Abstract
This study examined the positive modulatory properties of 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one (rac-BHFF) at γ-aminobutyric acid B (GABAB) receptors in different brain regions. Using quantitative autoradiography, we measured GABAB receptor-stimulated binding of guanosine 5′-O-(3-[35S]thiotriphosphate) ([35S]GTPγS) to G proteins in medial prefrontal cortex (mPFC), hippocampus, and cerebellum. CGP7930 and rac-BHFF enhanced baclofen-stimulated [35S]GTPγS binding similarly in mPFC and hippocampus, but were more effective in cerebellum. CGP7930 (100 μM) increased [35S]GTPγS binding stimulated by baclofen (30 μM) from 29 to 241% above basal in mPFC and from 13 to 1530% above basal in cerebellum. Likewise, rac-BHFF (10 μM) increased baclofen-stimulated [35S]GTPγS binding more in cerebellum (from 13 to 1778% above basal) than in mPFC (from 29 to 514% above basal). rac-BHFF (10 μM) in combination with γ-hydroxybutyrate (20 mM) increased [35S]GTPγS binding in cerebellum but not in mPFC. rac-BHFF also enhanced the effects of 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP35348). Consistent with its partial agonist properties, CGP35348 stimulated [35S]GTPγS binding in mPFC when given alone (to 18% above basal), but less extensively than baclofen (140% above basal), and antagonized baclofen when given together. CGP35348 (1 mM) in combination with rac-BHFF (100 μM) produced an increase in [35S]GTPγS binding that was larger in cerebellum (from 61 to 1260% above basal) than in mPFC (from 18 to 118% above basal). Taken together, the results show that GABAB receptor-positive modulators enhance [35S]GTPγS binding stimulated by GABAB receptor agonists in a brain region-dependent manner. This regionally selective enhancement is further evidence of pharmacologically distinct GABAB receptor populations, possibly allowing for more selective therapeutic targeting of the GABAB system.
Introduction
Metabotropic GABAB receptors are present throughout the central nervous system and have been implicated in various psychiatric disorders (Cryan and Kaupmann, 2005; Bowery, 2006), including drug dependence (Addolerato et al., 2009; Maccioni et al., 2010; Vlachou and Markou, 2010). These receptors are heterodimers composed of GABAB1a or GABAB1b subunits in combination with GABAB2 subunits (Calver et al., 2002; Bettler et al., 2004). GABAB receptors are coupled through Gαi/o to the inhibition of adenylyl cyclase, the closing of voltage-dependent calcium channels, and the opening of inwardly rectifying K+ channels (Bowery et al., 2002; Bettler et al., 2004). GABAB receptors function as autoreceptors and heteroreceptors, modulating neurotransmitter release and neuronal firing and influencing long-term changes in synaptic strength (Pinard et al., 2010).
Allosteric modulators at GABAB receptors are of great interest given the widespread distribution of GABAB receptors in the central nervous system and the critical role these receptors play in modulating neuronal excitability and synaptic plasticity. Allosteric modulators bind to regions on the receptor that are different from the orthosteric site where the endogenous ligand binds and act by enhancing or attenuating the response elicited by the endogenous transmitter or agonist (Jensen and Spalding, 2004; Conn et al., 2009). By altering the effects of activated receptors without affecting nonactivated receptors, allosteric modulators may have a broader therapeutic window than ligands that indiscriminately alter the activity of all receptors, thereby perhaps offering a potentially attractive alternative to conventional pharmacological agents (Jensen and Spalding, 2004; Pin and Prézeau, 2007; Conn et al., 2009).
Several compounds have been characterized as positive allosteric modulators of GABAB receptors. 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) enhances GABAB receptor-stimulated [35S]GTPγS binding in rat brain membranes and membranes from GABAB1b/2-expressing clonal cells in culture and has modulatory effects in cellular assays of GABAB receptor-mediated electrophysiological responses (Urwyler et al., 2001; Adams and Lawrence, 2007). In vitro studies also indicate that (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one (rac-BHFF) increases the potency and efficacy of GABA to stimulate [35S]GTPγS binding in membrane preparations from recombinant cells in culture and enhances electrophysiological responses to the GABAB receptor agonist baclofen in hippocampal slice preparations (Malherbe et al., 2008). In vivo, rac-BHFF and CGP7930 increase the loss of righting in mice induced by a subthreshold dose of baclofen (Carai et al., 2004; Malherbe et al., 2008). We have recently shown that in rats the GABAB receptor-positive modulators CGP7930 and rac-BHFF enhance the loss of righting induced by baclofen or γ-hydroxybutyrate (GHB), which also activates GABAB receptors (Koek et al., 2010). The hypothermic response induced by baclofen or GHB, however, is not modulated by CGP7930 and rac-BHFF. The data from this in vivo study suggest that GABAB receptor-positive modulators CGP7930 and rac-BHFF act as positive modulators at some, but not all, GABAB receptor populations (Koek et al., 2010).
The present study is part of an effort to examine the positive modulatory properties of CGP7930 and rac-BHFF at GABAB receptors in different brain regions. Using quantitative autoradiography, we measured GABAB receptor-stimulated binding of [35S]GTPγS to G proteins. This approach allowed us to determine the effects of rac-BHFF and CGP7930 on GABAB receptor function, specifically the capacity of GABAB receptors to activate G proteins, with a high degree of neuroanatomical resolution. We hypothesized that rac-BHFF and CGP7930, although not stimulating [35S]GTPγS binding alone, would increase [35S]GTPγS binding stimulated by the GABAB receptor agonist baclofen and by GHB, which has GABAB receptor agonist properties (e.g., Mathivet et al., 1997). Given that neurochemical and electrophysiological responses mediated by GABAB receptors have been characterized extensively in hippocampus and neocortex (Pinard et al., 2010) and cerebellum (Misgeld et al., 1995; Vigot and Batini, 1997; Tu et al., 2010), we chose to examine these brain areas in the present study. Taken together, the results show that GABAB receptor-positive modulators enhance [35S]GTPγS binding stimulated by GABAB receptor agonists in a brain region-dependent manner.
Materials and Methods
Animals.
C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were bred at the University of Texas Health Science Center at San Antonio. The current study used adult male mice that were group-housed and maintained on a 14:10-h day/night cycle with continuous access to food and water. These studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) as adopted and promulgated by the National Institutes of Health.
Tissue Preparation.
Mice were killed by rapid decapitation. Brains were rapidly removed, frozen on powdered dry ice, and then stored at −80°C until sectioning. Coronal sections of 20-μm thickness were cut at −17°C in a cryostat microtome at the level of the medial prefrontal cortex (mPFC; plates 11–12), anterior cingulate cortex (ACC; plates 21–22), dorsal hippocampus (plates 45–46), and cerebellum (plates 86–87) (Paxinos and Franklin, 1997). Sections were thaw-mounted onto gelatin-coated glass slides, desiccated at 4°C for 18 h under vacuum, and then stored at −80°C until they were used in the autoradiographic experiments.
[35S]GTPγS Autoradiography.
Autoradiography of agonist-stimulated [35S]GTPγS binding in brain sections was performed as described previously (Hensler and Durgam, 2001; Advani et al., 2007) with slight modifications. Slide-mounted sections were equilibrated in HEPES buffer (50 mM, pH 7.4), supplemented with 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, and 0.2 mM dithiothreitol for 10 min at 25°C. Sections were preincubated in HEPES buffer containing GDP (2 mM) and the adenosine A1 receptor antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; 1 μM) for 15 min at 25°C, and then incubated in HEPES buffer containing GDP (2 mM), DPCPX (1 μM), and 40 pM [35S]GTPγS, either in the absence or the presence of agonist, for 60 min at 25°C. Basal [35S]GTPγS binding was defined in the absence of agonist. Nonspecific [35S]GTPγS binding was defined in the absence of agonist and the presence of 10 μM GTPγS. The incubation was stopped by two washes for 5 min each in ice-cold 50 mM HEPES buffer, pH 7.4, followed by a brief immersion in ice-cold deionized water. Sections were dried on a slide warmer and exposed to Kodak Biomax MR film (Eastman Kodak, Rochester, NY) for 48 h.
Image Analysis.
Analysis of the digitized autoradiograms was performed with the image analysis program ImageJ, version 1.42q (National Institutes of Health, Bethesda, MD). Tissue sections were stained with thionin, and the brain areas were identified with the atlas of the mouse brain (Paxinos and Franklin, 1997). Autoradiograms of agonist-stimulated [35S]GTPγS binding were quantified by the use of simultaneously exposed [14C] standards (ARC-146; American Radiochemicals, St. Louis, MO). Standard curves were fit to pixel data obtained from [14C] standards, and tissue equivalent values (nanocuries/gram) were provided by American Radiochemicals and used to transform the actual regional densitometric values into relative radioactivity measures. Nonspecific binding of [35S]GTPγS was subtracted from basal binding and binding in the presence of agonist. Specific agonist-stimulated binding was expressed as the percentage above basal. Basal binding of [35S]GTPγS varied with brain region, ranging from 266 ± 10.7 nCi/g in cortical areas and 232 ± 6.02 nCi/g in hippocampus to 116 ± 8.51 nCi/g in cerebellum.
Drugs.
[35S]GTPγS (1250 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). GDP (disodium salt) was purchased from Sigma (St. Louis, MO). GTPγS (tetralithium salt) was purchased from Roche Diagnostics (Indianapolis, Indiana). Baclofen hydrochloride and DPCPX were purchased from Tocris Bioscience (Ellisville, Missouri). GHB was provided by the National Institute on Drug Abuse (Bethesda, MD). CGP7930 and rac-BHFF were synthesized by K. Cheng at the National Institute on Drug Abuse, and 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP35348) was synthesized by J. Agyin at the University of Texas Health Science Center (San Antonio, TX).
Data Analysis.
[35S]GTPγS binding was expressed as percentage above basal. Drug effects on this measure were tested by one-factor analysis of variance (followed by comparisons with vehicle control by Dunnett's test) when several doses were examined or by unpaired t tests if single doses were used. Differences from 0% basal were analyzed by one-sample t tests. [35S]GTPγS binding data obtained in cerebellum were compared with those obtained in mPFC by means of two-factor (brain region, drug treatment) analysis of variance, performed separately on the data obtained with baclofen, GHB, and rac-BHFF, followed by Bonferroni-corrected comparisons between regions.
Results
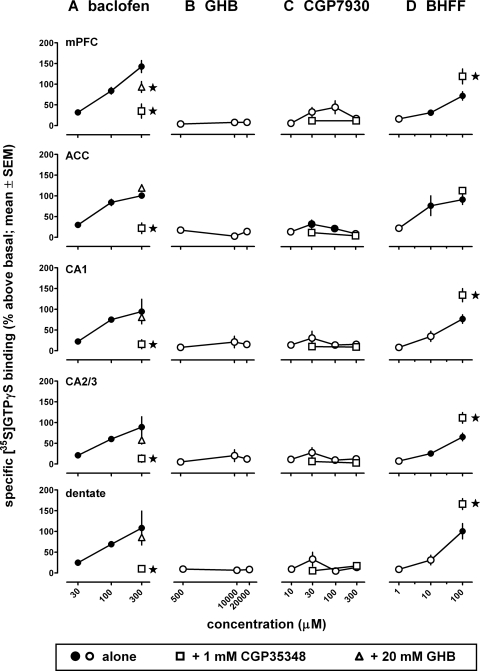
Before studying the effects of combining the GABAB receptor-positive modulators CGP7930 or rac-BHFF with compounds that possess GABAB receptor agonist properties (i.e., baclofen or GHB), each was examined alone for its ability to stimulate [35S]GTPγS binding. Baclofen (Fig. 1A) dose-dependently increased [35S]GTPγS binding in mPFC and ACC, as well as in subregions of dorsal hippocampus (CA1, CA2/3, dentate gyrus) (F2,17 ≥ 6.71; P < 0.01). The binding stimulated by 300 μM baclofen ranged from 89% above basal in CA1 region of hippocampus to 143% above basal in mPFC. The putative GABAB receptor antagonist CGP35348 (1 mM) completely blocked the effect of 300 μM baclofen. The results obtained with the combination of CGP35348 and baclofen differed significantly from the results obtained with baclofen alone in all regions (t6 ≥ 2.84; P ≤ 0.03), except dentate (t6 = 2.37; p = 0.056), and did not differ significantly from basal (t3 ≤ 2.02; P ≥ 0.14). When given alone at 1 mM, CGP35348 significantly increased [35S]GTPγS binding in the mPFC (mean ± S.E.M.: 18 ± 3.6% above basal; t3 versus 0% above basal = 4.83; p = 0.017), but not in the other regions (P ≥ 0.074) (data not shown), suggesting that CGP35348 acts as a weak partial agonist in mPFC to stimulate [35S]GTPγS binding.
Fig. 1.
Effects of the GABAB receptor agonist baclofen (A) or GHB (B) and the GABAB receptor-positive modulator CGP7930 (C) or rac-BHFF (D), alone or together with the GABAB receptor antagonist CGP35348, on [35S]GTPγS binding in regions of forebrain. Using quantitative autoradiography, [35S]GTPγS binding was assessed in mPFC and ACC, as well as in subregions of dorsal hippocampus (CA1, CA2/3, dentate gyrus). ●, significantly different from 0% basal; ○, not significantly different from basal. * in A, significantly different from 300 μM baclofen; * in D, significantly different from 100 μM rac-BHFF. Data are means ± S.E.M. (n = 4–8).
In contrast with baclofen, GHB did not significantly alter [35S]GTPγS binding from basal in any of the regions examined (F2,11 ≤ 1.39; p ≥ 0.29) (Fig. 1B). At the highest concentration tested alone (i.e., 20 mM), GHB significantly decreased the effects of baclofen, but did so only in the mPFC (t6 = 2.50; p = 0.047; other regions, p ≥ 0.09) and did so only partially (t3 versus 0% above basal = 6.98; p = 0.006) (Fig. 1A). Although GHB produces many of its effects by activating GABAB receptors (Carter et al., 2009), it was ineffective in stimulating [35S]GTPγS binding in the forebrain regions examined.
The GABAB receptor-positive modulators CGP7930 and rac-BHFF alone significantly stimulated [35S]GTPγS binding in the cortical areas and hippocampal subregions examined, with rac-BHFF being more potent and more efficacious than CGP7930. CGP7930 alone seemed to increase [35S]GTPγS binding in several regions (Fig. 1C), but only in ACC did this effect reach statistical significance (F3,14 = 4.74; p = 0.018; other regions: p ≥ 0.16). In ACC, [35S]GTPγS binding significantly differed from basal at 30 and 100 μM CGP7930 (t3 = 3.34, p = 0.045 and t5 = 6.82, p = 0.001), but not at the other concentrations tested (p ≥ 0.08), and attained a maximum of 33%. None of the results obtained when CGP7930 was combined with the putative GABAB receptor antagonist CGP35348 differed significantly from those obtained with CGP7930 alone (p ≥ 0.12). rac-BHFF dose-dependently increased [35S]GTPγS binding in all regions examined (Fig. 1D) (ACC: F2,10 = 5.51, p = 0.024; other regions: F2,11 ≥ 9.83, p ≤ 0.0034). The binding stimulated by 100 μM rac-BHFF ranged from 66% above basal in CA2/3 to 101% above basal in dentate gyrus. Thus, rac-BHFF stimulated [35S]GTPγS binding more potently and to a larger extent than CGP7930. It is noteworthy that [35S]GTPγS binding stimulated by the combination of CGP35348 (1 mM) and rac-BHFF (100 μM) was significantly greater than that obtained with rac-BHFF alone in all forebrain regions examined (p ≤ 0.033) except ACC (p = 0.22) (Fig. 1D). These data are consistent with the modulation of partial agonist activity of CGP35348 by rac-BHFF in mPFC and dorsal hippocampus.
Both positive modulators markedly enhanced the effect of baclofen (30 μM) to increase [35S]GTPγS binding, at concentrations lower than those that stimulated [35S]GTPγS binding when applied alone (Fig. 2). CGP7930 dose-dependently enhanced the effects of baclofen (F4,25 ≥ 3.04; p ≤ 0.036) (Fig. 2A), with 10 μM producing significant enhancement in three regions (i.e., mPFC, ACC, and hippocampal CA2/3) and 100 μM producing significant enhancement in these regions as well as in dentate gyrus. At 100 μM, the effects of CGP7930 combined with baclofen were significantly greater than those of CGP7930 alone in all regions examined (mPFC: t9 = 3.43, p = 0.0076; other regions: t10 ≥ 2.91, p ≤ 0.016) (Fig. 2A). CGP7930 enhanced baclofen-stimulated [35S]GTPγS binding at minimum significant concentrations of 10 μM in mPFC, ACC, and CA2/3 and 100 μM in CA1 and dentate. Binding stimulated by baclofen (30 μM) in the presence of CGP7930 (100 μM) ranged from 123% above basal in CA2/3 to 241% above basal in mPFC. rac-BHFF dose-dependently enhanced the effects of baclofen (F4,17 ≥ 5.83; p ≤ 0.0038) (Fig. 2B), with 1 and 10 μM significantly enhancing baclofen-stimulated [35S]GTPγS binding in all regions. As illustrated by the autoradiograms in Fig. 3, rac-BHFF at a concentration (1 μM) that did not stimulate [35S]GTPγS binding above basal markedly enhanced the effect of 30 μM baclofen. At concentrations of 1 and 10 μM, the effects of rac-BHFF combined with baclofen were significantly greater than those of rac-BHFF alone in all regions examined (mPFC: t9 = 3.43, p = 0.0076; other regions: t10 ≥ 2.91, p ≤ 0.016). Binding stimulated by 30 μM baclofen in the presence of 10 μM rac-BHFF ranged from 464% above basal in CA2/3 to 628% above basal in dentate. CGP7930 and rac-BHFF enhanced baclofen-stimulated [35S]GTPγS binding at concentrations at least 3- to 10-fold lower than those that had effects when given alone, and rac-BHFF enhanced baclofen-stimulated [35S]GTPγS binding at least 10-fold more potently and to a larger extent than CGP7930.
Fig. 2.
Effects of the GABAB receptor-positive modulators CGP7930 (A) or rac-BHFF (B) together with baclofen and GHB on [35S]GTPγS binding in regions of forebrain. Using quantitative autoradiography, [35S]GTPγS binding was assessed in mPFC and ACC, as well as in subregions of dorsal hippocampus (CA1, CA2/3, dentate gyrus). *, significantly different from 30 μM baclofen or 20 mM GHB. #, significantly different from the corresponding dose of CGP7930 (A) or rac-BHFF (B) given alone. Data are means ± S.E.M. (n = 4–6). Note that the scale of the abscissa is different from the scale used in Fig. 1.
Fig. 3.
rac-BHFF markedly enhances the effect of baclofen to stimulate the binding of [35S]GTPγS. Representative autoradiograms of [35S]GTPγS binding to coronal sections of brain taken through the dorsal hippocampus are shown. A, nonspecific binding was defined in the presence of 10 μM GTPγS. B, basal binding was determined in the absence of the GABAB receptor agonist baclofen or modulator rac-BHFF. C to E, the binding of [35S]GTPγS in the presence of rac-BHFF (1 μM) (C), baclofen (30 μM) (D), or rac-BHFF (1 μM) + baclofen (30 μM) (E). Data from these experiments are plotted in Fig. 2.
In contrast to what was observed with baclofen, the modulators did not seem to act in combination with GHB to increase [35S]GTPγS binding in cortical or hippocampal regions. CGP7930 at 100 μM, a concentration that markedly enhanced baclofen-stimulated [35S]GTPγS binding, did not significantly increase the binding of [35S]GTPγS above basal when combined with GHB (mPFC: t7 = 0.89, p = 0.40; other regions: t8 ≤ 1.19, p ≥ 0.27) (Fig. 2A). rac-BHFF in combination with GHB dose-dependently increased [35S]GTPγS binding (all regions except CA2/3: F2,11 ≥ 4.63, p ≤ 0.035; CA2/3: p = 0.073), with 10 μM having significant effects versus GHB alone in all regions (Fig. 2B). However, only in the CA2/3 region of hippocampus did [35S]GTPγS binding stimulated by 10 μM rac-BHFF in combination with GHB differ significantly from [35S]GTPγS binding stimulated by rac-BHFF alone (t6 = 2.51, p = 0.047; other regions: p ≥ 0.10).
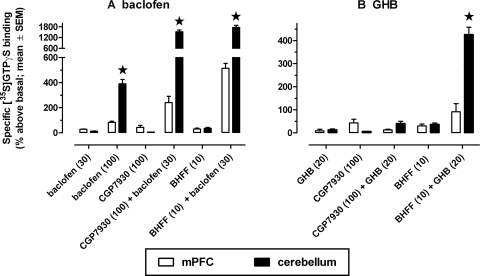
Although baclofen seemed to stimulate [35S]GTPγS binding to a similar extent in cortical and hippocampal regions (Figs. 1 and 2), it had a markedly greater effect in cerebellum (molecular layer) (Fig. 4A). Baclofen (100 μM) increased [35S]GTPγS binding in cerebellum to 390% above basal, which is significantly larger than its effect in, for example, the mPFC (i.e., 84% above basal). At a lower concentration (i.e., 30 μM), baclofen stimulated GTPγS binding only modestly and did so similarly in the mPFC and the cerebellum (29 and 13% above basal, respectively; p > 0.05). Thus, baclofen seemed to increase GTPγS binding in both brain regions with different efficacy. Although the effects of the modulators alone did not differ significantly between cerebellum and mPFC (p > 0.05), large regional differences became apparent when the modulators were given together with baclofen. CGP7930 (100 μM) increased [35S]GTPγS binding stimulated by baclofen (30 μM) significantly more in cerebellum (from 13 to 1530% above basal) than in mPFC (from 29 to 241% above basal) (p < 0.0001). Likewise, rac-BHFF (10 μM) increased baclofen-stimulated [35S]GTPγS binding significantly more in cerebellum (from 13 to 1778% above basal) than in mPFC (from 29 to 514% above basal) (p < 0.0001). Together, these results indicate that the modulators were more effective in cerebellum than in mPFC to enhance the effects of baclofen (see also Fig. 5).
Fig. 4.
Comparison of the effects of the GABAB receptor-positive modulator CGP7930 and rac-BHFF together with baclofen (A) or GHB (B) on [35S]GTPγS binding in cerebellum (molecular layer) and mPFC. Numbers in parentheses are concentrations in microliters, except the concentration of GHB, which is in milliliters. Main effects of brain region and treatment, and brain region × treatment interactions, were statistically significant for the results shown (p < 0.0001). *, significantly different from the corresponding results obtained in mPFC. Results obtained in mPFC are replotted from Figs. 1 and 2. Data are means ± S.E.M. (n = 4–6). Note that the scale of the abscissa in A is interrupted and differs from the scales used in B and Figs. 1 and 2.
Fig. 5.
rac-BHFF is more effective in cerebellum than in mPFC in enhancing the effects of baclofen. Representative autoradiograms of [35S]GTPγS binding to coronal sections of brain taken through the medial prefrontal cortex or cerebellum are shown. Nonspecific binding was defined in the presence of 10 μM GTPγS. Basal binding was determined in the absence of the GABAB receptor agonist baclofen or modulator rac-BHFF. The binding of [35S]GTPγS in the presence of rac-BHFF (10 μM), baclofen (30 μM), or rac-BHFF (10 μM) + baclofen (30 μM) is also shown. Data from these experiments are plotted in Fig. 4.
As was observed in cortical and hippocampal regions, GHB (20 mM) alone did not significantly affect [35S]GTPγS binding in the cerebellum (Fig. 4B). And as was observed in these forebrain regions, CGP7930 (100 μM) in combination with GHB did not increase [35S]GTPγS binding in the cerebellum (Fig. 4B). However, we did observe marked regional differences in the positive modulatory effect of rac-BHFF in combination with GHB comparing cerebellum and forebrain areas. rac-BHFF (10 μM) combined with GHB produced a marked increase in [35S]GTPγS binding, which was significantly larger in cerebellum (from 13 to 426% above basal) than, for example, in mPFC (from 7.1 to 91% above basal) (p < 0.0001) (Fig. 4B). It is noteworthy that this concentration of rac-BHFF by itself did not have significantly different effects in cerebellum than in mPFC.
It is noteworthy that we also observed marked regional differences in the effects of CGP35348 in combination with rac-BHFF on [35S]GTPγS binding in cerebellum versus mPFC (data not shown). CGP35348 (1 mM) significantly increased [35S]GTPγS binding to 18 ± 3.6% above basal in mPFC (p = 0.017) and nonsignificantly (p = 0.057) to 61 ± 20% above basal in cerebellum; the difference between the effects of CGP35348 in the two regions was not significant (p > 0.20). Although rac-BHFF (100 μM) stimulated [35S]GTPγS binding more in cerebellum (247 ± 40% above basal) than in mPFC (77 ± 9.7% above basal), this difference was not statistically significant (p = 0.15). CGP35348 (1 mM) in combination with rac-BHFF (100 μM) produced a marked increase in [35S]GTPγS binding, which was significantly larger in cerebellum (from 61 to 1260% above basal) than in mPFC (from 18 to 118% above basal) (p < 0.0001). Our data indicate that the putative GABAB receptor antagonist CGP35348 exhibits agonist activity to stimulate [35S]GTPγS binding above basal values, and these agonist effects are markedly enhanced by rac-BHFF, particularly in cerebellum.
Discussion
The main finding of the present study is that the responses to GABAB receptor-positive modulators, as well as to compounds with agonist activity at GABAB receptors, differed markedly among brain regions. Baclofen stimulated [35S]GTPγS binding to a greater extent in cerebellum than in cortical and hippocampal regions. CGP7930 and rac-BHFF were markedly more effective in enhancing the effects of baclofen in cerebellum than in other regions. Previously, we found CGP7930 and rac-BHFF enhance baclofen-induced loss of righting, but not hypothermia (Koek et al., 2010). Together, these results suggest that different GABAB receptor populations may differ in their susceptibility to GABAB receptor agonist- and -positive modulatory effects, possibly allowing for more selective therapeutic interventions of the GABAB system.
Baclofen increased [35S]GTPγS binding in cortical areas and subregions of dorsal hippocampus, an effect blocked by CGP35348, a putative antagonist at GABAB receptors. CGP35348 has been shown to antagonize the effect of baclofen to induce hypothermia and loss of righting in mice (Koek et al., 2010). In general, baclofen was more efficacious in stimulating [35S]GTPγS binding in cerebellum than in forebrain regions. Large regional differences were also apparent when the modulators CGP7930 or rac-BHFF were given together with baclofen, i.e., the modulators were markedly more effective in cerebellum than in mPFC in enhancing the effects of baclofen. In vivo, the positive GABAB receptor modulators CGP7930 and rac-BHFF enhance baclofen-induced loss of righting, but not hypothermia in mice (Koek et al., 2010). Together, these studies provide further evidence of pharmacologically distinct GABAB receptor populations.
In the present study CGP35348 seemed to have partial agonist activity in medial prefrontal cortex and cerebellum, producing a modest, but significant, increase in [35S]GTPγS binding above basal values. This effect of CGP35348 to stimulate [35S]GTPγS binding in these brain regions was markedly enhanced by the modulator rac-BHFF and to a greater extent in cerebellum than in mPFC. CGP35348 has partial agonist activity to inhibit adenylyl cyclase activity in intact recombinant CHO cells expressing GABAB receptors. The potency and efficacy of CGP35348 in this assay system is increased by the positive modulators CGP7930 and GS39783 (Urwyler et al., 2005). Although CGP35348 does not stimulate [35S]GTPγS binding in membrane preparations from these cells, CGP35348 is a low-efficacy partial agonist in the presence of the modulators (Urwyler et al., 2005). Thus, allosteric modulators seem to be useful in revealing the intrinsic properties of agonist ligands at the orthosteric site of GABAB receptors.
Positive allosteric modulators act synergistically with agonists to elicit an enhanced response, but have little or no intrinsic agonistic activity of their own (Jensen and Spalding, 2004; Pin and Prézeau, 2007; Conn et al., 2009). In the present study, both CGP7930 and rac-BHFF markedly enhanced the effect of baclofen to increase [35S]GTPγS binding at concentrations lower than those that stimulated [35S]GTPγS binding when applied alone. Likewise, CGP7930 and its analog 3,5-bis(1,1-dimethylethyl)-4-hydroxy-α,α-dimethylbenzenepropanal (CGP13501) (Urwyler et al., 2001) or rac-BHFF (Malherbe et al., 2008) enhance the biochemical and electrophysiological effects of GABA at concentrations that produce little or no GABAB receptor activation when applied alone. At higher concentrations, however, CGP7930 and rac-BHFF alone significantly stimulated [35S]GTPγS binding in forebrain regions, with rac-BHFF being more potent and more efficacious than CGP7930. It is noteworthy that the effects of the modulators by themselves did not differ significantly between cerebellum and mPFC. In recombinant CHO cells expressing GABAB receptors, the positive modulators CGP7930 and GS39783 alone produce a small inhibition of adenylyl cyclase (Urwyler et al., 2005). In cerebellar granular neurons in culture, CGP7930 has similar effects as baclofen to stimulate ERK1/2/CREB signaling (Tu et al., 2007) and biochemical pathways mediating neuroprotection (Tu et al., 2010). rac-BHFF has similar effects as GABA in stimulating [35S]GTPγS binding in CHO cells that stably overexpress human Gα16 and human GABAB1a and GABAB2a subunits (Malherbe et al., 2008). Thus under particular conditions, these GABAB receptor-positive modulators have intrinsic agonist activity in the absence of GABA. Our data, collected in brain sections that probably lack GABA due to equilibration and preincubation steps in the [35S]GTPγS binding assay, suggest that this agonist activity is not restricted to primary culture or recombinant systems and therefore may have more general relevance. Conceivably, intrinsic agonist activity of these GABAB receptor-positive modulators could be involved in their in vivo effects, such as anxiolytic- and antidepressant-like effects (Cryan and Kaupmann, 2005) and inhibition of the reinforcing effects of drugs of abuse (Vlachou and Markou, 2010).
In the present study, GHB was ineffective in stimulating [35S]GTPγS binding in the cerebellum and forebrain regions. GHB binds with weak affinity to the GABAB receptor (Mathivet et al., 1997; Lingenhoehl et al., 1999; Wu et al., 2004). Many of the behavioral and physiological effects of GHB seem to reflect weak agonist activity at GABAB receptors, in that they are prevented or attenuated by GABAB receptor antagonists (Carai et al., 2001; Kaupmann et al., 2003; Quéva et al., 2003; Carter et al., 2005; Koek et al., 2007, 2010). GHB-induced hypothermia and sedation are absent in GABAB receptor knockout mice (Quéva et al., 2003). Although GHB had no effect on the binding of [35S]GTPγS in any area examined in the current study, the combination of GHB and rac-BHFF resulted in increased [35S]GTPγS binding but only in the cerebellum. It is unlikely that these effects involve GHB binding sites distinct from GABAB receptors, because these sites, although prevalent in hippocampus and cortex, seem to be absent in cerebellum (Snead, 1996; Gould et al., 2003; Wu et al., 2004). Consistent with the involvement of GABAB receptors in the effects of the combination of GHB and rac-BHFF reported here, rac-BHFF enhances GHB-induced loss of righting (Koek et al., 2010), an effect that probably involves cerebellar GABAB receptors.
The marked differences between forebrain and cerebellum in the effect of compounds with GABAB receptor agonist properties to stimulate [35S]GTPγS binding, or the effects of positive modulators CGP7930 or rac-BHFF, can not be readily explained by a corresponding difference in GABAB receptor density. The density of GABAB receptors in the cerebellum (molecular layer) is comparable with that in frontal cortex and anterior cingulate cortex, with somewhat lower densities in the hippocampus (Chu et al., 1990). Instead of differences in receptor density, a family of auxiliary proteins that form high-molecular-mass complexes with GABAB receptors could conceivably be involved in the brain region-dependent effects reported here. These KCTD (potassium channel tetramerization domain-containing) proteins increase agonist potency and markedly alter the G protein signaling of GABAB receptors (Schwenk et al., 2010). The distinct regional expression profiles of KCTD proteins in the brain (Schwenk et al., 2010; Metz et al., 2011) raise the interesting possibility that these proteins in complex with GABAB receptors determine not only agonist potency and G protein signaling, but also the effects of positive allosteric modulators.
Positive allosteric modulators act synergistically with agonist or the endogenous neurotransmitter and hold several advantages to the actions of conventional agonists. For example, the modulator will only amplify the neural signal when the neurotransmitter is released into the synapse. The receptor may be less likely to desensitize upon sustained exposure to a positive allosteric modulator compared with an agonist. Because the orthosteric binding sites for a particular endogenous ligand are often highly conserved, it is difficult to achieve high selectivity for conventional ligands targeting this site among specific G protein-coupled receptor subtypes (Jensen and Spalding, 2004; Pin and Prézeau, 2007; Conn et al., 2009). Thus, allosteric modulators offer a potentially attractive alternative to conventional pharmacological agents. Our data suggest that different GABAB receptor populations differ in their sensitivity to the effects of positive GABAB receptor modulators, which may be related to regional differences in the expression of auxiliary GABAB receptor (e.g., KCTD) proteins. That different GABAB receptor populations seem to differ in their susceptibility to positive modulatory effects may allow for more selective therapeutic targeting of the GABAB receptor system.
Acknowledgments
We thank Marisela Valdez, Jason Persyn, Sonia Cano, and Bindumahi Sudaabattula for outstanding technical assistance.
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse [Grant DA15692]. The research of the Drug Design and Synthesis Section, Chemical Biology Research Branch, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism was supported by the National Institutes of Health Intramural Research Programs of the National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- CGP35348
- 3-aminopropyl(diethoxymethyl)phosphinic acid
- CGP7930
- 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol
- rac-BHFF
- (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one
- GHB
- γ-hydroxybutyrate
- DPCPX
- 1,3-dipropyl-8-cyclopentylxanthine
- [35S]GTPγS
- guanosine 5′-O-(3-[35S]thiotriphosphate)
- mPFC
- medial prefrontal cortex
- ACC
- anterior cingulate cortex
- KCTD
- potassium channel tetramerization domain-containing
- CHO
- Chinese hamster ovary
- CGP13501
- 3,5-bis(1,1-dimethylethyl)-4-hydroxy-α,α-dimethylbenzenepropanal.
Authorship Contributions
Participated in research design: Hensler, Advani, and Koek.
Conducted experiments: Advani and Burke.
Contributed new reagents or analytic tools: Cheng and Rice.
Performed data analysis: Hensler, Advani, and Koek.
Wrote or contributed to the writing of the manuscript: Hensler and Koek.
References
- Adams CL, Lawrence AJ. (2007) CGP7930: a positive allosteric modulator of the GABAB receptor. CNS Drug Rev 13:308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Cardone S, Ferruli A, Gasbarrini G. (2009) Role of the GABA(B) receptor system in alcoholism and stress: focus on clinical studies and treatment perspectives. Alcohol 43:559–563 [DOI] [PubMed] [Google Scholar]
- Advani T, Hensler JG, Koek W. (2007) Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6 mice. Int J Neuropsychopharmacol 10:595–607 [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. (2004) Molecular structure and physiological functions of GABAB receptors. Physiol Rev 84:835–867 [DOI] [PubMed] [Google Scholar]
- Bowery NG. (2006) GABAB receptor: a site of therapeutic benefit. Curr Opin Pharmacol 6:37–43 [DOI] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. (2002) International Union of Pharmacology. XXXIII. Mammalian γ-aminobutyric acid B receptors: structure and function. Pharmacol Rev 54:247–264 [DOI] [PubMed] [Google Scholar]
- Calver AR, Davies CH, Pangalos M. (2002) GABAB receptors: from monogamy to promiscuity. Neurosignals 11:299–314 [DOI] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Froestl W, Gessa GL. (2004) In vivo effectiveness of CGP7930, a positive allosteric modulator of the GABAB receptor. Eur J Pharmacol 504:213–216 [DOI] [PubMed] [Google Scholar]
- Carter LP, Koek W, France CP. (2009) Behavioral analyses of GHB: receptor mechanisms. Pharmacol Ther 121:100–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Wu H, Chen W, Matthews MM, Mehta AK, Hernandez RJ, Thomson JA, Ticku MK, Coop A, Koek W, et al. (2005) Novel γ-hydroxybutyric acid (GHB) analogs share some, but not all behavioral effects of GHB and GABAB receptor agonists. J Pharmacol Exp Ther 313:1314–1323 [DOI] [PubMed] [Google Scholar]
- Chu DC, Albin RL, Young AB, Penney JB. (1990) Distribution and kinetics of GABAB binding sites in rat central nervous system: a quantitative autoradiographic study. Neuroscience 34:341–357 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. (2009) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 8:41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K. (2005) Don't worry ‘B’ happy!: a role for GABAB receptors in anxiety and depression. Trends Pharmacol Sci 26:36–43 [DOI] [PubMed] [Google Scholar]
- Gould GG, Mehta AK, Frazer A, Ticku MK. (2003) Quantitative autoradiographic analysis of the new radioligand [3H](2E)-(5-hydroxy-5,7,8,9-tetrahydro-6H-benzo[a][7]annulen-6-ylidene) ethanoic acid ([3H]NCS-382) at γ-hydroxybutyric acid (GHB) binding sites in rat brain. Brain Res 979:51–56 [DOI] [PubMed] [Google Scholar]
- Hensler J, Durgam H. (2001) Regulation of 5-HT1A receptor-stimulated [35S]-GTPγS binding as measured by quantitative autoradiography following chronic agonist administration. Br J Pharmacol 132:605–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Jensen AA, Spalding TA. (2004) Allosteric modulation of G-protein coupled receptors. Eur J Pharm Sci 21:407–420 [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Cryan JF, Wellendorph P, Mombereau C, Sansig G, Klebs K, Schmutz M, Froestl W, van der Putten H, Mosbacher J, et al. (2003) Specific γ-hydroxybutyrate-binding sites but loss of pharmacological effects of γ-hydroxybutyrate in GABAB1-deficient mice. Eur J Neurosci 18:2722–2730 [DOI] [PubMed] [Google Scholar]
- Koek W, France CP, Cheng K, Rice KC. (2010) GABAB receptor-positive modulators: enhancement of GABAB receptor agonist effects in vivo. J Pharmacol Exp Ther 335:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A. (2007) Cataleptic effects of γ-hydroxybutyrate (GHB), its precursor γ-butyrolactone (GBL), and GABAB receptor agonists in mice: differential antagonism by the GABAB receptor antagonist CGP35348. Psychopharmacology 192:407–414 [DOI] [PubMed] [Google Scholar]
- Lingenhoehl K, Brom R, Heid J, Beck P, Froestl W, Kaupmann K, Bettler B, Mosbacher J. (1999) γ-Hydroxybutyrate is a weak agonist at recombinant GABAB receptors. Neuropharmacology 38:1667–1673 [DOI] [PubMed] [Google Scholar]
- Maccioni P, Fantini N, Froestl W, Carai MA, Gessa GL, Colombo G. (2008) Specific reduction of alcohol's motivational properties by the positive allosteric modulator of the GABAB receptor, GS39783–comparison with the effect of the GABAB receptor direct agonist, baclofen. Alcohol Clin Exp Res 32:1558–1564 [DOI] [PubMed] [Google Scholar]
- Malherbe P, Masciadri R, Norcross RD, Knoflach F, Kratzeisen C, Zenner MT, Kolb Y, Marcuz A, Huwyler J, Nakagawa T, et al. (2008) Characterization of (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one as a positive allosteric modulator of GABAB receptors. Br J Pharmacol 154:797–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivet P, Bernasconi R, De Barry J, Marescaux C, Bittiger H. (1997) Binding characteristics of γ-hydroxybutyric acid as a weak but selective GABAB receptor agonist. Eur J Pharmacol 321:67–75 [DOI] [PubMed] [Google Scholar]
- Metz M, Gassmann M, Fakler B, Schaeren-Wiemers N, Bettler B. (2011) Distribution of the auxiliary GABAB receptor subunits KCTD8, 12, 12b, and 16 in the mouse brain. J Comp Neurol 519:1435–1454 [DOI] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. (1995) A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol 46:423–462 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. (1997) The Mouse Brain in Stereotaxic Coordinates. Academic Press, New York [Google Scholar]
- Pin JP, Prézeau L. (2007) Allosteric modulators of GABAB receptors: Mechanism of action and therapeutic perspective. Curr Neuropharmacol 5:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinard A, Seddik R, Bettler B. (2010) GABAB receptors: physiological functions and mechanisms of diversity. Adv Pharmacol 58:231–255 [DOI] [PubMed] [Google Scholar]
- Quéva C, Bremner-Danielsen M, Edlund A, Ekstrand AJ, Elg S, Erickson S, Johansson T, Lehmann A, Mattsson JP. (2003) Effects of GABA agonists on body temperature regulation in GABAB(1)−/− mice. Br J Pharmacol 140:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk J, Metz M, Zolles G, Turecek R, Fritzius T, Bildl W, Tarusawa E, Kulik A, Unger A, Ivankova K, et al. (2010) Native GABAB receptors are heteromultimers with a family of auxiliary subunits. Nature 465:231–235 [DOI] [PubMed] [Google Scholar]
- Snead OC., 3rd (1996) Relation of the [3H] γ-hydroxybutyric acid (GHB) binding site to the γ-aminobutyric acid B (GABAB) receptor in rat brain. Biochem Pharmacol 52:1235–1243 [DOI] [PubMed] [Google Scholar]
- Tu H, Rondard P, Xu C, Bertaso F, Cao F, Zhang X, Pin JP, Liu J. (2007) Dominant role of GABAB2 and Gbetagamma for GABAB receptor-mediated-ERK1/2/CREB pathway in cerebellar neurons. Cell Signal 19:1996–2002 [DOI] [PubMed] [Google Scholar]
- Tu H, Xu C, Zhang W, Liu Q, Rondard P, Pin JP, Liu J. (2010) GABAB receptor activation protects neurons from apoptosis via IGF-1 receptor transactivation. J Neurosci 30:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwyler S, Gjoni T, Koljatić J, Dupuis DS. (2005) Mechanisms of allosteric modulation at GABAB receptors by CGP7930 and GS39783: effects on affinities and efficacies of orthosteric ligands with distinct intrinsic properties. Neuropharmacology 48:343–353 [DOI] [PubMed] [Google Scholar]
- Urwyler S, Mosbacher J, Lingenhoehl K, Heid J, Hofstetter K, Froestl W, Bettler B, Kaupmann K. (2001) Positive allosteric modulation of native and recombinant γ-aminobutyric acid B receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol Pharmacol 60:963–971 [PubMed] [Google Scholar]
- Vigot R, Batini C. (1997) GABAB receptor activation of Purkinje cells in cerebellar slices. Neurosci Res 29:151–160 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Markou A. (2010) GABAB receptors in reward processes. Adv Pharmacol 58:315–371 [DOI] [PubMed] [Google Scholar]
- Wu Y, Ali S, Ahmadian G, Liu CC, Wang YT, Gibson KM, Calver AR, Francis J, Pangalos MN, Carter Snead O., 3rd (2004) γ-Hydroxybutyric acid (GHB) and γ-aminobutyric acid B receptor (GABABR) binding sites are distinctive from one another: molecular evidence. Neuropharmacology 47:1146–1156 [DOI] [PubMed] [Google Scholar]