Abstract
Products containing naphthalen-1-yl-(1-pentylindol-3-yl) methanone (JWH-018) and naphthalen-1-yl-(1-butylindol-3-yl) methanone (JWH-073) are emerging drugs of abuse. Here, the behavioral effects of JWH-018 and JWH-073 were examined in one behavioral assay selective for cannabinoid agonism, rhesus monkeys (n = 4) discriminating Δ9-tetrahydrocannabinol (Δ9-THC; 0.1 mg/kg i.v.), and another assay sensitive to cannabinoid withdrawal, i.e., monkeys (n = 3) discriminating the cannabinoid antagonist rimonabant (1 mg/kg i.v.) during chronic Δ9-THC (1 mg/kg s.c. 12 h) treatment. Δ9-THC, JWH-018, and JWH-073 increased drug-lever responding in monkeys discriminating Δ9-THC; the ED50 values were 0.044, 0.013, and 0.058 mg/kg, respectively and the duration of action was 4, 2, and 1 h, respectively. Rimonabant (0.32–3.2 mg/kg) produced surmountable antagonism of Δ9-THC, JWH-018, and JWH-073. Schild analyses and single-dose apparent affinity estimates yielded apparent pA2/pKB values of 6.65, 6.68, and 6.79 in the presence of Δ9-THC, JWH-018, and JWH-073, respectively. In Δ9-THC-treated monkeys discriminating rimonabant, the training drug increased responding on the rimonabant lever; the ED50 value of rimonabant was 0.20 mg/kg. Δ9-THC (1–10 mg/kg), JWH-018 (0.32–3.2 mg/kg), and JWH-073 (3.2–32 mg/kg) dose-dependently attenuated the rimonabant-discriminative stimulus (i.e., withdrawal). These results suggest that Δ9-THC, JWH-018, and JWH-073 act through the same receptors to produce Δ9-THC-like subjective effects and attenuate Δ9-THC withdrawal. The relatively short duration of action of JWH-018 and JWH-073 might lead to more frequent use, which could strengthen habitual use by increasing the frequency of stimulus-outcome pairings. This coupled with the possible greater efficacy of JWH-018 at cannabinoid 1 receptors could be associated with greater dependence liability than Δ9-THC.
Introduction
Cannabis abuse and dependence remains a worldwide problem. A series of cannabinoids originally synthesized by Huffman et al., (2005), labeled as JWH, have entered the recreational market. Highly purified JWH compounds, which are more readily synthesized than plant-derived cannabinoids, are available from online vendors in gram quantities with no age verification. Herbal blends infused with these compounds (e.g., Spice) are being sold in “head shops” and smoked despite warnings that the products are not intended for human consumption. Several online vendors provide these drugs at similar purity to those from Sigma-Aldrich (St. Louis, MO) (Ginsburg et al., 2012), but for as little as 1/2000 of the price. In Germany, Japan, and the United Kingdom, so-called herbal cannabis substitutes contain mostly naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) (Lindigkeit et al., 2009; Hudson et al., 2010; Uchiyama et al., 2010). Samples confiscated by the U.S. Drug Enforcement Administration were found to contain JWH-018; the drug was apparently obtained from online suppliers in the United States, although production seems to be occurring in Asia (T. Boos, personal communication). Recently, the Drug Enforcement Administration used an emergency scheduling authority to temporarily control JWH-018, naphthalen-1-yl-(1-butylindol-3-yl)methanone (JWH-073), and three other cannabinoids (Gay, 2010). Nevertheless, because of easy access, low cost, and absence of a convenient test for human use, there has been a marked increase in use of JWH-018, JWH-073, and other synthetic cannabinoids.
Clinical and preclinical data on the effects of JWH-018 and JWH-073 are limited. In a case report (Zimmermann et al., 2009), a former regular user of hashish (a concentrated preparation of cannabis) switched to an herbal preparation containing JWH-018. After discontinuing use of the JWH-018-containing product, a severe withdrawal syndrome emerged, which was alleviated by resuming use of the herbal mixture. The subject reported the effects of JWH-018 to be stronger than hashish, but less euphoric. Recent case studies indicate that JWH-018 has effects similar to, although perhaps not identical with, those of cannabis and Δ9-tetrahydrocannabinol (Δ9-THC); in particular, JWH compounds seem to have effects on the cardiovascular system that are distinct from those observed after Δ9-THC administration (Simmons et al., 2011; Young et al., 2011). Δ9-THC and its actions at CB1 receptors are responsible primarily for the behavioral effects of cannabis (Wiley, 1999). Both JWH-018 and JWH-073 are reported to be CB1 receptor agonists in vitro, similar to Δ9-THC, although JWH-018 seems to have higher CB1 agonist efficacy than JWH-073 or Δ9-THC (Wiley et al., 1998; Huffman et al., 2005; Brents et al., 2011). For example, in one recent study, the maximal effect of Δ9-THC was less than 25% of that of JWH-018 (Brents et al., 2011). Although the in vivo effects of JWH-018 and JWH-073, which include hypoactivity, immobility, antinociception, and hypothermia in mice (Wiley et al., 1998), are consistent with a CB1 receptor agonist profile, there is currently no published evidence consistent with in vivo efficacy differences between these compounds.
Among behavioral assays, drug discrimination is an especially powerful tool for examining the in vivo effects of cannabinoids, not only because this approach is associated with a high degree of selectivity for CB1 receptor agonism, but also because it is highly predictive of the subjective effects of cannabis (Balster and Prescott, 1992). Typically any one of a variety of species, including pigeons, mice, rats, monkeys, and humans, responds on one manipulandum after receiving Δ9-THC and another after receiving vehicle (Henriksson et al., 1975; Wiley et al., 1993; McMahon, 2006, 2008; Lile et al., 2009). Once adequately trained, subjects respond on the Δ9-THC-associated manipulandum after receiving Δ9-THC or other CB1 receptor agonists and, in general, the vehicle-appropriate manipulandum after receiving noncannabinoids. Two assays were used here; in one, rhesus monkeys discriminated Δ9-THC (0.1 mg/kg i.v.) from vehicle. In a second assay, monkeys discriminated the CB1 antagonist rimonabant (1 mg/kg i.v.) while receiving a relatively large dose (1 mg/kg s.c.) of Δ9-THC every 12 h, which resulted in tolerance and dependence, with the latter being evidenced by rimonabant-induced withdrawal (Stewart and McMahon, 2010; McMahon, 2011).
JWH-018 and JWH-073 are alkylindole derivatives that seem to act as cannabinoid agonists. Recreational use of these and other similar compounds is becoming widespread, because of marketing that promotes them as alternatives to marijuana. Drug discrimination assays were conducted in nonhuman primates trained to discriminate Δ9-THC from vehicle to examine whether JWH-018 and JWH-073 exhibit a Δ9-THC-like behavioral profile. To assess the extent to which these discriminative stimulus effects are probably mediated by action at the CB1 receptor, quantitative (i.e., Schild) analyses of antagonism of JWH-018, JWH-073, and Δ9-THC by the CB1-selective antagonist rimonabant were conducted. In addition, Δ9-THC, JWH-018, and JWH-073 were examined for their capacity to modify the dose-response curve for rimonabant in Δ9-THC-treated monkeys.
Materials and Methods
Subjects.
One female and three male adult rhesus monkeys (Macaca mulatta) discriminated Δ9-THC from vehicle and one male and two female rhesus monkeys discriminated rimonabant during chronic Δ9-THC (1 mg/kg s.c. 12 h) treatment. Monkeys were housed individually on a 14-h light/10-h dark schedule and maintained at 95% free-feeding weight (range 5.8–10.4 kg) with a diet consisting of primate chow (High Protein Monkey Diet; Harlan Teklad, Madison, WI), fresh fruit, and peanuts; water was provided in the home cage. Monkeys received cannabinoids and noncannabinoids in previous studies (Stewart and McMahon 2010; McMahon, 2011). Monkeys were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
Surgery.
Monkeys were anesthetized with ketamine (10 mg/kg i.m.) followed by isoflurane (1.5–3.0% inhaled via facemask). A catheter (heparin-coated polyurethane; o.d. = 1.68 mm; i.d. = 1.02 mm; Instech Laboratories, Plymouth Meeting, PA) was inserted approximately 5 cm into a subclavian or femoral vein. Suture silk (coated vicryl; Ethicon Inc., Somerville, NJ) was used to anchor the catheter to the vessel and ligate the section of the vessel proximal to the catheter insertion. The other end of the catheter passed subcutaneously to the midscapular region of the back and was attached to a vascular access port (Mida-cbas-c50; Instech Laboratories).
Apparatus.
Monkeys were seated in chairs (model R001; Primate Products, Miami, FL) that provided restraint. Chairs were placed in ventilated, sound-attenuating chambers equipped with two levers; a light was positioned above each lever. Feet were placed in shoes containing brass electrodes to which a brief electric stimulus (3 mA, 250 ms) could be delivered from an A/C generator. The chambers were connected to a computer with an interface (MED Associates, St. Albans, VT); experimental events were controlled and recorded with Med-PC software (MED Associates).
Drug Discrimination Procedure.
Four monkeys discriminated Δ9-THC (0.1 mg/kg i.v.) from vehicle (1 part absolute ethanol, 1 part Emulphor-620, and 18 parts saline) while responding under a fixed ratio 5 (FR5) schedule of stimulus-shock termination. Three other monkeys received 1 mg/kg s.c. Δ9-THC (at 6:00 AM and 6:00 PM) and discriminated rimonabant (1 mg/kg i.v.) from the same vehicle at noon under an FR5 schedule of stimulus-shock termination. This reinforcer was chosen instead of food presentation because of a concern that the appetite-suppressant effects of rimonabant (Colombo et al., 1998) would interfere with responding in the rimonabant discrimination assay. Experimental sessions were divided into 10-min multiple cycles; each cycle began with a 5-min timeout. Responses during the timeout had no programmed consequence. The timeout was followed by a 5-min schedule of stimulus-shock termination, the beginning of which was signaled by illumination of red lights. Five consecutive responses on the correct lever extinguished the red lights, prevented delivery of an electric stimulus, and initiated a 30-s timeout. Otherwise, an electric stimulus was delivered every 40 s (Δ9-THC discrimination) or 10 s (rimonabant discrimination). Responding on the incorrect lever reset the response requirement on the correct lever. Determination of correct levers varied among monkeys (i.e., left lever associated with the training dose of the training drug; right lever associated with vehicle) and remained the same for that monkey for the duration of the study.
Training sessions were conducted by administering the training drug (Δ9-THC or rimonabant) or vehicle within the first minute of a cycle followed by vehicle or sham (dull pressure applied to the skin overlying the vascular access port) within the first minute of subsequent cycles. Drug training consisted of three cycles and was immediately preceded by zero to three vehicle-training cycles; some training sessions included vehicle or sham only at the beginning of three to six cycles. Completion of the FR on the correct lever was required for a reinforcer during each training cycle. Monkeys had previously satisfied the criteria for testing, i.e., at least 80% of the total responses occurred on the correct lever and fewer than five responses occurred on the incorrect lever before completion of the first FR on the correct lever within a cycle for all cycles during five consecutive or six of seven training sessions. Tests were conducted after performance for consecutive training sessions, including both vehicle and drug training sessions, satisfied the test criteria. The order of training with drug or vehicle varied nonsystematically.
During test sessions, five consecutive responses on either lever postponed the shock schedule. In monkeys discriminating Δ9-THC, dose-effect functions for Δ9-THC, JWH-018, and JWH-073 were determined by administering vehicle in the first cycle followed by doses increasing by 0.5 log unit in subsequent cycles. The dose-effect function included ineffective doses (i.e., doses producing responses predominantly on the vehicle lever) up to doses that produced more than 80% of responses on the Δ9-THC lever. To establish a time course, Δ9-THC (0.1 mg/kg) or JWH-018 (0.032 mg/kg) were administered at the beginning and in 1-h increments before a six-cycle test; vehicle was administered in the first cycle when drug was administered before the session and sham was administered at the beginning of subsequent cycles. Tests with rimonabant were conducted by administering an intravenous dose in the first cycle followed by cumulative doses of Δ9-THC, JWH-018, or JWH-073 in subsequent cycles. In Δ9-THC-treated monkeys, Δ9-THC (1–10 mg/kg), JWH-018 (0.32–3.2 mg/kg), and JWH-073 (3.2–32 mg/kg) were studied in combination with rimonabant by administering vehicle or a dose of agonist at the beginning of the first cycle followed by doses of rimonabant increasing by 0.25 or 0.5 log unit in subsequent cycles. Rimonabant was studied from ineffective doses up to doses that produced more than 80% of responses on the rimonabant lever or up to a dose of 5.6 mg/kg, whichever occurred first. Because of limitations in the solubility of rimonabant in the vehicle used for intravenous administration, 5.6 mg/kg was the largest dose studied.
Drugs.
Δ9-THC (100 mg/ml in absolute ethanol) and rimonabant (Research Technology Branch of the National Institute on Drug Abuse, Rockville, MD), JWH-018 (IU Chem Holding Co, Ltd., Shanghai, China), and JWH-073 (Research Chemical Supplier, Scottsdale, AZ) were dissolved in a mixture of 1 part absolute ethanol, 1 part Emulphor-620 (Rhodia Inc., Cranbury, NJ), and 18 parts physiologic saline and administered intravenously in a volume of 0.1 to 1 ml/kg. Doses were expressed as the weight of the forms listed above in milligrams per kilogram of body weight.
Data Analyses.
Discrimination data were expressed as a percentage of responses on the drug lever of total responses on both the drug and vehicle levers. Rate of responding on both levers (i.e., drug and vehicle) was calculated as responses per second excluding responses during timeouts. Rate of responding during a test was expressed as the percentage of the control response rate for individual animals. The control was defined as the average response rate for all cycles during the five previous vehicle training sessions excluding sessions during which the test criteria were not satisfied. Discrimination and rate data were averaged among subjects, separately for the four monkeys discriminating Δ9-THC and the three Δ9-THC-treated monkeys discriminating rimonabant, and plotted as a function of dose. The drug time courses were plotted as an average of data collected every two cycles (i.e., 20 min).
To estimate the ED50 value or dose producing 50% responding on the drug lever, individual dose-response data were analyzed with linear regression (Prism version 5.0 for Windows; GraphPad Software Inc., San Diego, CA). The analyses included doses spanning the linear portion of the dose-response curve, and a common, best-fitting slope was used for further analyses (Kenakin, 1997). Doses corresponding to the 50% level of effect (ED50 value), potency ratios, and their 95% confidence limits were calculated by parallel line analyses of data from individual subjects (Tallarida, 2000). Potencies were considered significantly different when the 95% confidence limits of the potency ratio did not include 1. The time-course data were converted to area under the curve per animal, and differences among Δ9-THC, JWH-018, and JWH-073 were analyzed with repeated-measures one-way analysis of variance and post hoc Tukey's multiple comparison test (p < 0.05) with Prism.
Schild plots were constructed by expressing the logarithm of the dose ratio −1 as a function of the negative logarithm of the molar dose of rimonabant for individual monkeys (Arunlakshana and Schild, 1959). Straight lines were simultaneously fit to individual Schild plots with the equation log(dose ratio −1) = −log(molar dose of rimonabant) × slope + intercept. Schild plots for rimonabant in combination with Δ9-THC and JWH-018 were compared by using two mathematical models: a simpler model (i.e., slope constrained to unity or −1) and a more complex model that allowed slopes for each Schild plot to vary. The two models were compared with an F-ratio test. If the calculated F value was not significant, then the pA2 value was calculated with both the constrained and unconstrained slope. For rimonabant in combination with JWH-073, a single-dose apparent affinity estimate was calculated for individual monkeys with the following equation: pKB = −log(B/dose ratio −1), with B expressed in moles per kilogram of body weight.
In Δ9-THC-treated monkeys discriminating rimonabant, the potencies of Δ9-THC, JWH-018, and JWH-073 were calculated by expressing the mean shift in the rimonabant dose-response curve (i.e., ED50 value determined in the presence of agonist divided by the control ED50 value) as a function of dose for individual monkeys. Linear regression of the individual data was used to estimate the dose of agonist producing a 2-fold rightward shift in the rimonabant dose-response curve.
The effects of drugs on response rate were examined with linear regression; a significant dose-dependent decrease in responding was evidenced by the slope being significantly different from 0 (p < 0.05). ED50 values and potency ratios were calculated when response rate was decreased to below 50%. If the slope was significantly different from 0 and response rate was not decreased to below 50%, then ED75 values and potency ratios were calculated.
Results
Effects of JWH-018 and JWH-073 in Monkeys Discriminating Δ9-THC.
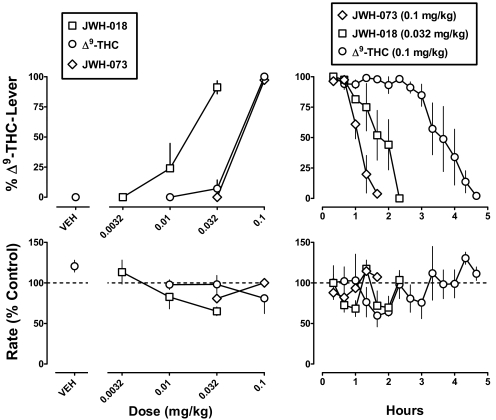
JWH-018 and JWH-073 dose-dependently increased mean responding on the Δ9-THC lever (Fig. 1 top left); maximum responding on the drug lever was 91% at 0.032 mg/kg JWH-018 and 97% at 0.1 mg/kg JWH-073. Likewise,Δ9-THC dose-dependently increased responding on the Δ9-THC lever, from 0% at a dose of 0.01 mg/kg to 100% at the training dose (0.1 mg/kg) (Fig. 1, top left, ○). After administration of vehicle, mean responding on the Δ9-THC lever was 0% (Fig. 1, top left, VEH). The slopes of the dose-response curves for Δ9-THC, JWH-018, and JWH-073, when determined alone and in combination with various doses of rimonabant, were not significantly different from each other (F9,88 = 1.19; p = 0.31). The ED50 values (95% confidence limits) calculated from the common slope were 0.044 (0.032–0.061) mg/kg for Δ9-THC, 0.013 (0.0091–0.019) mg/kg for JWH-018, and 0.058 (0.036–0.094) mg/kg for JWH-073. JWH-018 was 3.4- and 4.4-fold more potent than Δ9-THC and JWH-073, respectively (Table 1).
Fig. 1.
Discriminative stimulus effects of Δ9-THC, JWH-018, and JWH-073 as a function of dose (left) and time (right). Abscissae: vehicle or dose in milligram per kilogram of body weight (left) and time in hours (right). Ordinates: mean (± S.E.M.) percentage of responding on the Δ9-THC lever (top) and mean (± S.E.M.) response rate expressed as a percentage of control (VEH training days) rate [rate (% control)] (bottom).
TABLE 1.
ED50 values and 95% confidence limits (CL) for Δ9-THC, JWH-018, and JWH-073, alone and in combination with rimonabant, in rhesus monkeys discriminating Δ9-THC (0.1 mg/kg i.v.)
Potency ratios and 95% CLs are the ED50 values of the agonist in combination with rimonabant divided by the ED50 value of the agonist alone.
| Drug | ED50 Value (95% CL) | Potency Ratio (95% CL) |
|---|---|---|
| mg/kg | ||
| Δ9-THC | 0.044 (0.032–0.061) | 3.4 (2.1–5.3) vs. JWH-018** |
| + Rimonabant (0.32 mg/kg) | 0.24 (0.16–0.36)* | 5.5 (3.4–8.9) |
| + Rimonabant (1 mg/kg) | 0.43 (0.31–0.59)* | 9.7 (6.3–15) |
| + Rimonabant (3.2 mg/kg) | 1.3 (0.98–1.8)* | 30 (20–46) |
| JWH-018 | 0.013 (0.0091–0.019) | |
| + Rimonabant (0.32 mg/kg) | 0.070 (0.044–0.11)* | 5.4 (3.1–9.2) |
| + Rimonabant (1 mg/kg) | 0.15 (0.11–0.20)* | 11 (7.0–18) |
| + Rimonabant (3.2 mg/kg) | 0.44 (0.32–0.60)* | 33 (21–53) |
| JWH-073 | 0.058 (0.036–0.094) | 4.4 (2.6–7.7) vs. JWH-018** |
| + Rimonabant (1 mg/kg) | 0.89 (0.57–1.4)* | 15 (8.3–28) |
Significantly different from the respective controls (i.e., Δ9-THC alone or JWH-018 alone).
Significantly less potent than JWH-018.
When discriminative stimulus effects were examined over time, Δ9-THC was found to have a significantly longer duration of action compared with JWH-018 and JWH-073 (F2,9 = 33.5; p < 0.001). JWH-018 and JWH-073 did not significantly differ in their duration of action. All three drugs produced more than 80% drug-lever responding for up to 40 min (Fig. 1, right). Responding on the drug lever remained more than 80% for up to 3 h after Δ9-THC, whereas responding on the Δ9-THC lever was predominantly on the vehicle lever beginning at 2 h, 20 min for JWH-018 and 1 h, 40 min for JWH-073.
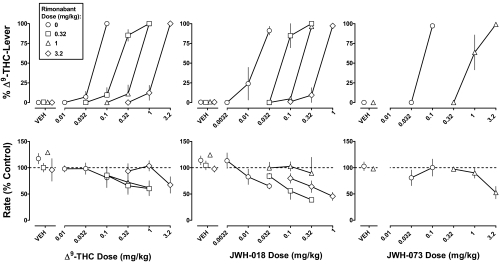
Rimonabant (0.32–3.2 mg/kg) alone produced a maximum of 1% responding on the Δ9-THC lever and antagonized the discriminative stimulus effects of Δ9-THC, JWH-018, and JWH-073 (Fig. 2, top). Rimonabant, at doses of 0.32, 1, and 3.2 mg/kg, increased the ED50 value of Δ9-THC 5.5-, 9.7-, and 30-fold, respectively; likewise, the ED50 value of JWH-018 was increased 5.4-, 11-, and 33-fold, respectively (Table 1). A dose of 1 mg/kg rimonabant produced a 15-fold increase in the ED50 value of JWH-073.
Fig. 2.
Discriminative stimulus effects of Δ9-THC (left), JWH-018 (center), and JWH-073 (right): antagonism by rimonabant. Abscissae: dose in milligram per kilogram of body weight or vehicle (VEH). Ordinates: mean (± S.E.M.) percentage of responses on the Δ9-THC lever (top) and mean (± S.E.M.) response rate expressed as a percentage of control (VEH training days) rate [rate (% control)]. The control dose-response curves for Δ9-THC, JWH-018, and JWH-073 are replotted from Fig. 1.
Absolute rates of responding for individual monkeys were 1.31, 1.75, 2.57, and 2.75 responses per second. Up to the largest doses tested, JWH-018 dose-dependently decreased the rate of responding (F1,10 = 8.21; p < 0.05), whereas Δ9-THC and JWH-073 alone did not significantly modify the response rate (Figs. 1, bottom left and 2, bottom). The ED75 value of JWH-018 to decrease the response rate was 0.018 mg/kg; the ED75 value was increased by 2.6- and 8.0-fold in the presence of 0.32 and 3.2 mg/kg rimonabant, respectively. The fold-shift in the dose-response curve for JWH-018 produced by a dose of 1 mg/kg rimonabant could not be calculated, i.e., the response rate was not decreased to less than 75% of the control.
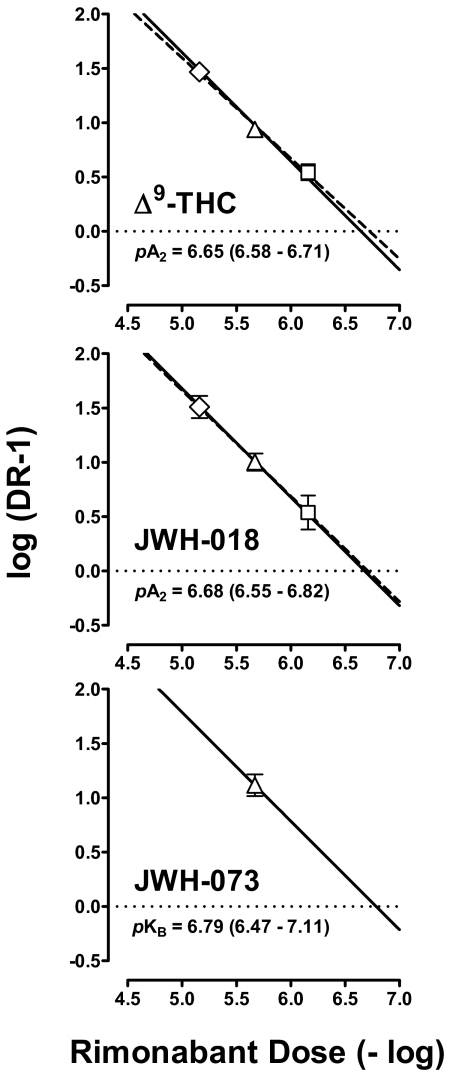
The Schild plots for antagonism of the discriminative stimulus effects of Δ9-THC and JWH-018 are shown in Fig. 3, top and middle, respectively; the coefficients of determination (r2) were 0.94 and 0.79, respectively, and the unconstrained slopes (95% confidence limits) were −0.93 (−1.08 to −0.77) and −0.97 (−1.32 to −0.62), respectively. Neither slope was significantly different from unity (i.e., −1; p = 0.32 and 0.86, respectively) nor from each other (p = 0.79). The apparent pA2 values of rimonabant calculated from the constrained slopes were 6.70 in the presence of Δ9-THC and 6.74 in the presence of JWH-018. When the slopes were constrained to −1, the apparent pA2 values (95% confidence limits) were 6.65 (6.58–6.71) in the presence of Δ9-THC and 6.68 (6.55–6.82) in the presence of JWH-018. The single-dose apparent affinity (pKB) value (95% confidence limits) of rimonabant (1 mg/kg) determined in the presence of JWH-073 was 6.79 (6.47–7.11) (Fig. 3, bottom).
Fig. 3.
Schild plots for Δ9-THC (top) and JWH-018 (middle) and a log (DR-1) value for JWH-073 (bottom) constructed from the mean data shown in Fig. 2. Abscissae: negative logarithm of the dose in moles per kilogram. Ordinates: mean (± S.E.M.) logarithm of the dose ratio −1. Schild plots were constructed from the unconstrained slopes (dashed lines) and by constraining the slopes to −1 (solid lines).
Effects of Δ9-THC, JWH-018, and JWH-073 in Δ9-THC-Treated Monkeys Discriminating Rimonabant.
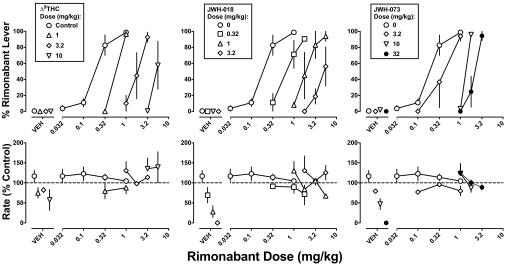
Rimonabant dose-dependently increased drug-lever responding, with 0.1 mg/kg producing relatively low levels of drug-lever responding (10%) and 0.32 and 1 mg/kg rimonabant producing higher levels of drug-lever responding (81 and 99%, respectively; Fig. 4 top, circles). The ED50 value of rimonabant was 0.20 mg/kg (Table 2). Vehicle produced 1% responding on the rimonabant lever and Δ9-THC (1–10 mg/kg), JWH-018 (0.32–3.2 mg/kg), and JWH-073 (3.2–32 mg/kg) produced 0% responding on the rimonabant lever. When monkeys received an acute intravenous dose of Δ9-THC in addition to the chronic Δ9-THC treatment before the session the rimonabant-discriminative stimulus was attenuated; doses of 1, 3.2, and 10 mg/kg Δ9-THC increased the ED50 value of rimonabant by 2.9-, 8.5-, and 29-fold, respectively. JWH-018 and JWH-073 produced similar dose-dependent increases in the ED50 value of rimonabant (Table 2).
Fig. 4.
Discriminative stimulus effects of rimonabant: attenuation by Δ9-THC (left), JWH-018 (center), and JWH-073 (right). Abscissae: dose of rimonabant in milligram per kilogram of body weight or vehicle (VEH). Ordinates: mean (± S.E.M.) percentage of responses on the rimonabant lever (top) and mean (± S.E.M.) response rate expressed as a percentage of control (VEH training days) rate [rate (% control)] (bottom). The control dose-response curve for rimonabant is the same in each panel.
TABLE 2.
ED50 values and 95% CLs for rimonabant, alone and in combination with Δ9-THC, JWH-018, and JWH-073, in Δ9-THC (1 mg/kg/12 h)-treated rhesus monkeys discriminating rimonabant (1 mg/kg i.v.)
Potency ratios and 95% CLs are the ED50 values of rimonabant in combination with the agonist divided by the ED50 value of rimonabant alone.
| Drug | ED50 Value (95% CL) | Potency Ratio (95% CL) |
|---|---|---|
| mg/kg | ||
| Rimonabant | 0.20 (0.14–0.29) | |
| + Δ9-THC (1 mg/kg) | 0.59 (0.53–0.66)* | 2.9 (1.9–4.4) |
| + Δ9-THC (3.2 mg/kg) | 1.7 (0.84–3.5)* | 8.5 (4.5–16) |
| + Δ9-THC (10 mg/kg) | 5.8 (0.93–52)* | 29 (17–70) |
| + JWH-018 (0.32 mg/kg) | 0.63 (0.41–0.97)* | 3.1 (1.9–5.0) |
| + JWH-018 (1 mg/kg) | 2.2 (1.2–4.1)* | 11 (6.1–20) |
| + JWH-018 (3.2 mg/kg) | 5.7 (2.5–13)* | 29 (16–50) |
| + JWH-073 (3.2 mg/kg) | 0.40 (0.14–1.2) | 2.0 (0.9–4.0) |
| + JWH-073 (10 mg/kg) | 1.3 (1.2–1.6)* | 6.5 (4.3–10) |
| + JWH-073 (32 mg/kg) | 2.1 (1.2–3.7)* | 11 (6.0–17) |
Significantly different from rimonabant alone.
Absolute rates of responding for individual monkeys were 0.79, 1.80, and 2.31 responses per second. Rimonabant alone did not alter response rates (Fig. 4, bottom). Likewise, Δ9-THC, when studied up to a dose of 10 mg/kg, did not significantly modify the response rate (Fig. 4, bottom left). However, across the dose range producing comparable rightward shifts in the rimonabant dose-response curve for discriminative stimulus effects, both JWH-018 and JWH-073 markedly and dose-dependently decreased the response rate (Fig. 4, bottom center and right, respectively). The ED50 values and 95% confidence limits were 0.58 (0.31–1.1) mg/kg for JWH-018 and 7.9 (5.6–11) mg/kg for JWH-073; JWH-018 was significantly more potent than JWH-073 by 14-fold, i.e., the 95% confidence limits of the potency ratio were 7.6 to 24. However, coadministration of rimonabant antagonized the rate-decreasing effects of every dose of JWH-018 and JWH-073 tested.
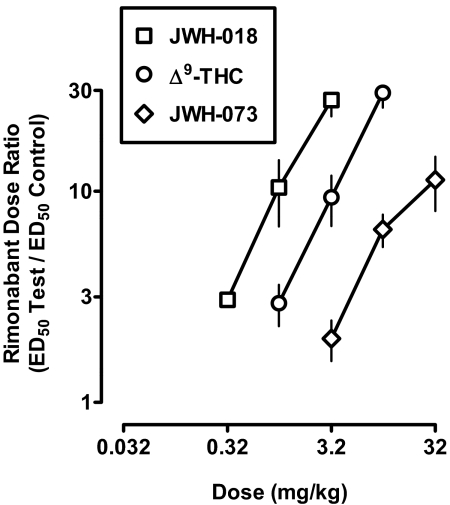
Figure 5 shows the magnitude of shift in the rimonabant dose-response curve expressed as a function of dose of JWH-018, Δ9-THC, and JWH-073. The slopes of the lines were not significantly different from each other (p = 0.33). Linear regression was used to estimate the dose of each agonist producing a 2-fold shift in the rimonabant dose-response curve; the values were 0.33 mg/kg for JWH-018, 1.1 mg/kg for Δ9-THC, and 3.2 mg/kg for JWH-073. The relative potency of JWH-018 and Δ9-THC to attenuate the rimonabant-discriminative stimulus and substitute for the Δ9-THC-discriminative stimulus were similar. In contrast, the relative potency of JWH-073 was somewhat less in Δ9-THC-treated monkeys discriminating rimonabant (i.e., 9.7-fold relative to JWH-018) compared with monkeys discriminating Δ9-THC (i.e., 4.4-fold relative to JWH-018).
Fig. 5.
Magnitude of rightward shift in the rimonabant dose-response function expressed as a function of JWH-018, Δ9-THC, and JWH-073 dose. Abscissa: dose in milligram per kilogram of body weight. Ordinate: mean (± S.E.M.) rightward shift in the rimonabant dose-response function, calculated as the rimonabant ED50 value after pretreatment with a cannabinoid agonist divided by the control rimonabant ED50 value.
Discussion
With the availability and abuse of novel synthetic cannabinoids on the rise, there is a need to understand the pharmacologic mechanisms of these novel compounds in vivo, especially with respect to that of the prototypic cannabinoid Δ9-THC. Here, JWH-018 and JWH-073 fully substituted for the discriminative stimulus effects of Δ9-THC, with JWH-018 being approximately 4-fold more potent than Δ9-THC and JWH-073, which were equipotent. The duration of action of intravenous Δ9-THC was longer (i.e., approximately 4 h) than that of JWH-018 (i.e., 2 h) and JWH-073 (i.e., 1 h). Rimonabant produced orderly antagonism of the discriminative stimulus effects of all three drugs. Up to the smallest dose producing near-maximal responding on the Δ9-THC lever, JWH-018, but not Δ9-THC or JWH-073, decreased response rates in monkeys discriminating Δ9-THC from vehicle, and this effect was antagonized by rimonabant. Schild analyses of drug discrimination data were consistent with a simple, competitive, and reversible interaction; the apparent pA2 or pKB values (i.e., potency) of rimonabant did not differ significantly among Δ9-THC, JWH-018, and JWH-073, suggesting that the agonists produce discriminative stimulus effects through the same receptors.
In a separate group of Δ9-THC-treated monkeys discriminating rimonabant, the discriminative stimulus effects of rimonabant were attenuated not only by Δ9-THC but also by JWH-018 and JWH-073. Although overall potency for attenuating the rimonabant-discriminative stimulus was less than potency in substituting for the Δ9-THC-discriminative stimulus, relative potency was similar across conditions, with the exception of JWH-073 being somewhat less potent than expected in Δ9-THC-treated monkeys discriminating rimonabant. Across the dose range producing comparable attenuation of the rimonabant-discriminative stimulus, JWH-018 and JWH-073, but not Δ9-THC, reduced response rate; these effects were abolished by even the lowest dose of rimonabant.
Results similar to those obtained in the current study were reported previously in rats, i.e., JWH-018 substituted for the discriminative stimulus effects of Δ9-THC at training doses of 1.8 and 3.0 mg/kg and also for the CB1 agonist methanandamide at a training dose of 10 mg/kg (Järbe et al., 2010, 2011). The ED50 values for JWH-018 were consistent with a greater potency compared with Δ9-THC or methanandamide. The ED50 value for JWH-018 generalization to Δ9-THC shifted more than 10-fold to the right by acute pretreatment with 1 mg/kg rimonabant, suggesting that CB1 receptors mediated discriminative stimulus effects in rats. Here, we demonstrate with Schild analyses that Δ9-THC, JWH-018, and JWH-073 exert overlapping discriminative stimulus effects via the same, and presumably CB1, receptors in nonhuman primates. Thus, the effects observed in rats and anecdotal evidence from humans are consistent with our present results in monkeys.
Although the Δ9-THC-discriminative stimulus is highly selective for CB1 receptors, actions of JWH-018 and JWH-073 at non-CB1- or Δ9-THC-insensitive receptors cannot be excluded. However, any actions at non-CB1 receptors do not seem to interfere with the capacity of JWH-018 and JWH-073 to share discriminative stimulus with Δ9-THC. Thus, experienced cannabis users are likely to find similarities between the subjective effects of JWH-018 or JWH-073 and cannabis. This is despite the large number of constituents in cannabis that are not present in the synthetic preparation, and the possibility that JWH-018 and JWH-073 exert actions at receptors not targeted by agents in cannabis. Thus, the growing popularity of Spice and other products containing JWH-018 or similar chemicals seems to be caused, in part, by their capacity to produce the subjective high produced by marijuana.
The duration of action of both synthetic agents is shorter than that of Δ9-THC. Because of a relatively short duration of action, JWH-018 and JWH-073 might be administered more frequently than Δ9-THC to achieve a similar time course of effect as Δ9-THC. Such frequent, repeated use could present additional abuse and dependence liability for these shorter-acting drugs by strengthening the association between stimulus and drug effects, thereby leading to more habitual use. Indeed, an inverse relationship between duration of action and the rate of self-administration has been established for psychostimulants, i.e., the shorter a drug effect lasts, the greater the rate of responding it maintains under limited access conditions (Lile, 2006). Furthermore, withdrawal from discontinuation of use of shorter-acting compounds is more robust than withdrawal associated with longer-acting compounds (Boisse and Okamoto, 1978). It is likely that similar relationships exist for other drugs of abuse, including cannabinoids. The short duration of action coupled with the potentially greater efficacy of JWH-018 or other similar compounds could lead to greater dependence liability and more severe withdrawal effects.
Δ9-THC is a low-efficacy agonist in vitro (Breivogel and Childers, 2000) and as such its discriminative stimulus effects are not expected to be particularly sensitive to differences in CB1 receptor agonist efficacy inasmuch as higher efficacy agonists fully mimic the effects of Δ9-THC. Receptor theory predicts that decreases in CB1 receptor function, either from decreased receptor number, desensitization, or both, increases the efficacy required for agonist activity. This can be achieved by treating animals chronically with Δ9-THC, and the functional consequences include greater tolerance and cross-tolerance to lower efficacy agonists than to higher efficacy agonists in mice (Fan et al., 1994; Falenski et al., 2010; Singh et al., 2011) and rhesus monkeys (McMahon, 2011). In the current study, chronic Δ9-THC treatment did not seem to differentially alter the potency of Δ9-THC, JWH-018, and JWH-073 as evidenced by attenuation of the rimonabant-discriminative stimulus (i.e., compared with substitution for the Δ9-THC-discriminative stimulus).
In earlier work from our laboratory, the full-efficacy agonists 2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]-5-(2-methyloctan-2-yl)phenol (CP 55940) and (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone (WIN 55212-2), but not Δ9-THC, dose-dependently decreased response rates in monkeys treated chronically with Δ9-THC and trained to discriminate rimonabant from vehicle at the same doses that reversed the rimonabant discrimination (Stewart and McMahon, 2010). Yet these same drugs did not affect the response rates in a separate group of monkeys trained to discriminate Δ9-THC from vehicle (McMahon, 2006). Here, we saw a similar pattern for JWH-073: doses that reversed the rimonabant discrimination in monkeys chronically treated with Δ9-THC produced pronounced rate reductions, but doses that substituted for Δ9-THC in a Δ9-THC discrimination assay did not affect response rate. In contrast, JWH-018 produced rate reductions in both assays, making it unique among the cannabinoid agonists we have studied with these procedures to date. The implications of these results remain unclear; however, greater CB1 receptor efficacy or alternative sites of action for JWH-018 compared with the other CB1 agonists are possible reasons for these findings, though in vitro studies suggest similar efficacies among CP 55940, WIN 55212-2, and JWH-018 (Wiley et al., 1998).
We have previously shown that the potency of the CB1 receptor agonist WIN 55212-2 in attenuating cannabinoid withdrawal was less than expected based on its relative potency with Δ9-THC and other cannabinoids in monkeys not dependent or tolerant to Δ9-THC (Stewart and McMahon, 2010). This same discrepancy between the potency for producing Δ9-THC-discriminative stimulus effects and reversing cannabinoid withdrawal was observed for JWH-073, but not JWH-018. Because both JWH compounds and WIN 55212-2 are alkylindoles, this discrepancy cannot be explained by differences among the chemical class of the CB1 agonist. Furthermore, JWH-018 and JWH-073 differ in their structures by only a single methyl group on a side chain. Thus, this discrepancy is unexpected based on such a subtle difference in the structures of the two JWH compounds. The mechanism and implications for this feature of WIN 55212-2 and JWH-073 activity remains unclear. Another recent study showed that antagonism of the Δ9-THC-like discriminative stimulus effect of WIN 55212-2 by rimonabant in rats was less pronounced compared with JWH-018 (Järbe et al., 2011). Those authors interpret this result in the context of potential differences in the way various CB1 receptor agonists interact with rimonabant, and that this might suggest a difference in the mode of action among the drugs.
Taken together, the present results demonstrate that JWH-018 and JWH-073 exhibit similar discriminative stimulus effects to Δ9-THC. Furthermore, these compounds attenuate rimonabant-induced Δ9-THC withdrawal in monkeys. This represents the first published report of the discriminative stimulus effects of this class of drug that is growing in popularity among recreational users in nonhuman primates. Schild analyses suggest that the discriminative stimulus effects all are mediated via a common receptor, probably the CB1 receptors. JWH-018 and JWH-073 have substantially shorter durations of action than Δ9-THC, which could enhance their abuse liabilities relative to Δ9-THC. Discrimination studies did not reveal any differences among the drugs that might reflect reported differences in efficacy in vitro, although the parameters in the present study were not optimized to identify such differences. However, differences in rate-reducing effects of the drugs between the Δ9-THC and rimonabant discrimination assays might be caused, in part, by efficacy differences. Likewise, the discrepancy between the relative potency of JWH-073 to produce Δ9-THC-appropriate responding in the Δ9-THC discrimination and attenuate rimonabant-appropriate responding in the rimonabant discrimination might reflect efficacy differences between JWH-073 and JWH-018 at a common site or differential binding to alternative, yet unidentified, sites of action. Indeed anecdotal reports of intoxication with JWH compounds include effects that are not typical of CB1 agonists, such as elevated blood pressure, which could owe to alternative sites of action. In summary, JWH-018 and JWH-073 exhibit a discriminative stimulus profile similar to Δ9-THC and seem to exert agonist activity at CB1 receptors, consistent with in vitro results, limited work in rodents, and anecdotal reports from humans. These drugs pose a mounting concern for health-care practitioners, because JWH-018 and JWH-073 are likely to have abuse liability that is similar to and perhaps even greater than that of Δ9-THC.
Acknowledgments
We thank C. Cunningham for assistance with statistical analysis.
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA19222, R01-DA26781].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- JWH-018
- naphthalen-1-yl-(1-pentylindol-3-yl) methanone
- JWH-073
- naphthalen-1-yl-(1-butylindol-3-yl) methanone
- Δ9-THC
- Δ9-tetrahydrocannabinol
- CB
- cannabinoid
- FR
- fixed ratio
- CL
- confidence limit
- CP 55940
- 2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol
- WIN 55212-2
- (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone.
Authorship Contributions
Participated in research design: Ginsburg and McMahon.
Conducted experiments: Schulze and Hruba.
Performed data analysis: Schulze, Hruba, and McMahon.
Wrote or contributed to the writing of the manuscript: Ginsburg and McMahon.
References
- Arunlakshana O, Schild HO. (1959) Some quantitative uses of drug antagonists. Br J Pharmacol Chemother 14:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. (1992) Δ9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev 16:55–62 [DOI] [PubMed] [Google Scholar]
- Boisse NR, Okamoto M. (1978) Physical dependence to barbital compared to pentobarbital. IV. Influence of elimination kinetics. J Pharmacol Exp Ther 204:526–540 [PubMed] [Google Scholar]
- Breivogel CS, Childers SR. (2000) Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther 295:328–336 [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. (2011) Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One 6:e21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. (1998) Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci 63:PL113–PL117 [DOI] [PubMed] [Google Scholar]
- Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH, Selley DE, Lichtman AH, Sim-Selley LJ. (2010) FAAH−/− mice display differential tolerance, dependence, and cannabinoid receptor adaptation after Δ9-tetrahydrocannabinol and anandamide administration. Neuropsychopharmacology 35:1775–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. (1994) Development of cross-tolerance between Δ9-tetrahydrocannabinol, CP 55, 940 and WIN 55, 212. J Pharmacol Exp Ther 271:1383–1390 [PubMed] [Google Scholar]
- Gay M. (2010) Synthetic marijuana spurs state bans. The New York Times; July 11, 2010; p A17 [Google Scholar]
- Ginsburg BC, McMahon LR, Herrera CR, Javors M. (2012) Purity of synthetic cannabinoids sold online for recreational use. J Anal Toxicol, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson BG, Johansson JO, Järbe TU. (1975) Δ9-Tetrahydrocannabinol produced discrimination in pigeons. Pharmacol Biochem Behav 3:771–774 [DOI] [PubMed] [Google Scholar]
- Hudson S, Ramsey J, King L, Timbers S, Maynard S, Dargan PI, Wood DM. (2010) Use of high-resolution accurate mass spectrometry to detect reported and previously unreported cannabinomimetics in “herbal high” products. J Anal Toxicol 34:252–260 [DOI] [PubMed] [Google Scholar]
- Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, Thompson AL, Bushell S, Tartal C, Hurst DP, et al. (2005) Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB1 and CB2 receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB2 receptor agonists. Bioorg Med Chem 13:89–112 [DOI] [PubMed] [Google Scholar]
- Järbe TU, Deng H, Vadivel SK, Makriyannis A. (2011) Cannabinergic aminoalkylindoles, including AM678=JWH018 found in ‘Spice’, examined using drug (Δ9-tetrahydrocannabinol) discrimination for rats. Behav Pharmacol 22:498–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TU, Li C, Vadivel SK, Makriyannis A. (2010) Discriminative stimulus functions of methanandamide and Δ9-THC in rats: tests with aminoalkylindoles (WIN55,212-2 and AM678) and ethanol. Psychopharmacology (Berl) 208:87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. (1997) Pharmacologic Analysis of Drug-Receptor Interaction, Lippincott-Raven, Philadelphia, PA [Google Scholar]
- Lile JA. (2006) Pharmacological determinants of the reinforcing effects of psychostimulants: relation to agonist substitution treatment. Exp Clin Psychopharmacol 14:20–33 [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. (2009) Substitution profile of Δ9-tetrahydrocannabinol, triazolam, hydromorphone, and methylphenidate in humans discriminating Δ9-tetrahydrocannabinol. Psychopharmacology (Berl) 203:241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, Beuerle T. (2009) Spice: a never ending story? Forensic Sci Int 191:58–63 [DOI] [PubMed] [Google Scholar]
- McMahon LR. (2006) Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther 319:1211–1218 [DOI] [PubMed] [Google Scholar]
- McMahon LR. (2011) Chronic Δ9-tetrahydrocannabinol treatment in rhesus monkeys: differential tolerance and cross-tolerance among cannabinoids. Br J Pharmacol 162:1060–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. (2008) Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Δ9-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 198:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2003) Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research, National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- Simmons J, Cookman L, Kang C, Skinner C. (2011) Three cases of “spice” exposure. Clin Toxicol (Phila) 49:431–433 [DOI] [PubMed] [Google Scholar]
- Singh H, Schulze DR, McMahon LR. (2011) Tolerance and cross-tolerance to cannabinoids in mice: schedule-controlled responding and hypothermia. Psychopharmacology 215:665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. (2010) Rimonabant-induced Δ9-tetrahydrocannabinol withdrawal in rhesus monkeys: discriminative stimulus effects and other withdrawal signs. J Pharmacol Exp Ther 334:347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. (2000) Drug Synergism and Dose-Effect Data Analysis, Chapman and Hall/CRC, Boca Raton, FL [Google Scholar]
- Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y. (2010) Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int 198:31–38 [DOI] [PubMed] [Google Scholar]
- Wiley JL. (1999) Cannabis: discrimination of “internal bliss”? Pharmacol Biochem Behav 64:257–260 [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Balster RL, Martin BR. (1993) Tolerance to the discriminative stimulus effects of Δ9-tetrahydrocannabinol. Behav Pharmacol 4:581–585 [PubMed] [Google Scholar]
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR. (1998) Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther 285:995–1004 [PubMed] [Google Scholar]
- Young AC, Schwarz E, Medina G, Obafemi A, Feng SY, Kane C, Kleinschmidt K. (2011) Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med http://dx.doi.org/10.1016/j.ajem.2011.05.013 [DOI] [PubMed]
- Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. (2009) Withdrawal phenomena and dependence syndrome after the consumption of “spice gold.” Dtsch Arztebl Int 106:464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]