Abstract
In overdose acetaminophen (APAP) is hepatotoxic. Toxicity occurs by metabolism to N-acetyl-p-benzoquinone imine, which depletes GSH and covalently binds to proteins followed by protein nitration. Nitration can occur via the strong oxidant and nitrating agent peroxynitrite, formed from superoxide and nitric oxide (NO). In hepatocyte suspensions we reported that an inhibitor of neuronal nitric-oxide synthase (nNOS; NOS1), which has been reported to be in mitochondria, inhibited toxicity and protein nitration. We recently showed that manganese superoxide dismutase (MnSOD; SOD2) was nitrated and inactivated in APAP-treated mice. To understand the role of nNOS in APAP toxicity and MnSOD nitration, nNOS knockout (KO) and wild-type (WT) mice were administered APAP (300 mg/kg). In WT mice serum alanine aminotransferase (ALT) significantly increased at 6 and 8 h, and serum aspartate aminotransferase (AST) significantly increased at 4, 6 and 8 h; however, in KO mice neither ALT nor AST significantly increased until 8 h. There were no significant differences in hepatic GSH depletion, APAP protein binding, hydroxynonenal covalent binding, or histopathological assessment of toxicity. The activity of hepatic MnSOD was significantly lower at 1 to 2 h in WT mice and subsequently increased at 8 h. MnSOD activity was not altered at 0 to 6 h in KO mice but was significantly decreased at 8 h. There were significant increases in MnSOD nitration at 1 to 8 h in WT mice and 6 to 8 h in KO mice. Significantly more nitration occurred at 1 to 6 h in WT than in KO mice. MnSOD was the only observed nitrated protein after APAP treatment. These data indicate a role for nNOS with inactivation of MnSOD and ALT release during APAP toxicity.
Introduction
Acetaminophen (APAP; N-acetyl-p-aminophenol), a commonly used analgesic/antipyretic drug, is believed to be safe at therapeutic doses. However, in overdose it produces a centrilobular hepatic necrosis. APAP-induced toxicity occurs by initial hepatic metabolism of APAP by cytochrome P450 to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) (Dahlin et al., 1984). After a therapeutic dose NAPQI is efficiently detoxified by GSH, but when taken in overdose NAPQI reacts with GSH, leading to its depletion (Mitchell et al., 1973), and the metabolite covalently binds to hepatic cellular proteins to form 3-(cystein-S-yl)-acetaminophen (APAP-Cys) adducts (Hoffmann et al., 1985). These events are followed by oxidative stress, and toxicity has been postulated to be by oxidative stress. Previous studies have implicated the role of peroxynitrite, a reactive nitrogen species formed from nitric oxide and superoxide, in the development of oxidative stress. Peroxynitrite is both a nitrating and an oxidizing agent. Immunohistochemical analysis of livers from APAP-treated mice indicated that nitrated proteins were colocalized with APAP-protein adducts in the hepatocytes of the centrilobular regions undergoing necrosis (Hinson et al., 1998). Because iNOS is known to be in hepatocytes and Kupffer cells (Stolz et al., 2002) it was initially postulated to be the source of NO leading to peroxynitrite. However, APAP was found to be equally hepatotoxic in iNOS knockout (KO) mice and wild-type (WT) mice in two different laboratories (Michael et al., 2001; Bourdi et al., 2007). Moreover, a pharmacological inhibitor of iNOS, aminoguanidine, did not decrease APAP hepatotoxicity in mice (Hinson et al., 2002). These data suggested that iNOS was not important in the development of toxicity in the wild-type mice.
To further understand the mechanism of APAP toxicity and the source of NO, we examined mechanisms of APAP toxicity in a hepatocyte suspension assay. In freshly isolated mouse hepatocytes APAP toxicity occurs in two phases. There is an initial metabolism phase (0–2 h), which is characterized by GSH depletion and covalent binding to proteins, but little toxicity. Subsequent washing of the hepatocytes to remove APAP and incubation with media alone results in toxicity in the reincubation phase (3–5 h) (Boobis et al., 1986; Grewal and Racz, 1993). We found that APAP toxicity occurred with mitochondrial permeability transition (MPT) (Reid et al., 2005). Likewise APAP toxicity was reported by others to occur with MPT in cultured hepatocytes (Kon et al., 2004). MPT is an abrupt increase in the permeability of the inner mitochondrial membrane to ions and small molecular weight solutes. It is promoted by oxidative stress and calcium and leads to a large increase in oxidative stress. MPT occurs with the loss of the ability of the mitochondria to produce ATP, a lethal event for the cell (Byrne et al., 1999; Zorov et al., 2000). In the hepatocyte suspension assay we showed that the MPT inhibitor cyclosporine A and the antioxidant N-acetylcysteine added in the reincubation phase blocked toxicity, loss of mitochondrial membrane potential, the large increase in oxidative stress (2′,7′-dichlorofluorescin oxidation), and MPT (Reid et al., 2005).
Subsequently, we examined the role of peroxynitrite in the increased oxidative stress in APAP toxicity in the hepatocyte suspension assay. It was shown that APAP toxicity was associated with the increased nitration of proteins. Toxicity, protein nitration, loss of mitochondrial membrane potential, and dichlorodihydrofluorescein oxidation were blocked by addition in the reincubation phase of the MPT inhibitor cyclosporine A, the antioxidant N-acetylcysteine, and the nNOS inhibitor 7-nitroindazole but not by iNOS inhibitors (Burke et al., 2010). nNOS has been reported previously to be in mitochondria as mitochondrial NOS (Brookes, 2004; Ghafourifar and Cadenas, 2005). It was postulated that in the liver APAP-induced MPT is mediated by the activation of nNOS with increased NO formation, which reacts with superoxide to form peroxynitrite followed by nitration of proteins.
In our recent work we have investigated the role of mitochondrial manganese superoxide dismutase (MnSOD; SOD2) in APAP toxicity (Agarwal et al., 2011). It was shown that hepatic MnSOD activity was inhibited, and the protein was nitrated early in the course of APAP toxicity. Inhibition of activity correlated with the relative amount of nitration of MnSOD (Agarwal et al., 2011). This protein was previously shown to be nitrated and inactivated during renal transplant injury (MacMillan-Crow et al., 1996; MacMillan-Crow and Cruthirds, 2001).
In the present work we investigated APAP-induced hepatotoxicity in nNOS KO and WT mice. It was postulated that if nNOS present in hepatic mitochondria was mechanistically important in nitration, leading to toxicity, then nNOS KO mice would not develop the hepatic toxicity.
Materials and Methods
Reagents.
APAP and xanthine oxidase were obtained from Sigma (St. Louis, MO). Coomassie Plus Protein Assay Reagent and SuperSignal chemiluminescent substrate reagent were obtained from Thermo Fisher Scientific (Waltham, MA). Protease inhibitor cocktail was obtained from Roche Diagnostics (Mannheim, Germany). Hybond-ECL nitrocellulose membranes for Western blotting were obtained from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). Anti-3-nitrotyrosine and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were obtained from Millipore Bioscience Research Reagents (Temecula, CA). 4-Hydroxynonenal (4-HNE) antibody was obtained from Novus Biologicals, Inc. (Littleton, CO), and actin antibody was obtained from Abcam, Inc. (Cambridge, MA). Peroxidase-labeled goat anti-rabbit IgG, goat anti-mouse IgG, and rabbit anti-goat IgG antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and Thermo Fisher Scientific. All chemicals were the highest grade commercially available.
Animals.
Eight-week-old male B6129SF2/J mice (WT) and B6129S4Nos1tm1Plh/J mice (nNOS KO), all having mean weight of 24.4 g, were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were fed ad libitum and housed in individual cages in a room at a constant temperature (22°C) with a 12-h light/dark cycle. All animal experimentation and protocols were approved by the institutional animal care and use committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) as adopted by the National Institute of Health. Mice were acclimatized for 1 week before the planned experiments. On the day before experiments, mice were fasted overnight. Control mice received saline only, and treated mice received APAP in 0.4 ml of saline (intraperitoneally). Mice were administered APAP (300 mg/kg) (n = 4 per time point). At the designated time, mice were anesthetized in a carbon dioxide chamber; blood was withdrawn by cardiac puncture, and animals were subsequently euthanized under carbon dioxide followed by cervical dislocation. Livers were removed and weighed. Approximately 200 mg of liver was preserved in GSH homogenization buffer, a sample was preserved in buffered formalin, and the remaining tissue was snap-frozen in liquid nitrogen and stored at −80°C for later analysis.
Serum Assays for Liver Toxicity.
Toxicity was determined by the quantitation of serum alanine aminotransferase (ALT) and serum aspartate aminotransferase (AST) in the blood, which occurred as a result of liver damage. Collected blood was allowed to coagulate at room temperature for at least 1 h, after centrifugation for 20 min at 3000g in a microcentrifuge. The serum was collected and kept on ice until it was used to determine ALT and AST measurements with a colorimetric endpoint method using diagnostic reagent kits (Pointe Scientific Inc., Lincoln Park, MI) according to the manufacturer's protocol by using a Roche Cobas Mira Classic Chemistry Analyzer (Roche Diagnostics).
Isolation of Liver Mitochondria and Cytoplasm.
Liver mitochondrial and cytosolic fractions were isolated by differential centrifugation after removal of the nuclear fraction. In brief, livers were excised and homogenized in an isotonic buffer (10 mM HEPES, pH 7.8, 0.25 M sucrose, 1 mM EGTA, and 25 mM KCl) containing a protease inhibitor cocktail (Sigma) and 1 mM p-amidinophenyl methanesulfonyl fluoride by using a Dounce homogenizer. Homogenate was centrifugation at 1000g for 10 min, and the pellet representing nuclear fraction was removed. The resulting supernatant was centrifuged at 10,000g for 20 min to separate the mitochondrial (pellet) and cytosolic fractions (supernatant).
Hepatic Assays.
MnSOD activity in liver homogenates was measured by a cytochrome c reduction method using 1 mM potassium cyanide to inactivate copper-zinc SOD (SOD1) and extracellular SOD (SOD3) as described previously (McCord and Fridovich, 1969). GSH content in total liver homogenate, mitochondria, and a cytosolic fraction was determined by using a modification of the Elman procedure as described previously (Mitchell et al., 1973a).
Histology.
Livers samples were fixed in 10% neutral buffered formalin, processed, embedded into paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. Slides were examined by using an Olympus (Tokyo, Japan) BX40 microscope under bright-field illumination. Hemorrhage, hepatocellular vacuolization, and hepatocellular necrosis were scored from 0 (no lesion) to 4 (severe change), and lobular localization was noted for all lesions.
APAP-Cysteine Protein Adducts.
APAP-Cys adducts formed by covalent binding of NAPQI with liver proteins were measured by initial protease treatment of liver homogenates followed by a high-performance liquid chromatography-electrochemical detection method as described previously (Muldrew et al., 2002).
Western Blot Analysis.
Liver homogenate preparation and Western blot analysis were performed as described previously (Agarwal et al., 2011). In brief, after separation using SDS-polyacrylamide gel electrophoresis under reducing conditions, proteins were transferred to nitrocellulose membranes. Membranes were blocked 30 min for 4-HNE and overnight for nitrotyrosine (NT) in blocking buffer (5% milk in Tris-buffered saline and 0.1% Tween 20). The blocked immunoblots were incubated for 120 min with anti-4-HNE (1:1000) or anti-3-NT (1:500). Membranes for 3-NT were next incubated with peroxidase-labeled goat anti-mouse IgG, and for 4-HNE they were incubated with peroxidase-labeled rabbit anti-goat IgG for 60 min. Membranes were developed by using SuperSignal chemiluminescent substrate reagent, and the relative amount of protein in the blots was determined by densitometry analysis using a Fluorchem 8900 imaging densitometer (Alpha Innotech, San Leandro, CA). Subsequently, all blots were stripped, blocked for 30 min, and reprobed with anti-GAPDH (1:1000) or anti-actin (1:1000) for 120 min. Next, membranes for GAPDH were incubated with peroxidase-labeled goat anti-mouse IgG; for actin they were incubated with peroxidase-labeled goat anti-rabbit IgG for 60 min and visualized, and densitometric analysis was performed.
Statistical Analysis.
All animal experiments used four animals per treatment group. Toxicology results (increase in serum ALT levels as well as GSH assay) were confirmed by two different time-course experiments. Confirmation of analytical results was obtained by repeated analyses (two or three times). For statistical analyses Western blots of liver homogenates from each liver (100 μg) were performed, and the density of the blots was determined as described above. Data are reported as mean ± S.E and were analyzed by using one-way analysis of variance followed by the Tukey Honestly Significant Difference post hoc test. PASW Statistics Student version 18 (SPSS Inc., Chicago, IL) was used for statistical analyses. Results were considered statistically significant at p ≤ 0.05.
Results
Effect of a Toxic Dose of APAP on Biomarkers of Toxicity in WT and nNOS KO Mice.
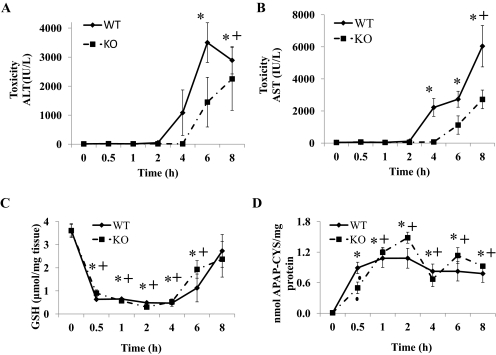
To understand the potential role of nNOS in APAP toxicity, WT and nNOS KO mice were administered a toxic dose of APAP (300 mg/kg i.p.). Control mice were treated with saline. In a preliminary experiment it was determined that the APAP-induced increases in serum ALT levels were significantly delayed in KO mice. At 6 h serum ALT levels in the WT mice were 7854 ± 2751 IU/liter, whereas in the KO mice serum ALT was significantly less at 2431 ± 609 IU/liter. However, by 8 h serum ALT levels were not different (4811 ± 572 IU/liter in WT mice and 4850 ± 362 IU/liter in KO mice). This experiment was repeated with more extensive analyses. Mice were again administered 300 mg/kg APAP and sacrificed at 0.5, 1, 2, 4, 6, and 8 h after APAP administration. The development of hepatotoxicity was observed by the determination of serum ALT and AST levels and histopathological evaluation. The time course for development of APAP-induced increases in serum levels of ALT is shown in Fig. 1A. Consistent with previous observations in WT mice, ALT levels were significantly increased at 6 and 8 h. In KO mice, ALT levels were not significantly increased until 8 h. By comparison in the KO mice, serum ALT levels paralleled those observed in the WT mice but seemed to be delayed. The serum ALT levels at 6 h in the KO mice were significantly less than those observed in the WT mice. Because an elevated serum AST level occurs with liver toxicity, AST levels were also determined in WT and KO mice (Fig. 1B). In WT mice, AST levels were significantly increased at 4, 6, and 8 h. In KO mice, AST levels showed a time-dependent increase but were only significantly increased at 8 h. AST levels in WT mice were significantly higher than that in KO mice at 4 and 8 h.
Fig. 1.
Time course for the effect of APAP on serum ALT, serum AST, hepatic GSH, and formation of APAP-cysteine adducts in WT and KO mice. Mice (n = 4) were treated with a 300 mg/kg dose of APAP and sacrificed at the indicated times, and serum and liver were collected. The 0 time is the saline-treated control mice in both WT and KO. A, ALT levels in serum (hepatotoxicity). B, AST levels in serum (hepatotoxicity). C, GSH levels in liver. D, APAP-cysteine adducts in liver. The data are presented as mean ± S.E. * and + indicate significant difference of WT and KO from their respective saline controls, and ▴ indicates a significant difference between WT and KO at a particular time point, p ≤ 0.05. Only two animals were used for study at 0.5 h in the KO group.
Hepatic GSH levels were determined to ascertain whether there were differences in its depletion in the APAP-treated WT and KO mice. In both WT and KO mice GSH levels were maximally depleted by 0.5 h and remained maximally depleted at 1, 2, and 4 h. At 8 h, GSH levels had recovered to levels comparable with that of saline-treated animals in the liver of both APAP-treated WT and KO mice. No significant differences in hepatic GSH levels were observed between WT and KO mice (Fig. 1C). Covalent binding of the reactive metabolite NAPQI to protein as 3-cystein-S-yl-acetaminophen or APAP-Cys is known to be a biomarker of toxicity that correlates with development of toxicity. Therefore, liver homogenates from WT and KO mice were analyzed for APAP-Cys by high-performance liquid chromatography-electrochemical detection. Adducts were detected at all time points after the administration of APAP (Fig. 1D). Comparison of the relative amount of APAP covalent binding between WT and KO mice showed a parallel time-dependent increase in APAP-Cys formation with no significant difference between the two groups. These data indicate that APAP covalently bound to protein at equal rates and amounts in both WT and KO mice. To determine whether delay in toxicity may be a result of a difference in mitochondrial GSH depletion another time-course experiment was performed. WT and KO mice were administered a toxic dose of APAP (300 mg/kg i.p.) and euthanized after 2 and 4 h. Control mice were treated with saline. Mitochondrial as well as cytosolic GSH levels were significantly depleted by 2 h and remained significantly depleted at 4 h in both WT and KO mice. GSH depletion was not significantly different for both mitochondrial and cytosolic fractions. Mitochondrial hepatic GSH levels were depleted by 70 ± 17% in KO mice at both 2 and 4 h and by 77 ± 13% in WT mice at both 2 and 4 h.
Comparison of a Toxic Dose of APAP to WT and nNOS KO Mice on Liver Pathology.
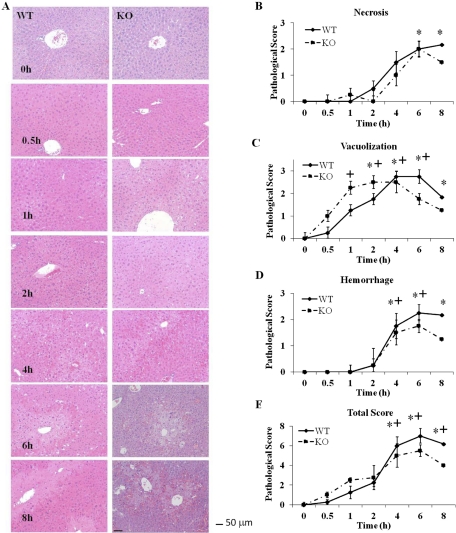
Necrosis was most obvious in centrilobular regions at 4, 6, and 8 h after APAP treatment, and statistically significant differences between WT and KO mice were not apparent at any time point (Fig. 2B). Necrosis was significantly elevated in WT animals compared with controls at 6 and 8 h. Hepatocellular vacuolization was first evident in three of four KO animals and one of four WT animals at 0.5 h in centrilobular hepatocytes (Fig. 2C). By 4 h vacuolization was more prominent in midzonal hepatocytes in both groups. Hemorrhage was first noted in the midzonal region at 2 h in both WT and KO animals and became more severe and moved to the centrilobular location at later time points (Fig. 2D). Hemorrhage scores were similar between KO and WT animals at all time points and were elevated for both groups compared with controls at 4 and 6 h. The hemorrhage score remained elevated at 8 h for WT animals compared with controls, but was decreased in KO animals at that time, although the decrease did not reach statistical relevance.
Fig. 2.
Histopathological analysis for hepatic necrosis in livers from APAP-treated WT and KO mice. Livers from mice (n = 4) were treated with acetaminophen (300 mg/kg) or saline control. A, hematoxylin and eosin staining. Liver sections were prepared at the indicated times and stained with hematoxylin and eosin. One representative picture is shown per time point. Magnification, 200×. Bar equals 50 μm. B, necrosis. C, vacuolization. D, hemorrhage. E, total score. The data are presented as mean ± S.E. * and + indicate significant difference of WT and KO from their respective saline controls at a particular time point, p ≤ 0.05.
Total additive pathology score for vacuolization, hemorrhage, and necrosis was significantly increased over 0 h at 4, 6, and 8 h for both KO and WT mice (Fig. 2E). Although scores tended to be higher for WT mice at these time points, low sample size precluded statistical relevance. A subsequent experiment was performed to determine toxicity at 24 h. In this experiment all of the KO mice died (four) and half of the WT mice (two of four) died. Thus, even though the toxicity was delayed it did not change the lethality of APAP at this dose.
Effect of a Toxic Dose of APAP on the Activity and Nitration of Hepatic MnSOD in WT and nNOS KO Mice.
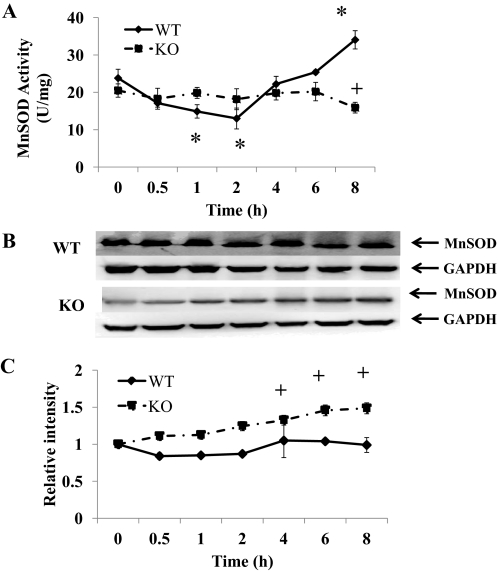
To understand the role of nNOS and nitration of MnSOD in APAP toxicity, MnSOD activity and relative amounts were determined in liver homogenates from KO and WT mice (Fig. 3). We reported previously that nitration of MnSOD results in a decrease in its activity (Agarwal et al., 2011). In the liver homogenates of the WT mice MnSOD activity was significantly decreased at 1 to 2 h with a nadir at 2 h and subsequently increased to control level at 6 h and significantly increased by 8 h (Fig. 3A). In the liver homogenates of the KO mice MnSOD activity was not significantly different from control levels from 0 to 6 h; however, at 8 h it significantly decreased. The amount of protein in the livers of WT mice did not change over the time course, but there was an increase in protein in the livers of the KO mice (Fig. 3, B and C).
Fig. 3.
Effect of APAP on MnSOD in livers of WT and KO mice. Mice (n = 4) were treated with 300 mg/kg dose of APAP and sacrificed at the indicated times, and liver was collected. The 0 time is the saline-treated control mice in both WT and KO. A, effect on MnSOD activity. B, Western blot analysis of combined homogenates for MnSOD in livers of WT and KO mice. GAPDH was used as a loading control. C, time course for MnSOD protein in livers of WT and KO mice. Western blot analysis was performed on each homogenate by using MnSOD antibody. GAPDH was used as a loading control. The data are presented as mean ± S.E. of the relative density of the 24-kDa MnSOD protein divided by the density of 38-kDa GAPDH protein. * and + indicate significant differences of WT and KO from respective saline, and ▴ indicates significant differences between WT and KO at particular time points, p ≤ 0.05.
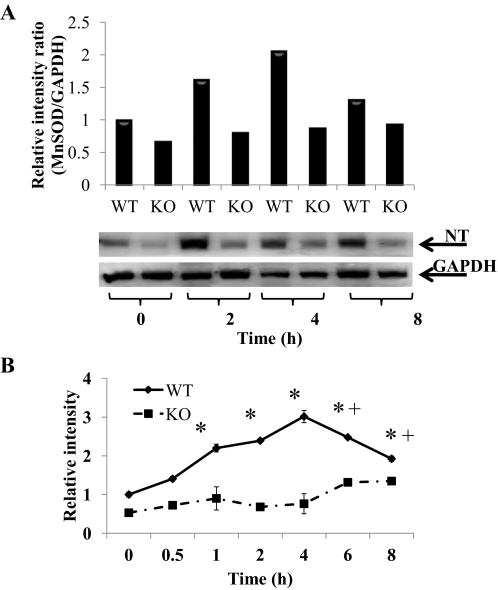
We previously presented evidence that decreased activity of hepatic MnSOD in APAP toxicity occurred with nitration. Thus, the data in Fig. 3A, showing greater MnSOD inactivation in WT mice compared with KO mice, suggested that there was significantly more MnSOD nitration in the liver of the APAP-treated WT mice than in the KO mice. Therefore, additional analyses were performed to determine nitration of hepatic MnSOD in the WT and KO mice (Fig. 4). The combined liver homogenates of the 0-, 2-, 4-, and 8-h groups of the WT and KO mice were analyzed for nitration of MnSOD on a single gel. As shown in Fig. 4A, there seems to be substantially more MnSOD nitration in the WT liver homogenate at 0, 2, 4, and 8 h compared with the liver homogenate from the KO mice. To confirm this finding Western blotting analyses were performed where all samples were analyzed on multiple blots with overlapping samples on the blots for comparison purposes (Fig. 4B), and the data were statistically analyzed. MnSOD nitration in the WT mice was significantly greater than in the KO mice at 1 to 6 h. In the WT mice liver homogenates nitration of MnSOD was significantly increased by 1 h after APAP administration and remained significantly higher until 8 h in WT mice. However, in the KO mice a significant increase in hepatic MnSOD nitration was not observed until 6 h and remained significantly high at 8 h.
Fig. 4.
Effect of APAP on nitration of MnSOD protein in livers of WT and KO mice. Mice (n = 4) were treated with 300 mg/kg dose of APAP and sacrificed at the indicated times, and liver was collected. The 0 time is the saline-treated control mice in both WT and KO. A, nitrated MnSOD (NT) levels in liver of WT and KO mice at 0, 2, 4, and 8 h. GAPDH was used as a loading control. The data are presented as the relative density of the 24-kDa MnSOD protein divided by the density of 38-kDa GAPDH protein. Representative gels are shown above the density values. B, time course for nitration of MnSOD protein in livers of WT and KO mice. GAPDH was used as a loading control. The data are presented as mean ± S.E. of the relative density of the 24-kDa MnSOD protein divided by the density of 38-kDa GAPDH protein. * and + indicate significant differences of WT and KO from respective saline control, and ▴ indicates significant differences between WT and KO at particular time points, p ≤ 0.05.
Effect of a Toxic Dose of APAP on Oxidative Stress in Liver in WT and nNOS KO Mice.
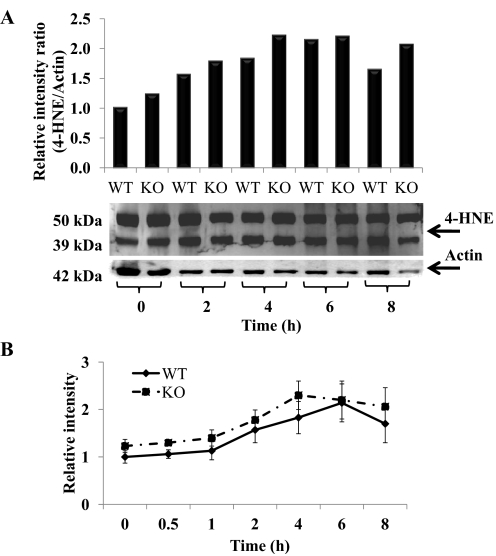
Oxidative stress also leads to lipid peroxidation, a major mechanism of cellular damage with some chemicals. 4-HNE is a lipid peroxidation-derived aldehyde that covalently binds to protein and has been used as a biomarker of lipid peroxidation (Esterbauer, 1996). To determine the role of lipid peroxidation Western blot analysis for 4-HNE covalent binding was performed by using anti-4-HNE. In the hepatic homogenates from the APAP-treated WT and KO mice two bands of 50 and 39 kDa were detected in the control lane and APAP-treated samples in both WT and KO mice (Fig. 5A). Densitometric analysis of the time course suggested an increase in 4-HNE protein adducts after APAP treatment in both WT and KO animals (Fig. 5B); however, no significant increases with time were observed. Even though there was smearing of bands at 6 and 8 h, densitometric analysis indicated that there was no significant difference between WT and KO (Fig. 5B).
Fig. 5.
Hepatic lipid peroxidation in APAP-treated WT and KO mice. Hepatic tissue was collected at the indicated time points from APAP-treated WT and KO mice. The 0 time is the saline-treated control mice in both WT and KO mice. Hepatic lipid peroxidation was assessed by 4-HNE Western blotting. A, 4-HNE levels in livers of WT and KO mice at 0, 2, 4, 6, and 8 h. Actin was used as a loading control. The data are presented as the relative ratio of the combined density of 50- and 39-kDa HNE proteins divided by the density of 42-kDa actin protein. Representative gels are shown above the density values. B, time course for lipid peroxidation in livers of WT and KO mice. Actin was used as a loading control. The data are presented as mean ± S.E. of the ratio of relative combined density of 50- and 39-kDa HNE proteins divided by the density of 42-kDa actin protein.
Assay of Liver Homogenates of WT and nNOS KO Mice for Total Protein Nitration.
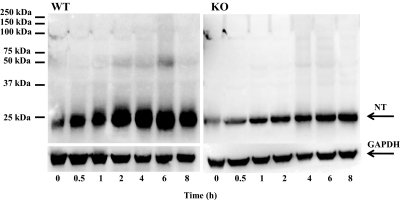
To examine the possible effect of nNOS deletion on nitration of proteins other than MnSOD, additional Western blot analyses were performed on homogenates of the livers from the APAP-treated WT and KO mice. As shown in Fig. 6, only one 3-nitrotyrosine protein adduct with a molecular mass the same as that of MnSOD (24 kDa) was detected in the livers of APAP-treated WT and KO mice.
Fig. 6.
Nitration of proteins in liver homogenates of APAP-treated WT (left) and KO (right) mice. Mice (n = 4) were treated with a 300 mg/kg dose of APAP and sacrificed at the indicated times, and livers were collected. The 0 time is the saline-treated control mice in both WT and KO mice. Hepatic homogenates were analyzed by Western blotting for the presence of nitrotyrosine (NT) protein adducts by using anti-3-nitrotyrosine antibody. GAPDH was used as a loading control. One representative gel is shown.
Discussion
The objective of the current study was to examine the role of nNOS in APAP-induced hepatotoxicity in mice by determining toxicity in nNOS KO and WT mice. After APAP treatment hepatotoxicity was detected in both KO and WT mice. In the WT mice serum ALT levels were significantly increased at 6 and 8 h (Fig. 1A) and AST was significantly increased at 4, 6, and 8 h (Fig. 1B). However, in KO mice increases in ALT and AST were significantly delayed (Fig. 1). These differences were not observed in the histopathological evaluation of toxicity (Fig. 2). Histopathological evaluation is the gold standard for toxicity; however, it is not as sensitive a measure of toxicity as the appearance of transaminases in serum. The finding that increases in serum ALT and AST levels were delayed suggests that the development of hepatic toxicity in the KO mice was delayed compared with the WT mice. Even though the hepatic toxicity as measured by transaminase elevations was delayed it did not convey a protective effect against lethality. In a 24-h experiment it was determined that all of the KO mice died; however, only half of the WT mice died.
Determination of the effect of APAP on MnSOD activity in the liver of WT mice indicated that its activity was significantly decreased at 1 and 2 h followed by a recovery phase at 4 to 8 h (Fig. 3A). These data are similar to what was observed previously (Agarwal et al., 2011). MnSOD activity in the KO mice was not significantly altered until 8 h at which time it was significantly decreased (Fig. 3). The relative amount of protein did not significantly change in the livers of the WT mice consistent with what we observed previously (Agarwal et al., 2011); however, the amount of protein in the livers of the KO mice increased slightly (Fig. 3, B and C). The combined liver homogenates in the time groups of 0, 2, 4, and 8 h of the WT and KO mice groups were analyzed on a single gel for nitrated proteins (Fig. 4A). There was significantly more nitrated MnSOD in the liver homogenates of WT mice than in the KO mice. Consistent with our previous observations, there was a background level of nitration in the nontreated mice (Agarwal et al., 2011), which suggests a background level of peroxynitrite formation. The differences were statistically analyzed by using multiple gels with overlapping samples (Fig. 4B). After APAP administration nitration was significantly increased in the livers of WT mice at 1 to 8 h but was significantly increased in KO mice only at 6 and 8 h. There was significantly more nitration in the WT mice compared with KO mice at 1 to 6 h (Fig. 4B). Thus, nNOS in hepatic mitochondria seems to be a major source of NO leading to nitration of MnSOD. However, nitration occurs in the hepatic mitochondria of KO mice, which is consistent with NO formed by other NOS forms in the liver such as endothelial NOS or iNOS (NO is freely diffusible). There was a significant increase in nitration of MnSOD in the liver homogenates of the KO mice at 6 and 8 h (Fig. 4B). This increase coincided with a significant decrease in MnSOD activity at 8 h (Fig. 3) and a significant increase in ALT in serum at 8 h (Fig. 1A). The increased nitration and the decreased MnSOD activity may be mechanistically important, and even in the KO mice the toxicity may be mediated by peroxynitrite. However, the alterations in hepatic MnSOD activity may not be completely a result of nitration but may have occurred by other mechanisms. Ozden et al. (2011) have shown that MnSOD activity is regulated by the reversible acetylation of specific, evolutionarily conserved lysines in the protein.
We and others have postulated a role for peroxynitrite in APAP hepatotoxicity (Hinson et al., 1998; James et al., 2003; Jaeschke et al., 2003). We found that in hepatocytes the nNOS inhibitor 7-nitroindazole inhibited APAP-induced nitration and toxicity (Burke et al., 2010). The finding that in APAP hepatotoxicity MnSOD is nitrated with loss of activity coupled with the finding that MnSOD heterozygote mice (Fujimoto et al., 2009) and MnSOD knockdown rats (Yoshikawa et al., 2009) are much more sensitive to APAP hepatotoxicity than the wild type further supports a role for peroxynitrite in the toxicity. MnSOD limits peroxynitrite formation, and with loss of activity upon nitration, additional peroxynitrite would be formed. These findings suggest that the inhibition of MnSOD activity in APAP toxicity contributes to the toxicity.
A critical question that has not been answered in this article is what is the mechanism of initiation of peroxynitrite formation? Conceivably either an increase in superoxide or NO may have been the initiating event. One possibility is that MPT may have been initiated by signal transduction events involving c-Jun NH2-terminal kinase (Kaplowitz et al., 2008). Alternatively, because nNOS can be induced by cytosolic calcium it is conceivable that increased cytosolic calcium was mechanistically important in toxicity, leading to increased NO and formation of peroxynitrite. Altered cytosolic calcium concentrations have been postulated to be important in APAP hepatotoxicity (Moore et al., 1985; Tirmenstein and Nelson, 1989; Boobis et al., 1990).
To more fully understand the oxidative stress in APAP toxicity hepatic lipid peroxidation in APAP toxicity in nNOS knockout mice was determined. Whereas peroxidation of lipids may occur by a Fenton reaction (ferrous plus peroxide), it may also be mediated by peroxynitrite (Rubbo et al., 1994). Lipid peroxidation has been reported to occur in APAP hepatotoxicity (Younes et al., 1986; Hinson et al., 2002); however, this is controversial (Kamiyama et al., 1993). Lipid peroxidation in the APAP-treated WT and KO mice was determined by assaying for 4-HNE covalent binding to liver homogenate. 4-HNE is produced by the peroxidation of lipids and binds to nucleophilic groups on proteins. Covalent binding of 4-HNE to proteins is a biomarker of lipid peroxidation (Esterbauer, 1996; Waeg et al., 1996). As shown in Fig. 5, 4-HNE covalent binding to protein did not significantly increase with time in the liver homogenates of either WT or KO mice. In addition, there were no significant differences observed between 4-HNE binding in the livers of WT and KO mice. Thus, there is no evidence that nNOS contributes to this mechanism of oxidative stress in APAP toxicity.
Previously, we reported one major nitrated protein, presumably MnSOD, in hepatic homogenates of APAP-treated iNOS knockout and WT mice; however, we did observe a number of minor nitrated proteins (Michael et al., 2001). Abdelmegeed et al., (2010) administered CYP2E1 KO mice and the corresponding WT mice a toxic dose of APAP and reported major nitrated proteins at, 60, 50, and 25 kDa and a number of minor nitrated proteins. The relative amount of nitration correlated with toxicity. Thus, the finding of only one primary nitrated protein at 24 kDa (MnSOD) in this study was surprising. However, peroxynitrite is not only a nitrating agent; it is an oxidizing agent, and oxidative mechanisms may be important in toxicity. For example, Schöpfer et al. (2000) have pointed out that electron transfer in the mitochondria via ubihydroquinone (ubiquinol) can be oxidized by peroxynitrite, leading to formation of the semiquinone. The semiquinone can react with oxygen, forming superoxide. This leads to disruption of electron transfer. By this mechanism initiation of toxicity would be a result of induction of NO synthesis, which reacts with superoxide to form peroxynitrite, and GSH depletion, which normally detoxifies peroxynitrite. Thus, the reaction is self-propagating. In support of this mechanism, Amimoto et al. (1995) showed a time-dependent decrease in hepatic-reduced coenzyme Q9 and Q10 (ubihydroquinones) after administration of toxic doses of APAP to mice. Thus, both nitration and oxidation mechanisms may play an important role in APAP toxicity.
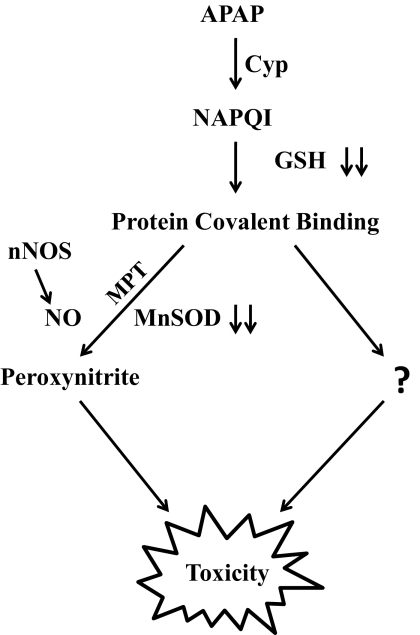
In conclusion, the finding that nitration of MnSOD is much greater in livers of APAP-treated WT mice than in livers of the KO mice establishes a role for nNOS in the nitration of MnSOD in APAP toxicity. These data are consistent with nNOS being a mitochondrial protein. In addition, the nitration of MnSOD observed in the liver of APAP-treated nNOS KO mice is evidence for other NOS forms contributing to protein nitration. The delay in the onset of toxicity (serum ALT and AST) in the nNOS KO mice compared with the WT mice suggests that nNOS plays an important role in initiation of toxicity. However, the observation that there was a similar amount of toxicity by 8 h in the WT and KO mice suggests that, although nitration and inactivation of MnSOD was delayed, alternative mechanisms are also important, which may lead to toxicity after APAP treatment, and these mechanisms are independent of nNOS. As discussed above, one possible mechanism for the activation of nNOS is by increased cytosolic calcium. In the absence of nNOS the increased cytosolic calcium may produce toxicity by other mechanisms. The postulated mechanism of APAP toxicity is presented in Fig. 7.
Fig. 7.
Postulated mechanism of APAP toxicity.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants R01-DK079008, R01-DK059872, R01-DK75936].
This work was presented in part previously: Agarwal R, Hennings L, Rafferty TM, Letzig LG, McCullough S, James LP, MacMillan-Crow LA, and Hinson JA (2011) Acetaminophen (APAP)-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase (nNOS) knockout mice, at Experimental Biology Meeting; 2011 April 9–13; Washington DC. American Society for Pharmacology and Experimental Therapeutics, Bethesda, MD.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- APAP
- acetaminophen
- APAP-Cys
- 3-cystein-S-yl-acetaminophen
- NAPQI
- N-acetyl-p-benzoquinone imine
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- NO
- nitric oxide
- nNOS
- neuronal NO synthase (NOS1)
- iNOS
- inducible NO synthase (NOS2)
- SOD
- superoxide dismutase
- MnSOD
- manganese SOD (SOD2)
- MPT
- mitochondrial permeability transition
- WT
- wild type
- KO
- knockout
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- 4-HNE
- 4-hydroxynonenal
- NT
- nitrotyrosine.
Authorship Contributions
Participated in research design: Agarwal, Rafferty, James, and Hinson.
Conducted experiments: Agarwal, Rafferty, Letzig, McCullough, and Hinson.
Performed data analysis: Agarwal, Hennings, Rafferty, and Hinson.
Wrote or contributed to the writing of the manuscript: Agarwal, Hennings, James, MacMillan-Crow, and Hinson.
References
- Abdelmegeed MA, Moon KH, Chen C, Gonzalez FJ, Song BJ. (2010) Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochem Pharmacol 79:57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA. (2011) Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther 337:110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amimoto T, Matsura T, Koyama SY, Nakanishi T, Yamada K, Kajiyama G. (1995) Acetaminophen-induced hepatic injury in mice: the role of lipid peroxidation and effects of pretreatment with coenzyme Q10 and α-tocopherol. Free Radic Biol Med 19:169–176 [DOI] [PubMed] [Google Scholar]
- Boobis AR, Seddon CE, Nasseri-Sina P, Davies DS. (1990) Evidence for a direct role of intracellular calcium in paracetamol toxicity. Biochem Pharmacol 39:1277–1281 [DOI] [PubMed] [Google Scholar]
- Boobis AR, Tee LB, Hampden CE, Davies DS. (1986) Freshly isolated hepatocytes as a model for studying the toxicity of paracetamol. Food Chem Toxicol 24:731–736 [DOI] [PubMed] [Google Scholar]
- Bourdi M, Eiras DP, Holt MP, Webster MR, Reilly TP, Welch KD, Pohl LR. (2007) Role of IL-6 in an IL-10 and IL-4 double knockout mouse model uniquely susceptible to acetaminophen-induced liver injury. Chem Res Toxicol 20:208–216 [DOI] [PubMed] [Google Scholar]
- Brookes PS. (2004) Mitochondrial nitric oxide synthase. Mitochondrion 3:187–204 [DOI] [PubMed] [Google Scholar]
- Burke AS, MacMillan-Crow LA, Hinson JA. (2010) Reactive nitrogen species in acetaminophen-induced mitochondrial damage and toxicity in mouse hepatocytes. Chem Res Toxicol 23:1286–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne AM, Lemasters JJ, Nieminen AL. (1999) Contribution of increased mitochondrial free Ca2+ to the mitochondrial permeability transition induced by tert-butylhydroperoxide in rat hepatocytes. Hepatology 29:1523–1531 [DOI] [PubMed] [Google Scholar]
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. (1984) N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A 81:1327–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H. (1996) Estimation of peroxidative damage. A critical review. Pathol Biol (Paris) 44:25–28 [PubMed] [Google Scholar]
- Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, Yamoto T, Manabe S. (2009) Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol 37:193–200 [DOI] [PubMed] [Google Scholar]
- Ghafourifar P, Cadenas E. (2005) Mitochondrial nitric oxide synthase. Trends Pharmacol Sci 26:190–195 [DOI] [PubMed] [Google Scholar]
- Grewal KK, Racz WJ. (1993) Intracellular calcium disruption as a secondary event in acetaminophen-induced hepatotoxicity. Can J Physiol Pharmacol 71: 26–33 [DOI] [PubMed] [Google Scholar]
- Hinson JA, Bucci TJ, Irwin LK, Michael SL, Mayeux PR. (2002) Effect of inhibitors of nitric oxide synthase on acetaminophen-induced hepatotoxicity in mice. Nitric Oxide 6:160–167 [DOI] [PubMed] [Google Scholar]
- Hinson JA, Pike SL, Pumford NR, Mayeux PR. (1998) Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol 11:604–607 [DOI] [PubMed] [Google Scholar]
- Hoffmann KJ, Streeter AJ, Axworthy DB, Baillie TA. (1985) Identification of the major covalent adduct formed in vitro and in vivo between acetaminophen and mouse liver proteins. Mol Pharmacol 27:566–573 [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Jaeschke H, Knight TR, Bajt ML. (2003) The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett 144:279–288 [DOI] [PubMed] [Google Scholar]
- James LP, Mayeux PR, Hinson JA. (2003) Acetaminophen-induced hepatotoxicity. Drug Metab Dispos 31:1499–1506 [DOI] [PubMed] [Google Scholar]
- Kamiyama T, Sato C, Liu J, Tajiri K, Miyakawa H, Marumo F. (1993) Role of lipid peroxidation in acetaminophen-induced hepatotoxicity: comparison with carbon tetrachloride. Toxicol Lett 66:7–12 [DOI] [PubMed] [Google Scholar]
- Kaplowitz N, Shinohara M, Liu ZX, Han D. (2008) How to protect against acetaminophen: don't ask for JUNK. Gastroenterology 135:1047–1051 [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. (2004) Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40:1170–1179 [DOI] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. (1996) Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A 93:11853–11858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Cruthirds DL. (2001) Invited review: manganese superoxide dismutase in disease. Free Radic Res 34:325–336 [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055 [PubMed] [Google Scholar]
- Michael SL, Mayeux PR, Bucci TJ, Warbritton AR, Irwin LK, Pumford NR, Hinson JA. (2001) Acetaminophen-induced hepatotoxicity in mice lacking inducible nitric oxide synthase activity. Nitric Oxide 5:432–441 [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. (1973a) Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther 187:185–194 [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. (1973) Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 187:211–217 [PubMed] [Google Scholar]
- Moore M, Thor H, Moore G, Nelson S, Moldéus P, Orrenius S. (1985) The toxicity of acetaminophen and N-acetyl-p-benzoquinone imine in isolated hepatocytes is associated with thiol depletion and increased cytosolic Ca2+. J Biol Chem 260:13035–13040 [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. (2002) Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos 30:446–451 [DOI] [PubMed] [Google Scholar]
- Ozden O, Park SH, Kim HS, Jiang H, Coleman MC, Spitz DR, Gius D. (2011) Acetylation of MnSOD directs enzymatic activity responding to cellular nutrient status or oxidative stress. Aging 3:102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. (2005) Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther 312:509–516 [DOI] [PubMed] [Google Scholar]
- Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. (1994) Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 269:26066–26075 [PubMed] [Google Scholar]
- Schöpfer F, Riobó N, Carreras MC, Alvarez B, Radi R, Boveris A, Cadenas E, Poderoso JJ. (2000) Oxidation of ubiquinol by peroxynitrite: implications for protection of mitochondria against nitrosative damage. Biochem J 349:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz DB, Zamora R, Vodovotz Y, Loughran PA, Billiar TR, Kim YM, Simmons RL, Watkins SC. (2002) Peroxisomal localization of inducible nitric oxide synthase in hepatocytes. Hepatology 36:81–93 [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. (1989) Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3′-hydroxyacetanilide, in mouse liver. J Biol Chem 264:9814–9819 [PubMed] [Google Scholar]
- Waeg G, Dimsity G, Esterbauer H. (1996) Monoclonal antibodies for detection of 4-hydroxynonenal modified proteins. Free Radic Res 25:149–159 [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Morita M, Hosomi H, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. (2009) Knockdown of superoxide dismutase 2 enhances acetaminophen-induced hepatotoxicity in rat. Toxicology 264:89–95 [DOI] [PubMed] [Google Scholar]
- Younes M, Cornelius S, Siegers CP. (1986) Ferrous ion supported in vivo lipid peroxidation induced by paracetamol–its relation to hepatotoxicity. Res Commun Chem Pathol Pharmacol 51:89–99 [PubMed] [Google Scholar]
- Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. (2000) Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192:1001–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]