Abstract
3,4-Methylenedioxymethamphetamine (MDMA) is known to enhance tactile sensory perception, an effect that contributes to its popularity as a recreational drug. The neurophysiological basis for the effects of MDMA on somatosensation are unknown. However, MDMA interactions with the serotonin transporter (SERT) and subsequent enhancement of serotonin neurotransmission are well known. The rat trigeminal somatosensory system receives serotonergic afferents from the dorsal raphe nucleus. Because these fibers express SERT, they should be vulnerable to MDMA-induced effects. We found that administration of a challenge injection of MDMA (3 mg/kg i.p.) after repeated MDMA treatment (3 mg/kg per day for 4 days) elicits both serotonin and norepinephrine efflux in the ventral posterior medial (VPM) thalamus of Long-Evans hooded rats, the main relay along the lemniscal portion of the rodent trigeminal somatosensory pathway. We evaluated the potential for repeated MDMA administration to modulate whisker-evoked discharge of individual neurons in this region. After surgically implanting stainless steel eight-wire multichannel electrode bundles, we recorded spike train activity of single cells while activating the whisker pathway using a piezoelectric mechanical stimulator. We found that repeated MDMA administration increased the spontaneous firing rate but reduced both the magnitude and duration of whisker-evoked discharge in individual VPM thalamic neurons. The time course of drug action on neuronal firing patterns was generally consistent with fluctuations in neurotransmitter efflux as shown from our microdialysis studies. On the basis of these results, we propose that single use and repeated administration of MDMA may “distort,” rather than enhance, tactile experiences in humans, in part, by disrupting normal spike firing patterns through somatosensory thalamic relay circuits.
Introduction
In human subjects the popular recreational drug “ecstasy” (MDMA) produces powerful, yet poorly understood, effects on somatosensation (for review, see Starr et al., 2008). We reported previously that single-dose (naive subject) systemic administration of MDMA suppresses the transmission of sensory signals through the rat somatosensory thalamus (Starr et al., 2008). Because human users of MDMA rarely consume the drug only once, in the present study we used the same rodent model to examine the effects of repeated MDMA administration on serotonin (5-HT) and norepinephrine (NE) efflux and somatosensory-evoked discharge in the ventral posterior medial nucleus (VPM) of rat thalamus.
In the previous report (Starr et al., 2008), we found that single-dose MDMA administration led to a significant, rapid dose-dependent increase of 5-HT levels in the VPM thalamus of waking male Long-Evans Hooded rats. However, single-dose administration caused increased NE efflux in this region only at the highest dose tested (10 mg/kg). In conjunction with these results, we found that injection of 3 mg/kg MDMA leads to significant increases in plasma levels of MDMA and its major metabolite methylenedioxyamphetamine. In addition, we determined that single-dose (3 mg/kg) MDMA administration led to decreased responsiveness of individual neurons to whisker stimulation.
The methods used in the present study are identical to those described in the previous report (Starr et al., 2008) with the exception of the repeated drug regimen. In brief, we used the rat trigeminal somatosensory pathway as a model to investigate the effects of repeated MDMA administration on 5-HT and NE efflux and sensory-evoked discharge. This system transmits tactile information from the rat's facial whiskers to the contralateral somatosensory cortex. Sensory information from individual whiskers is first relayed to the brainstem and then projected to the contralateral VPM thalamus. Whisker-related information is then relayed to the somatosensory cortex. All regions along this pathway receive serotonergic and noradrenergic projections (Simpson et al., 1997; Kirifides et al., 2001) and thus are vulnerable to the pharmacological effects of MDMA.
We found that administration of a challenge injection (3 mg/kg i.p.) of MDMA after repeated MDMA treatment elicits both 5-HT and NE efflux in the VPM thalamus. Repeated systemic MDMA administration led to decreases in magnitude and timing of individual sensory neuron responses to whisker stimulation. Of importance, after repeated MDMA administration, the effects of the drug on spontaneous firing rate are blunted compared with the effects of single-dose treatment. The net effect of suppressed evoked discharge and increased spontaneous firing is a pronounced reduction in the signal/noise ratio for sensory signals. Therefore, it appears that the net effect of both single and repeated MDMA administration is to reduce neuronal responsiveness to sensory afferents. Overall, there is evidence of changes in response to MDMA or baseline neuronal activity that suggest persistent drug effects in repetitively treated animals. Although psychophysical studies are needed, we speculate that the blunted electrophysiological effects observed after repeated MDMA treatment could potentially help explain tolerance to MDMA's positive effects as reported by many human MDMA users (Parrott, 2005).
Materials and Methods
Animals
Male, Long-Evans hooded rats (Charles River Laboratories, Inc., Wilmington, MA) weighing 250 to 300 g at the start of the experiment were housed individually with ad libitum access to food and water. The animal facility was maintained at 21°C with a 12-h light/dark cycle with the light period beginning at 7:00 AM. All procedures were conducted in accordance with the guidelines published in the National Institutes of Health Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). All protocols were approved by the Drexel University College of Medicine Institutional Animal Care and Use Committee.
Dialysis Probe Construction and Calibration
Vertical concentric microdialysis probes were used (Abercrombie et al., 1988). In brief, a piece of fused silica (Polymicro Technologies, Phoenix, AZ) was inserted through PE10 tubing (Clay Adams, Parsippany, NJ) and a semipermeable membrane of hollow cuprammonium rayon fibers with a 224-μm o.d. and 35,000 molecular weight cutoff (C series; Terumo Medical Corp., Somerset, NJ) was fixed over the fused silica into the PE10 tubing with epoxy. The open end of the dialysis fiber was sealed with a 0.5-mm epoxy plug, and a region was coated with epoxy, leaving an active area of 1 mm for exchange across the membrane. The in vitro recovery rate was determined by placing the probe in a beaker filled with artificial cerebrospinal fluid containing a known concentration of NE or 5-HT standard. The concentration of NE or 5-HT in the perfusate was compared with the amount in the bath. Probes that did not correspond to the typical range of recovery (11–21%) were identified and eliminated. Because the diffusion properties of neurochemicals in brain tissue are probably different from those in in vitro conditions, dialysate values reported were not corrected for the recovery of the probe.
Operations
Microdialysis Studies.
Animals were allowed to acclimate to the animal facility for at least 1 week before surgery. The day before an experiment, rats were anesthetized with halothane (0.75% in oxygen), positioned in a stereotaxic apparatus with the skull flat, and allowed to breathe spontaneously. Under anesthesia, microdialysis probes were surgically implanted into the VPM (anteroposterior −3.5, mediolateral −2.9, and dorsoventral −5.5) thalamus. The rats were then placed in cylindrical Plexiglas containers lined with bedding material, connected to a liquid swivel by a spring tether, and allowed to recover overnight.
Electrophysiology Studies.
Adult male (150–300 g) Long-Evans Hooded rats were anesthetized with sodium pentobarbital (17.5 mg/kg i.p.) and choral hydrate (400 mg/kg i.p.). The animal's body temperature was monitored using a rectal probe and maintained at 37°C by a heating pad. Anesthesia was maintained throughout the surgery such that animals were not responsive to foot pinch, the blinking reflex was absent, and the breathing was slow and regular. Supplemental doses of sodium pentobarbital and choral hydrate were alternately administered as needed. Rats were positioned in a stereotaxic apparatus with the skull flat. An incision was made down the scalp, and the skull and dura mater overlying the VPM thalamus were removed to expose the brain tissue. Lidocaine gel was used to locally anesthetize incised skin. A small hole was drilled in the skull, centered 3.5 mm posterior and 2.9 mm lateral to bregma. Four stainless-steel screws were fixed to the skull to serve as electrical grounds and anchors for cementing the electrodes. Microelectrode bundles (eight microwires per bundle) were implanted in the VPM thalamus. The microelectrode bundles were placed initially using stereotaxic coordinates (anterior 3.5, lateral 2.9, and dorsoventral 5.5); however, the final position of each bundle was determined using electrophysiological verification that the site contained cells that met established criteria for VPM thalamic neurons. Microelectrode bundles were permanently attached to the head using a connector and dental cement. The wound was sutured, and the animal was administered topical antibiotics to prevent infection. All animals were allowed to recover for 1 week before the beginning of unit recording sessions.
Repeated Drug Administration and Microdialysis Experiments
We used a repeated drug administration protocol similar to that described by Kalivas et al. (1998). Animals were given injections of MDMA (3 mg/kg i.p.) once per day for 4 days. Of importance, intraperitoneal injection of 3 mg/kg MDMA results in peak plasma levels of MDMA (322.5 ± 19.738 ng/ml) that are similar to those seen in humans (223 ± 48 ng/ml) after an oral dose of 100 mg (Pizarro et al., 2002). On day 5, microdialysis probes were surgically implanted into the VPM thalamus (as described above). Baseline sample collection began the following morning (day 6). Rats received a challenge injection of MDMA (3 mg/kg i.p.) or vehicle after 2 h of baseline sampling. Dialysate samples were collected every 20 min for 7 h and frozen at −80°C for subsequent HPLC analysis.
HPLC
5-HT Detection and Quantification.
Samples of 15 μl (each sample was divided in half to analyze both 5-HT and NE) were injected directly onto the HPLC column using an autosampler (model 542; ESA Inc., Chelmsford, MA). The HPLC system consisted of an ESA solvent delivery system and an ESA column (model MD-150; 150 × 2 mm). The mobile phase consisted of 32 mM NaH2PO4, 0.67 μM EDTA, 0.43 mM octyl sulfate, and 19% methanol adjusted to a pH of 5.6. The detection system consisted of an ESA Coulochem II electrochemical detector with two electrodes in series, the guard cell set at +150 mV and the compounds of interest quantified at the analytical cell (model 5041; ESA, Inc.), which was set at +500 mV. Peak heights were measured and compared with the peak height of a 10−8 M standard calibrated daily. The detection limit, defined as the sample amount producing a peak height that was twice the height of background noise, was approximately 0.5 pg of 5-HT.
NE Detection and Quantification.
Samples of 15 μl were injected directly onto the HPLC column using an autosampler (model 542; ESA, Inc.). The HPLC system consisted of an ESA solvent delivery system, and an ESA column (model MD-150; 150 × 2 mm). The mobile phase consisted of 60 mM sodium phosphate buffer (pH 4.2) with 100 μm EDTA, 1.5 mM sodium octyl sulfate, and 3.5% (v/v) methanol. The detection system consisted of an ESA Coulochem II electrochemical detector with two electrodes in series, the applied potential of the guard cell set at −150 mV and the compounds of interest quantified at the analytical cell (model 5041; ESA, Inc.), which was set at +220 mV. Peak heights were measured and compared with the peak height of a 10−8 M standard calibrated daily. The detection limit, defined as the sample amount producing a peak height that was twice the height of background noise, was approximately 0.5 pg of NE.
Extracellular Unit Recordings
After 1 week, recovery animals were anesthetized with isoflurane, positioned in a stereotaxic apparatus, and allowed to breathe spontaneously. Previously implanted microelectrode bundles were used to record the extracellular activity from ensembles of single neurons in the VPM thalamus. Output from the electrodes was amplified and displayed on an oscilloscope and sent to a window discriminator for spike isolation. Neuronal activity was recorded from the eight microelectrodes simultaneously using the multichannel acquisition processor hardware and real-time acquisition system programs for unit timing in neuroscience (RASPUTIN; Plexon Inc., Dallas, TX). Data acquisition parameters (amplification, filtering, and threshold of detection) were set independently for each channel. Multiple methods for real-time spike sorting online were available, such as time-voltage window discrimination and template-based discrimination. As an alternative, we stored all waveforms that crossed the detection threshold and sorted them later using an off-line sorter. Output from the window discriminator was sent to a digital computer to build real-time poststimulus time histograms (PSTHs) and raster records of neuronal activity.
Single-unit recordings in our data were verified off-line by analyzing waveforms, as well as interspike interval histograms. A recording was identified as a single unit if it met all three of the following criteria: 1) reproducible waveforms being present; 2) interspike interval histograms showing <5% of the interspike intervals being shorter than 1.2 ms; and 3) the amplitude of the waveform being at least three times the average background noise levels.
We generated PSTHs in NeuroExplorer (Plexon), using the whisker pad stimulation as the reference event and a bin width of 1 ms. Two hundred 2-s trials (20 min total) were used to build PSTHs. Each recording session consisted of one 20-min control period, one 20-min period immediately after a saline (0.09% i.p.) injection, and 11 20-min periods after a challenge injection of MDMA (3 mg/kg i.p.). All animals received repeated MDMA treatment (3 mg/kg i.p., once per day for 4 days). The duration of the chronic recordings was determined from the results of our microdialysis studies. We chose recording periods based on the time course of MDMA-induced increases in extracellular 5-HT and NE.
The response to the whisker deflection (see below) usually consisted of a short latency excitation (E1), followed by a long latency excitation (E2). Numerical data from PSTHs were sent to MATLAB (The MathWorks, Inc., Natick, MA) for the determination of the amplitude of the whisker-evoked response and the spontaneous firing rate (SFR) during the 400 ms preceding whisker deflection. The amplitude of E1 (peak E1) was defined as the maximum amplitude during the first 25 ms after whisker stimulation minus the SFR. The onset, offset, latency, and duration of whisker-evoked responses were determined using a Gaussian 95% confidence interval. The onset (onset E1) and offset (offset E1) of the whisker-evoked response were defined as the time during which the firing rate increased and decreased above and below the 95% confidence interval limit, respectively. The duration of E1 was determined by subtracting onset E1 from offset E1. The latency of E1 was defined as the time in milliseconds (beginning 2 ms after whisker stimulation to account for any potential artifact from the whisker stimulator) that it took to reach the peak amplitude of the E1 response. The onset of E1 occurred between 4 and 9 ms after whisker stimulation, and the latency of E1 was between 4 and 20 ms.
Thalamic field potentials were collected throughout the duration of each recording to determine the effects of MDMA administration on the animal's level of arousal. Power spectrum analysis was performed for each 20-min interval using a maximum value of 100 Hz and 128 frequency values.
Vibrissae Stimulation
Somatosensory afferent pathways were activated by mechanical displacement of single whiskers on the side of the muzzle contralateral to recording sites in the VPM thalamus. Initially a cell's receptive field was characterized by deflecting individual whiskers with a hand-held probe while listening to spike discharge on an audio monitor. After initial characterization of a cell's receptive field, a multi-angular piezoelectric stimulator was used to produce uniform displacement of individual whiskers. Activation of the piezoelectric stimulator triggered computer collection of spike train data. This device has the ability to reliably generate a range of small whisker deflections (1–1000 μm) in any direction (360°) or velocity (1–1440°/s) of motion as a linear function of input voltage. To determine whether MDMA administration differentially affects responsiveness of VPM thalamic neurons to whisker stimulation, we stimulated the primary whisker using two randomly presented stimulation intensities that we termed “medium” and “high.” Medium- and high-intensity whisker stimulation caused whisker deflection of ∼200 and 500 μm, respectively. Deflection of the whisker with the medium intensity stimulus produced a less robust response in VPM thalamic neurons compared with high-intensity whisker stimulation. In all cases, single whisker deflection was achieved without perturbation of surrounding whiskers. For detailed information on this device, see Armstrong-James and Fox (1987).
Histology
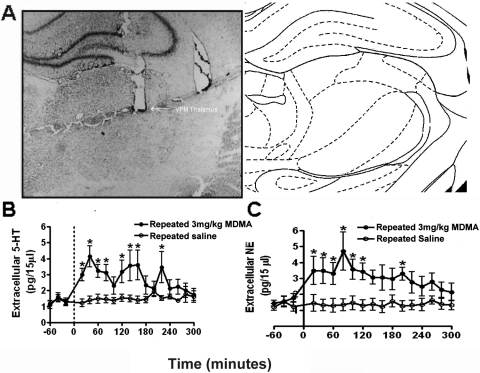
After each experiment, the probe location was marked by infusion of 2% pontamine sky blue dye through the microdialysis probe. After euthanasia, the brains were removed, sectioned, and stained with neutral red for histological verification of probe or electrode placement within the VPM thalamus (Fig. 1A).
Fig. 1.
A, coronal section of the rat brain illustrating electrode placement in the VPM thalamus. Low-power photomicrograph demonstrating the tip of the microelectrode bundle in the VPM thalamus. The section was stained with neutral red. B, effects of repeated systemic administration of MDMA on extracellular 5-HT levels in the rat VPM thalamus. Serotonin levels were measured using in vivo microdialysis with HPLC. Significant prolonged fluctuating increases in extracellular 5-HT were elicited by a challenge injection of MDMA after repeated MDMA administration (n = 6 animals). Repeated administration of saline (n = 4 animals) did not elicit any significant changes in extracellular 5-HT. C, effects of repeated systemic administration of MDMA on extracellular NE levels in the rat VPM thalamus. Norepinephrine levels in the rat VPM thalamus were measured using in vivo microdialysis with HPLC. Significant increases in extracellular NE were elicited by a challenge injection of MDMA after repeated MDMA administration (n = 6 animals). Repeated administration of saline (n = 4 animals) did not elicit any significant changes in extracellular NE. All data are presented as the mean ± S.E.M.; *, significant changes (Dunnett's post hoc test, p > 0.05).
Data Analysis
Microdialysis Experiments.
The baseline value against which MDMA administration was compared was derived from the average of three samples immediately before drug injection. Extracellular neurotransmitter levels are expressed as the mean ± S.E.M. The overall effect of MDMA on 5-HT and NE efflux and locomotor activity were analyzed using two-way analysis of variance (ANOVA) with repeated measures. The absolute amount of neurotransmitter measured in dialysates (picograms per 15 μl) was used as the dependent variable for assessment of within-group effects, and the absolute change was measured using a one-way ANOVA with a Dunnett's post hoc test.
Electrophysiology Experiments
Computer-generated PSTHs and cumulative raster records were used to characterize stimulus-evoked responses and quantitate evoked activity as both spikes/stimulus (excitations only) and percentage of baseline spontaneous firing rate (excitations and inhibitions). Rasters and histograms were generated before and after intraperitoneal administration of MDMA. Equal numbers of stimuli were used to compute each histogram.
Changes in evoked and spontaneous activity were calculated for each cell by comparing discharges in identical portions of histograms computed during saline and MDMA postinjection periods. Spontaneous and evoked discharge rates were calculated as described above. These rates were computed from control and post-MDMA injection histograms and compared, and the difference is expressed as percentage increase or decrease from control response. Spikes per stimulus comparisons were made between histograms using a similar method. Changes in stimulus-bound activity between saline and MDMA postinjection periods were assessed for statistical significance using one-way ANOVA. Such analytical procedures have been used previously in our laboratory to define 5-HT and NE actions on rodent somatosensory and visual cortical neurons.
Results
Effects of Repeated MDMA Administration on 5-HT Efflux in VPM Thalamus.
A challenge injection of MDMA after repeated systemic MDMA administration (3 mg/kg once per day for 4 days) led to a rapid prolonged increase in 5-HT efflux in the VPM thalamus (F6,15 = 13.53, p = 0.0001, one-way repeated-measures ANOVA) (Fig. 1B). MDMA (3 mg/kg) increased the concentration of extracellular 5-HT in dialysates from 1.337 ± 0.145 to 4.145 ± 0.649 pg/15 μl. These effects were evident within 20 min of drug administration. 5-HT levels peaked 40 min after MDMA administration. Levels returned to baseline within 3 h and 20 min (Dunnett's post hoc test, p < 0.05). Thus, a challenge injection of MDMA after repeated treatment leads to prolonged increases in 5-HT efflux compared with that in single-treatment animals (Fig. 2A) (Starr et al., 2008). Extracellular levels of 5-HT did not change significantly after saline injection (1.341 ± 0.250 to 1.416 ± 0.228 pg/15 μl) (Fig. 1B).
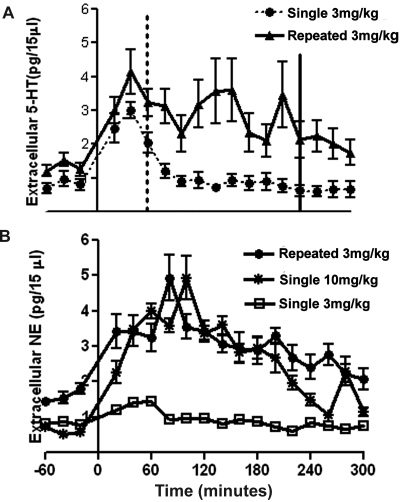
Fig. 2.
A, comparison of the effects of single (acute) versus repeated MDMA administration on 5-HT efflux in the VPM thalamus. Both single (n = 6 animals) and repeated (n = 6 animals) MDMA administration led to significant increases in 5-HT efflux. However, prolonged fluctuating increases in extracellular 5-HT were elicited by a challenge injection of MDMA in repetitively treated animals compared with those receiving a single administration of the drug, The dashed and solid vertical lines indicate the time at which 5-HT levels returned to baseline after single and repeated MDMA administration, respectively. B, effects of single versus repeated MDMA administration on NE efflux in the VPM thalamus. Single low-dose (3 mg/kg; n = 6 animals) MDMA administration had no significant effects on NE efflux, whereas single high-dose (10 mg/kg; n = 6 animals) and repeated low-dose (3 mg/kg once per day for 4 days; n = 6 animals) MDMA administration produced significant prolonged increases in NE efflux. All data are presented as the mean ± S.E.M. (Single administration data are taken from Starr et al., 2008, in which it was reported as acute administration; hence, the labeling remains the same in this figure. Repeated administration data are from Fig. 1.).
Effects of Repeated MDMA Administration on NE Efflux in VPM Thalamus.
A challenge injection of MDMA after repeated systemic MDMA administration led to a rapid prolonged increase in NE efflux in the VPM thalamus (F5,15 = 19.31, p = 0.0001, one-way repeated measures ANOVA) (Fig. 1C). MDMA (3 mg/kg) increased the concentration of extracellular NE in dialysates from 1.338 ± 0.171 to 4.715 ± 1.205 pg/15 μl. These effects were evident within 20 min of drug administration and peaked 1 h and 20 min after MDMA administration. NE levels returned to baseline within 3 h and 20 min (Dunnett's post hoc test, p < 0.05). Extracellular levels of NE did not change significantly after saline injection (1.326 ± 0.291 to 1.459 ± 0.518 pg/15 μl). (Fig. 1C). Thus, administration of a challenge injection after repeated drug treatment elicits both 5-HT and NE efflux in the VPM thalamus. This result was in contrast to the selective increase in 5-HT efflux that was observed after a single injection of the same dose (3 mg/kg i.p.) in naive animals (Fig. 2, A and B) (Starr et al., 2008). In addition, a challenge injection of MDMA after repeated low-dose treatment produces effects on NE efflux similar to those of a single high-dose (10 mg/kg i.p.) injection of MDMA (Starr et al., 2008) (Fig. 2B).
Effects of Repeated MDMA Administration on VPM Thalamic Unit Responsiveness (Peak E1) to Whisker Deflection.
A challenge injection of MDMA (3 mg/kg) after repeated MDMA treatment (3 mg/kg × 4 days) led to a reduction in the magnitude of short latency responses (peak E1) to medium- and high-intensity whisker stimulation (F66,12 = 4.783, p < 0.0001, one-way ANOVA and F66,12 = 3.844, p < 0.0001, one-way ANOVA, respectively) (Fig. 3, A and B). Unlike its effects in naive animals treated with a single dose of the drug, MDMA's suppressant effects on whisker responsiveness to high-intensity whisker stimulation (n = 7 animals, 45 cells, 67 responses) were blunted after repeated drug treatment (Fig. 5A). Peak E1 was defined as the maximum firing rate that occurs from 2 to 25 ms after stimulus onset minus the spontaneous firing rate.
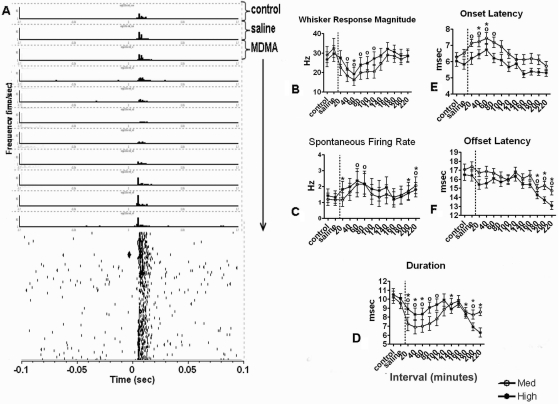
Fig. 3.
A, representative example of the effects of a challenge injection of MDMA (3 mg/kg) after repeated MDMA administration (3 mg/kg once per day for 4 days) on VPM thalamic neuron responsiveness to mechanical whisker stimulation. Peri-event histograms (above) and raster records (below) illustrate the response of a single VPM neuron to whisker deflection, before (control) and after challenge injections of saline and MDMA. Time 0 represents stimulus onset. Each vertical dash in the raster record represents the occurrence of a single action potential. Peri-event histograms represent cell activity during 20-min intervals. The black diamond in the raster record corresponds to the time of MDMA injection. Spikes were collected for 3 h and 20 min after drug injection. B, effects of repeated MDMA administration on VPM thalamic unit responsiveness (peak E1) to mechanical whisker deflection. Repeated MDMA (3 mg/kg for 4 days) administration led to decreased responsiveness to medium- and high-intensity whisker stimulation. C, effects of repeated MDMA administration on the SFR of neurons in the VPM thalamus. Repeated MDMA administration led to an increased level of spontaneous firing during medium- and high-intensity whisker stimulation, followed by a return to control levels. D, effects of repeated MDMA administration on the duration of the response. MDMA administration decreased the duration of the whisker-evoked response during medium- and high-intensity whisker stimulation. E, effects of repeated MDMA administration on the onset latency of the response. Repeated MDMA administration led to an increase in the onset latency during medium- and high-intensity whisker stimulation. F, effects of repeated MDMA administration on the offset latency of the response. There was a significant decrease in the offset latency after repeated drug treatment, but this effect was observed only during the last 60 min of the recording session. B–F, * and ○, significant changes from control (Dunnett's post hoc test, p > 0.05) for high-intensity versus medium-intensity whisker stimulation, respectively. The vertical dashed lines indicate the time of challenge MDMA injection.
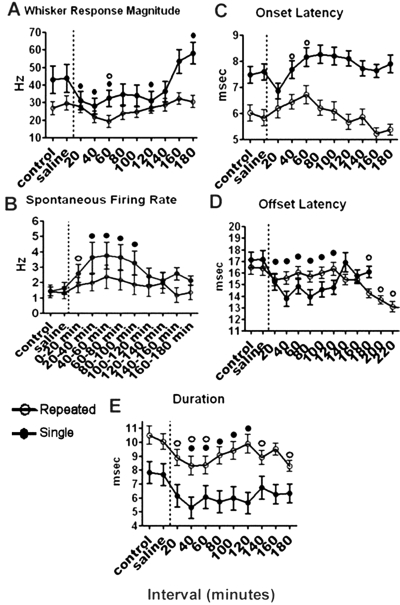
Fig. 5.
A, effects of single versus repeated MDMA administration on VPM thalamic unit responsiveness (peak E1) to mechanical whisker deflection. A single (3 mg/kg) MDMA administration decreased responsiveness to medium- and high (data not shown)-intensity whisker stimulation 10 to 120 min after drug and increased responsiveness at 160 to 180 min after drug administration. A challenge injection of MDMA (3 mg/kg) after repeated drug administration (3 mg/kg for 4 days) led to a reduction in the response magnitude (peak E1) to medium- and high (data not shown)-intensity whisker stimulation, respectively. Unlike the single drug treatment condition, MDMA's effects on whisker responsiveness to high-intensity whisker stimulation were blunted after repeated MDMA administration. B, effects of single versus repeated MDMA administration on the SFR of neurons in the VPM thalamus. Acute MDMA administration led to an increased level of spontaneous firing during medium- and high (data not shown)-intensity whisker stimulation, followed by a return to control levels. Repeated MDMA administration increased the spontaneous firing rate of individual neurons during medium- and high (data not shown)-intensity whisker stimulation, followed by a return to control levels, respectively. However, after repeated MDMA administration, the effects on spontaneous firing were blunted compared with those for acute, single-dose treatment. C, effects of single versus repeated MDMA administration on the onset latency of the response. Single administration of MDMA had no significant effect on the onset latency of the whisker-evoked response. A challenge injection of MDMA after repeated MDMA administration led to an increase in the onset latency during medium- and high (data not shown)-intensity whisker stimulation, respectively. However, the onset latency to medium- and high (data not shown)-intensity whisker stimulation was shorter during the control period after repeated MDMA administration compared with that observed in the drug-naive animal. D, effects of single versus repeated MDMA administration on the offset latency of the response. MDMA administration led to an initial decrease in the offset latency during medium- and high (data not shown)-intensity whisker stimulation. A challenge injection of MDMA after repeated MDMA administration led to a significant decrease in the offset latency after repeated MDMA administration, but this effect was observed only during the last 60 min of the recording session. MDMA's effects on offset latency appear to be greater in the single-treatment animals. E, effects of single versus repeated MDMA administration on the duration of the response. Single administration of MDMA decreased the duration of the whisker-evoked response during medium- and high (data not shown)-intensity whisker stimulation. Repeated MDMA administration also decreased the duration of the whisker-evoked response during medium- and high (data not shown)-intensity whisker stimulation. The dashed vertical lines indicate the time of MDMA injection. ● and ○, significant changes from control after single versus repeated MDMA administration, respectively (Dunnett's post hoc test, p > 0.05). (Single administration data are taken from Starr et al., 2008.).
Effects of Repeated MDMA Administration on the SFR of Neurons in the VPM Thalamus.
A challenge injection of MDMA after repeated MDMA treatment increased the spontaneous firing rate of individual neurons during medium- and high-intensity whisker stimulation, followed by a return to control levels (F37,12 = 2.088, p < 0.01, one-way ANOVA and F44,12 = 2.43, p < 0.004, one-way ANOVA, respectively) (Fig. 3C). In addition, a challenge injection led to a second significant increase in the spontaneous firing rate 180 to 220 min postdrug (Fig. 3C). Of interest, the effects on spontaneous firing were blunted compared with single MDMA treatment conditions (Fig. 5B). SFR was defined as the average firing rate 400 ms before stimulation onset. Scatterplots were generated to illustrate the change in peak E1 and SFR for each unit. Percentage change in peak E1 and SFR was calculated using the following formulas: percentage change peak E1 = control peak E1 − peak E1 (during peak drug effect) × 100; and percentage change SFR = control SFR − drug SFR (during peak drug effect) × 100, respectively. After a challenge injection of MDMA, the majority of points plotted from this analysis lie above a 45° equivalence line, indicating cases in which MDMA suppressed evoked discharge and increased the spontaneous firing rate of the recorded neuron (Fig. 4).
Fig. 4.
Scatterplot diagram summarizing the effects of a challenge injection of MDMA after repeated MDMA administration on responsiveness to medium- and high-intensity whisker stimulation. A, effects of a challenge injection of MDMA on responsiveness to medium-intensity whisker stimulation. B, effects of chronic MDMA administration on responsiveness to high-intensity whisker stimulation. The results shown here are taken from 40 to 60 min after MDMA administration, when the drug's strongest effects on whisker responsiveness were observed. In both cases, the majority of cells lie above the 45% line, which indicates that MDMA suppresses evoked discharge and increases the spontaneous firing rate of VPM neurons.
Effects of Repeated MDMA Administration on the Onset Latency of the Whisker-Evoked Response.
A challenge injection of MDMA after repeated MDMA treatment led to an increase in the onset latency during medium- and high-intensity whisker stimulation (F66,12 = 8.345, p < 0.0001, one-way ANOVA and F66,12 = 9.561, p < 0.0001, one-way ANOVA, respectively) (Fig. 3E). However, the onset latency to medium- and high-intensity whisker stimulation was shorter during the control period after repeated drug treatment compared with that observed in the single-treatment, drug-naive animal (control period on day 1) (t66 = 2.343, p = 0.0227 and t66 = 2.392, p = 0.0195, respectively) (Fig. 5C). Onset latency was defined as the time in milliseconds from stimulation onset to when the firing rate first crosses the 95% Gaussian confidence interval minus 2 ms to control for stimulation-related artifact.
Effects of Repeated MDMA Administration on the Offset Latency of the Whisker-Evoked Response.
A challenge injection of MDMA after repeated drug treatment led to a significant decrease in the offset latency after repeated drug treatment, but this effect was observed only during the last 60 min of the recording session (Dunnett's post hoc test, p < 0.05) (Fig. 3F). Overall MDMA's effects on offset latency appear to be greater in the single-treatment, drug-naive animals described in Starr et al. (2008) (Fig. 5D). Offset latency was defined as the time in milliseconds from stimulation onset to when the firing rate first falls below the 95% Gaussian confidence interval (after the peak response) minus 2 ms to control for stimulation-related artifact.
Effects of Repeated MDMA Administration on the Duration of the Whisker-Evoked Response.
Similar to the effect in the single-treatment, drug-naive animals described in the previous study (Starr et al., 2008), a challenge injection of MDMA after repeated drug treatment also reduced the duration of the whisker-evoked response during medium- and high-intensity whisker stimulation (F66,12 = 5.439, p < 0.0001, one-way ANOVA and F66,12 = 10.44, p < 0.0001, one-way ANOVA, respectively) (Fig. 3D). For a decrease in duration of the whisker-evoked response to occur, either the onset latency alone must increase, the offset latency alone must decrease, or the onset latency must increase combined with a decrease in the offset latency. It appears that single MDMA administration reduces the duration of the whisker-evoked response by causing a decrease in the offset latency, whereas repeated MDMA treatment increases the onset latency of the response (Fig. 5E). Duration was defined as the offset latency minus the onset latency in milliseconds.
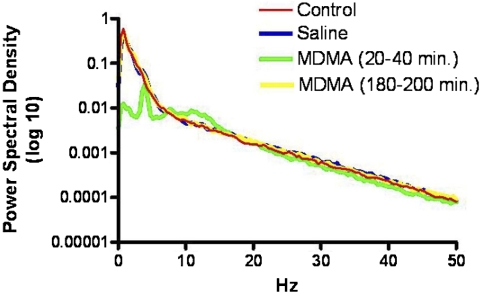
Effects of Repeated MDMA Administration on Thalamic Field Potential Activity.
Power spectral density (PSD) analysis was performed to determine the effects of repeated MDMA administration on thalamic field potential activity. Similar to the effects of single MDMA administration in drug-naive animals, a challenge injection of MDMA after repeated MDMA administration also led to decreases in the mean PSD values (Fig. 6). In other words, MDMA administration causes a reduction in high-frequency field potential activity compared with control periods. This suggests that administration of MDMA to an anesthetized animal causes the animal to transition to a “lighter” level of anesthesia and, as a result, more excitable sensory pathways and increased spontaneous discharge as reported by Chapin et al. (1981). Instead, we observed suppression of sensory-evoked discharge in the VPM thalamus after MDMA administration. Time had no effect on the mean PSD values in the thalamus (Fig. 7A from Starr et al., 2008).
Fig. 6.
Representative example showing the effects of a challenge injection of MDMA after repeated MDMA administration on thalamic field potential activity. Power spectral density analysis was performed at 20-min intervals before and after a challenge injection of MDMA. The halothane level remained constant throughout the recording session. A challenge injection of MDMA led to a reduction in low-frequency activity (n = 6 animals).
Discussion
Summary of Findings.
First, we found that administration of a challenge injection (3 mg/kg i.p.) of MDMA after repeated MDMA treatment elicits both 5-HT and NE efflux in the VPM thalamus. Of importance, extracellular 5-HT levels remain elevated longer in the repeated-treatment rats compared with the single-treatment animals as described in the previous report (Starr et al., 2008). Furthermore, the observed increases in NE efflux after challenge injection in repeated-treatment animals were similar in time course and magnitude to those with a single high-dose injection of MDMA (10 mg/kg) in naive animals. Because we conducted the electrophysiology recordings in the anesthetized rat, we performed a series of control microdialysis experiments using an anesthetized preparation to ensure that MDMA-induced efflux of 5-HT and NE in the VPM thalamus is comparable to that observed in the waking rat. Of importance, both 5-HT and NE efflux were increased by a challenge dose of MDMA in the VPM thalamus of a repetitively treated, anesthetized animal (Supplemental Fig. 1).
Second, we demonstrated that a challenge injection of MDMA after repeated systemic MDMA administration leads to decreases in magnitude and timing of individual sensory neuron responses to whisker stimulation. However, the increase in responsiveness to whisker stimulation 160 to 180 min after drug administration that was observed in the single-treatment animals was absent after a challenge injection of MDMA in animals receiving repeated drug treatment. Of interest, the MDMA's suppressant effects on whisker responsiveness to high-intensity whisker stimulation are blunted after repeated drug treatment. After repeated MDMA administration, the effects of the drug on spontaneous firing rate are also blunted compared with single-dose treatment. The picture that emerges is one in which repetitive drug treatment sensitizes neurochemical responses and desensitizes electrophysiological responses in the brain to subsequent drug administration. Nevertheless, as with single-dose drug administration in naive animals, the net effect of suppressed evoked discharge and increased spontaneous firing after challenge injections in repetitively treated animals is a pronounced reduction in the signal/noise ratio for sensory signals within the VPM thalamus.
MDMA Pharmacokinetics After Repeated MDMA Administration.
In the present study, we did not measure plasma levels of MDMA after challenge doses of the drug in repetitively treated animals. However, other studies have reported nonlinear pharmacokinetics of MDMA (de la Torre et al., 2000; Mueller et al., 2008; Baumann et al., 2009) and suppressed liver function after repeated MDMA administration (Beitia et al., 2000; Moon et al., 2008; Cerretani et al., 2011). In combination after several days of repeated MDMA administration, these effects might result in elevated circulating MDMA levels after challenge drug injection and subsequent enhancement of neurochemical and electrophysiological effects.
Impact of Repeated MDMA on VPM Neuronal Responses to Sensory Input.
The initial decrease in response latency and decrease in offset latency that was observed in the single-treatment animals was absent after a challenge injection of MDMA after repeated drug treatment. However, similar to single-dose MDMA administration, repeated drug treatment also decreases the duration of the whisker-evoked response. In single- and repeated-treatment animals, MDMA administration decreases the duration of the whisker-evoked response through a reduction in the offset latency and increase in the onset latency, respectively. Therefore, it appears that the net effect of both single and repeated MDMA administration is to reduce neuronal responsiveness to sensory-driven afferent inputs. Furthermore, there is evidence of changes in response to MDMA or baseline neuronal activity that suggests persistent drug effects in repeated-treatment animals.
The observation that many of the electrophysiological effects observed with single-dose drug administration are blunted after repetitive MDMA treatment could help explain tolerance to MDMA's positive effects as reported by human MDMA users (Parrott, 2005). In humans, tolerance to MDMA's positive effects occurs within the first few drug-taking sessions (Greer and Tolbert, 1986); thus, it is possible that the blunted neurophysiological effects of MDMA after our repeated dosing regimen represents a similar form of tolerance in the rat.
Of importance, our microdialysis studies demonstrated that MDMA selectively increases 5-HT efflux when administered singly at low doses (3 mg/kg i.p.) and increases both 5-HT and NE efflux in the VPM thalamus when a challenge injection of MDMA (3 mg/kg i.p. once per day for 4 days) is administered after repeated drug treatment. Therefore, the current study was the first to examine indirectly, via repeated systemic MDMA administration, the electrophysiological effects of simultaneous 5-HT and NE efflux in the VPM thalamus. Because it was shown previously that 5-HT increases the spontaneous firing rate and suppresses sensory-evoked discharge and because NE was shown to produce the opposite effects (Waterhouse and Woodward, 1980; Waterhouse et al., 1986, 1990), it is possible that efflux of each transmitter after challenge doses in repetitively treated animals acts to counterbalance the effects of the other on neuronal responsiveness. Such mitigating actions could explain the blunting of electrophysiological effects in repetitively treated rats and some aspects of tolerance to drug effects reported by human recreational users. In general, this effect is consistent with evidence of tolerance to various MDMA effects after repeated drug administration in humans (Parrott, 2005), nonhuman primates (Frederick et al., 1995; Fantegrossi, 2008), and rats (Callaway and Geyer, 1992; Marston et al., 1999; Baumann et al., 2008; Reveron et al., 2010).
To our knowledge this is the first demonstration of sensitization of noradrenergic neurotransmission after repeated MDMA administration. However, there was a previous report (Kalivas et al., 1998) of behavioral (increased motor activity) and neurochemical (dopamine transmission in the nucleus accumbens) sensitization to repeated MDMA administration. These findings are generally consistent with other reports of changes in catecholamine transmitter responses after repeated exposure to psychostimulant drugs (Pierce and Kalivas, 1998; Cadoni et al., 2000). In our study, we observed increased monoamine neurotransmission and blunted electrophysiological responses to MDMA challenge in animals receiving repeated administrations of MDMA. One might expect parallel increases in these measures of brain activity; however, the complex interplay and net outcome of drug-induced increases in NE and 5-HT neurotransmission on neuronal discharge in intact animals is difficult to predict. As described above, increased release of NE after challenge MDMA administration in repetitively treated animals could offset the 5-HT-mediated effects on spontaneous and evoked discharge, thereby providing the appearance of blunted electrophysiological responses.
MDMA and Sensory Perception in Human Subjects.
At first glance, it appears that there is a paradox between the results presented here and human reports of MDMA's effects on tactile sensations. Why do humans report feelings of enhanced tactile sensations, when the results here and previously (Starr et al., 2008) show that MDMA administration ultimately results in suppressed responsiveness of VPM thalamocortical neurons to tactile stimulation? Although there is considerable risk in extrapolating from single-unit recording studies in animal models to drug influences on human perception, there are several points worth mentioning in the context of the current study.
First, as mentioned previously, it is not known what effects MDMA has on other components of the primary somatosensory pathway, e.g., somatosensory cortical, posterior medial/nucleus reticularis thalamic, and trigeminal ganglion neurons. Despite MDMA's potent effects on VPM thalamocortical neurons, the perceived effects of the drug on tactile awareness may emerge from combinatorial effects on multiple brain regions. This is likely, considering that MDMA affects neurotransmitter systems (i.e., serotonergic and noradrenergic) that send widespread projections to multiple levels of the neuraxis, including all levels of the trigeminal somatosensory pathway.
Second, it must be taken into account that there could be a discrepancy between human subjective reports and what is actually occurring in sensory circuits after MDMA consumption. Although humans report an enhanced sense of tactile awareness after MDMA consumption, the reality may be a “distortion” of tactile sensations deriving from reduced neuronal responsiveness to sensory-driven synaptic input. This distortion may be perceived as enhancement by virtue of the fact that it represents an altered sensory experience but not necessarily an augmented signal. By analogy, humans sometimes report that they can drive a vehicle better when they are intoxicated (Flemen, 1997), but, in fact, many studies have demonstrated that the opposite is true (Ferrara et al., 1994; Ogden and Moskowitz, 2004).
Third, although we demonstrated MDMA-induced suppression of sensory-evoked VPM thalamic responses, we also showed that the basal discharge of these cells increased. This means that there is a generalized increase in thalamocortical transmission after MDMA administration, albeit nonspecific with respect to the stimulus. This increase in the amount of “noise” reaching the cortex could, in part, be responsible for the distorted tactile sensations reported by human recreational users.
In summary, both single-dose and repetitive administration of MDMA alters levels of monoamine transmitters across broad regions of the brain and specifically modifies transmission of somatosensory signals through the thalamus. A question remaining for future study is why such actions are not prominently expressed in other sensory modalities.
Supplementary Material
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants 1-F31-DA018469-01A, R21-DA023711].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- MDMA
- 3,4-methylenedioxymethamphetamine
- 5-HT
- serotonin
- NE
- norepinephrine
- VPM
- ventral posterior medial
- HPLC
- high-performance liquid chromatography
- PSTH
- poststimulus time histogram
- SFR
- spontaneous firing rate
- ANOVA
- analysis of variance
- PSD
- power spectral density.
Authorship Contributions
Participated in research design: Starr, Page, and Waterhouse.
Conducted experiments: Starr.
Performed data analysis: Starr.
Wrote or contributed to the writing of the manuscript: Starr, Page, and Waterhouse.
References
- Abercrombie ED, Keller RW, Jr, Zigmond MJ. (1988) Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience 27:897–904 [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K. (1987) Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol 263:265–281 [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB. (2008) Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience 152:773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. (2009) Effects of dose and route of administration on pharmacokinetics of (+ or −)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos 37:2163–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitia G, Cobreros A, Sainz L, Cenarruzabeitia E. (2000) Ecstasy-induced toxicity in rat liver. Liver 20:8–15 [DOI] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Di Chiara G. (2000) Psychostimulant sensitization: differential changes in accumbal shell and core dopamine. Eur J Pharmacol 388:69–76 [DOI] [PubMed] [Google Scholar]
- Callaway CW, Geyer MA. (1992) Tolerance and cross-tolerance to the activating effects of 3,4-methylenedioxymethamphetamine and a 5-hydroxytryptamine 1B agonist. J Pharmacol Exp Ther 263:318–326 [PubMed] [Google Scholar]
- Cerretani D, Bello S, Cantatore S, Fiaschi AI, Montefrancesco G, Neri M, Pomara C, Riezzo I, Fiore C, Bonsignore A, Turillazzi E, Fineschi V. (2011) Acute administration of 3,4-methylenedioxymethamphetamine (MDMA) induces oxidative stress, lipoperoxidation and TNFα-mediated apoptosis in rat liver. Pharmacol Res 64:517–527 [DOI] [PubMed] [Google Scholar]
- Chapin JK, Waterhouse BD, Woodward DJ. (1981) Differences in cutaneous sensory response properties of single somatosensory cortical neurons in awake and halothane anesthetized rats. Brain Res Bull 6:63–70 [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Ortuño J, Mas M, Brenneisen R, Roset PN, Segura J, Camí J. (2000) Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br J Clin Pharmacol 49:104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE. (2008) In vivo pharmacology of MDMA and its enantiomers in rhesus monkeys. Exp Clin Psychopharmacol 16:1–12 [DOI] [PubMed] [Google Scholar]
- Ferrara SD, Zancaner S, Giorgetti R. (1994) Low blood alcohol concentrations and driving impairment. A review of experimental studies and international legislation. Int J Legal Med 106:169–177 [DOI] [PubMed] [Google Scholar]
- Flemen K. (1997) Smoke and Whispers: Drugs and Youth Homelessness in Central London, p 64, Hungerford Drug Project, London [Google Scholar]
- Frederick DL, Ali SF, Slikker W, Jr, Gillam MP, Allen RR, Paule MG. (1995) Behavioral and neurochemical effects of chronic methylenedioxymethamphetamine (MDMA) treatment in rhesus monkeys. Neurotoxicol Teratol 17:531–543 [DOI] [PubMed] [Google Scholar]
- Greer G, Tolbert R. (1986) Subjective reports of the effects of MDMA in a clinical setting. J Psychoactive Drugs 18:319–327 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Kalivas PW, Duffy P, White SR. (1998) MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacology 18:469–479 [DOI] [PubMed] [Google Scholar]
- Kirifides ML, Simpson KL, Lin RC, Waterhouse BD. (2001) Topographic organization and neurochemical identity of dorsal raphe neurons that project to the trigeminal somatosensory pathway in the rat. J Comp Neurol 435:325–340 [DOI] [PubMed] [Google Scholar]
- Marston HM, Reid ME, Lawrence JA, Olverman HJ, Butcher SP. (1999) Behavioural analysis of the acute and chronic effects of MDMA treatment in the rat. Psychopharmacology 144:67–76 [DOI] [PubMed] [Google Scholar]
- Moon KH, Upreti VV, Yu LR, Lee IJ, Ye X, Eddington ND, Veenstra TD, Song BJ. (2008) Mechanism of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy)-mediated mitochondrial dysfunction in rat liver. Proteomics 8:3906–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Peters FT, Maurer HH, McCann UD, Ricaurte GA. (2008) Nonlinear pharmacokinetics of (+/−)3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) and its major metabolites in squirrel monkeys at plasma concentrations of MDMA that develop after typical psychoactive doses. J Pharmacol Exp Ther 327:38–44 [DOI] [PubMed] [Google Scholar]
- Ogden EJ, Moskowitz H. (2004) Effects of alcohol and other drugs on driver performance. Traffic Inj Prev 5:185–198 [DOI] [PubMed] [Google Scholar]
- Parrott AC. (2005) Chronic tolerance to recreational MDMA (3,4-methylenedioxymethamphetamine) or Ecstasy. J Psychopharmacol 19:71–83 [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. (1998) Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J Neurosci 17:3254–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro N, Ortuño J, Farré M, Hernández-López C, Pujadas M, Llebaria A, Joglar J, Roset PN, Mas M, Segura J, et al. (2002) Determination of MDMA and its metabolites in blood and urine by gas chromatography-mass spectrometry and analysis of enantiomers by capillary electrophoresis. J Anal Toxicol 26:157–165 [DOI] [PubMed] [Google Scholar]
- Reveron ME, Maier EY, Duvauchelle CL. (2010) Behavioral, thermal and neurochemical effects of acute and chronic 3,4-methylenedioxymethamphetamine (“Ecstasy”) self-administration. Behav Brain Res 207:500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KL, Altman DW, Wang L, Kirifides ML, Lin RC, Waterhouse BD. (1997) Lateralization and functional organization of the locus coeruleus projection to the trigeminal somatosensory pathway in rat. J Comp Neurol 385:135–147 [PubMed] [Google Scholar]
- Starr MA, Page ME, Waterhouse BD. (2008) MDMA (3,4-methylenedioxymethamphetamine)-mediated distortion of somatosensory signal transmission and neurotransmitter efflux in the ventral posterior medial thalamus. J Pharmacol Exp Ther 327:20–31 [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Azizi SA, Burne RA, Woodward DJ. (1990) Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res 514:276–292 [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. (1986) Interaction of serotonin with somatosensory cortical neuronal responses to afferent synaptic inputs and putative neurotransmitters. Brain Res Bull 17:507–518 [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Woodward DJ. (1980) Interaction of norepinephrine with cerebrocortical activity evoked by stimulation of somatosensory afferent pathways in the rat. Exp Neurol 67:11–34 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.