophysiol 20:803–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli W, Parsons RW, Colatsky TJ. (1992) Effects of WAY-123,398, a new class III antiarrhythmic agent, on cardiac refractoriness and ventricular fibrillation threshold in anesthetized dogs: a comparison with UK-68798, E-4031, and dl-sotalol. J Cardiovasc Pharmacol 20:913–922 [DOI] [PubMed] [Google Scholar]
- Szél T, Koncz I, Jost N, Baczkó I, Husti Z, Virág L, Bussek A, Wettwer E, Ravens U, Papp JG, et al. (2011) Class I/B antiarrhythmic property of ranolazine, a novel antianginal agent, in dog and human cardiac preparations. Eur J Pharmacol 662:31–39 [DOI] [PubMed] [Google Scholar]
- Wang GK, Calderon J, Wang SY. (2008) State- and use-dependent block of muscle Nav1.4 and neuronal Nav1.7 voltage-gated Na+ channel isoforms by ranolazine. Mol Pharmacol 73:940–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettwer E, Hála O, Christ T, Heubach JF, Dobrev D, Knaut M, Varró A, Ravens U. (2004) Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation 110:2299–2306 [DOI] [PubMed] [Google Scholar]
- Whalley DW, Wendt DJ, Grant AO. (1995) Basic concepts in cellular cardiac electrophysiology: Part II: Block of ion channels by antiarrhythmic drugs. PACE 18:1686–1704 [DOI] [PubMed] [Google Scholar]
- Wiesfeld AC, De Langen CD, Crijns HJ, Bel KJ, Hillege HL, Wesseling H, Lie KI. (1996) Rate-dependent effects of the class III antiarrhythmic drug almokalant on refractoriness in the pig. J Cardiovasc Pharmacol 27:594–600 [DOI] [PubMed] [Google Scholar]
- Wilson SR, Scirica BM, Braunwald E, Murphy SA, Karwatowska-Prokopczuk E, Buros JL, Chaitman BR, Morrow DA. (2009) Efficacy of ranolazine in patients with chronic angina observations from the randomized, double-blind, placebo-controlled MERLIN-TIMI (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Segment Elevation Acute Coronary Syndromes) 36 Trial. J Am Coll Cardiol 53:1510–1516 [DOI] [PubMed] [Google Scholar]
- Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. (2004) Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther 310:599–605 [DOI] [PubMed] [Google Scholar]
- Zygmunt AC, Nesterenko VV, Rajamani S, Hu D, Barajas-Martinez H, Belardinelli L, Antzelevitch C. (2011) Mechanisms of atrial-selective block of Na+ channel by ranolazine I. Experimental analysis of the use-dependent block. Am J Physiol Heart Circ Physiol 301:H1606–H1614 [DOI] [PMC free article] [PubMed] [Google Scholar]