Abstract
Adenosine is increased in ischemic tissues where it serves a protective role by activating adenosine receptors (ARs), including the A3 AR subtype. We investigated the effect of N-{2-[(3,4-dichlorophenyl)amino]quinolin-4-yl}cyclohexanecarboxamide (LUF6096), a positive allosteric modulator of the A3 AR, on infarct size in a barbital-anesthetized dog model of myocardial ischemia/reperfusion injury. Dogs were subjected to 60 min of coronary artery occlusion and 3 h of reperfusion. Infarct size was assessed by macrohistochemical staining. Three experimental groups were included in the study. Groups I and II received two doses of vehicle or LUF6096 (0.5 mg/kg i.v. bolus), one administered before ischemia and the other immediately before reperfusion. Group III received a single dose of LUF6096 (1 mg/kg i.v. bolus) immediately before reperfusion. In preliminary in vitro studies, LUF6096 was found to exert potent enhancing activity (EC50 114.3 ± 15.9 nM) with the canine A3 AR in a guanosine 5′-[γ-[35S]thio]triphosphate binding assay. LUF6096 increased the maximal efficacy of the partial A3 AR agonist 2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide and the native agonist adenosine more than 2-fold while producing a slight decrease in potency. In the dog studies, administration of LUF6096 had no effect on any hemodynamic parameter measured. Pretreatment with LUF6096 before coronary occlusion and during reperfusion in group II dogs produced a marked reduction in infarct size (∼50% reduction) compared with group I vehicle-treated dogs. An equivalent reduction in infarct size was observed when LUF6096 was administered immediately before reperfusion in group III dogs. This is the first study to demonstrate efficacy of an A3 AR allosteric enhancer in an in vivo model of infarction.
Introduction
Allosteric modulators of G protein-coupled receptors (GPCRs) are ligands that interact with a binding site on the receptor that is topologically distinct from the orthosteric site to which the native ligand binds (Gao and Jacobson, 2006; May et al., 2007; Göblyös and IJzerman, 2011). Binding of an allosteric ligand causes a conformational change that influences the behavior of the receptor in response to its orthosteric ligand. Allosteric modulators can increase [positive allosteric modulator (PAM)] or decrease (negative allosteric modulator) the pharmacological response of orthosteric ligands by modifying their binding affinity and/or their efficacy. Currently, there is great interest in the development of allosteric enhancers of GPCRs, because it is envisioned they will provide therapeutic advantages over conventional orthosteric agonists (Gao and Jacobson, 2006; May et al., 2007; Göblyös and IJzerman, 2011). For instance, PAMs as therapeutic agents are predicted to produce a better clinical response with fewer side effects, because they modulate responses to an endogenous ligand in tissues in a spatially and temporally specific manner rather than producing widespread, persistent receptor activation. Benzodiazepines such as diazepam are a good example of the clinical advantage provided by pharmacological allosterism. These agents, which act as allosteric enhancers of the ion channel-coupled γ-aminobutyric acid type A receptor, are widely used to treat disorders of the central nervous system, including anxiety and insomnia. In contrast, direct-acting γ-aminobutyric acid type A agonists have proven to be much less useful in part because of their potential to produce undesirable side effects. Cinacalcet is the first allosteric modulator of a GPCR to be approved for clinical use. This agent effectively treats certain forms of hyperparathyroidism and functions by allosterically modulating the Ca2+-sensing receptor involved in parathyroid hormone release.
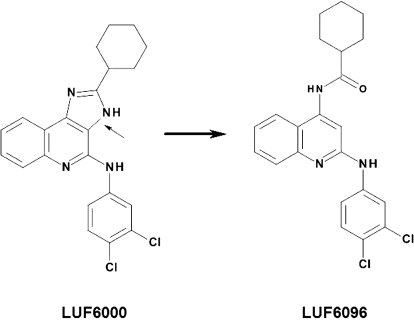
Allosteric enhancers for the A3 adenosine receptor (AR) subtype have been identified and characterized, including the imidazoquinolinamine derivative N-(3,4-dichlorophenyl)-2-cyclohexyl-1H-imidazo[4,5-c]quinolin-4-amine (LUF6000) and the 2,4-di-substituted quinoline derivative N-{2-[(3,4-dichlorophenyl)amino]quinolin-4-yl}cyclohexanecarboxamide (LUF6096) (Gao et al., 2001, 2002, 2008, 2011; Göblyös et al., 2006; Heitman et al., 2009; Kim et al., 2009). These modulators increase the maximal efficacy of orthosteric agonists with the human A3 AR heterologously expressed in Chinese hamster ovary cells, in some assays by more than 2-fold. Unlike other related A3 AR modulators that have been characterized [i.e., 1H-imidazo[4,5-c]quinolin-4-amine (DU124183), in an earlier generation of imidazoquinolinamine series], LUF6000 and LUF6096 increase the maximal efficacy of orthosteric ligands without decreasing potency (Göblyös et al., 2006; Gao et al., 2008, 2011; Heitman et al., 2009; Kim et al., 2009; Göblyös and IJzerman, 2011). LUF6096, which is structurally similar to LUF6000, differing only by opening of the imidazoquinolinamine heterocyclic ring system to leave an amide bridge in place of the imidazole ring (Fig. 1), provides greater A3 AR selectivity versus the A1 AR in terms of enhancing activity and exhibits almost negligible orthosteric effects on A1, A2A, and A3 ARs (Heitman et al., 2009; Göblyös and IJzerman, 2011).
Fig. 1.
Chemical structures of the PAMs of the A3 AR, LUF6000 (left) and LUF6096 (right). The arrow points to the bond deleted in LUF6000 that led to LUF6096.
The production of adenosine is increased substantially in tissues under ischemic conditions because of the breakdown of ATP where it is well known to provide cytoprotection and promote tissue repair via a variety of mechanisms involving all four AR subtypes, including the A3 AR (Ely and Berne, 1992; Auchampach and Bolli, 1999; Headrick and Lasley, 2009). Local enhancement during ischemia/reperfusion injury using an A3 AR PAM may be an effective means to magnify the protective actions of adenosine and further minimize tissue injury. In the present study, we have examined whether administration of LUF6096 provides protection against myocardial ischemia/reperfusion injury by using a well established in vivo dog model of infarction (Gross and Auchampach, 1992; Mizumura et al., 1996; Auchampach et al., 2003).
Materials and Methods
Materials.
Cell culture reagents were purchased from Invitrogen (Carlsbad, CA). Guanosine 5′-[γ-[35S]thio]triphosphate ([35S]GTPγS); specific activity 1250 Ci/mmol), 2-[p-(2-carboxyethyl)phenethylamino]-5′-N-[3H]ethylcarboxamidoadenosine ([3H]CGS 21680; 40.5 Ci/mmol), Na125I (2200 Ci/mmol), and carbonized plastic 141Ce-radiolabeled microspheres (15 μm diameter) were from PerkinElmer Life and Analytical Sciences (Waltham, MA). LUF6096 was synthesized in house. Adenosine deaminase (ADA) was obtained from Roche Applied Science (Indianapolis, IN), and all remaining drugs and reagents were purchased from Sigma-Aldrich (St. Louis, MO). C18 Bond Elute SPE cartridges (6 ml, 500 mg) were obtained from Varian, Inc. (Palo Alto, CA), and glass fiber filters (GF/B and GF/C) were obtained from Whatman (Sanford, ME).
Animals.
Adult mongrel dogs of either sex weighing 15 to 25 kg were obtained from licensed vendors. All experiments involving animals were approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin and complied with the procedures established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Cell Culture and Membrane Preparation.
Human embryonic kidney (HEK) 293 cells stably expressing recombinant dog ARs were cultured in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum, 100 units/ml penicillin, 100 mg/ml streptomycin, and 400 μg/ml G418 at 37°C in a humidified environment consisting of room air containing 5% CO2 (Auchampach et al., 1997a).
To prepare cell membranes, HEK 293 cells expressing ARs were washed in phosphate-buffered saline followed by homogenization in 50 mM Tris-HCl buffer, pH 7.4 containing 1 mM EDTA and 5 mM MgCl2, and then centrifuged at 27,000g for 30 min at 4°C. Cell pellets were washed twice in the same buffer after which the resultant pellets were resuspended in 50 mM Tris-HCl buffer containing 10% sucrose and stored at −80°C.
[35S]GTPγS Binding Assays.
[35S]GTPγS binding was measured in 200 μl of buffer containing 50 mM Tris-HCl, pH 7.4, 1 mM EGTA, 10 mM MgCl2, 1 μM GDP, 1 mM dithiothreitol, 100 mM NaCl, 3 U/ml ADA, 0.2 nM [35S]GTPγS, 300 nM 8-[4-[4-(4-chlorophenyl)piperazide-1-sulfonyl)phenyl]]-1-propylxanthine (PSB-603) to block A2B ARs expressed endogenously in HEK 293 cells (Gao et al., 1999), 0.005% CHAPS, and 0.5% bovine serum albumin (Gao et al., 2008). Incubations were started by the addition of the membrane suspension (5 μg of protein) to the test tubes and carried out in quadruplicate for 2 h at 25°C. The reactions were stopped by rapid filtration through Whatman GF/B filters presoaked in 50 mM Tris-HCl buffer, pH 7.4 containing 5 mM MgCl2 and 0.02% CHAPS. The filters were washed three times with 3 ml of wash buffer (10 mM Tris-HCl and 1 mM MgCl2, pH 7.4), and radioactivity trapped in the filters was measured by liquid scintillation counting. Nonspecific binding of [35S]GTPγS was measured in the presence of 10 μM unlabeled GTPγS.
Equilibrium Radioligand Binding Assays.
Membranes prepared from HEK 293 cells expressing ARs were incubated with radioligands in 100 μl of binding buffer consisting of 50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 10 mM MgCl2, and 3 units/ml ADA (except where indicated). After incubation at 25°C for 2 to 3 h, bound and free radioligand were separated by rapid filtration through GF/C glass fiber filters. Radioactivity trapped in the filters was measured by using a γ-counter. Nonspecific binding was determined in the presence of 200 μM adenosine-5′-N-ethylcarboxamide. N6-(4-amino-3-[125I]iodobenzyl)adenosine-5′-N-methylcarboxamide ([125I]I-AB-MECA) was used in assays with the A1 and A3 ARs, and [3H]CGS 21680 was used in assays with A2A ARs. [125I]I-AB-MECA was prepared by radioiodination of precursor AB-MECA using the chloramine-T method and purified by high-performance liquid chromatography (Auchampach et al., 1997a).
Anesthetized Dog Model of Infarction.
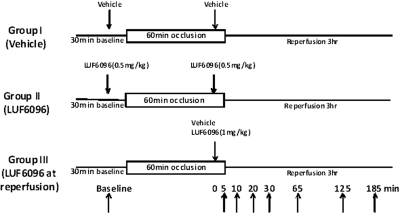
An established barbital-anesthetized, open-chest dog model of infarction was used as described previously in detail (Gross and Auchampach, 1992; Mizumura et al., 1996; Auchampach et al., 2003). Dogs were randomly assigned to three experimental groups and subjected to the experimental protocols depicted in Fig. 2. All dogs were subjected to 60 min of left anterior descending (LAD) coronary artery occlusion and 3 h of reperfusion. Dogs in experimental groups I and II received two doses of either vehicle (1 ml of 50% dimethyl sulfoxide in normal saline) or LUF6096 (0.5 mg/kg i.v. bolus), respectively. The first dose was given 10 min before coronary occlusion, and the second dose was given 5 min before reperfusion. Dogs in experimental group III received a single dose of LUF6096 (1 mg/kg i.v. bolus) 5 min before reperfusion. In all experimental groups, hemodynamic measurements (systemic arterial pressure and left ventricular pressure) were collected before occlusion, at 30 min of occlusion, and every hour after reperfusion via a double-tipped Millar pressure transducer catheter placed into the left ventricular cavity by way of the left carotid artery. LAD coronary artery blood flow was continually measured by using a flow probe, and regional myocardial blood flow was measured at 30 min into the 60-min occlusion period by the radioactive microsphere technique to assess collateral blood flow (Gross and Auchampach, 1992; Mizumura et al., 1996; Auchampach et al., 2003). Throughout the ischemia/reperfusion experiments, heart rate was maintained at 150 beats/min by left atrial pacing.
Fig. 2.
Schematic illustration of the experimental protocols. The arrows to the right in the protocol for group III indicate the time after drug administration that blood samples were collected from the femoral vein for determination of the plasma concentration of LUF6096.
After 3 h of reperfusion, the anatomic area at risk (AAR) and the nonischemic area were demarcated by staining with Evan's blue dye (Gross and Auchampach, 1992; Auchampach et al., 2003). The hearts were electrically fibrillated, removed, and prepared for infarct size determination by using triphenyltetrazolium chloride staining and regional myocardial blood flow measurements (Gross and Auchampach, 1992; Auchampach et al., 2003). Infarcted and noninfarcted tissues within the AAR were separated and determined gravimetrically (Gross and Auchampach, 1992; Mizumura et al., 1996; Auchampach et al., 2003). Regional myocardial blood flow was assessed in the subendocardial, midmyocardial, and epicardial regions of the ischemic and nonischemic regions (Gross and Auchampach, 1992; Mizumura et al., 1996; Auchampach et al., 2003). Dogs were excluded from the study if subendocardial collateral blood flow was >0.15 ml/min/g or more than three consecutive attempts were required to convert ventricular fibrillation with low-energy direct current pulses (Gross and Auchampach, 1992; Mizumura et al., 1996; Auchampach et al., 2003).
Measurement of LUF6096 Plasma Concentrations by Liquid Chromatography-Mass Spectrometry.
Blood was collected into heparinized tubes from the femoral vein in dogs from experimental group III at the times indicated in Fig. 2. The blood samples were immediately centrifuged (1000g for 10 min at 4°C), and the plasma was stored at −80°C until assayed by LC-MS. Samples collected at each time point from all of the dogs in group III were pooled and assayed in triplicate.
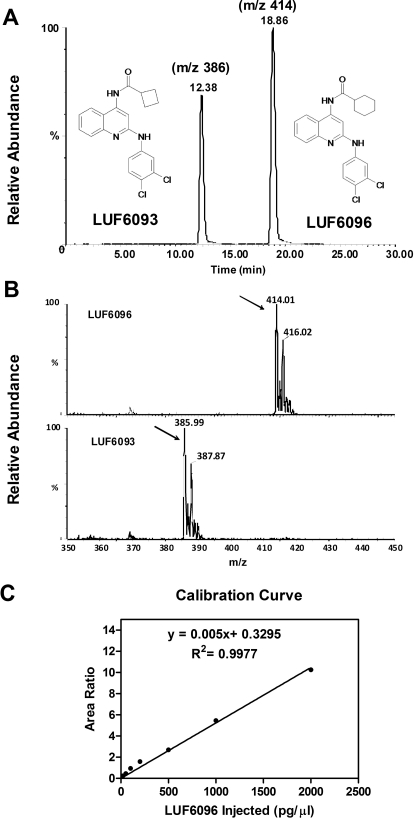
Before analysis, the samples were subjected to a solid-phase extraction procedure as follows. LUF6093 (80 ng), a structural homolog of LUF6096 (see Fig. 3), was added into each plasma sample (1 ml) as an internal standard to normalize for sample recovery. After the addition of ethanol (333 μl) and glacial acetic acid (20 μl), the plasma samples were centrifuged (1000g for 10 min at 4°C), and the supernatant was applied to a C18 Bond Elute SPE cartridge that had been preconditioned with 5 ml of ethanol and 15 ml of deionized water. The cartridges were subsequently washed with 5 × 5 ml of deionized water after which the retained material was eluted with 6 ml of ethyl acetate. The column eluate was dried under a stream of nitrogen, redissolved in 400 μl of 100% acetonitrile, and transferred to a sample vial for LC-MS analyses. Calibration standards were prepared by dissolving 200 pg/μl of LUF6093 and increasing concentrations of LUF6096 in 100% acetonitrile. The standard curve consisted of seven concentrations of LUF6096 ranging from 20 to 2000 pg/μl (Fig. 3).
Fig. 3.
Measurement of LUF6096 plasma levels by LC-MS. A, chromatogram showing the retention times of LUF6096 and the internal standard LUF6093 after separation by high-performance liquid chromatography and detection by MS. B, mass spectra of LUF6093 and LUF6096. The arrows point to strong (M + H) signals at 414.01 for LUF6096 and 385.99 for LUF6093. C, calibration curve used to calculate the plasma levels of LUF6096.
The measurements were carried out with a Waters (Milford, MA) liquid chromatograph-electrospray Micromass Quattro triple quadrupole mass spectrometer. The samples were separated on a reverse-phase C18 column (Kromasil 2.0 × 250 mm, 5 μm; Phenomenex, Torrance, CA) using a water-acetonitrile (containing 0.1% formic acid) mobile phase with a linear gradient of 50 to 80% acetonitrile over 30 min at a flow rate of 0.2 ml/min. Under these conditions, the retention times of LUF6096 and LUF6093 were ∼19 and ∼12 min, respectively (Fig. 3). Positive ion electrospray conditions and the single ion recording mode were used for the analyses where mass-to-charge ratios (m/z) of 414 and 386 were used for the two compounds (Fig. 3).
Data and Statistical Analyses.
All values are presented as the mean ± S.E.M. For the [35S]GTPγS assays, EC50 and Emax values were calculated by fitting the data to E = (Emax × x)/(EC50 + x), in which x is the concentration of the test compound. In the dog infarction studies, hemodynamic variables were analyzed by two-way repeated-measures analysis of variance. If global tests showed a main effect, post hoc contrasts between time points or treatments were performed with Student's t test for unpaired or paired data, as appropriate, with the Bonferroni correction. Infarct sizes and AAR sizes were compared by using a one-way analysis of variance followed by Student's t test for unpaired data. Linear regression analysis was used to compare the relationship between infarct size and regional collateral blood flow in the different experimental groups. Pharmacokinetic parameters of LUF6096 [rate constant (k) and half-life (t1/2)] were calculated by fitting the data to: y = span × e(−k × x) + plateau, in which x is the concentration of LUF6096 in plasma. The t1/2 was obtained from 0.693/k.
Results
Characterization of the Modulatory Activity of LUF6096 with the Canine A3 AR.
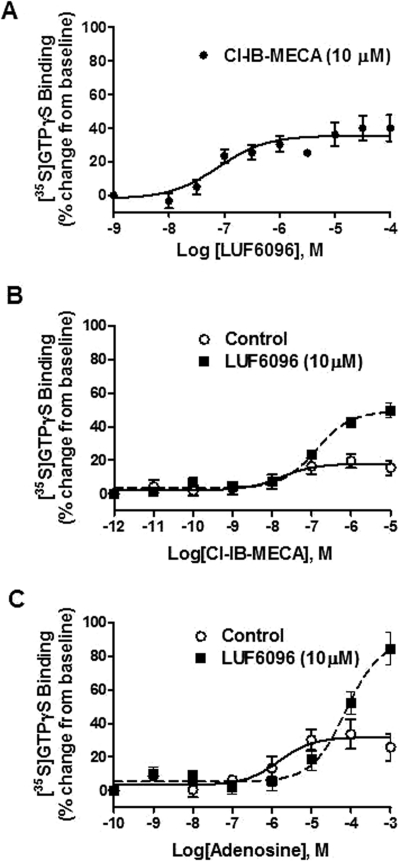
Because the activity of A3 AR allosteric modulators has been assessed only with respect to the human A3 AR (Gao et al., 2001, 2002, 2008, 2011; Göblyös et al., 2006; Heitman et al., 2009; Kim et al., 2009), we conducted preliminary assays to characterize the modulatory actions of LUF6096 on the canine A3 AR by using stably transfected HEK 293 cells and a [35S]GTPγS binding assay (Gao et al., 2008), which directly reflects receptor-mediated activation of G proteins. Similar to previous reports with the human A3 AR (Heitman et al., 2009; Göblyös and IJzerman, 2011), we found that LUF6096 produced substantial enhancing activity with the canine A3 AR (Fig. 4). LUF6096 enhanced the maximal efficacy of the partial agonist 2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide (Cl-IB-MECA; Fig. 4, A and B) more than 2.5-fold. The enhancing activity was concentration-dependent, producing a detectable effect at 30 nM and a maximal effect between 0.3 and 1 μM (EC50 114.3 ± 15.9 nM; Fig. 4A). At a concentration of 10 μM, LUF6096 only slightly reduced the potency of Cl-IB-MECA to stimulate [35S]GTPγS binding (56.1 ± 27.0 versus 106.0 ± 19.7 nM; P < 0.05; Fig. 4B). LUF6096 also enhanced [35S]GTPγS binding in assays using adenosine as the agonist, demonstrating it is a PAM of the native ligand of the A3 AR (Fig. 4C). At a concentration of 10 μM, LUF6096 increased the Emax of adenosine from ∼30% of baseline activity to more than 80% of baseline (2.7-fold increase) and increased the EC50 from 2.1 ± 0.7 to 41.0 ± 16.4 μM.
Fig. 4.
Effect of LUF6096 on [35S]GTPγS binding in assays with the canine A3 AR. Incubations were started by the addition of the membrane suspension (5 μg of protein) as described under Materials and Methods and carried out in quadruplicate for 2 h at 25°C. The membrane solutions were preincubated with LUF6096 for 30 min at 25°C before the addition of ∼0.1 nM [35S]GTPγS. A, the effect of increasing concentrations of LUF6096 on [35S]GTPγS binding in response to a maximal concentration of Cl-IB-MECA (10−5 M) (n = 9). B, stimulation of [35S]GTPγS binding by increasing concentrations of Cl-IB-MECA in the absence (control) or presence of LUF6096 (10 μΜ) (n = 6). C, stimulation of [35S]GTPγS binding in response to increasing concentrations of adenosine in the absence (control) or presence of LUF6096 (10 μΜ) (n = 5).
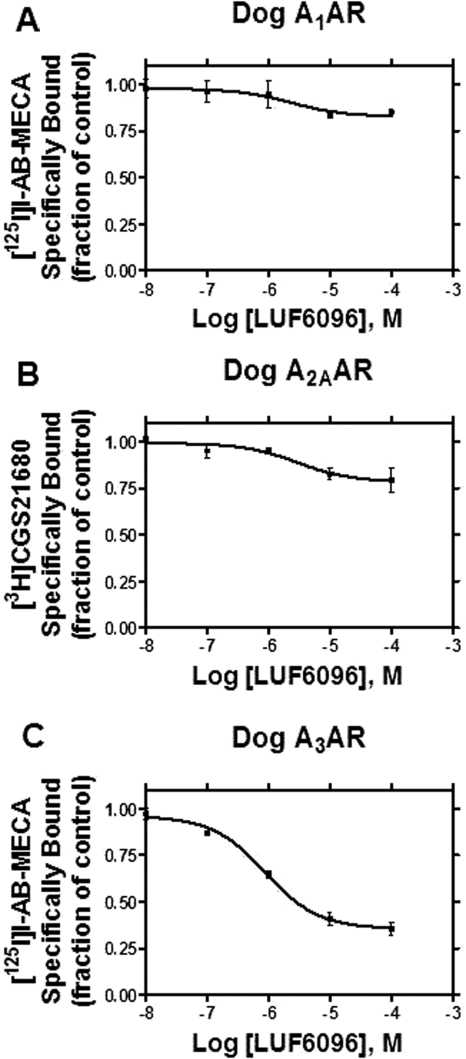
LUF6096 was further tested in equilibrium displacement assays performed with HEK 293 cell membranes expressing canine ARs and the orthosteric agonist radioligands [125I]I-AB-MECA (A1 and A3 ARs) and [3H]CGS21680 (A2A ARs). In assays with the canine A1 and A2A ARs, LUF6096 produced negligible displacement of specific binding at a concentration as high as 100 μM, whereas it significantly reduced specific binding in assays with the canine A3 AR by ∼70% (Fig. 5). Displacement of binding to the dog A3 AR by LUF6096 occurred within the same concentration range (0.1–10 μM) that also increased efficacy in the [35S]GTPγS binding assays (EC50 ∼0.11 μM; Fig. 4).
Fig. 5.
Effect of LUF6096 on equilibrium orthosteric radioligand binding to the canine A1 (A), A2A (B), and A3 (C) ARs. Membranes (50 μg) were incubated with [125I]I-AB-MECA (∼0.5 nM; A1 and A3 ARs) or [3H]CGS21680 (∼10 nM; A2A ARs) for 2 h at room temperature in the presence of increasing concentrations of LUF6096. Nonspecific binding was determined with 200 μM adenosine-5′-N-ethylcarboxamide. The data shown are representative of three independent experiments.
Treatment with LUF6096 Protects against Ischemia/Reperfusion Injury.
The dog ischemia/reperfusion experiments were designed to determine whether LUF6096 potentially protects against infarction by reducing injury that occurs during ischemia or reducing reperfusion-mediated injury by altering the timing of drug administration (Fig. 2). A total of 25 dogs were initially included in the study. One dog was excluded from the vehicle-treated group (group I) because of intractable ventricular fibrillation that developed after release of the occlusion, and one dog was excluded from an LUF6096-treated group (group II) because of high collateral blood flow. Thus, a total of 23 dogs were included in the data analyses.
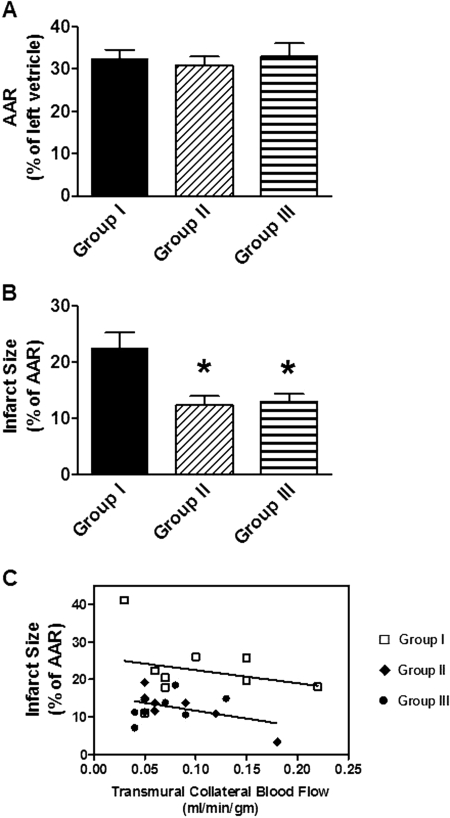
Figure 6 summarizes the infarct size data. It was found that infarct size expressed as a percentage of the AAR was significantly smaller in groups II and III that were treated with LUF6096 compared with the vehicle-treated control group (Fig. 6B). Infarct size expressed as a percentage of the left ventricle was also significantly smaller in the LUF6096-treated groups (group II, 3.8 ± 0.5%; group III, 3.9 ± 0.5%) than in the vehicle-treated group (group I, 7.2 ± 0.7%). Remarkably, the reduction in infarct size was equivalent in magnitude (∼50%) in group III dogs treated with LUF6096 when it was administered only during the reperfusion phase of the study compared with group II dogs given two doses of LUF6096. Among the treatment groups, there were no differences with respect to the weight of the left ventricle (data not shown). It is noteworthy that there were also no differences among the three experimental groups with respect to the size of the area at risk (Fig. 6A). Figure 6C illustrates the relationship between infarct size and transmural collateral blood flow measured at 30 min into the occlusion period in the three experimental groups. In the control group (group I), there was an inverse relationship between infarct size and collateral blood flow. This relationship was shifted downward in a parallel fashion (Fig. 6C) in groups II and III, indicating that the smaller infarcts observed in the LUF6096-treated dogs occurred independently of changes in collateral blood flow. Collectively, these data show that treatment with LUF6096 provided protection from ischemia/reperfusion injury and was effective at mitigating reperfusion-mediated injury.
Fig. 6.
Myocardial infarct size data. Dogs in experimental groups I and II received two doses of either vehicle (1 ml of 50% dimethyl sulfoxide in normal saline) or LUF6096 (0.5 mg/kg i.v. bolus), respectively. The first dose was given 10 min before coronary occlusion, and the second dose was given 5 min before reperfusion. Dogs in experimental group III received a single dose of LUF6096 (1 mg/kg i.v. bolus) 5 min before reperfusion. A, the AAR expressed as a percentage of the left ventricle. B, infarct size expressed as a percentage of the AAR. C, plot of infarct size expressed as a percentage of the AAR versus transmural collateral blood flow measured 30 min after coronary occlusion. The data shown in C were fitted by linear regression analysis: group l, y = −35.49 x + 26.57, r2 = 0.67 (n = 8); groups II and III (pooled), y = −42.25 x + 16.20, r2 = 0.17 (n = 15). *, P < 0.05 versus Group I.
Bolus administration of LUF096 at 0.5 or 1.0 mg/kg produced no immediate changes in heart rate, arterial blood pressure, left ventricular pressure, or LAD coronary artery blood flow in the anesthetized dogs (data not shown). Table 1 summarizes the hemodynamic data collected throughout the ischemia/reperfusion experiments. There were no significant differences in hemodynamics between any of the treatment groups. There were also no differences in collateral blood flow within any region of the ischemic myocardium (Table 2). Thus, cardioprotection provided by LUF6096 was not related to favorable changes in the hemodynamic status of the dogs or an increase in collateral blood flow.
TABLE 1.
Hemodynamic data
| Baseline | Occlusion, 30 min | Occlusion, 60 min | Reperfusion, 1 h | Reperfusion, 2 h | Reperfusion, 3 h | |
|---|---|---|---|---|---|---|
| Group I (n = 8) | ||||||
| HR, beats/min | 164 ± 4 | 164 ± 6 | 162 ± 8 | 156 ± 5 | 155 ± 5 | 157 ± 5 |
| MBP, mm Hg | 123 ± 7 | 114 ± 4 | 111 ± 7 | 106 ± 8 | 110 ± 7 | 108 ± 8 |
| LVdP/dt, mm Hg/s | 2550 ± 361 | 2213 ± 226 | 2119 ± 259 | 1819 ± 164† | 1819 ± 180† | 1856 ± 179† |
| Group II (n = 8) | ||||||
| HR, beats/min | 154 ± 6 | 150 ± 3 | 151 ± 3 | 155 ± 3 | 154 ± 3 | 151 ± 4 |
| MBP, mm Hg | 139 ± 8 | 131 ± 9 | 129 ± 11 | 113 ± 4† | 118 ± 6† | 121 ± 7† |
| LVdP/dt, mm Hg/s | 2893 ± 349 | 2507 ± 394 | 2614 ± 393 | 2193 ± 169† | 2400 ± 284 | 2507 ± 275 |
| Group III (n = 7) | ||||||
| HR, beats/min | 161 ± 6 | 159 ± 6 | 158 ± 5 | 154 ± 4 | 159 ± 4 | 162 ± 3 |
| MBP, mm Hg | 121 ± 10 | 121 ± 8 | 118 ± 10 | 104 ± 9† | 109 ± 6 | 115 ± 7 |
| LVdP/dt, mm Hg/s | 1950 ± 191 | 1992 ± 175 | 1843 ± 211 | 1500 ± 135 | 1586 ± 146 | 1436 ± 117† |
HR, heart rate; MBP, mean arterial blood pressure; LVdP/dt, maximal left ventricular dP/dt.
P < 0.05 versus respective baseline value within groups.
TABLE 2.
Regional myocardial blood flow data
Nonischemic refers to the region perfused by the left circumflex coronary artery. Ischemic refers to the region perfused by the left anterior descending coronary artery.
| Epicardium |
Midmyocardium |
Endocardium |
Transmural |
|||||
|---|---|---|---|---|---|---|---|---|
| Nonischemic | Ischemic | Nonischemic | Ischemic | Nonischemic | Ischemic | Nonischemic | Ischemic | |
| ml/min/g | ||||||||
| Group I | 1.41 ± 0.18 | 0.15 ± 0.04 | 1.52 ± 0.13 | 0.08 ± 0.02 | 1.50 ± 0.12 | 0.07 ± 0.01 | 1.48 ± 0.13 | 0.10 ± 0.02 |
| Group II | 1.14 ± 0.11 | 0.12 ± 0.03 | 1.19 ± 0.09 | 0.08 ± 0.02 | 1.31 ± 0.14 | 0.06 ± 0.01 | 1.24 ± 0.11 | 0.08 ± 0.02 |
| Group III | 1.30 ± 0.19 | 0.10 ± 0.03 | 1.51 ± 0.22 | 0.06 ± 0.01 | 1.54 ± 0.19 | 0.05 ± 0.01 | 1.45 ± 0.19 | 0.07 ± 0.01 |
Plasma Concentrations of LUF6096.
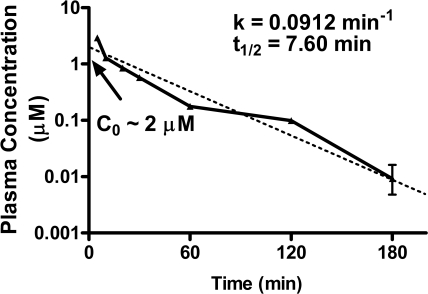
Blood samples were collected during the reperfusion period in group III dogs for measurement of LUF6096 in plasma by using a sensitive LC-MS procedure. The data fit best to a single-phase exponential decay model with a plasma t1/2 of 7.60 min (k = 0.0912 min−1; Fig. 7). From the data shown in Fig. 7, the theoretical plasma concentration of LUF6096 at time 0 min (Co) was extrapolated to be ∼2 μM; using this value and a half-life of 7.60 min, the apparent volume of distribution (Vd = dose/Co) was estimated to be ∼1.21 L/kg (∼24 liters for a 20-kg dog) and clearance (Cl = Vd × k) was determined to be 0.11 L/kg · min−1. Despite a short plasma half-life, bolus administration of LUF6096 at a dose of 1.0 mg/kg in group III dogs resulted in sufficient plasma concentrations expected to provide some degree of A3 AR-enhancing activity for the first 2 to 3 h of the reperfusion period.
Fig. 7.
Plasma concentrations of LUF6096 in group III dogs determined by LC-MS. Samples collected at each time point from all of the dogs in group III were pooled and assayed in triplicate. The rate constant (k) and half-life (t1/2) were calculated by fitting the data to a one-phase exponential equation as described under Materials and Methods.
Discussion
ARs are prime targets for pharmacological manipulation by an allosteric mechanism, because adenosine is produced locally in tissues during hypoxia, ischemia, and tissue injury/inflammation where it functions to reduce cellular injury and promote tissue repair (Ely and Berne, 1992; Auchampach and Bolli, 1999; Headrick and Lasley, 2009). Local enhancement of the actions of adenosine might provide greater benefit compared with orthosteric agonists while avoiding unwanted side effects caused by the stimulation of ARs expressed in other tissues (Gao and Jacobson, 2006; May et al., 2007; Göblyös and IJzerman, 2011). In this study, we found that treatment with LUF6096, a selective PAM of the A3 AR, significantly reduced infarct size in a barbital-anesthetized dog model of myocardial ischemia/reperfusion injury. LUF6096 produced equivalent cardioprotection whether it was present during ischemia or present exclusively during the reperfusion phase. Administration of LUF6096 to dogs at a dose of up to 1.0 mg/kg was found to be hemodynamically inert during barbital anesthesia, producing no measureable effects on systemic blood pressure, left ventricular pressure, coronary artery blood flow, or collateral blood flow during the ischemic period. Although LUF6096 was found to have a relatively short plasma half-life, the dogs tolerated large doses of the drug, resulting in plasma concentrations that were expected to provide a sustained therapeutic effect.
Because of the large size of the dogs, we were not able to administer A3 AR antagonists to confirm the specificity of LUF6096 in this study. This will have to await further investigation in smaller animal species or potentially in gene-target animals. Nevertheless, there is substantial circumstantial evidence supporting the involvement of an A3 AR-mediated mechanism. First, preliminary studies established that LUF6096 exhibits enhancing activity toward the canine A3 AR. LUF6096 was found to potently (EC50 120 nM) increase the maximal efficacy of the partial A3 AR agonist Cl-IB-MECA as well as the native agonist adenosine more than 2-fold in [35S]GTPγS binding assays using cell membranes expressing the canine A3 AR. Second, we established that LUF6096 is present in plasma at concentrations that would be expected to provide A3 AR-enhancing activity. Finally, the cardioprotective profile of LUF6096 was found to be similar to that observed with orthosteric agonists for the A3 AR such as N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide, Cl-IB-MECA, and (2S,3S,4R,5R)-3-amino-5-[6-(2,5-dichlorobenzylamino)purin-9-yl]-4-hydroxytetrahydrofuran-2-carboxylic acid methylamide (CP532,903) (Auchampach et al., 1997b; Black et al., 2002; Ge et al., 2006, 2010; Wan et al., 2008). Like orthosteric A3 AR agonists, LUF6096 was able to reduce infarct size by ∼50% and protect against reperfusion-mediated injury by a mechanism that is unrelated to changes in systemic hemodynamics. The large size of the dog also precluded extensive studies to investigate potential molecular mechanisms by which LUF6096 provides protection from ischemia/reperfusion injury. Earlier studies suggest that A3 AR activation may promote cell survival by facilitating opening of the KATP channel in cardiac myocytes and suppressing the robust inflammatory response that occurs during reperfusion (Auchampach et al., 1997b; Black et al., 2002; Ge et al., 2006, 2010; Wan et al., 2008).
Although we found that LUF6096 functioned effectively as a PAM of the dog A3 AR, its pharmacological profile versus the dog receptor was slightly different from that observed previously with the human A3 AR (Heitman et al., 2009; Göblyös and IJzerman, 2011). Using the dog A3 AR in the [35S]GTPγS binding assay, we found that LUF6096 robustly increased the intrinsic efficacy of Cl-IB-MECA while slightly reducing its potency (∼2-fold increase in the EC50). Similar results on potency and efficacy were obtained with LUF6096 when using adenosine as the orthosteric ligand. In equilibrium binding assays, LUF6096 displaced binding at the orthosteric site of the canine A3 AR within the same concentration range that produced enhancement, suggesting that the reduction in potency in the functional assays is related to a decrease in orthosteric binding affinity. In contrast, we observed previously that LUF6096 not only increased the intrinsic efficacy of Cl-IB-MECA in functional assays (inhibition of forskolin-stimulated cAMP accumulation) with the human A3 AR, but that it also increased potency 3-fold (Heitman et al., 2009; Göblyös and IJzerman, 2011). Based on these findings, it is apparent that the actions of allosteric modulators of the A3 AR are likely to be species-dependent, similar to that observed previously with orthosteric ligands for this AR subtype (Linden, 1994).
We do not have an explanation for the rapid disappearance of LUF6096 from the plasma in the present study. It is possible that it is rapidly metabolized or excreted without chemical modification. The relatively large apparent volume of distribution of LUF6096 and its high lipophilicity suggest that it might be partitioning into certain tissues or is subject to protein binding. As a consequence, the plasma concentration might not reflect its concentration at the site of action. Considering that A3 AR PAMs have potential utility in treating chronic inflammatory diseases, it will be important to further study the pharmacokinetic characteristics of LUF6096. Because LUF6096 (2,4-disubstituted quinolone) and LUF6000 (imidazoquinolinamine) are structurally distinct (Heitman et al., 2009; Göblyös and IJzerman, 2011), it will be interesting to determine the in vivo pharmacokinetic parameters of LUF6000 in future animal studies as well.
In summary, this study represents the first reported efficacy of an A3 AR PAM in an in vivo experimental animal model of myocardial infarction. Our results demonstrate that LUF6096 is well tolerated in anesthetized dogs and is effective at reducing injury caused by myocardial ischemia and reperfusion. This proof-of-concept study supports the possibility that A3 AR enhancers are effective in treating acute ischemia/reperfusion injury as well as other diseases associated with ischemia, tissue injury, and inflammation. Findings from clinical trials with the orthosteric A3 AR agonist N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide indicate that dosing is limited by hemodynamic side effects, including tachycardia (van Troostenburg et al., 2004). These actions may be caused by A3 AR activation within the cardiovascular system (Yang et al., 2010) or nonspecific interactions with other AR subtypes (Murphree et al., 2002). Considering that PAMs function more selectively in a site- and event-specific manner and have the potential to produce fewer adverse effects compared with orthosteric agonists, the allosteric enhancer approach for targeting the A3 AR for disease treatment might prove to be superior.
Acknowledgments
We thank Jeannine Moore, Anna Hsu, and Marilyn Isbell for technical assistance.
This research was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants R01-HL077707, R37-HL074314] and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- GPCR
- G protein-coupled receptor
- AAR
- area at risk
- ADA
- adenosine deaminase
- AR
- adenosine receptor
- [3H]CGS 21680
- 2-[p-(2-carboxyethyl)phenethylamino]-5′-N-[3H]ethylcarboxamidoadenosine
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- Cl-IB-MECA
- 2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide
- CP532,903
- (2S,3S,4R,5R)-3-amino-5-[6-(2,5-dichlorobenzylamino)purin-9-yl]-4-hydroxytetrahydrofuran-2-carboxylic acid methylamide
- DU124183
- 1H-imidazo[4,5-c]quinolin-4-amine
- [35S]GTPγS
- guanosine 5′-[γ-[35S]thio]triphosphate
- HEK
- human embryonic kidney
- [125I]I-AB-MECA
- N6-(4-amino-3-[125I]iodobenzyl)adenosine-5′-N-methylcarboxamide
- LAD
- left anterior descending
- LC-MS
- liquid chromatography-mass spectroscopy
- LUF6000
- N-(3,4-dichlorophenyl)-2-cyclohexyl-1H-imidazo[4,5-c]quinolin-4-amine)
- LUF6096
- N- {2-[(3,4-dichlorophenyl)amino]quinolin-4-yl}cyclohexanecarboxamide
- NECA
- adenosine-5′-N-ethylcarboxamide
- PAM
- positive allosteric modulator
- PSB-603
- 8-[4-[4-(4-chlorophenyl)piperazide-1-sulfonyl)phenyl]]-1-propylxanthine.
Authorship Contributions
Participated in research design: Du, Nithipatikom, Gross, and Auchampach.
Conducted experiments: Du.
Contributed new reagents or analytic tools: Gao, IJzerman, van Veldhoven, and Jacobson.
Performed data analysis: Du, Nithipatikom, and Auchampach.
Wrote or contributed to the writing of the manuscript: Du and Auchampach.
References
- Auchampach JA, Bolli R. (1999) Adenosine receptor subtypes in the heart: therapeutic opportunities and challenges. Am J Physiol Heart Circ Physiol 276:H1113–H1116 [DOI] [PubMed] [Google Scholar]
- Auchampach JA, Ge ZD, Wan TC, Moore J, Gross GJ. (2003) A3 adenosine receptor agonist IB-MECA reduces myocardial ischemia-reperfusion injury in dogs. Am J Physiol Heart Circ Physiol 285:H607–H613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. (1997a) Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2B receptor. Mol Pharmacol 52:846–860 [DOI] [PubMed] [Google Scholar]
- Auchampach JA, Rizvi A, Qiu Y, Tang XL, Maldonado C, Teschner S, Bolli R. (1997b) Selective activation of A3 adenosine receptors with N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide protects against myocardial stunning and infarction without hemodynamic changes in conscious rabbits. Circ Res 80:800–809 [DOI] [PubMed] [Google Scholar]
- Black RG, Jr, Guo Y, Ge ZD, Murphree SS, Prabhu SD, Jones WK, Bolli R, Auchampach JA. (2002) Gene dosage-dependent effects of cardiac-specific overexpression of the A3 adenosine receptor. Circ Res 91:165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely SW, Berne RM. (1992) Protective effects of adenosine in myocardial ischemia. Circulation 85:893–904 [DOI] [PubMed] [Google Scholar]
- Gao Z, Chen T, Weber MJ, Linden J. (1999) A2B adenosine and P2Y2 receptors stimulate mitogen-activated protein kinase in human embryonic kidney-293 cells. Cross-talk between cyclic AMP and protein kinase c pathways. J Biol Chem 274:5972–5980 [DOI] [PubMed] [Google Scholar]
- Gao ZG, Jacobson KA. (2006) Keynote review: allosterism in membrane receptors. Drug Discov Today 11:191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Kim SG, Soltysiak KA, Melman N, IJzerman AP, Jacobson KA. (2002) Selective allosteric enhancement of agonist binding and function at human A3 adenosine receptors by a series of imidazoquinoline derivatives. Mol Pharmacol 62:81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Van Muijlwijk-Koezen JE, Chen A, Müller CE, IJzerman AP, Jacobson KA. (2001) Allosteric modulation of A3 adenosine receptors by a series of 3-(2-pyridinyl)isoquinoline derivatives. Mol Pharmacol 60:1057–1063 [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Verzijl D, Zweemer A, Ye K, Göblyös A, IJzerman AP, Jacobson KA. (2011) Functionally biased modulation of A3 adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers. Biochem Pharmacol 82:658–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Ye K, Göblyös A, IJzerman AP, Jacobson KA. (2008) Flexible modulation of agonist efficacy at the human A3 adenosine receptor by the imidazoquinoline allosteric enhancer LUF6000. BMC Pharmacol 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge ZD, Peart JN, Kreckler LM, Wan TC, Jacobson MA, Gross GJ, Auchampach JA. (2006) Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. J Pharmacol Exp Ther 319:1200–1210 [DOI] [PubMed] [Google Scholar]
- Ge ZD, van der Hoeven D, Maas JE, Wan TC, Auchampach JA. (2010) A3 adenosine receptor activation during reperfusion reduces infarct size through actions on bone marrow-derived cells. J Mol Cell Cardiol 49:280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göblyös A, Gao ZG, Brussee J, Connestari R, Santiago SN, Ye K, IJzerman AP, Jacobson KA. (2006) Structure-activity relationships of new 1H-imidazo[4,5-c]quinolin-4-amine derivatives as allosteric enhancers of the A3 adenosine receptor. J Med Chem 49:3354–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göblyös A, IJzerman AP. (2011) Allosteric modulation of adenosine receptors. Biochim Biophys Acta 1808:1309–1318 [DOI] [PubMed] [Google Scholar]
- Gross GJ, Auchampach JA. (1992) Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res 70:223–233 [DOI] [PubMed] [Google Scholar]
- Headrick JP, Lasley RD. (2009) Adenosine receptors and reperfusion injury of the heart. Handb Exp Pharmacol 193:189–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman LH, Göblyös A, Zweemer AM, Bakker R, Mulder-Krieger T, van Veldhoven JP, de Vries H, Brussee J, IJzerman AP. (2009) A series of 2,4-disubstituted quinolines as a new class of allosteric enhancers of the adenosine A3 receptor. J Med Chem 52:926–931 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Kim Y, de Castro S, Gao ZG, IJzerman AP, Jacobson KA. (2009) Novel 2- and 4-substituted 1H-imidazo[4,5-c]quinolin-4-amine derivatives as allosteric modulators of the A3 adenosine receptor. J Med Chem 52:2098–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. (1994) Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends Pharmacol Sci 15:298–306 [DOI] [PubMed] [Google Scholar]
- May LT, Leach K, Sexton PM, Christopoulos A. (2007) Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 47:1–51 [DOI] [PubMed] [Google Scholar]
- Mizumura T, Auchampach JA, Linden J, Bruns RF, Gross GJ. (1996) PD 81,723, an allosteric enhancer of the A1 adenosine receptor, lowers the threshold for ischemic preconditioning in dogs. Circ Res 79:415–423 [DOI] [PubMed] [Google Scholar]
- Murphree LJ, Marshall MA, Rieger JM, MacDonald TL, Linden J. (2002) Human A2A adenosine receptors: high-affinity agonist binding to receptor-G protein complexes containing Gβ4. Mol Pharmacol 61:455–462 [DOI] [PubMed] [Google Scholar]
- van Troostenburg AR, Clark EV, Carey WD, Warrington SJ, Kerns WD, Cohn I, Silverman MH, Bar-Yehuda S, Fong KL, Fishman P. (2004) Tolerability, pharmacokinetics and concentration-dependent hemodynamic effects of oral CF101, an A3 adenosine receptor agonist, in healthy young men. Int J Clin Pharmacol Ther 42:534–542 [DOI] [PubMed] [Google Scholar]
- Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, Gross GJ, Kwok WM, Auchampach JA. (2008) The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3′-aminoadenosine-5′-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J Pharmacol Exp Ther 324:234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JN, Wang Y, Garcia-Roves PM, Bjornholm M, Fredholm BB. (2010) Adenosine A3 receptors regulate heart rate, motor activity and body temperature. Acta Physiol (Oxf) 199:221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]