Abstract
Our recent study demonstrated that central cannabinoid receptor 1 (CB1R) activation caused dose-related pressor response in conscious rats, and reported studies implicated the brainstem phosphatidylinositol 3-kinase (PI3K)/Akt-extracellular signal-regulated kinase 1/2 (ERK1/2) pathway in blood pressure control. Therefore, in this study, we tested the hypothesis that the modulation of brainstem PI3K/Akt-ERK1/2 signaling plays a critical role in the central CB1R-mediated pressor response. In conscious freely moving rats, the pressor response elicited by intracisternal (i.c.) (R)-(+)-[2,3-dihydro-5-methyl-3[(4-morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate salt (WIN55,212-2) (15 μg) was associated with significant increases in ERK1/2 phosphorylation in the rostral ventrolateral medulla (RVLM) and the nucleus tractus solitarius (NTS). In contrast, Akt phosphorylation was significantly reduced in the same neuronal pools. Pretreatment with the selective CB1R antagonist N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) (30 μg i.c.) attenuated the neurochemical responses elicited by central CB1R activation. Furthermore, pretreatment with the ERK/mitogen-activated protein kinase kinase inhibitor 2′-amino-3′-methoxyflavone (PD98059) (5 μg i.c.) abrogated WIN55,212-2-evoked increases in blood pressure and neuronal ERK1/2 phosphorylation but not the reduction in Akt phosphorylation. On the other hand, prior PI3K inhibition with wortmannin (0.4 μg i.c.) exacerbated the WIN55,212-2 (7.5 and 15 μg i.c.) dose-related increases in blood pressure and ERK1/2 phosphorylation in the RVLM. The present neurochemical and integrative studies yield new insight into the critical role of two brainstem kinases, PI3K and ERK1/2, in the pressor response elicited by central CB1R activation in conscious rats.
Introduction
The molecular mechanisms that underlie the central CB1R-mediated pressor response are poorly understood (Niederhoffer and Szabo, 1999; Padley et al., 2003; Pfitzer et al., 2004). Our recent finding that implicated GABAergic inhibition in the central CB1R-mediated pressor response in conscious rats (Ibrahim and Abdel-Rahman, 2011) raised the interesting possibility that neuronal GABA modulators such as PI3K/Akt and ERK1/2 (Wang et al., 2003; Bell-Horner et al., 2006) might play important roles in central CB1R-evoked pressor response. It is noteworthy that reported findings, mostly obtained in vitro, showed that CB1R activation caused increases or decreases in PI3K/Akt-ERK1/2 signaling (Galve-Roperh et al., 2002; Derkinderen et al., 2003; Sánchez et al., 2003; Ellert-Miklaszewska et al., 2005; Greenhough et al., 2007; Ozaita et al., 2007), which suggests tissue/organ specificity for the responses of these kinases. To date, there are no integrative or neurochemical studies on the role of PI3k/Akt and ERK1/2 signaling, in cardiovascular controlling neuronal pools, in the central CB1R-evoked pressor response.
Brainstem ERK1/2 signaling plays an important role in the central control of blood pressure, but such a role seems to be influenced by changes in its upstream modulators. For example, prolonged inhibition of RVLM ERK1/2 phosphorylation gradually lowered blood pressure in both normotensive and hypertensive rats (Seyedabadi et al., 2001), and rapid activation of RVLM ERK1/2 underlies the angiotensin II-mediated pressor response (Chan et al., 2005, 2007). On the other hand, our previous studies showed that ERK1/2 phosphorylation in the RVLM plays a causal role in the acute hypotensive response elicited by central α2A adrenergic or imidazoline receptor activation (Zhang and Abdel-Rahman, 2005; Nassar and Abdel-Rahman, 2008). Moreover, PI3K/Akt activation evokes a depressor response and underlies the hypotensive response elicited by intra-NTS insulin (Huang et al., 2004). It is noteworthy that PI3K signaling is up-regulated in central cardiovascular regulatory areas in the brainstem (RVLM and NTS) and hypothalamus of hypertensive rats (Veerasingham et al., 2005; Zubcevic et al., 2009). Collectively, the role of PI3/Akt-ERK signaling in mediating a blood pressure response seems to depend, at least partly, on the type of the targeted receptor within the central nervous system. It is imperative, therefore, to predict that changes in these inter-related kinases in cardiovascular controlling nuclei might constitute a critical step in the neurochemical events that underlie the pressor response mediated by central CB1R activation.
The aim of the present study was to test the hypothesis that brainstem PI3K/Akt and/or ERK1/2 signaling contributes to the central CB1R-mediated pressor response. To test this hypothesis, we investigated the effect of intracisternal (R)-(+)-[2,3-dihydro-5-methyl-3[(4-morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate salt (WIN55,212-2) on MAP and HR and the associated changes in the phosphorylation of Akt (pAkt), a PI3K downstream effector, and ERK1/2 (pERK1/2) in the RVLM and NTS. To ascertain causal involvement of the observed neurochemical responses of these signaling molecules in the central CB1R-mediated pressor response, we investigated the effects of prior central CB1R blockade [N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251)], PI3K inhibition [(1S,6br,9aS,11R, 11bR)11-(acetyloxy)-1,6b,7,8,9a,10,11,11b-octahydro-1-(metho xymethyl)-9a,11b-dimethyl-3H-furo[4,3,2-de]indeno[4,5,-h][2-h]-2-benzopyran-3,6,9-trione (wortmannin)], or MEK/ERK1/2 inhibition [2′-amino-3′-methoxyflavone (PD98059)] on the neurochemical (pAkt and pERK1/2) and blood pressure responses elicited by central CB1R activation. The integrative studies were conducted in conscious unrestrained rats, and the brains were collected at the conclusion of cardiovascular measurements for undertaking the neurochemical studies.
Materials and Methods
Male Sprague-Dawley rats (300–350 g; Charles River Laboratories, Raleigh, NC) were housed two per cage in a room with controlled environment at a constant temperature of 23 ± 1°C, humidity of 50 ± 10%, and a 12-h light/dark cycle. Food (Prolab Rodent Chow, Prolab RMH 3000; Granville Milling, Creedmoor, NC) and water were provided ad libitum. All surgical, experimental, and animal-care procedures were performed in accordance with, and approved by, the Institutional Animal Care and Use Committee and in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Intra-Arterial and Intracisternal Cannulation.
These surgeries were performed as in our previous studies (Nassar and Abdel-Rahman, 2006; Ibrahim and Abdel-Rahman, 2011). In brief, 5 days before the experiment, rats were anesthetized with ketamine (9 mg/100 g) and xylazine (1 mg/100 g i.p.), and a polyethylene catheter (PE50 connected to PE10) was placed in the abdominal aorta via the femoral artery for blood pressure measurement. For intracisternal drug administration, a stainless-steel guide cannula (23G; Small Parts, Inc., Miramar, FL) was implanted into the cisterna magna. The guide cannula was passed between the occipital and the cerebellum through a hole drilled 1 to 1.5 mm distal to the caudal edge of the occipital bone so that the guide cannula tip protruded into the cisterna magna. The cannula was secured in place with small metal screws and dental acrylic cement (Durelon; Thompson Dental Supply, Raleigh, NC). The patency of the guide cannula was verified when a spontaneous flow of cerebrospinal fluid was observed and by gross postmortem histological verification after routine injection of 2 μl of fast green dye (EM Sciences, Cherry Hill, NJ) at the end of the experiment.
Blood Pressure and Heart Rate Measurements.
On the day of the experiment, the arterial catheter was flushed with heparinized saline (100 IU/ml) and connected to a Gould-Statham (Oxnard, CA) pressure transducer. BP was recorded by ML870 (PowerLab 8/30) and analyzed by using LabChart (v.6) pro software (ADInstruments, Colorado Springs, CO). Heart rate was extracted from BP recording by using the LabChart (v.6) blood pressure analysis module, and both variables were continuously recorded and stored for offline analysis. BP and HR were allowed to stabilize for at least 60 min. Data collected during the 30 min that preceded drug administration represented basal MAP and HR.
Western Blot Analysis.
A modified protocol from our recent studies (Bender and Abdel-Rahman, 2009; Nassar et al., 2011) was used to measure changes in Akt and ERK1/2 phosphorylation. Animals were euthanized, and brains were removed, flash-frozen, and stored at −80°C until used. Tissues were collected bilaterally from RVLM at −12.8 to −11.8 mm and NTS at −13.92 to −12.84 mm relative to bregma (Paxinos and Watson, 2005) by using a 0.75-mm punch instrument (Stoelting Co., Wood Dale, IL). Collected specimens were homogenized in cell lysis buffer, and protein was quantified by using Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). An equal amount of protein from each sample was denatured at 97°C for 5 to 10 min, separated by gel electrophoresis, and transferred to nitrocellulose membranes following standard procedures. Membranes were blocked in Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 h and then incubated for 48 h at 4°C with a mixture of mouse monoclonal antiphospho-Akt antibody (pSer473) (1:1000) and rabbit polyclonal anti-Akt antibody (1:1000) or mouse monoclonal antiphospho-ERK1/2 (T202/Y204) antibody (1:1000) and rabbit polyclonal anti-ERK antibody (1:1000). All four antibodies were purchased from Cell Signaling Technology (Danvers, MA). Membranes were washed four times with phosphate-buffered saline containing 0.1% Tween 20 then incubated for 60 min with mixture containing IRDye680-conjugated goat anti-rabbit and IRDye800-conjugated goat anti-mouse (1:1500; LI-COR Biosciences). After washing with phosphate-buffered saline containing 0.1% Tween 20, bands representing phosphorylated and total protein were detected simultaneously by using Odyssey Infrared Imager and analyzed with Odyssey application software v.3 (LI-COR Biosciences). All data were averaged values of integrated density ratio of phosphorylated protein (pAkt or pERK1/2) normalized to its corresponding total protein (tAkt or tERK1/2), and expressed as percentage of control (vehicle-treated rats) as in our previous studies (Nassar et al., 2011).
Drugs.
WIN55,212-2, wortmannin, PD98059, and DMSO were purchased from Sigma-Aldrich (St. Louis, MO). AM251 was purchased from Cayman Chemical (Ann Arbor, MI). Alkamus was purchased from Rhone-Poulenc (Cranbury, NJ). WIN55,212-2 and AM251 (30 μg/rat) were dissolved in a mixture of DMSO/alkamus/sterile saline (1:1:18). Wortmannin was dissolved in 10% DMSO in saline (1 nmol ∼0.4 μg/rat) as reported previously (Narita et al., 2002). PD98059 was dissolved in DMSO (5 μg/rat) as in our previous study (Zhang and Abdel-Rahman, 2005). Each vehicle (control) was tested in at least three animals. Because none of these vehicles significantly changed the basal levels of MAP and HR we refer to all of them as untreated control (vehicle).
Protocols and Experimental Groups.
Drug or vehicle injections were made into the cisterna magnum of conscious unrestrained rats as in our previous study (Ibrahim and Abdel-Rahman, 2011) via a 30-gauge stainless-steel injector extended 2.0 mm beyond the tip of the previously implanted guide cannula and connected to a 20-μl Hamilton (Reno, NV) syringe through a PE-10. Drugs and/or vehicles were loaded together and separated by air bubbles to avoid any potential problems as a result of multiple drug injections and removal of the injector. Injections were delivered in a total volume of 5 μl by hand over a period of 1 min.
Effects of Intracisternal WIN55,212-2 on NTS and RVLM ERK/Akt Phosphorylation.
We investigated the effects of intracisternal WIN55,212-2 on Akt and ERK1/2 phosphorylation in the NTS and RVLM. Brain tissues were collected from animals sacrificed 5 min after intracisternal WIN55,212-2 (15 μg/rat) or vehicle (n = 4–7) and prepared for Western blotting as detailed above to detect changes in phosphorylated Akt (pAkt) and ERK1/2 (pERK1/2) in the RVLM and NTS. The chosen dose of WIN55,212-2 (15 μg/rat i.c.) and the sacrifice time (5 min), which preceded the peak of the WIN55,212-2 evoked pressor response, were based on our recent study (Ibrahim and Abdel-Rahman, 2011).
Effect of PI3K or MEK-ERK1/2 Inhibition on the Pressor Response Elicited by Intracisternal WIN55,212-2.
In the first part of this experiment, six groups of rats (n = 5–8) were used to elucidate the effect of central PI3K/Akt inhibition (wortmannin) on intracisternal WIN55,212-2-evoked pressor response. Three groups received intracisternal wortmannin (0.4 μg), and the other three groups received equal volumes of vehicle. Thirty minutes after wortmannin or vehicle pretreatment, the rats received intracisternal WIN55,212-2 (7.5 or 15 μg) or its vehicle. The dose of wortmannin was selected based on reported studies (Narita et al., 2002). In the second part of this experiment, we investigated the effect of central MEK-ERK1/2 inhibition on the WIN55,212-2-mediated pressor response in two groups of rats (n = 5–8) that received the pharmacological MEK-ERK1/2 inhibitor PD98059 (5 μg/rat i.c.) 20 min before WIN55,212-2 (15 μg/rat i.c.) or its vehicle. The selected PD98059 dose was used in our previous studies (Zhang and Abdel-Rahman, 2005). Cardiovascular measurements were continued for 30 min after WIN55,212-2 or the vehicle in both parts of this experiment.
At the conclusion of the integrative studies, we investigated the changes in NTS and RVLM pAkt and pERK1/2 levels (Western blot) caused by intracisternal WIN55,212-2 in the absence or presence of CB1R blockade (AM251; 30 μg/rat i.c.), PI3K inhibition (wortmannin), or MEK-ERK1/2 inhibition (PD98059). The neurochemical studies on AM251 were conducted in the brains of animals used in our recent studies, which demonstrated abrogation of WIN55,212-2-evoked pressor response in the same animal model (Ibrahim and Abdel-Rahman, 2011).
Statistical Analysis.
MAP was calculated as: diastolic pressure + one-third (systolic pressure − diastolic pressure). MAP and heart rate are expressed as mean ± S.E.M. change from their respective baseline values after the pretreatment and before WIN55,212-2 or vehicle injection. Data were then analyzed by repeated-measures ANOVA using SPSS 16.0 statistical package for Windows (SPSS Inc., Chicago, IL) for differences in time and treatment trends followed by a one-way ANOVA to assess individual differences at different time points among different groups. Tukey's (equal variance) and Games Howell (unequal variance) tests were used for post hoc analysis. Western blot data for each protein were expressed as percentage of control (vehicle alone) value and analyzed by unpaired t test (Fig. 1) using Prism V5 for Windows (GraphPad Software Inc., San Diego, CA). Furthermore, one-way ANOVA, followed by Tukey's multiple comparison post hoc, was conducted to determine the effects of WIN55,212-2 alone and in the presence of AM251, wortmannin, or PD98059 on Akt (see Fig. 5) and ERK1/2 (see Fig. 6) phosphorylation. To achieve this goal, WIN55,212-2 values were compared with those of the control (vehicle alone) and to the corresponding values in animals pretreated with AM251, wortmannin, or PD98059. P < 0.05 was considered significant.
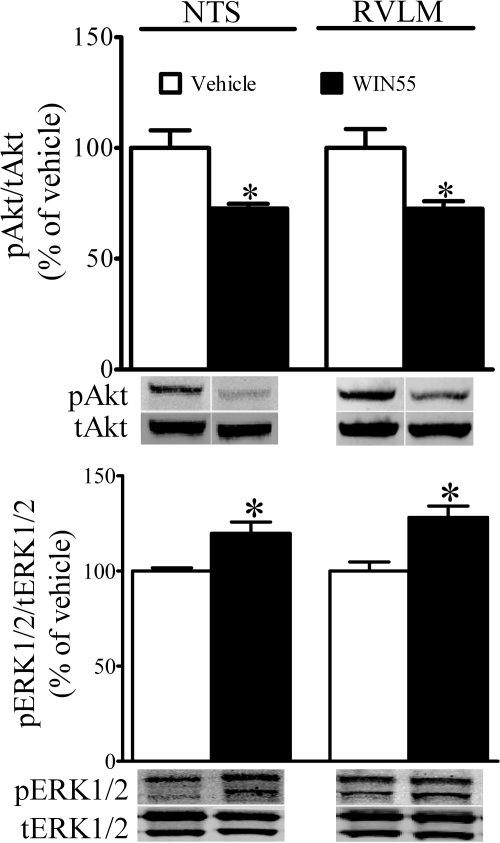
Fig. 1.
Effects of WIN55,212-2 on Akt and ERK1/2 phosphorylation in NTS and RVLM. Changes in rat NTS and RVLM phosphorylation of Akt (pAkt) and ERK1/2 (pERK1/2) elicited by WIN55,212-2 are shown; brains were collected 5 min after WIN55,212-2 or its vehicle. Data are presented as integrated density ratio of pAkt or pERK1/2 to its respective total protein (tAkt) or tERK1/2, and expressed as percentage of control (vehicle). Values are mean ± S.E.M. of four to seven observations. *, P < 0.05 versus respective (vehicle) values.
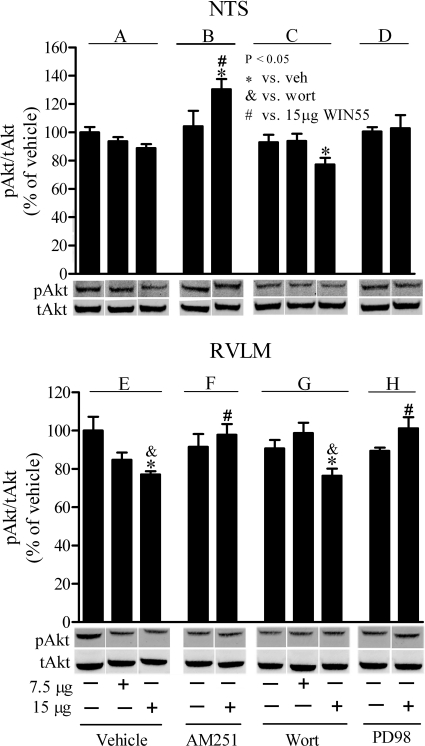
Fig. 5.
Effects of WIN55,212-2 on pAkt in the presence or absence of AM251, wortmannin, or PD98059 in rat NTS and RVLM. Changes in pAkt in rat NTS (A–D) and RVLM (E–H) elicited by intracisternal WIN55,212-2 in animals pretreated with vehicle (control) group (A and E), the CB1R antagonist AM251(30 μg/rat; B and F), the PI3K inhibitor wortmannin (Wort; 0.4 μg/rat; C and G), or the MEK/ERK1/2 inhibitor PD98059 (PD98; 5 μg/rat; D and H) are shown. Data are presented as integrated density ratio of phosphorylated Akt (pAkt) to total Akt (tAkt) and expressed as percentage of vehicle (control) value. Values are mean ± S.E.M. of four to five observations. Symbols denote statistical significance (P < 0.05) based on comparing the responses elicited by WIN55,212-2 alone or in the presence of the pharmacological intervention versus vehicle as well as treatment control, AM251, wortmannin, or PD98059 values.
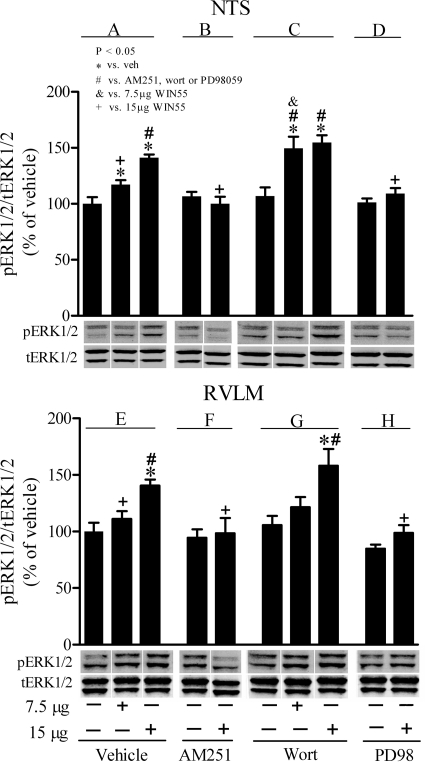
Fig. 6.
Effects of WIN55,212-2 on pERK1/2 in the presence or absence of AM251, wortmannin, or PD98059 in rat NTS and RVLM. Changes in pERK1/2 in rat NTS (A-D) and RVLM (E-H) elicited by intracisternal WIN55,212-2 in animals pretreated with vehicle (control) (A and E), the CB1R antagonist AM251(30 μg/rat; B and F), the PI3K inhibitor wortmannin (Wort; 0.4 μg/rat; C and G), or the MEK/ERK1/2 inhibitor PD98059 (PD98; 5 μg/rat; D and H) are shown. Data are presented as the integrated density ratio of phosphorylated ERK1 (pERK1/2) to total ERK1/2 (tERK1/2) and expressed as percentage of vehicle (control) value. Values are mean ± S.E.M. of four to five observations. Symbols denote statistical significance (P < 0.05) based on comparing the responses elicited by WIN55,212-2 alone or in the presence of the pharmacological intervention versus vehicle as well as treatment control, AM251, wortmannin, or PD98059 values.
Results
Baseline MAP and HR were similar in all groups of rats used in the study (Table 1). Furthermore, MAP and HR were similar after different pretreatments (vehicle, wortmannin, or PD98095) at the time of WIN55,212-2 or vehicle injection (Table 1).
TABLE 1.
Baseline MAP (mm Hg) and HR (bpm) values before and after pretreatment with different drugs that were administered before WIN55,212-2 or its vehicle
Values are means ± S.E.M.
| Treatment | n | MAP |
HR |
||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Vehicle + vehicle | 6 | 115.0 ± 6.0 | 113.3 ± 5.0 | 395 ± 12 | 400 ± 12 |
| Vehicle + 7.5 μg WIN55,212-2 | 6 | 111.3 ± 3.0 | 108.0 ± 5.0 | 375 ± 10 | 350 ± 11 |
| Vehicle + 15 μg WIN55,212-2 | 8 | 113.3 ± 5.0 | 115.0 ± 3.0 | 370 ± 10 | 368 ± 17 |
| Wortmannin + vehicle | 5 | 112.0 ± 6.0 | 117.0 ± 6.0 | 356 ± 15 | 376 ± 15 |
| Wortmannin + 7.5 μg WIN55,212-2 | 8 | 112.0 ± 6.0 | 111.0 ± 5.0 | 360 ± 11 | 380 ± 10 |
| Wortmannin + 15 μg WIN55,212-2 | 8 | 111.3 ± 3.0 | 112.0 ± 3.0 | 375 ± 10 | 363 ± 13 |
| PD98059 + vehicle | 5 | 113.0 ± 4.0 | 110.0 ± 4.0 | 398 ± 16 | 390 ± 20 |
| PD98059 + 15 μg WIN55,212-2 | 8 | 117.0 ± 6.0 | 115.0 ± 4.0 | 400 ± 12 | 410 ± 12 |
Effect of WIN55,212-2 on Akt and ERK1/2 Phosphorylation in NTS and RVLM.
In this study, we investigated the neurochemical responses at a time, 5 min, that preceded WIN55,212-2 (15 μg/rat, i.c.)-evoked peak pressor response based on our recent study (Ibrahim and Abdel-Rahman, 2011). Compared with the control group (vehicle alone) (n = 7), WIN55,212-2 (n = 4) significantly (P < 0.05; unpaired t test) increased ERK1/2 and inhibited Akt phosphorylation in the NTS and RVLM. Representative pERK1/2 and pAkt bands along with total protein bands from WIN55,212-2- or vehicle-treated groups are shown in Fig. 1.
PI3K Inhibition Exacerbates the Pressor Response Elicited by Intracisternal WIN55,212-2.
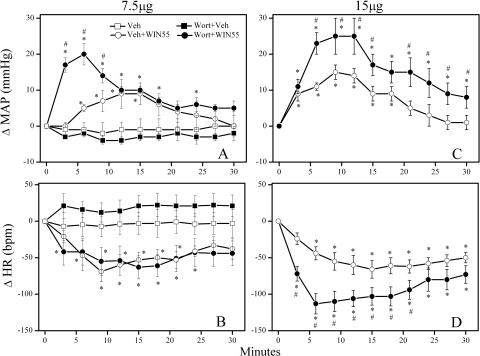
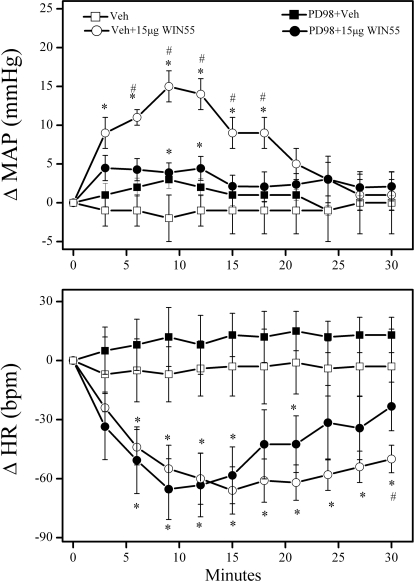
Pretreatment with the PI3K inhibitor wortmannin (0.4 μg/rat i.c.) did not significantly alter baseline MAP or HR (Table 1). However, intracisternal wortmannin significantly (P < 0.05) enhanced the WIN55,212-2 (15 μg/rat i.c.)-evoked pressor and bradycardic responses (Figs. 2, C and D and 4B). To further confirm the PI3K-Akt signaling involvement in the WIN55,212-2-evoked pressor response, we investigated the effect of wortmannin pretreatment on a modest pressor response elicited by a lower dose (7.5 μg/rat i.c.) of WIN55,212-2 in an additional group of rats. As shown in Fig. 2, A and B, wortmannin significantly (P < 0.05) enhanced the pressor, but had no effect on the bradycardic, response elicited by the lower dose of WIN55,212-2.
Fig. 2.
PI3K inhibition exacerbates the pressor response elicited by central CB1R activation. Time-course changes in mean arterial pressure (ΔMAP; A and C) and heart rate (ΔHR; B and D) evoked by intracisternal WIN55,212-2 (WIN55; 7.5 μg/rat, A and B or 15 μg/rat, C and D) in conscious rats pretreated, 30 min earlier, with the PI3K inhibitor wortmannin (Wort; 0.4 μg/rat i.c.) or an equal volume of its vehicle. Values are mean ± S.E.M. of five to eight observations. *, P < 0.05 versus control (Veh); #, P < 0.05 comparing Wort + WIN55 data with Veh + WIN55. The vehicle (Veh) or Wort + Veh alone (shown in A and B) were also used for statistical comparisons for data shown in C and D.
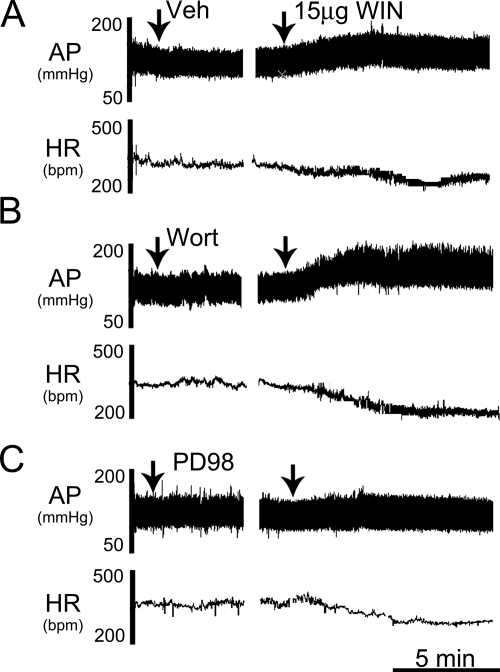
Fig. 4.
Representative tracings depicting the changes in AP and HR elicited by intracisternal WIN55,212-2 (15 μg/rat) in conscious animals pretreated with vehicle (A), the PI3K inhibitor wortmannin (Wort; 0.4 μg/rat) (B), or the MEK/ERK1/2 inhibitor PD98059 (PD98; 5 μg/rat) (C).
Inhibition of ERK1/2 Phosphorylation Attenuates WIN55,212-2-Evoked Pressor Response.
To investigate the role of brainstem ERK1/2 phosphorylation in the central CB1R-mediated pressor response, conscious rats were pretreated with the MEK/ERK/1/2 inhibitor PD98059 (5 μg/rat i.c.) before WIN55,212-2 (15 μg/rat i.c.). PD98059 had no significant effects on baseline MAP and HR before WIN55,212-2 administration (Table 1). However, PD98059 pretreatment significantly (P < 0.05) attenuated the pressor, but not the bradycardic, response elicited by central CB1R activation (Figs. 3 and 4C).
Fig. 3.
Inhibition of ERK1/2 phosphorylation (PD98059) attenuates the pressor response elicited by central CB1R activation. Time-course changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) evoked by intracisternal WIN55,212-2 (WIN55; 15 μg/rat) or an equal volume of its vehicle in conscious rats pretreated, 20 min earlier, with the MEK/ERK1/2 inhibitor PD98059 (PD98; 5 μg/rat i.c.) or equal volume of its vehicle are shown. Values are mean ± S.E.M. of five to eight observations. * or #, P < 0.05 versus vehicle (Veh) or PD98 + 15 μg WIN55 values, respectively.
Effect of Central CB1R Activation on Brainstem pAkt in the Absence or Presence of AM251, Wortmannin, and PD98059.
These molecular studies were undertaken to complement the pharmacological findings described above and more directly elucidate the role of the PI3K/Akt-ERK1/2 pathway in the pressor response elicited by central CB1R activation. Compared with vehicle (control), none of the pharmacological interventions (AM251, wortmannin, or PD98059) had any effect on pAkt levels in the NTS or RVLM (Fig. 5). It is noteworthy that the lower dose (7.5 μg) of WIN55,212-2 reduced pAkt levels in both brainstem areas, but the reduction was not statistically significant and was not altered by wortmannin. Therefore, the results discussed below pertain to the higher dose of WIN55,212-2. Compared with vehicle, WIN55,212-2 reduced Akt phosphorylation, and such reduction was significant in the RVLM (P < 0.05) but not in the NTS (Fig. 5, A and E). Prior CB1R blockade (AM251) fully abrogated WIN55,212-2-evoked reduction in pAkt levels when comparisons were made with the vehicle or AM251 values (Fig. 5, B and F). In the NTS, in the presence of wortmannin, WIN55,212-2 evoked reduction in pAkt was significant (P < 0.05) only compared with vehicle but not to WIN55,212-2 or wortmannin alone (Fig. 5, A and C). In the RVLM, WIN55,212-2-evoked pAkt reduction in wortmannin-pretreated rats was comparable with the reduction caused by WIN55,212-2 alone and was significant (P < 0.05) compared with vehicle or wortmannin alone (Fig. 5, E and G). Finally, PD98059 fully abrogated WIN55,212-2-evoked reductions in pAkt in the RVLM (Fig. 5H).
Effect of Central CB1R Activation on Brainstem pERK1/2 in the Absence or Presence of AM251, Wortmannin, and PD98059.
Compared with vehicle (control) values, none of the tested drugs (AM251, wortmannin, or PD98059) caused any change in pERK1/2 level in the NTS or RVLM when administered alone (Fig. 6). WIN55,212-2 elicited significant (P < 0.05) dose-related increases in ERK1/2 phosphorylation in RVLM and NTS (Fig. 6, A and E). After AM251 (Fig. 6, B and F) or PD98059 (Fig. 6, D and H), WIN55,212-2-evoked increases in pERK1/2 were fully abrogated compared with the corresponding values in the AM251- or PD98059-pretreated rats. By contrast, the WIN55,212-2-evoked enhancements of ERK1/2 phosphorylation in the RVLM and NTS were significantly (P < 0.05) exacerbated after wortmannin compared with the vehicle or wortmannin values (Fig. 6, C and G). These neurochemical responses paralleled the changes in blood pressure elicited by intracisternal WIN55,212-2 in the absence or presence of the pharmacological interventions (Figs. 2–6).
Discussion
In this study, we tested the hypothesis that brainstem PI3K/Akt-ERK1/2 signaling pathway plays a crucial role in the central CB1R-mediated pressor response. Our most important findings are: 1) before the peak pressor response, WIN55,212-2 significantly enhanced ERK1/2 and reduced Akt phosphorylation in the NTS and RVLM; 2) WIN55,212-2-evoked neurochemical responses were attenuated by central CB1R blockade (AM251); 3) prior inhibition of central PI3K (wortmannin) exacerbated the WIN55,212-2-evoked increases in blood pressure and ERK1/2 phosphorylation, and 4) in contrast, prior MEK-ERK inhibition (PD98059) abrogated WIN55,212-2-evoked increases in blood pressure and brainstem ERK1/2 phosphorylation and reversed the associated reduction in RVLM Akt phosphorylation. Collectively, these findings implicate differential alterations in brainstem PI3K/Akt (reduced) and ERK1/2 (enhanced) signaling as potential molecular mechanisms for the central CB1R-evoked pressor response in conscious rats.
The CB1R-mediated pressor response fully agrees with reported findings, including ours, which implicated central sympathoexcitation in such response (Niederhoffer and Szabo, 1999, 2000; Padley et al., 2003; Pfitzer et al., 2004; Ibrahim and Abdel-Rahman, 2011). We tested the intriguing hypothesis that brainstem PI3K/Akt-ERK1/2 signaling plays a critical role in the central CB1R-mediated pressor response. We focused on these signaling molecules because their role, particularly in the RVLM and NTS, in blood pressure control is well documented (Seyedabadi et al., 2001; Huang et al., 2004; Chan et al., 2005, 2007; Nassar and Abdel-Rahman, 2008). Furthermore, CB1R activation modulates the activity of these signaling molecules in cultured cells and behavioral studies (Galve-Roperh et al., 2002; Ellert-Miklaszewska et al., 2005; Greenhough et al., 2007).
We present the first evidence that the pressor response elicited by central CB1R (WIN55,212-2) activation is paralleled with a reduction in Akt and enhancement of ERK1/2 phosphorylation in the NTS and RVLM. The findings that the neurochemical responses preceded the peak pressor response inferred an important role for the differential alteration in Akt and ERK1/2 phosphorylation in the central CB1R-mediated pressor response. It is noteworthy that our findings are consistent with a CB1R-mediated PI3K/Akt inhibition in glioma cells and colorectal tumor cells but are contrary to an up-regulated PI3K/Akt signaling in U373 MG human astrocytoma cells and mouse hippocampal slices (Galve-Roperh et al., 2002; Derkinderen et al., 2003). This discrepancy might relate, at least partly, to differences in the response of Akt and other related molecules to CB1R activation in different tissues/organs. To ascertain a causal role for the inhibition of brainstem Akt phosphorylation in the central CB1R-mediated pressor response, we used the selective pharmacological PI3K inhibitor wortmannin.
There are no reported studies on the acute blood pressure effects of central PI3K inhibition (wortmannin) in conscious rats. However, the lack of a change in blood pressure after intracisternal wortmannin agrees with a similar finding after intra-RVLM wortmannin (Seyedabadi et al., 2001). It is imperative to note that blood pressure responses depend on pAkt basal level and its response to pharmacological interventions in different neuronal pools. For example, inhibition of an overactive PI3K in the spontaneously hypertensive rat (Veerasingham et al., 2005) in the NTS (Zubcevic et al., 2009) and the RVLM (Seyedabadi et al., 2001) elicits pressor and depressor response, respectively. Furthermore, insulin enhancement of NTS Akt phosphorylation causes hypotension in normotensive rats (Huang et al., 2004). Although the potential confounding effect of anesthesia in these reported studies cannot be ignored, the findings highlight a role for changes in Akt phosphorylation in blood pressure responses. Consistent with this view are our novel findings that prior inhibition of central PI3K (wortmannin) exacerbated WIN55,212-2-evoked pressor response (Figs. 2 and 4B). Equally important, the concomitant enhancement of ERK1/2 phosphorylation in the same neuronal pools (Fig. 5, C and G) inferred: 1) ERK1/2 phosphorylation occurred, at least partly, secondary to CB1R-mediated inhibition of brainstem PI3K/Akt, and 2) phosphorylation of brainstem ERK1/2 plays a pivotal role in the central CB1R-evoked pressor response. It is possible that, in the presence of wortmannin, the higher WIN55,212-2 dose caused nonspecific effects; notably, in the absence of wortmannin, CB1R blockade (AM251) abrogated the pressor response elicited by the higher dose of WIN55,212-2 (Ibrahim and Abdel-Rahman, 2011). Furthermore, WIN55,212-2's effect on heart rate may involve complex cross-talk between cardiac sympathetic and parasympathetic components as supported by the uncovering of the dose-related bradycardia by WIN55,212-2 only after wortmannin.
Consistent with a critical role for ERK1/2 phosphorylation in CB1R-mediated physiological responses, we provide the first evidence that enhanced brainstem ERK1/2 phosphorylation is involved in central CB1R-mediated pressor response. Intracisternal WIN55,212-2 significantly elevated pERK1/2 levels in the NTS and RVLM, two nuclei that are heavily involved in the central CB1R-evoked hemodynamic effects (Niederhoffer and Szabo, 2000; Padley et al., 2003; Rademacher et al., 2003; Seagard et al., 2004, 2005). Our findings inferred that the elevation in brainstem pERK1/2 obtained at 5 min contributed to the CB1R-mediated pressor response. However, the possibility exists that at peak pressor response (10–12 min) the pERK1/2 level could be lower than its peak because in cultured N18TG2 cells CB1R caused peak elevation in pERK1/2 at 5 min, which was followed by a decline at 10 min (Dalton and Howlett, 2011). In the latter study, the pERK1/2 level remained higher than basal level at 30 min. Likewise, in the present study, we demonstrated pERK1/2 elevation at 30 min (Fig. 6), and our pERK1/2 findings are supported by: 1) CB1R-evoked ERK1/2 phosphorylation peaked at 5 min and was sustained at 10 and 15 min in hippocampal tissues (Derkinderen et al., 2003); and 2) pERK1/2 elevation in the same neuronal pool (RVLM) was evident at 5, 15, and 30 min and contributed to angiotensin II-evoked pressor response (Chan et al., 2005). Finally, we showed that CB1R blockade (AM251) or ERK1/2 inhibition (PD98059) abrogated the WIN55,212-2-evoked increases in pERK1/2 (Fig. 6, B and F) and blood pressure. Collectively, these findings support a causal role for ERK1/2 activation in the RVLM in the CB1R-mediated pressor response.
It is important to reconcile the discrepancies in brainstem pERK1/2 elevation and the subsequent blood pressure response because pERK1/2 elevation in the RVLM contributed to α2-adrenergic or imidazoline I1 receptor-mediated hypotension (Zhang and Abdel-Rahman, 2005; Nassar and Abdel-Rahman, 2008). It is highly likely that the activation of brainstem ERK1/2 can lead to sympathoinhibition or sympathoexcitation; the final BP response might depend on the targeted receptor/neuronal pool and perhaps on the balance between ERK1/2 activation in RVLM (sympathoexcitatory) versus NTS (sympathoinhibitory). Therefore, in our model system, the CB1R-mediated RVLM ERK1/2 phosphorylation seems to prevail and mediates sympathoexcitation/pressor response. In support of this premise, localized activation of CB1R in the RVLM (Padley et al., 2003) and the NTS (Seagard et al., 2004, 2005; Brozoski et al., 2005) elicited sympathoexcitatory and sympathoinhibitory responses, respectively. However, no neurochemical measurements were made in these reported studies. Furthermore, it is likely that the ability of pERK1/2 to inhibit (Bell-Horner et al., 2006) and pAkt to enhance (Wang et al., 2003) GABA neurotransmission explains, at least partly, our recent findings that implicated central GABA inhibition in the central CB1R-evoked pressor response (Ibrahim and Abdel-Rahman, 2011). The present and reported findings suggest that CB1R-evoked ERK1/2 activation is a complex process and might involve other systems such as orexins, angiotensin II, and dopamine (Valjent et al., 2001; Hilairet et al., 2003; Ellis et al., 2006; Rozenfeld et al., 2011). For instance, tetrahydrocannabinol-evoked ERK1/2 activation in the striatum was abolished by prior dopamine receptor blockade (Valjent et al., 2001). Further studies are warranted to more critically delineate the role of ERK1/2 phosphorylation in the disparate blood pressure response elicited by central CB1R activation. Finally, pERK1/2 does not contribute to the CB1R-evoked bradycardia because it was not affected by prior MEK/ERK inhibition. Our findings fully agree with the ability of intracerebroventricular PD98059 to attenuate the acute urotensin-mediated pressor response but not the associated bradycardia (Lin et al., 2004).
Careful analysis of the neurochemical changes in pAkt and pERK1/2 revealed that although wortmannin had no effect on basal brainstem pERK1/2 level it substantially enhanced WIN55,212-2-induced pERK1/2 and the pressor response (Figs. 2 and 6). By contrast, prior inhibition of ERK1/2 phosphorylation had no impact on WIN55,212-2-evoked inhibition of Akt phosphorylation in the brainstem and abrogated the pressor response (Figs. 3–5). Collectively, these findings suggest that the enhancement of pERK1/2 caused by central CB1R activation occurs, at least partly, as a result of a reduction in PI3K-Akt signaling in brainstem cardiovascular controlling nuclei. However, because the drugs were administered intracisternally, our findings do not preclude the involvement of other brain structures in the observed responses.
In summary, the present study highlights a novel role for two important brainstem kinases, PI3K and ERK1/2, in the central CB1R-evoked pressor response in conscious rats. We conclude that differential modulation of these two kinases (inhibition of PI3K and activation of ERK1/2) in brainstem cardiovascular controlling areas underlies, at least partly, the pressor response caused by central CB1R activation. This conclusion gains credence from new integrative and neurochemical findings. First, blockade of central CB1R (AM251) abrogated WIN55,212-2-evoked neurochemical responses along with the pressor response. Second, inhibition of central PI3k/Akt signaling (wortmannin) exacerbated brainstem ERK1/2 phosphorylation and the pressor response elicited by central CB1R activation. Third, inhibition of brainstem ERK1/2 phosphorylation (PD98059) abrogated the elevations in brainstem ERK1/2 phosphorylation and blood pressure, but not the reduction in Akt phosphorylation, caused by central CB1R activation. Collectively, these novel findings suggest that central CB1R activation mediates a pressor response by triggering neurochemical responses in the brainstem that encompass, at least partly, the inhibition of PI3K and a subsequent activation of its downstream effector ERK1/2. Further studies are needed to elucidate the signaling pathways between PI3K/Akt and ERK1/2 as well as other neuromodulators of sympathetic tone after central CB1R activation.
Acknowledgments
We thank Kui Sun for technical assistance.
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant 2R01-AA07839-18] and in part by the El-Minia Faculty of Pharmacy via a scholarship provided by the Egyptian Government (Scholarship Missions Program, Ministry of Higher Education) (to B.M.I.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- CB1R
- cannabinoid receptor 1
- ANOVA
- analysis of variance
- AP
- arterial pressure
- MAP
- mean AP
- BP
- blood pressure
- DMSO
- dimethyl sulfoxide
- ERK1/2
- extracellular signal-regulated kinase 1/2
- pERK1/2
- phosphorylated ERK1/2
- GABA
- γ-aminobutyric acid
- HR
- heart rate
- i.c.
- intracisternal
- MEK
- mitogen-activated protein kinase kinase
- NTS
- nucleus tractus solitarius
- pAkt
- phosphorylated Akt
- PI3K
- phosphatidylinositol 3-kinase
- RVLM
- rostral ventrolateral medulla
- WIN55,212-2
- (R)-(+)-[2,3-dihydro-5-methyl-3[(4-morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate salt
- PD98059
- 2′-amino-3′-methoxyflavone
- AM251
- N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide.
Authorship Contributions
Participated in research design: Ibrahim and Abdel-Rahman.
Conducted experiments: Ibrahim.
Performed data analysis: Ibrahim.
Wrote or contributed to the writing of the manuscript: Ibrahim and Abdel-Rahman.
References
- Bell-Horner CL, Dohi A, Nguyen Q, Dillon GH, Singh M. (2006) ERK/MAPK pathway regulates GABAA receptors. J Neurobiol 66:1467–1474 [DOI] [PubMed] [Google Scholar]
- Bender TS, Abdel-Rahman AA. (2009) α2A-adrenergic receptor signaling underlies synergistic enhancement of ethanol-induced behavioral impairment by clonidine. Alcohol Clin Exp Res 33:408–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski DT, Dean C, Hopp FA, Seagard JL. (2005) Uptake blockade of endocannabinoids in the NTS modulates baroreflex-evoked sympathoinhibition. Brain Research 1059:197–202 [DOI] [PubMed] [Google Scholar]
- Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. (2005) NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res 97:772–780 [DOI] [PubMed] [Google Scholar]
- Chan SH, Wang LL, Tseng HL, Chan JY. (2007) Up-regulation of AT1 receptor gene on activation of protein kinase Cβ/nicotinamide adenine dinucleotide diphosphate oxidase/ERK1/2/c-fos signaling cascade mediates long-term pressor effect of angiotensin II in rostral ventrolateral medulla. J Hypertens 25:1845–1861 [DOI] [PubMed] [Google Scholar]
- Dalton GD, Howlett AC. (2011) CB1 cannabinoid receptors transactivate multiple receptor tyrosine kinases and regulate multiple serine/threonine kinases to achieve ERK activation in neuronal cells. Br J Pharmacol http://dx.doi.org/10.1111/j.1476-5381.2011.01455.x [DOI] [PMC free article] [PubMed]
- Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, Trzaskos J, Caboche J, Girault JA. (2003) Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci 23:2371–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellert-Miklaszewska A, Kaminska B, Konarska L. (2005) Cannabinoids down-regulate PI3K/Akt and Erk signalling pathways and activate proapoptotic function of Bad protein. Cell Signal 17:25–37 [DOI] [PubMed] [Google Scholar]
- Ellis J, Pediani JD, Canals M, Milasta S, Milligan G. (2006) Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J Biol Chem 281:38812–38824 [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Rueda D, Gómez del Pulgar T, Velasco G, Guzmán M. (2002) Mechanism of extracellular signal-regulated kinase activation by the CB(1) cannabinoid receptor. Mol Pharmacol 62:1385–1392 [DOI] [PubMed] [Google Scholar]
- Greenhough A, Patsos HA, Williams AC, Paraskeva C. (2007) The cannabinoid Δ9-tetrahydrocannabinol inhibits RAS-MAPK and PI3K-AKT survival signalling and induces BAD-mediated apoptosis in colorectal cancer cells. Int J Cancer 121:2172–2180 [DOI] [PubMed] [Google Scholar]
- Hilairet S, Bouaboula M, Carrière D, Le Fur G, Casellas P. (2003) Hypersensitization of the Orexin 1 receptor by the CB1 receptor: evidence for cross-talk blocked by the specific CB1 antagonist, SR141716. J Biol Chem 278:23731–23737 [DOI] [PubMed] [Google Scholar]
- Huang HN, Lu PJ, Lo WC, Lin CH, Hsiao M, Tseng CJ. (2004) In situ Akt phosphorylation in the nucleus tractus solitarii is involved in central control of blood pressure and heart rate. Circulation 110:2476–2483 [DOI] [PubMed] [Google Scholar]
- Ibrahim BM, Abdel-Rahman AA. (2011) Role of brainstem GABAergic signaling in central cannabinoid receptor evoked sympathoexcitation and pressor response in conscious rats. Brain Res 1414:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Lin Y, Matsumura K, Tsuchihashi T, Fukuhara M, Fujii K, Iida M. (2004) Role of ERK and Rho kinase pathways in central pressor action of urotensin II. J Hypertens 22:983–988 [DOI] [PubMed] [Google Scholar]
- Narita M, Ohnishi O, Nemoto M, Yajima Y, Suzuki T. (2002) Implications of phosphoinositide 3-kinase in the μ- and δ-opioid receptor-mediated supraspinal antinociception in the mouse. Neuroscience 113:647–652 [DOI] [PubMed] [Google Scholar]
- Nassar N, Abdel-Rahman AA. (2006) Central adenosine signaling plays a key role in centrally mediated hypotension in conscious aortic barodenervated rats. J Pharmacol Exp Ther 318:255–261 [DOI] [PubMed] [Google Scholar]
- Nassar N, Abdel-Rahman AA. (2008) Brainstem phosphorylated extracellular signal-regulated kinase 1/2-nitric-oxide synthase signaling mediates the adenosine A2A-dependent hypotensive action of clonidine in conscious aortic barodenervated rats. J Pharmacol Exp Ther 324:79–85 [DOI] [PubMed] [Google Scholar]
- Nassar NN, Li G, Strat AL, Abdel-Rahman AA. (2011) Enhanced hemeoxygenase activity in the rostral ventrolateral medulla mediates exaggerated hemin-evoked hypotension in the spontaneously hypertensive rat. J Pharmacol Exp Ther 339:267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhoffer N, Szabo B. (1999) Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br J Pharmacol 126:457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhoffer N, Szabo B. (2000) Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J Pharmacol Exp Ther 294:707–713 [PubMed] [Google Scholar]
- Ozaita A, Puighermanal E, Maldonado R. (2007) Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem 102:1105–1114 [DOI] [PubMed] [Google Scholar]
- Padley JR, Li Q, Pilowsky PM, Goodchild AK. (2003) Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anaesthetised rats. Br J Pharmacol 140:384–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2005) The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press, Boston [Google Scholar]
- Pfitzer T, Niederhoffer N, Szabo B. (2004) Central effects of the cannabinoid receptor agonist WIN55212-2 on respiratory and cardiovascular regulation in anaesthetised rats. Br J Pharmacol 142:943–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Patel S, Hopp FA, Dean C, Hillard CJ, Seagard JL. (2003) Microinjection of a cannabinoid receptor antagonist into the NTS increases baroreflex duration in dogs. Am J Physiol Heart Circ Physiol 284:H1570–H1576 [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Gupta A, Gagnidze K, Lim MP, Gomes I, Lee-Ramos D, Nieto N, Devi LA. (2011) AT1R-CB1R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J 30:2350–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez MG, Ruiz-Llorente L, Sánchez AM, Díaz-Laviada I. (2003) Activation of phosphoinositide 3-kinase/PKB pathway by CB1 and CB2 cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell Signal 15:851–859 [DOI] [PubMed] [Google Scholar]
- Seagard JL, Dean C, Patel S, Rademacher DJ, Hopp FA, Schmeling WT, Hillard CJ. (2004) Anandamide content and interaction of endocannabinoid/GABA modulatory effects in the NTS on baroreflex-evoked sympathoinhibition. Am J Physiol Heart Circ Physiol 286:H992–H1000 [DOI] [PubMed] [Google Scholar]
- Seagard JL, Hopp FA, Hillard CJ, Dean C. (2005) Effects of endocannabinoids on discharge of baroreceptive NTS neurons. Neurosci Lett 381:334–339 [DOI] [PubMed] [Google Scholar]
- Seyedabadi M, Goodchild AK, Pilowsky PM. (2001) Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension 38:1087–1092 [DOI] [PubMed] [Google Scholar]
- Valjent E, Pagès C, Rogard M, Besson MJ, Maldonado R, Caboche J. (2001) Δ9-Tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci 14:342–352 [DOI] [PubMed] [Google Scholar]
- Veerasingham SJ, Yamazato M, Berecek KH, Wyss JM, Raizada MK. (2005) Increased PI3-kinase in presympathetic brain areas of the spontaneously hypertensive rat. Circ Res 96:277–279 [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. (2003) Control of synaptic strength, a novel function of Akt. Neuron 38:915–928 [DOI] [PubMed] [Google Scholar]
- Zhang J, Abdel-Rahman AA. (2005) Mitogen-activated protein kinase phosphorylation in the rostral ventrolateral medulla plays a key role in imidazoline (i1)-receptor-mediated hypotension. J Pharmacol Exp Ther 314:945–952 [DOI] [PubMed] [Google Scholar]
- Zubcevic J, Waki H, Diez-Freire C, Gampel A, Raizada MK, Paton JF. (2009) Chronic blockade of phosphatidylinositol 3-kinase in the nucleus tractus solitarii is prohypertensive in the spontaneously hypertensive rat. Hypertension 53:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]