Abstract
Transient receptor potential cation channel subfamily V member 1 (TRPV1) is a high-conductance, nonselective cation channel strongly expressed in nociceptive primary afferent neurons of the peripheral nervous system and functions as a multimodal nociceptor gated by temperatures greater than 43°C, protons, and small-molecule vanilloid ligands such as capsaicin. The ability to respond to heat, low pH, vanilloids, and endovanilloids and altered sensitivity and expression in experimental inflammatory and neuropathic pain models made TRPV1 a major target for the development of novel, nonopioid analgesics and resulted in the discovery of potent antagonists. In human clinical trials, observations of hyperthermia and the potential for thermal damage by suppressing the ability to sense noxious heat suggested that full-scale blockade of TRPV1 function can be counterproductive and subtler pharmacological approaches are necessary. Here we show that the dihydropyridine derivative 4,5-diethyl-3-(2-methoxyethylthio)-2-methyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (MRS1477) behaves as a positive allosteric modulator of both proton and vanilloid activation of TRPV1. Under inflammatory-mimetic conditions of low pH (6.0) and protein kinase C phosphorylation, addition of MRS1477 further increased sensitivity of already sensitized TPRV1 toward capsaicin. MRS1477 does not affect inhibition by capsazepine or ruthenium red and remains effective in potentiating activation by pH in the presence of an orthosteric vanilloid antagonist. These results indicate a distinct site on TRPV1 for positive allosteric modulation that may bind endogenous compounds or novel pharmacological agents. Positive modulation of TRPV1 sensitivity suggests that it may be possible to produce a selective analgesia through calcium overload restricted to highly active nociceptive nerve endings at sites of tissue damage and inflammation.
Introduction
TRPV1 is a key ion channel for sensing noxious stimuli, is activated by capsaicin, protons or heat, and is highly expressed in nociceptive Aδ and C-fiber sensory afferent neurons of the peripheral nervous system (Caterina et al., 1997; Tominaga et al., 1998; Mitchell et al., 2010). These fibers are responsible for both the initiation and the transmission of nociceptive signals, and pathological pain states often correlate with their altered functioning (Gracely et al., 1992; Costigan et al., 2009; Bruehl, 2010). Increased amounts of TRPV1 in nerve endings at sites of neurogenic inflammation and in models of neuropathic pain and sensitization of TRPV1 by nerve growth factor released from inflamed tissues are consistent with the idea that TRPV1 plays a key role in chronic pain and hyperalgesia (Cortright and Szallasi, 2004). Observations that TRPV1 agonists such as RTX or capsaicin can either outright ablate (Karai et al., 2004; Brown et al., 2005) or render nociceptive afferent nerve endings unresponsive (Neubert et al., 2003) to a broad palette of noxious stimuli led to the development of therapeutic approaches by localized delivery. These include intrathecal administration of RTX to control pain in advanced cancer (Karai et al., 2004) and the topical use of high concentrations of capsaicin for postherpetic neuralgia (Backonja et al., 2008), complex regional pain syndrome (Robbins et al., 1998), and experimental inflammatory or neuropathic pain models (Nolano et al., 1999; Neubert et al., 2003; Bates et al., 2010).
Blockade of TRPV1 at the orthosteric capsaicin site also has therapeutic potential, and considerable effort has been directed to development of orally available TRPV1 antagonists as analgesics by the pharmaceutical industry and academia (Wong and Gavva, 2009). These efforts spanning the last decade, although producing extraordinarily potent and specific antagonists, fell short of producing the expected analgesic drugs because of side effects such as unpredictable levels of hyperthermia and whole-body suppression of thermosensation in the 48–50°C range, increasing the chance of burns associated with their use (Wong and Gavva, 2009; Rowbotham et al., 2011). Structural modifications to produce peripherally restricted antagonists and thereby eliminate potential actions on central nervous system thermoregulatory mechanisms did not solve the problem of hyperthermia (Tamayo et al., 2008). The aforementioned problems underscore the necessity of exploring novel pharmacological approaches in the development of TPRV1-targeted analgesics.
Allosteric agonism is a well known quality of TRPV1 because protons and small-molecule ligands with a vanilloid moiety activate the ion channel at spatially distinct and structurally divergent domains (Gavva et al., 2005; Ryu et al., 2007). Moderate proton concentrations of a slightly acidic extracellular milieu (pH ∼6.0) will not gate the channel but will increase its sensitivity toward vanilloid ligands (Jordt et al., 2000), an example of positive allosteric modulation. Thus, a logical next step is to explore existing chemical libraries for compounds that are allosteric modulators of TRPV1, rather than direct inhibitors of the orthosteric capsaicin binding site. Here we characterize in detail the 1,4-dihydropyridine (DHP)-derived compound 4,5-diethyl-3-(2-methoxyethylthio)-2-methyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (MRS1477) (Supplemental Fig. 1), previously identified as an enhancer of capsaicin activation of TRPV1 (Roh et al., 2008). We show that it is a generalized potentiator of vanilloid and/or proton activation of TRPV1. Studies using different classes of TRPV1 inhibitors suggest that this compound (MRS1477) does not interact directly with ligands targeting the vanilloid binding site or with the channel pore. We propose that MRS1477 is a proof of concept compound showing the feasibility of small-molecule positive allosteric modulation of TRPV1. The results also suggest the presence of sites on TRPV1 that exhibit activity- or state-dependent properties and potentially novel analgesic pharmacology.
Materials and Methods
Cell Culture.
The HEK293 cell line expressing rat TRPV1 (HEK293-TRPV1) (Caterina et al., 1997) was maintained following American Type Culture Collection (Manassas, VA) guidelines for HEK293 cells, supplemented with 200 μg/ml G418 sulfate.
45Ca2+ Uptake Assays.
Cells were seeded a day before the experiment to 96-well plates at a density of 4 × 104 cells/well. Drugs were diluted on separate 96-well plates using 45Ca2+-containing (0.5 μCi/well) buffer in a total volume of 100 μl/well. All experiments followed the same pattern and were conducted using a Biomek FX liquid handling robot (Beckman Coulter, Fullerton, CA): 1) removing cell culture medium, 2) washing the cells using assay buffer, 3) simultaneous transfer of drugs and 45Ca2+ to all 96 wells, 4) 5 to 8 min of incubation at room temperature, depending on agonist, 5) removing 45Ca2+ and drugs, 6) washing the cells, 7) lysis in hypotonic lysis buffer (1% Triton X-100 and 1% SDS), 8) transfer of 75 μl of lysate to a 96-well OptiPlate (white, PerkinElmer Life and Analytical Sciences, Waltham, MA) already containing 125 μl of MicroScint-40 (PerkinElmer Life and Analytical Sciences) scintillation cocktail. We used a TopCount NX (PerkinElmer Life and Analytical Sciences) liquid scintillation counter to quantify the 45Ca2+signal. Treatment and control experiments were run on the same plate; we also included a full, six- to seven-point capsaicin dose-response curve for within-plate normalization and to check the plate-to-plate consistency of our data.
Intracellular Ca2+ Imaging.
Cells were seeded on a coverslip the day before the experiment. A 4 μM solution of the ratiometric Ca2+ dye Fura4F-AM was used to load cells for 45 min at room temperature, followed by a 15-min period to permit complete cleavage of the AM groups from the dye. The imaging apparatus consisted of an Olympus BX60 microscope equipped with a Hamamatsu ORCA 12-bit monochrome charge-coupled device camera. Illumination was provided by a Lambda LS xenon arc lamp light source, and switching between excitation and emission filters was performed with a Lambda 10-2 filter wheel controller, all from Sutter Instrument Company (Novato, CA). Coverslips with cells were mounted on the microscope stage using the p21B perfusion chamber and P2 platform (both from Warner Instruments, Hamden, CT) and then imaged using the Olympus UApo/340 20× objective. Image acquisition and filter switching during the experiment were controlled through the MetaFluor (Molecular Devices, Sunnyvale, CA) software package. Perfusion speed was 0.732 ml/min using a Minipuls 3 peristaltic pump (Gilson, Inc., Middleton, WI); drug injection was done by manual operation of a high-performance liquid chromatography valve linked to a 0.5-ml sample loop.
Electrophysiology.
HEK293-TRPV1 cells were plated on 35-mm dishes at a density of 105 cells/dish. Electrophysiological experiments were performed on cells at room temperature using the whole-cell patch-clamp recording technique. Currents were recorded using an Axopatch 200B patch clamp amplifier (Molecular Devices) and were filtered at 2 kHz using a low-pass Bessel filter. Patch electrodes were fabricated from borosilicate glass (type 1B150F3; World Precision Instruments, Inc., Sarasota, FL) on a Brown Flaming horizontal puller (P87; Sutter Instrument Company) and heat-polished to a final tip resistance of 2 to 4 MΩ. All current records were captured and stored using the pClamp9 software packages in conjunction with a Digidata 1322A analog-to-digital converter (Molecular Devices). Patch electrodes were filled with a solution containing the following: 142 mM NaCl, 1 mM MgCl2, 10 mM EGTA, and 10 mM HEPES; pH was adjusted to 7.35 using 10 M NaOH. The osmolarity of the internal solutions was 306 mOsM. The bath solution contained the following: 148 mM NaCl, 3 mM KCl, 2 mM MgCl2, 10 mM glucose, and 10 mM HEPES; pH was adjusted to 7.35 using 10 M NaOH. No calcium was included in the extracellular solution to prevent receptor desensitization. The osmolarity of this solution was 295 to 305 mOsM. Capsaicin, MRS1477, and acidic solutions were prepared daily in bath buffer and applied using a fast gravity-driven rapid solution changer (RSC-200; Bio-Logic SAS, Claix, France). The current responses were recorded from single cells clamped at −60 mV or in 3-s voltage ramps ranging from −80 to +80 mV.
Core Body Temperature Measurements.
Procedures followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the National Institute of Dental and Craniofacial Research Animal Care and Use Committee. Core body temperature was measured using intraperitoneal implanted telemetric temperature probes (Data Sciences International, St. Paul, MN), and the telemeter signal was processed using a model RPC-1 receiver, data exchange matrix, and DATA Quest ART acquisition system (Data Sciences International). Telemetry devices were placed in the abdominal cavity of C57BL/6 mice (2–4 months old, 20–30 g) as described previously (Mishra et al., 2011). Experiments were initiated at least 2 weeks after surgery. Drugs were administered by intraperitoneal injection in a solution of 10% ethanol and 0.5% Tween in physiological saline. To facilitate dispersion, MRS1477 was sonicated before injection.
Solutions and Buffers.
The assay buffer used for 45Ca2+ assays contained 1× Ca2+, Mg2+-free Hanks' balanced salt solution (diluted from 10× Hanks' balanced salt solution, Gibco 14815; Invitrogen, Carlsbad, CA) supplemented with 0.8 mM MgCl2 and 10 mM glucose with the pH set to 7.4 using 10 mM HEPES. The same buffer was used for diluting drugs and 45Ca2+. When pH gradients for proton activation experiments were prepared, an unbuffered physiological salt solution was made consisting of 138 mM NaCl, 5.33 mM KCl, 0.8 mM MgCl2, 10 mM glucose, and no buffering agent; pH was set by mixing the sodium salt of HEPES and MES hydrate to a total concentration of 10 mM. Osmolality of the buffers was always set to 325mOsM using sucrose. All solutions containing capsaicin also contained 1 mM ascorbic acid to protect it from oxidation. For intracellular Ca2+ imaging, the assay buffer was supplemented with 2.5 mM CaCl2.
Drugs and Stock Solutions.
Capsaicin (Sigma-Aldrich, St. Louis, MO) was prepared as a 10 mM stock solution in 100% ethanol; 1 to 2 mM intermediate concentrations were prepared fresh on the day of the experiments in 75% ethanol and 2 mM ascorbic acid. This solution was used to prepare working concentrations of capsaicin (1–2 μM, ∼0.08% ethanol), which were always discarded at the end of the day; the ascorbic acid was contributed by the assay buffer (see above). NADA (Tocris Bioscience, Ellisville, MO) was prepared as a 12 mM stock solution and resiniferatoxin (LC Laboratories, Woburn, MA) was prepared as a 3.18 mM stock solution, both in 100% ethanol. Ruthenium red (RR) (Tocris Bioscience) was prepared as a 50 mM stock solution in Ultra Pure Water (K · D Medical, Columbia, MD). MRS1477 (Roh et al., 2008), capsazepine (CPZ) (Tocris Bioscience), and phorbol 12-myristate 13-acetate (PMA) (Tocris Bioscience) stock solutions were prepared at 20, 50, and 50 mM concentrations, respectively, in 100% DMSO. Drugs used in 45Ca2+ uptake, intracellular calcium imaging, and electrophysiology experiments were diluted from these stock solutions at least 500-fold, with final DMSO and ethanol concentrations not exceeding 0.15% for DMSO and 0.08% for ethanol in buffers during experiments. The highest concentrations of MRS1477 in the above vehicle produced no effect on calcium uptake (Supplemental Fig. 1).
Statistical Analyses.
Student's t test was used for pairwise comparisons, and one-way analysis of variance was used for multiple comparisons with the Bonferroni post hoc test except for the analysis of voltage-ramp results for which Dunnett's post hoc test was used. Differences were considered significant when P < 0.05. Curve fit of dose-response experiments was done using Prism 5.0 (GraphPad Software Inc., San Diego, CA) least-squares fit.
Results
MRS1477 Positively Modulates Both Vanilloid and Proton Activation of TRPV1.
1,4-DHP compounds are known to interact with calcium channels (Triggle et al., 1989), and MRS1477 was identified as a small-molecule potentiator of TRPV1 activation by capsaicin upon screening a library of 1,4-DHP compounds (Roh et al., 2008). In the present study our initial objectives were to probe the generality of the TRPV1-enhancing effect by 1) expanding the range of vanilloid ligands and 2) determining whether proton activation of TRPV1 could also be positively modulated. For this, 45Ca2+ uptake assays were conducted using HEK293-TRPV1 cells) (a kind gift from Dr. M. Caterina). We subsequently explored combinations of inflammatory-mimetic agents and the pharmacological actions of MRS1477 using electrophysiological, cell biological, and in vivo approaches.
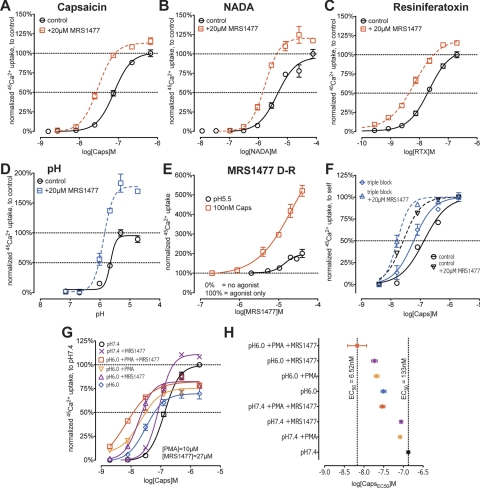
Capsaicin-induced calcium uptake through TRPV1 shows sensitivity enhancement with a decrease in the EC50 of capsaicin from 77.7 ± 3.72 nM, HS 1.97 ± 0.19 nM to 30.2 ± 1.46 nM, HS 2.01 ± 0.19 nM in the presence of 20 μM MRS1477 (Fig. 1A). Potentiation of vanilloid agonist activity is a generalized feature of MRS1477 that is independent of agonist potency because both NADA, a proposed endogenous ligand with low potency (Fig. 1B), and the ultra-potent agonist RTX (Fig. 1C) show comparable potentiation in the presence of MRS1477, reducing their respective EC50 values from 4.48 ± 0.66 μM, HS 1.34 ± 0.07 μM and 21.5 ±1.82 nM, HS 1.33±0.08 nM to 1.71 ± 0.13 μM, HS 1.72 ± 0.2 μM and 6.59 ± 0.57 nM, HS 1.09 ± 0.05 nM, respectively. Besides sensitization, MRS1477 also produces a small but consistent increase in the maximum response of TRPV1 at saturating doses of vanilloid agonists (∼10% enhancement in the presence of 20 μM MRS1477) (Fig. 1, A–C).
Fig. 1.
MRS1477 positively modulates vanilloid and proton activation of TRPV1 when studied using 45Ca2+ uptake of HEK293-TRPV1 cells. A, capsaicin activation undergoes sensitization with a slight increase in 45Ca2+ uptake at saturating concentrations of agonists in the presence of 20 μM MRS1477. B, activation of TRPV1 by NADA shows a similar enhancement in the presence of MRS1477. C, positive modulation of TRPV1 sensitivity toward vanilloid ligands is also observed when the ultra-potent agonist RTX is used. D, the potentiation of proton activation rather resembles efficacy modulation: the EC50 value for proton activation changes only slightly whereas maximum 45Ca2+ uptake almost doubles, compared with that of controls. E, modulation by MRS1477 reaches saturation at ∼20 μM concentration when enhancing proton activation, whereas enhancement of capsaicin activation is limited only by the solubility of the test compound. Potentiation by MRS1477 is independent of major calcium efflux mechanisms. F, inhibition of major plasma membrane Ca2+ pumps and channels resulted in a left shift of the capsaicin dose-response curve (control versus triple block control). This apparent sensitization did not interfere with modulation by MRS1477 that remained comparable both under control (control versus +20 μM MRS1477) and triple block conditions (triple block control versus triple block + 20 μM MRS1477). Positive modulation by MRS1477 is independent of sensitization by protons or serine phosphorylation. G, TRPV1 is sensitized toward vanilloid ligands by slightly acidic pH (pH 7.4 versus pH 6.0). This sensitization is further enhanced both by the PKC activator PMA (pH 6.0 + PMA) and by MRS1477 (pH 6.0 + MRS1477). The positive modulation by these three agents (pH 6.0 + PMA + MRS1477) is cumulative. H, EC50 values for capsaicin in the presence of various positive modulators and their combinations, as shown in G.
Proton activation of TRPV1 is also enhanced by MRS1477: calcium uptake is progressively potentiated as pH decreases, producing between 90 and 80% enhancement of Ca2+ uptake at pH values of 5.5 and 4.8, respectively (Fig. 1D). The EC50 value for proton activation also shows a small but significant leftward shift from pH 5.65 ± 0.03 under control conditions to pH 5.87 ± 0.03 in the presence of 20 μM MRS1477; however, the effect resembles an increase in efficacy rather than a change in sensitivity as seen with vanilloid agonists.
Dose-response studies performed with MRS1477 also reveal modality-dependent differences in potentiation. Low micromolar concentrations of MRS1477 produced potentiation of a fixed concentration (100 nM) of capsaicin. The potentiation exhibited a steady increase, with no sign of saturation, up to the maximum MRS1477 concentration of 40 μM (100 nM Caps, EC50 22.4 ± 14.1 μM; Fig. 1E). The potentiation observed upon activation by protons (pH 5.5) requires slightly higher concentrations of MRS1477 and the potentiation shows saturation for doses greater than 20 μM (pH 5.5, EC50 14.2 ± 2.82 μM; Fig. 1E). In the absence of TRPV1 agonists, MRS1477 did not elicit 45Ca2+ uptake at any concentration (Supplemental Fig. 2).
MRS1477 Potentiation Is Not Influenced by Calcium Efflux Mechanisms.
The generalized positive modulation of TRPV1 activation, regardless of the vanilloid compound or modality of activation used, raised the question of whether the action was directly on TRPV1 or indirectly on molecules such as calcium pumps. The 1,4-DHP structure is a chemical scaffold for L-type calcium channel antagonists (Triggle et al., 1989), and we conducted several experiments to determine whether the enhanced 45Ca2+ uptake observed was due to off-target inhibition of the plasma membrane Ca2+-ATPase (PMCA) and sodium-calcium exchanger (NCX) that could result in increased retention of 45Ca2+ after TRPV1 activation. We performed 45Ca2+ uptake assays under “triple block” conditions, simultaneously inhibiting the majority of calcium efflux mechanisms of HEK293-TRPV1 cells. Our triple block buffer was a modified assay buffer in which NaCl was replaced with LiCl (to block NCX), pH was set to 9.0 (to reduce PMCA efficiency), and 1 μM Gd3+ was added to inhibit capacitative calcium entry (Duman et al., 2008). Although we observed an expected leftward shift in the capsaicin dose-response curve when inhibiting calcium efflux using triple block conditions (control versus triple block, EC50 values: 132.7 ± 14.9 nM, HS 1.11 ± 0.13 nM versus 59.6 ± 7.13 nM, HS 1.29 ± 0.19 nM; Fig. 1F), the degree of potentiation by MRS1477 is unaffected and remains comparable between normal and blocked conditions (Fig. 1F).
Sensitization by Serine Phosphorylation and Extracellular Protons Is Further Enhanced by MRS1477.
Serine phosphorylation by protein kinase C (PKC) on residues S502 and S800 sensitizes TRPV1(Mandadi et al., 2006) and produces a leftward shift of the capsaicin dose-response curve similar to that with MRS1477 (Bhave et al., 2003). Likewise, a low concentration of extracellular protons corresponding to pH ∼6.0 will not activate the receptor but will sensitize it to vanilloid ligands (Jordt et al., 2000). To determine whether positive modulation by protons, serine phosphorylation, and MRS1477 produce additive effects or share mutually exclusive mechanisms, we compared the ability of MRS1477 to sensitize TRPV1 in the presence or absence of PMA, both at neutral and at slightly acidic pH (pH 6.0). We found that MRS1477 is as effective in potentiating capsaicin-stimulated 45Ca2+ uptake at either pH 7.4 (compare pH 7.4 versus pH 7.4 + MRS1477; Fig. 1G) or pH 6.0 (compare pH 6.0 versus pH 6.0 + MRS1477; Fig. 1G). Potentiation of vanilloid activation by serine phosphorylation appeared independent of and additive with pH 6.0 sensitization in the 45Ca2+ uptake assay, resulting in a reduction of capsaicin EC50 values from 133 nM at pH 7.4 through 31.4 nM at pH 6.0 to 21 nM at pH 6.0 with 10 μM PMA present. This reduction could be further enhanced by the addition of MRS1477, reducing the EC50 of capsaicin to 6.52 nM (pH 6.0 + PMA + MRS1477; Fig. 1G). Figure 1H is a graphic depiction of the EC50 shifts in the combination conditions tested in Fig. 1G (detailed curve-fit data are presented in Supplemental Table 1), illustrating enhanced TRPV1 sensitivity to the same concentration of vanilloid agonist with the progressive combination of low pH, serine phosphorylation, and MRS1477. These data suggest the possibility that the addition of a positive allosteric modulating agent may “overdrive” TRPV1 under inflammatory conditions. This overdrive could produce local nerve terminal inactivation and analgesia through calcium overload, similar to what has been observed with subcutaneous injections of direct-acting TRPV1 agonists such as capsaicin or resiniferatoxin (Neubert et al., 2003; Mitchell et al., 2010).
Electrophysiology and Calcium Imaging Disclose a Rapid Action for MRS1477.
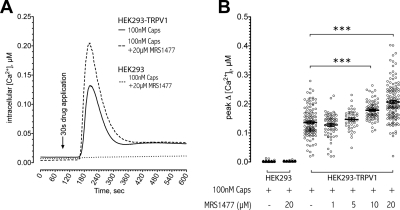
Intracellular Ca2+ imaging experiments revealed that coapplication of MRS1477 significantly increases the peak intracellular calcium concentration ([Ca2+]i) after capsaicin activation. The kinetics of the rise and the decay of the transient do not show substantial alterations apart from the increase in peak height (Fig. 2A). As with 45Ca2+ uptake, the potentiation by MRS1477 occurred in a dose-dependent manner (Fig. 2B). Application of MRS1477, regardless of the presence of capsaicin, does not elicit a detectable change in the [Ca2+]i of untransfected HEK293 cells (HEK293, 100 nM Caps + 20 μM MRS1477; Fig. 2A), confirming the results of 45Ca2+ uptake experiments under the triple block condition, showing that MRS1477 does not interfere with intracellular calcium homeostasis.
Fig. 2.
Intracellular calcium imaging. A, we recorded the intracellular calcium levels for individual cells over the course of experiments and plotted the average [Ca2+]i of the population at each time point to obtain a kinetic trace. Nontransfected HEK293 cells did not show any change in their [Ca2+]i when exposed to 100 nM capsaicin and 20 μM MRS1477 (dotted line). The increase of [Ca2+]i in HEK293-TRPV1 cells was more important in the presence of 100 nM capsaicin and 20 μM MRS1477 (dashed line) than in the presence of 100 nM capsaicin only (continuous line). B, plotting the maximum increase in [Ca2+]i of individual cells after the administration of 100 nM capsaicin reveals a dose-dependent increase in average peak height when increasing concentrations of MRS1477 are present. Significant differences are present at 10 μM MRS1477 and higher (P < 0.001, number of cells per treatment, from left to right: 59, 90, 55, 74, 44, 85, and 139, respectively).
The experimental methods used so far provided us either with endpoint data of calcium entry through activated TRPV1 present in the plasma membrane (45Ca2+ uptake assays) or with real-time low-frequency readout of cytoplasmic calcium concentrations, regardless of whether it is the result of calcium release from the endoplasmic reticulum or entry through plasma membrane calcium channels (intracellular calcium imaging) (Kárai et al., 2004). To obtain better temporal resolution, we studied the interaction between MRS1477 and TRPV1 using the whole-cell patch-clamp method.
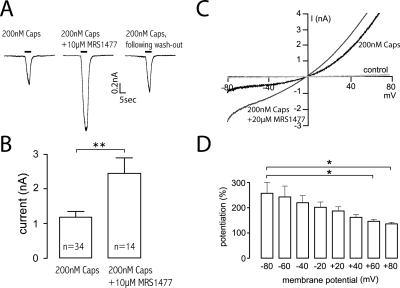
As we observed in calcium imaging studies, MRS1477 significantly increased peak currents activated by the administration of 200 nM capsaicin. Application of the agonist alone over a 5-s period resulted in a strong inward current (200 nM Caps; Fig. 3A); the peak amplitude of this current increased ∼2-fold in the presence of 20 μM MRS1477 (200 nM Caps + 20 μM MRS1477; Fig. 3A). This increase was highly significant with a peak current of ∼1 nA on average when capsaicin only was applied compared with >2 nA when 20 μM MRS1477 was coapplied (P < 0.01) (Fig. 3B). Preincubation with MRS1477 was not necessary to observe potentiation, and the positive modulation was reversible by a 3-min washing step, resulting in currents comparable to those of controls when capsaicin only was applied (Fig. 3A).
Fig. 3.
Electrophysiology experiments demonstrate increased current through TRPV1. A, whole-cell patch-clamp experiments using HEK293-TRPV1 cells show that coapplication of 10 μM MRS1477 results in a 2-fold increase in the peak current activated by 200 nM capsaicin. The action of MRS1477 is rapid and reversible; it does not require preincubation and can be washed out. B, statistical analysis of peak currents shows that this increase is highly significant (P < 0.01). C, voltage-ramp experiments show the generalized enhancement of capsaicin-activated currents with no apparent change in the reversal potential of the current. In the absence of both capsaicin and MRS1477 (control), there was no measurable current. D, the enhancement is more important at negative holding potentials; the difference is significant when the extreme values of the holding potentials examined are compared.
Applying a voltage ramp to the capsaicin-activated receptor revealed no change in the reversal potential of the activated current when MRS1477 was coapplied, indicating that MRS1477 does not change the relative permeability of TRPV1 to cations. However, current flow through the ion channel shows a generalized increase both below and above the reversal potential suggesting that the action of MRS1477 is allosteric nature (Fig. 3C). Although the potentiation was observed at all membrane potentials, the magnitude of this effect was significantly higher at negative potentials (Fig. 3D), suggesting that the allosteric site for MRS1477 might be influenced by the electric field of the channel.
MRS1477 Enhances Capsaicin-Induced Hypothermia In Vivo.
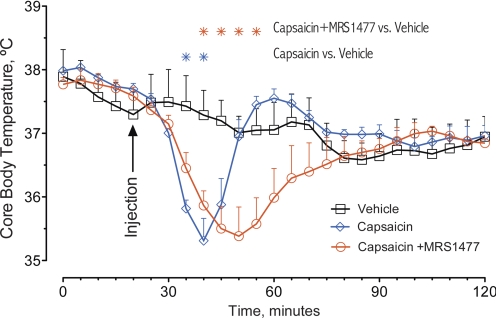
The ability of MRS1477 to enhance the actions of capsaicin in vivo was tested using capsaicin-evoked hypothermia. Intraperitoneal administration of 5 μg of capsaicin provoked a drop in core body temperature (CBT) and statistically significant hypothermia (compared with vehicle) at time points 15 and 20 min after drug injection (Capsaicin; Fig. 4). Coinjection of 200 μg of MRS1477 extended the duration of hypothermia by a factor of 2 with CBT significantly below control values at time points 20 to 35min (Capsaicin + MRS1477; Fig. 4). The presence of MRS1477 had no effect on the amplitude of the peak decrease in CBT. Minimum CBTs were 35.31 and 35.38°C for capsaicin and capsaicin with MRS1477, respectively. These data show that MRS1477 extended the duration but not the amplitude of capsaicin-evoked hypothermia.
Fig. 4.
Capsaicin-induced hypothermia is enhanced by the coadministration of MRS1477. Intraperitoneal injection of 5 μg of capsaicin provoked a significant drop in CBT. Hypothermia started to develop 10 min after the injection, peaked within the following 10 min, and then returned to control values during the subsequent 15-min period (Capsaicin). Coadministration of MRS1477 significantly extends the duration of the hypothermia with no change in amplitude of the drop in CBT (Capsaicin + MRS1477). Mice receiving vehicle only did not show significant changes in their CBT (Vehicle). CBT values were recorded every 5 min, starting 20 min before the injection of drugs, for 120 min total.
Positive Modulation Is Independent of the Vanilloid-Binding Site.
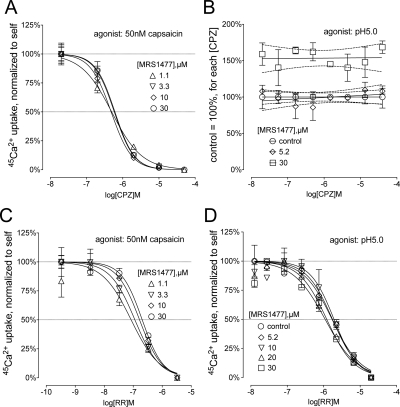
We tested whether MRS1477 interacts with the channel pore or with the vanilloid binding site and determined dose-inhibition profiles of CPZ and RR in the presence of increasing concentrations of MRS1477. The results of the dose-response studies are presented in two formats. First, we show dose inhibition data normalized to their respective minimums and maximums to better visualize any change in the IC50 values of inhibitors (Fig. 5; see Supplemental Table 2 for detailed curve-fit data). We also included the same set of results normalized to the minimum and maximum values of their respective controls to show the potentiation by increasing doses of MRS1477 (Supplemental Fig. 3; see Supplemental Table 3 for detailed curve-fit data).
Fig. 5.
MRS1477 does not affect TRPV1 inhibition by capsazepine or ruthenium red. A, increasing concentrations of MRS1477 potentiate activation of TRPV1 by 50 nM capsaicin up to 3-fold (Supplemental Fig. 3A). The CPZ interaction with TRPV1 remains unaffected, as the normalized dose-inhibition curves show no change in CPZ IC50 values. B, CPZ is a partial inhibitor of TRPV1 activation by protons, yielding incomplete dose-inhibition curves that show a linear increase in inhibition with increasing doses of CPZ (Supplemental Fig. 3B). Normalizing calcium uptake to each CPZ concentration without MRS1477 produces a horizontal line at 100% for control. Potentiation is apparent at all CPZ concentrations, ∼50% above control in the presence of 30 μM MRS1477 (dashed lines represent upper and lower 95% CI). C, RR inhibition of capsaicin-activated TRPV1 in the presence of increasing amounts of MRS1477 shows a progressive potentiation of the TRPV1 response (Supplemental Fig. 3C). Normalized curves show a small but nonsignificant reduction in RR IC50 values with high concentrations of MRS1477. D, RR is also an inhibitor of proton activation of TRPV1. It does not interfere with potentiation by MRS1477 (Supplemental Fig. 3D), whereas normalized dose-inhibition curves of RR show no change in RR potency in the presence of MRS1477.
CPZ is a competitive (orthosteric) vanilloid antagonist, capable of inhibiting capsaicin activation with an IC50 in the 10−7 M range. However, it is an inefficient antagonist of low pH activation at similar concentrations (Gavva et al., 2005). Although the TRPV1 response to 50 nM capsaicin shows gradual potentiation in the presence of increasing concentrations of MRS1477 (>3-fold with 30 μM MRS1477) (Supplemental Fig. 3), normalized results reveal no change in CPZ IC50 values (Fig. 5A). Inhibition of proton activation by CPZ is only partial and we could not obtain a full dose-inhibition curve (Supplemental Fig. 3B), but normalization of calcium uptake at each CPZ concentration to CPZ only (control) values revealed that the extent of potentiation by 30 μM MRS1477 is not altered by different concentrations of this antagonist, with 50% enhancement observed at any concentration of CPZ (Fig. 5B).
RR is a nonspecific calcium channel inhibitor binding directly to the extracellular side of the pore, inhibiting all modalities of TRPV1 (Caterina et al., 1997; García-Martínez et al., 2000). Similar to results obtained with CPZ, ascending concentrations of MRS1477 produced robust potentiation of capsaicin and proton-induced calcium uptake (Supplemental Fig. 3, C and D). No substantial differences are seen in the RR inhibition profiles for either capsaicin or pH activation (Fig. 5, C and D), although there is a small decrease in RR potency inhibiting capsaicin. These data are consistent with the idea that MSR1477 does not interact directly with either the CPZ or RR binding sites.
Discussion
The present studies describe the positive allosteric modulator effects of a 1,4-DHP on the vanilloid receptor TRPV1. We show ligand-specific positive modulation using three independent methodologies: 45Ca2+ uptake assays, ratiometric calcium imaging, and patch-clamp recording. Positive modulation of a capsaicin-induced physiological response was also confirmed using an in vivo assay. Dose-response results from 45Ca2+ uptake experiments suggest that vanilloid agonists undergo sensitivity modulation regardless of their potencies; in addition, the maximum response from proton-induced activation is increased. Such generalized positive modulation of TRPV1 has been observed in slightly acidic extracellular environments (Jordt et al., 2000), in response to elevated concentrations of extracellular divalent cations (Ahern et al., 2005) or as the result of direct phosphorylation from constitutive PKC activation (Bhave et al., 2003). All of these conditions are present during inflammation or tissue damage. Although there are known orthosteric vanilloid antagonists that potentiate receptor activation by protons (Wong and Gavva, 2009), MRS1477 is the first known small-molecule positive modulator of TRPV1 without detectable agonist or antagonist activity.
Multiple factors can affect TRPV1 sensitivity, especially in inflammatory conditions. The combined effects of multiple sensitizing agents are expected to be additive if the underlying mechanisms are independent. We observed decreases of capsaicin EC50 values upon treatment with either PMA, low pH, low pH with PMA, or MRS1477 alone. However, under inflammatory-mimetic conditions (low pH + PMA), MRS1477 caused a further 3.1-fold reduction of the capsaicin EC50 value, reaching a cumulative EC50 decrease 20-fold less than that of the control (Fig. 1H). This result shows that MRS1477 can act independently of, but also in concert with, either proton- or PKC-mediated sensitization and is consistent with observations that the potency of TRPV1 ligands and endovanilloids can vary greatly, depending on the physiological or pathophysiological context (i.e., at sites of inflammation and tissue damage) (Olah et al., 2001). Of importance, their actions can be further enhanced by small-molecule compounds.
Interference with mechanisms of active calcium extrusion (PMCA and NCX) or the stimulation of capacitative calcium entry by MRS1477 could result in an apparent increase of 45Ca2+ uptake in our assay. However, inhibition of these major calcium transport mechanisms did not affect the capacity of MRS1477 to potentiate TRPV1 activation. Intracellular calcium imaging experiments show that increasing doses of MRS1477 correlate with increasing peak [Ca2+]i after TRPV1 activation but have no effect on other kinetic parameters of the calcium transient such as peak rise and decay times. Calcium imaging also showed that MRS1477 alone does not induce detectable changes in baseline [Ca2+]i. Whole-cell patch-clamp experiments using HEK293-TRPV1 cells show increased amplitude of capsaicin-activated current in the presence of MRS1477 with no change in other kinetic parameters. MRS1477 alone causes no detectable transmembrane current, and no effects were seen on the parental cell line. These three methods encompass a broad range of temporal resolution and consistently demonstrate positive modulation of TRPV1 by MRS1477 with no detectable effect on calcium transport mechanisms involved in Ca2+ homeostasis.
In our initial evaluations of the in vivo activity of MRS1477 using acute nociceptive tests, we found that nociceptive behavioral responses evoked by acute capsaicin application, such as the eye wipe test or intraplantar injection, lack assay sensitivity for detecting enhancement by MRS1477. In place of an acute pain assay, we used capsaicin-evoked hypothermia as a test model, because we were able to accurately measure small changes in CBT during this more gradually evolving physiological response. A reduction in CBT after systemic administration of capsaicin occurs over several minutes, and this extended onset provided a less volatile baseline. Coadministration of MRS1477 with capsaicin significantly extends the duration of hypothermia. Although recovery time was prolonged with MRS1477, we did not observe a significant change in the amplitude of CBT. Because administration of 20 μg of capsaicin can provoke a further drop of 1°C in CBT, the results with 5 μg of capsaicin are within the dynamic range of the assay. Although it is difficult to extrapolate from our in vitro observations which parameters—duration, intensity, or both—should have been affected, nevertheless, the nature of the effect, an enhancement of capsaicin-evoked hypothermia by MRS1477, is consistent with our in vitro findings. It is also interesting to note that a previous study showed a similar prolongation of hypothermia, without a change in amplitude, when the TRPV1 agonists RTX and capsaicin were compared (de Vries and Blumberg, 1989).
Additional evidence for an allosteric mechanism derives from our studies with TRPV1 inhibitors. The decrease in the EC50 values we observe with vanilloid ligands suggests that MRS1477 may act by altering the affinity of the vanilloid binding site. However, increasing concentrations of the positive modulator did not affect CPZ inhibition of capsaicin activation in 45Ca2+ uptake experiments. This apparent contradiction can be explained by the fact that the 45Ca2+ uptake assay is a functional assay and measures TRPV1 channel opening over time as opposed to directly quantifying receptor-ligand interactions as with radioligand-binding assays. The lack of effect on capsaicin-capsazepine interaction is consistent with the idea that MRS1477 does not substantially alter the vanilloid binding site. This being the case, then positive modulation by MRS1477 probably is due to modulation of channel gating. It is important to note that the present studies were conducted with CPZ concentrations between 10−8 and 10−5 M. Some studies have shown that high micromolar concentrations of CPZ can inhibit voltage-gated calcium channels in cultured rat dorsal root ganglia (Docherty et al., 1997) and nicotinic acetylcholine receptors in rat trigeminal ganglia (Liu and Simon, 1997). However, on the basis of our dose-inhibition curves, this nonspecific antagonism is not sufficient to explain our results obtained using HEK cells expressing high levels of TRPV1.
Although the crystal structure of TRPV1 is not known, homology models based either on the structure of the Kv1.2 Shaker K+ channel (Salazar et al., 2009) or the Streptomyces lividans K+ channel (Ryu et al., 2007) are regularly used to model the pore region of TRPV1. These models use sequences encompassing transmembrane helices (TM) 5 and 6 with the pore-loop sequence in between. The structure of the S. lividans K+ channel also served as the prototype structure for the homology model of the pore region of 1,4-DHP-sensitive L-type calcium channels (Huber et al., 2000). 1,4-DHP binding was localized to a pocket formed by sequences that correspond to TM 5 and 6 in TRPV1 (Ryu et al., 2007). In light of the above findings, combined with our results showing no interaction between MRS1477 and CPZ or RR, we hypothesize that MRS1477 interacts with the pore-forming TM 5 and 6, directly influencing channel gating regardless of the potency of agonists. Further experiments examining the dynamics of open and closed states of TRPV1 at the single-channel level will be used to further explore the mechanism of action of this compound.
The potentiation of vanilloid and endovanilloid agonists and low pH suggests a new potential mechanism for amplification of nociceptive stimuli in primary afferent endings at sites of peripheral inflammation and tissue damage. This possibility may be operative if the MRS1477 allosteric site is a target for endogenous compounds generated during tissue damage or inflammation. Positive allosteric modulation of TRPV1 may provide an additional mechanism for peripheral sensitization processes, to sustain TRPV1 transduction capabilities in chronic pain states, or to override a desensitized TRPV1 to allow the neuron to respond to new changes in status at sites of peripheral inflammation or pathology. However, lessons from capsaicin and RTX demonstrate that a balance needs to occur between active and “overactive” TRPV1 to avoid complete incapacitation of the nerve ending due to calcium overload. Peripheral application of vanilloid agonists has been abundantly demonstrated to produce a selective, TRPV1-mediated axonopathy and prolonged analgesia (Robbins et al., 1998; Nolano et al., 1999; Neubert et al., 2003; Karai et al., 2004; Backonja et al., 2008; Mitchell et al., 2010). For therapeutic use, ‘A TRPV1 PAM could produce just such a calcium overload in highly active endings, causing a pharmacological transition to an inactive nerve terminal. Studies of capsaicin or RTX action suggest that this acute effect would last for several days or longer until the ending is functionally reconstituted (Bates et al., 2010), thereby providing a defined, long-duration analgesic action after a single administration. The TRPV1 PAM approach is predicated on the participation of a sufficiently potent endogenous agonist, combined with a potent PAM, to drive the afferent ending into an inactive state, rather than prolonging or exacerbating nociceptive processes. However, the very same nociceptive processes seen in inflammation or tissue damage also bias the system to potential MRS1477 actions on TRPV1.
Supplementary Material
Acknowledgments
We thank Dr. M. Caterina (Cellular and Molecular Medicine, Johns Hopkins Medicine, Baltimore MD) for providing us with the cell line HEK293-TRPV1.
This work was supported by the National Institutes of Health National Institute of Dental and Craniofacial Research, Division of Intramural Research; the National Institutes of Health National Institute of Child Health and Human Development; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases; and the National Institutes of Health National Institute of Mental Health [Grant R03-MH089480-01A1].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- TRPV1
- transient receptor potential cation channel subfamily V member 1
- RTX
- resiniferatoxin
- DHP
- dihydropyridine
- MRS1477
- 4,5-diethyl-3-(2-methoxyethylthio)-2-methyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate
- HEK
- human embryonic kidney
- MES
- 4-morpholineethanesulfonic acid
- NADA
- N-arachidonyldopamine
- CPZ
- capsazepine
- PMA
- phorbol 12-myristate 13-acetate
- DMSO
- dimethyl sulfoxide
- RR
- ruthenium red
- HS
- Hill slope
- Caps
- capsaicin
- PMCA
- plasma membrane Ca2+ ATPase
- NCX
- sodium-calcium exchanger
- PKC
- protein kinase C
- CBT
- core body temperature
- TM
- transmembrane helix
- PAM
- positive allosteric modulator.
Authorship Contributions
Participated in research design: Kaszas, Keller, Coddou, Mishra, Hoon, Stojilkovic, and Iadarola.
Conducted experiments: Kaszas, Coddou, Mishra, Hoon.
Contributed new reagents or analytic tools: Jacobson.
Performed data analysis: Kaszas, Coddou, Mishra, Hoon.
Wrote or contributed to the writing of the manuscript: Kaszas, Keller, Coddou, Mishra, Hoon, Jacobson, and Iadarola.
References
- Ahern GP, Brooks IM, Miyares RL, Wang XB. (2005) Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J Neurosci 25:5109–5116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backonja M, Wallace MS, Blonsky ER, Cutler BJ, Malan P, Jr, Rauck R, Tobias J, and NGX-4010 C116 Study Group (2008) NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol 7:1106–1112 [DOI] [PubMed] [Google Scholar]
- Bates BD, Mitchell K, Keller JM, Chan CC, Swaim WD, Yaskovich R, Mannes AJ, Iadarola MJ. (2010) Prolonged analgesic response of cornea to topical resiniferatoxin, a potent TRPV1 agonist. Pain 149:522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th (2003) Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc Natl Acad Sci USA 100:12480–12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, Olah Z, Mannes AJ. (2005) Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology 103:1052–1059 [DOI] [PubMed] [Google Scholar]
- Bruehl S. (2010) An update on the pathophysiology of complex regional pain syndrome. Anesthesiology 113:713–725 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824 [DOI] [PubMed] [Google Scholar]
- Cortright DN, Szallasi A. (2004) Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem 271:1814–1819 [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. (2009) Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32:1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries DJ, Blumberg PM. (1989) Thermoregulatory effects of resiniferatoxin in the mouse: comparison with capsaicin. Life Sci 44:711–715 [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Piper AS. (1997) Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br J Pharmacol 121:1461–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman JG, Chen L, Hille B. (2008) Calcium transport mechanisms of PC12 cells. J Gen Physiol 131:307–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Martínez C, Morenilla-Palao C, Planells-Cases R, Merino JM, Ferrer-Montiel A. (2000) Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J Biol Chem 275:32552–32558 [DOI] [PubMed] [Google Scholar]
- Gavva NR, Tamir R, Klionsky L, Norman MH, Louis JC, Wild KD, Treanor JJ. (2005) Proton activation does not alter antagonist interaction with the capsaicin-binding pocket of TRPV1. Mol Pharmacol 68:1524–1533 [DOI] [PubMed] [Google Scholar]
- Gracely RH, Lynch SA, Bennett GJ. (1992) Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain 51:175–194 [DOI] [PubMed] [Google Scholar]
- Huber I, Wappl E, Herzog A, Mitterdorfer J, Glossmann H, Langer T, Striessnig J. (2000) Conserved Ca2+-antagonist-binding properties and putative folding structure of a recombinant high-affinity dihydropyridine-binding domain. Biochem J 347 (Pt 3):829–836 [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Jordt SE, Tominaga M, Julius D. (2000) Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci USA 97:8134–8139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. (2004) Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest 113:1344–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kárai LJ, Russell JT, Iadarola MJ, Oláh Z. (2004) Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J Biol Chem 279:16377–16387 [DOI] [PubMed] [Google Scholar]
- Liu L, Simon SA. (1997) Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganglia. Neurosci Lett 228:29–32 [DOI] [PubMed] [Google Scholar]
- Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, Roufogalis BD, Tominaga M. (2006) Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCε-mediated phosphorylation at S800. Pain 123:106–116 [DOI] [PubMed] [Google Scholar]
- Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. (2011) TRPV1-lineage neurons are required for thermal sensation. EMBO J 30:582–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K, Bates BD, Keller JM, Lopez M, Scholl L, Navarro J, Madian N, Haspel G, Nemenov MI, Iadarola MJ. (2010) Ablation of rat TRPV1-expressing Aδ/C-fibers with resiniferatoxin: analysis of withdrawal behaviors, recovery of function and molecular correlates. Mol Pain 6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert JK, Karai L, Jun JH, Kim HS, Olah Z, Iadarola MJ. (2003) Peripherally induced resiniferatoxin analgesia. Pain 104:219–228 [DOI] [PubMed] [Google Scholar]
- Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. (1999) Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain 81:135–145 [DOI] [PubMed] [Google Scholar]
- Olah Z, Karai L, Iadarola MJ. (2001) Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J Biol Chem 276:31163–31170 [DOI] [PubMed] [Google Scholar]
- Robbins WR, Staats PS, Levine J, Fields HL, Allen RW, Campbell JN, Pappagallo M. (1998) Treatment of intractable pain with topical large-dose capsaicin: preliminary report. Anesth Analg 86:579–583 [DOI] [PubMed] [Google Scholar]
- Roh EJ, Keller JM, Olah Z, Iadarola MJ, Jacobson KA. (2008) Structure-activity relationships of 1,4-dihydropyridines that act as enhancers of the vanilloid receptor 1 (TRPV1). Bioorg Med Chem 16:9349–9358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham MC, Nothaft W, Duan WR, Wang Y, Faltynek C, McGaraughty S, Chu KL, Svensson P. (2011) Oral and cutaneous thermosensory profile of selective TRPV1 inhibition by ABT-102 in a randomized healthy volunteer trial. Pain 152:1192–1200 [DOI] [PubMed] [Google Scholar]
- Ryu S, Liu B, Yao J, Fu Q, Qin F. (2007) Uncoupling proton activation of vanilloid receptor TRPV1. J Neurosci 27:12797–12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar H, Jara-Oseguera A, Hernández-García E, Llorente I, Arias-Olguín II, Soriano-García M, Islas LD, Rosenbaum T. (2009) Structural determinants of gating in the TRPV1 channel. Nat Struct Mol Biol 16:704–710 [DOI] [PubMed] [Google Scholar]
- Tamayo N, Liao H, Stec MM, Wang X, Chakrabarti P, Retz D, Doherty EM, Surapaneni S, Tamir R, Bannon AW, et al. (2008) Design and synthesis of peripherally restricted transient receptor potential vanilloid 1 (TRPV1) antagonists. J Med Chem 51:2744–2757 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531–543 [DOI] [PubMed] [Google Scholar]
- Triggle DJ, Langs DA, Janis RA. (1989) Ca2+ channel ligands: structure-function relationships of the 1,4-dihydropyridines. Med Res Rev 9:123–180 [DOI] [PubMed] [Google Scholar]
- Wong GY, Gavva NR. (2009) Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Res Rev 60:267–277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.