Abstract
Telomerase ribonucleoprotein (RNP) employs an RNA subunit to template the addition of telomeric repeats onto chromosome ends. Previous studies have suggested that a region of the RNA downstream of the template may be important for telomerase activity and that the region could fold into a pseudoknot. Whether the pseudoknot motif is formed in the active telomerase RNP and what its functional role is have not yet been conclusively established. Using single-molecule FRET, we show that the isolated pseudoknot sequence stably folds into a pseudoknot. However, in the context of the full-length telomerase RNA, interference by other parts of the RNA prevents the formation of the pseudoknot. The protein subunits of the telomerase holoenzyme counteract RNA-induced misfolding and allow a significant fraction of the RNPs to form the pseudoknot structure. Only those RNP complexes containing a properly folded pseudoknot are catalytically active. These results not only demonstrate the functional importance of the pseudoknot but also reveal the critical role played by telomerase proteins in pseudoknot folding.
Telomeres shorten with each round of DNA replication. Once telomeres reach a critical length, they become vulnerable to DNA damage response, which triggers cellular senescence and chromosome fusion (1). Telomerase protects telomeres from this replication-induced DNA erosion by addition of short G-rich repeats to the DNA ends (2). The telomerase enzyme is an attractive drug target in both cancer and regenerative medicine, because most highly proliferative cells, including stem and cancer cells, rely on its activity to maintain genomic integrity (3). Furthermore, multiple human diseases are known to be caused by mutations in telomerase subunits (4–6), underscoring the importance of understanding the enzyme’s structure and function.
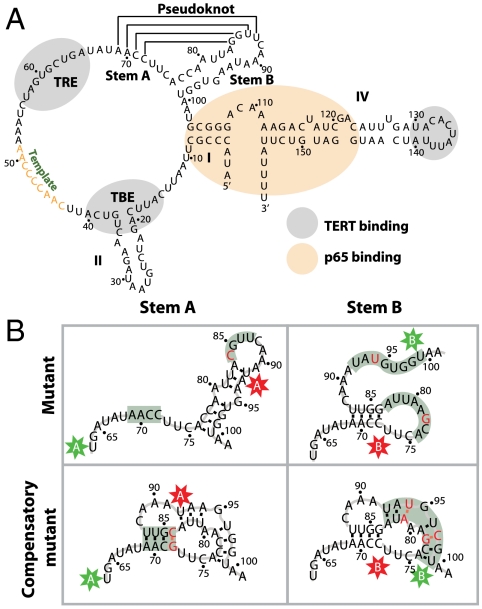
The ciliate Tetrahymena thermophila has long been used as a model system to study telomerase activity. Tetrahymena telomerase is comprised of a 159-nucleotide RNA subunit, a 133-kDa telomerase reverse transcriptase (TERT), and multiple protein cofactors (7). The RNA has a conserved secondary structure (Fig. 1A) that contains a template region, which serves as template for the telomeric repeat synthesis, flanked upstream by the template boundary element (TBE) and downstream by a putative pseudoknot, as well as a highly structured region made of stems I and IV (8–10). TERT, the catalytic subunit, binds telomerase RNA in the TBE region, the template recognition element, and the loop of stem IV (10–13) (Fig. 1A) . Of the multiple Tetrahymena telomerase cofactors identified to date, only p65 binds the RNA directly in the stem I-proximal stem IV region (Fig. 1A) and promotes the hierarchical telomerase RNP assembly (14–16).
Fig. 1.
Tetrahymena telomerase RNA pseudoknot. (A) Telomerase RNA contains a potential pseudoknot consisting of stems A and B, located downstream from the template. The TBE, template recognition element (TRE), and stemloop IV (IV) constitute TERT binding sites and are shown in gray. Stem I (I) and proximal stem IV bind p65 and are shown in beige. (B) Labeling and mutation schemes used to test the formation of the pseudoknot. Two sets of labeling sites, A sites (U63 and U92) and B sites (U73 and U99), were designed to flank stems A and B, respectively. Each set includes a FRET donor (Cy3) and acceptor (Cy5) sites marked by the colored stars. Nucleotide substitutions designed to either disrupt or recover base-pairing potential of the two stems are marked in red. Stems targeted by the mutations are highlighted in gray.
Past studies have suggested that the putative pseudoknot region downstream of the template is a conserved and functionally important segment of telomerase RNA. Phylogenetic analyses of ciliate (9, 17), yeast (18–20), and vertebrate (21) RNAs suggest that this region may fold into a pseudoknot structure. NMR and single-molecule force studies of the isolated pseudoknot sequence from human show that this sequence can indeed form a pseudoknot structure (22, 23). Mutation analyses indicate that disruption of potential base pairs in the motif correlates with telomerase assembly and activity defects in multiple organisms (18, 24, 25), but in vitro telomerase activity assays give varying results on the functional importance of the motif (12, 13, 26–28). Footprinting studies and FRET measurements suggest conformational change in this region of the telomerase RNA upon RNP assembly (12, 29–31). However, direct structural proof for the pseudoknot fold in the full-length telomerase RNA and RNP has not yet been demonstrated. Moreover, the conformation adopted by the sequence within the active telomerase enzyme and its behavior during catalysis remain incompletely understood, though it has been hypothesized to act as a “molecular switch” in promoting enzyme translocation during processive repeat addition (17, 29, 32, 33).
In this work, we used single-molecule FRET (34–36) to interrogate the conformation adopted by the Tetrahymena pseudoknot sequence and monitored its behavior during catalysis. To determine whether the sequence can fold into a pseudoknot, we excised the region from the rest of the telomerase RNA and characterized its conformation in isolation, demonstrating that the sequence can indeed fold into a stable pseudoknot. Within the full-length telomerase RNA, however, the pseudoknot is not folded. We further show that assembly with holoenzyme proteins p65 and TERT promotes folding of the pseudoknot, that only those telomerase complexes with a properly folded pseudoknot are active, and that this motif is stable during both single-nucleotide extension and processive repeat addition.
Results
Telomerase RNA Sequence Forms a Stable Pseudoknot in Isolation.
A pseudoknot is a structural motif composed of two end-to-end stacked helices, stems A and B (Fig. 1A). To show that this motif is folded, it is necessary to demonstrate that both stems are formed simultaneously. To this end, we compared conformations of wild-type stems A and B with those of mutants and compensatory mutants, in which base pairing of the stems was disrupted and rescued, respectively. Specifically, we designed RNA constructs comprised of two parts: a 3′ core and a 5′ extension. The 3′ core was based on the A65-A101 sequence of Tetrahymena telomerase RNA (Fig. 1A) that spanned the putative pseudoknot region and, in the case of the mutants, contained the appropriate base substitutions (Fig. 1B). The 5′ extension was a 21-nucleotide sequence that base-paired with a biotinylated DNA tether, which was used to anchor the RNA on a microscope slide for single-molecule detection (Fig. 2A). We labeled the construct with two separate FRET schemes as shown in Fig. 1B: (i) to test whether stem A is formed, we placed the FRET donor (Cy3) and acceptor (Cy5) on the A sites (U63, U92), and (ii) to test whether stem B is formed, we placed the dyes on the B sites (U73, U99). Stem formation should bring the two dyes closer and result in a higher FRET signal as compared to that of an unstructured sequence.
Fig. 2.
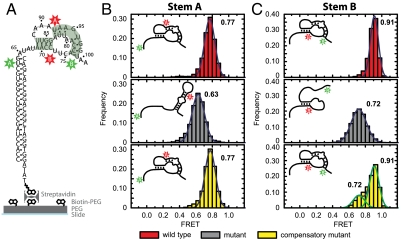
The pseudoknot sequence forms a stable pseudoknot in isolation. (A) The isolated pseudoknot sequence (Left, shown as a fully formed pseudoknot) was anchored to the quartz slide through a 21-nt extension that base-paired with a biotinylated DNA tether and a biotin-streptavidin linkage. (B) Formation of stem A was probed with FRET between Cy3 and Cy5 labeling the A sites as defined in Fig. 1B. The red, gray, and yellow FRET histograms correspond to the wild-type sequence, the mutant disrupting stem A, and the compensatory mutant restoring base pairing in stem A, respectively. The mutations are also defined in Fig. 1B. (C) Similar to B, but with stem B probed by the B labeling sites and mutations in stem B, as defined in Fig. 1B. The numbers next to the histograms indicate the peak positions of the FRET distributions derived from Gaussian fitting (blue and green outlines). The diagrams in the upper left corners depict the folding states of stems A and B, as derived from the FRET measurements.
As shown in Fig. 2B, wild-type stem A adopted a single, stable conformation exhibiting FRET centered at 0.77 when the FRET donor and acceptor were placed on the A sites. To determine whether this FRET value corresponds to folded or unfolded stem A, we compared it to one obtained from a mutant pseudoknot sequence containing a single-nucleotide substitution (G84C, Fig. 1B). The mutant sequence, predicted to disrupt stem A folding, yielded a lower FRET of 0.63 (Fig. 2B). Furthermore, a compensatory mutation (G84C and C72G, Fig. 1B), designed to restore stem A base-pairing potential, recovered the wild-type FRET of 0.77 (Fig. 2B). Together, these results indicate that the isolated wild-type pseudoknot sequence forms a stably folded stem A.
We used the same strategy to determine if stem B was formed by placing the dyes on the B sites. Wild-type stem B exhibited a stable FRET of 0.91 (Fig. 2C). A mutant sequence predicted to disrupt stem B folding (C78G and A94U, Fig. 1B) yielded a lower FRET value of 0.72 (Fig. 2C), whereas compensatory mutants (C78G, A94U, U81A, and G97C, Fig. 1B) largely recovered the wild-type FRET value of 0.91 (Fig. 2C). The compensatory mutant additionally exhibited a minor peak at FRET = 0.72, which originated from molecules transiently converting to the unfolded conformation. This incomplete rescue of the pseudoknot fold is possibly due to the disruption of a base triplex (37, 38) or the weakening of base stacking caused by the U81A and A94U substitutions in the compensatory mutant.
Next, we verified that the stable base pairing observed in wild-type stem A was not disrupted by substitutions in stem B, and vice versa. The mutant and compensatory mutant of stem B that were labeled at the A sites showed that stem A was formed stably regardless of the mutations; similarly, all stem A mutants that were labeled at the B sites showed that stem B was stably folded (Fig. S1).
Taken together, these results indicate that the Tetrahymena pseudoknot sequence, when in isolation, folds into a stable pseudoknot. The conformation of each stem can be determined from its characteristic FRET signature: The folded and unfolded stem A give rise to FRET = 0.77 and 0.63, respectively, whereas the folded and unfolded stem B result in FRET = 0.91 and 0.72, respectively. Below, we use these characteristic FRET values to determine the pseudoknot conformation within (i) the full-length, protein-free telomerase RNA, (ii) the reconstituted telomerase RNPs, and (iii) the catalytically active telomerase complexes.
Pseudoknot Is Not Formed in Full-Length, Protein-Free Telomerase RNA.
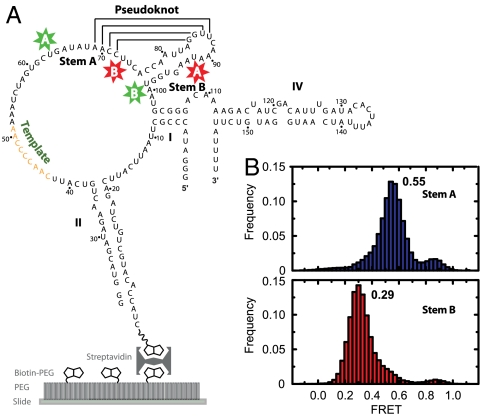
We engineered full-length RNA constructs labeled at sites flanking stem A (U63-Cy3, U92-Cy5) and stem B (U73-Cy5, U99-Cy3) similar to the ones used for the isolated pseudoknot sequence. A biotinylated extension of stem II anchored the molecules on microscope slides (Fig. 3A) and allowed us to image single molecules. Although this anchoring scheme disrupted the loop at the distal end of stem II, previous studies have shown that extension, mutation, and disruption of the distal stem loop II do not inhibit enzyme assembly and function (16, 26, 27).
Fig. 3.
Pseudoknot is not formed in full-length, protein-free telomerase RNA. (A) Wild-type telomerase RNAs labeled at the A and B sites (stars) to probe the formation of stems A and B, respectively, were anchored to slides through a biotinylated extension of stem II. (B) FRET histograms corresponding to Cy3 and Cy5 attached to A sites (blue) or B sites (red). The numbers next to the histograms indicate the major peak positions of the FRET distributions.
Protein-free, stem-A-labeled and stem-B-labeled telomerase RNA gave rise to unexpected FRET values of 0.55 and 0.29, respectively (Fig. 3B). These values were substantially lower than those observed for the folded stem A and B conformations of the isolated pseudoknot sequence, implying that this region did not fold into a pseudoknot in full-length, protein-free RNA. These FRET values were also lower than the ones observed for the unfolded stem A and B conformations of the isolated pseudoknot sequence, suggesting that these stems were not simply open but were involved in competing interactions with other RNA regions.
Pseudoknot Is Stably Formed in Telomerase RNPs and Is Required for Activity.
Considering that protein subunits in an RNP can influence the folding of its associated RNA, we further determined whether pseudoknot folding relies on assembly with telomerase proteins. We first attempted to image the conformation of the pseudoknot region within slide-anchored telomerase RNPs. Full-length, stem-A-labeled and stem-B-labeled telomerase RNAs were assembled in a rabbit reticulocyte lysate system with p65 and FLAG-tagged TERT. To control for potential assembly and activity defects due to dye labeling, we compared activity profiles of RNPs reconstituted with labeled and unlabeled telomerase RNAs. The dye labeling did not perturb telomerase assembly or function substantially (Fig. S2). To anchor the RNPs on microscope slides, we coated the slide surface with biotinylated PEG, streptavidin, and biotinylated protein G in a sequential manner. Anti-FLAG M2 antibodies were then bound to these slides through antibody-protein G interactions. Finally, the FLAG-tagged telomerase RNPs were attached to the slides through the anti-FLAG antibodies (Fig. S3A). As shown in Fig. S3B, both stem-A-labeled and stem-B-labeled constructs exhibited broad FRET distributions, likely due to heterogeneous populations present in the reconstituted RNPs.
To identify the species representing the functional fold, we used a recently developed single-molecule structure–function assay, which enabled us to observe the behavior of the pseudoknot as telomerase catalyzed primer extension (39). The assay consisted of two phases: a telomerase binding phase, followed by a catalytic outcome detection phase. During the binding phase, stem-A- or stem-B-labeled telomerase RNPs were flowed over a slide carpeted with anchored primers (Fig. S4A). Primer binding brought individual RNP complexes within the detection range of our total internal reflection fluorescence (TIRF) imaging setup, allowing us to observe the structure and dynamics of the enzyme in real time by FRET. Of those enzymes that were assembled properly enough to bind the substrate, some catalyzed primer extension. We identified such productive binding events in the subsequent activity detection phase by observing the oligonucleotide length difference between catalytically extended and unreacted primers. This task was accomplished by exploiting distinct hybridization stabilities between a heptameric detection oligonucleotide (DO) and primers of different lengths. Specifically, after the telomerase reaction, the enzyme was removed and the primers anchored on the slides were labeled with the FRET donor Cy3 by a labeling oligonucleotide (LO) (Fig. S4A). Then, FRET acceptor Cy5-labeled DO was flowed over the slide (Fig. S4A). Unextended primers could form only five base pairs with the DO, resulting in extremely transient interactions rarely detected at our data acquisition rate of 30 Hz (Fig. S4B, Top). Primers extended by a single nucleotide (i.e., the last G of the first telomeric repeat by design, Fig. S4A) formed transient interactions with the DO, which gave rise to spikes in the FRET time trajectories (Fig. S4A, Middle). Lastly, primers with two or more nucleotides added bound the DO for an extended period of time (Fig. S4B, Bottom). These primers represent the products of processive repeat addition, as the second nucleotide corresponds to the beginning of a new repeat and its addition requires translocation of the enzyme back to the origin of the template. The presence of chain-terminating dideoxyribonucleotide TTP (ddTTP) in the reaction ensured that primer extension did not extend beyond the second telomeric repeat such that the DO can only bind the primer in one configuration. Quantitative criteria for distinguishing these three types of products are described in the caption of Fig. S4. By correlating the activity detection outcome with the FRET observed during the enzyme binding phase, we were able to (i) identify active telomerase complexes, (ii) derive the functional conformation of the pseudoknot, and (iii) observe its behavior during catalysis.
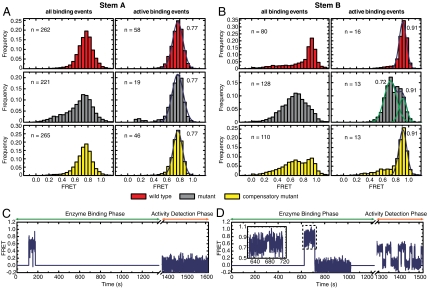
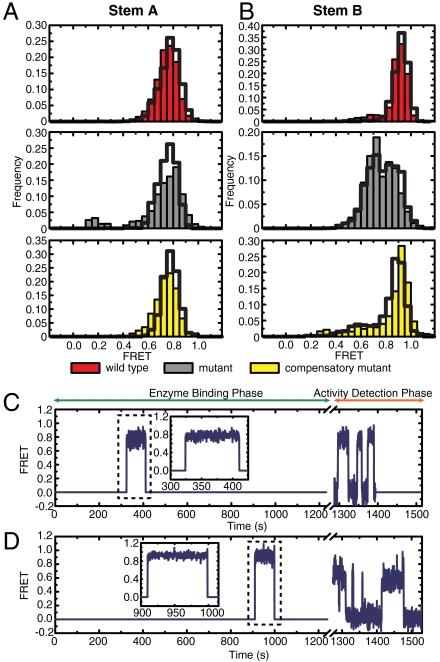
The assay showed that wild-type stem-A-labeled telomerase RNPs bound primers with a stably folded stem A, characterized by a FRET histogram centered around FRET = 0.77 (Fig. 4A). The fraction of complexes that catalyzed primer extension also displayed stable FRET of 0.77 (Fig. 4A), demonstrating that stem A is formed in the active telomerase enzyme. Data from the disruptive and compensatory mutants (Fig. 4A) allowed us to further determine whether telomerase requires stem A formation in order to bind its substrate and to catalyze primer extension. Because the disruptive mutant bound primers in both folded and unfolded conformations (represented by the peak at FRET = 0.77 and the broad shoulder at lower FRET, respectively), and at a similar frequency as the wild-type after normalization over enzyme concentration, we concluded that binding of telomerase to its substrate does not require a folded stem A. However, among the binding events, only those with FRET = 0.77 resulted in primer extension, indicating that a properly formed stem A is required for activity. The compensatory mutant displayed a similar behavior to that of the wild-type.
Fig. 4.
Pseudoknot is formed in active telomerase RNP. (A) FRET between stem-A-labeling sites for the wild-type telomerase (red), mutants that disrupt stem A (gray), and compensatory mutants that restore base-pairing potential in stem A (yellow). (Left) FRET histograms of all primer binding events. (Right) FRET histograms of active binding events, which resulted in primer extension. The number of binding events used in the FRET analysis is denoted by n. The number next to the histograms in the right panels denotes the peak position derived from Gaussian fitting. (B) Similar to A, except that FRET dyes are placed at the stem-B-labeling sites and the mutations are in stem B. Among the active binding events, 35%, 20%, and 26% resulted in processive repeat addition for the wild-type telomerase, stem A disruption mutant, and compensatory mutant, respectively. Similar trend was found for the stem B constructs. (C) Representative FRET trajectory of a single primer that did not undergo catalytic extension during binding of a mutant stem B complex. Stem B is in an unfolded conformation with FRET substantially lower than 0.91. (D) Same as C, except that the primer was extended processively. Stem B is rapidly converting between folded (FRET = 0.91) and open (FRET = 0.72) conformations. Inset shows expanded view of the enzyme binding event (dashed box).
Wild-type stem-B-labeled telomerase RNPs bound primers with stem B folded, as indicated by the hallmark FRET peak of 0.91 (Fig. 4B). The fraction of complexes that resulted in active primer extension also had stem B formed (Fig. 4B). Nonetheless, disruptive mutants bound to the primers at a similar frequency as the wild type and the binding events exhibited a broad FRET peak with values substantially lower than 0.91 (Fig. 4 B and C) indicating that telomerase also does not require a folded stem B in order to bind a primer. As expected, the binding events of the compensatory mutant showed a higher occupancy of the 0.91 FRET state. Despite the ability of telomerase to bind to primer with unfolded stem B, only those RNPs that managed to form the stem at least transiently catalyzed primer extension. This notion is supported by two observations: (i) Compared to the FRET distributions constructed from all binding events, the distributions from events that resulted in primer extension were highly enriched in the FRET = 0.91 state. (ii) Although the FRET distribution derived from the active binding events of the disruptive mutant showed two major peaks at FRET = 0.72 and 0.91, it arises from equilibrium conversion between these two states, as observed in the FRET traces of individual molecules. Importantly, every active binding event had reached the FRET = 0.91 state at least transiently (exemplified by Fig. 4D), and conversely, those binding events that remained at the lower FRET state for the entire binding duration did not result in primer extension (Fig. 4C). Our observations thus show that telomerase activity rests on a properly folded stem B as well. Taken together, the above results demonstrate that the pseudoknot, including both stems A and B, is essential for telomerase activity.
Pseudoknot Is Stable During Telomerase Catalysis.
The repeated formation and disruption of either stem A or B (or both) in the pseudoknot has been proposed as a molecular switch mechanism to relieve the strain imparted on telomerase RNA by repeat synthesis, allowing the enzyme to catalyze multiple repeat additions more efficiently (17, 29, 32, 33). We examined this hypothesis first by separating the active binding events into two groups: one that resulted in primer extension by a single nucleotide (the last nucleotide of the repeat by design) and one that resulted in the addition of more than one nucleotide due to enzyme translocation, the latter representing processive repeat addition. We reason that, if the pseudoknot changed conformation as telomerase translocated on a primer during processive repeat addition, this change could be detectable when comparing the FRET histograms constructed from the above two categories of active binding events. However, no significant difference was observed between these two groups for either stem-A- or stem-B-labeled RNPs (Fig. 5 A and B). Furthermore, we analyzed individual single-molecule traces of the processive primer extension events. If the pseudoknot were undergoing conformational rearrangements related to translocation, we would expect to observe corresponding FRET changes during these enzyme binding events. However, neither stem-A- nor stem-B-labeled wild-type RNP showed any discernible FRET changes during the binding events that resulted in processive repeat addition (Fig. 5 C and D). Therefore, conformational change of the pseudoknot is unlikely to be required for the translocation step of the telomerase catalytic cycle.
Fig. 5.
Pseudoknot is stable during repeat translocation (A) Active events from all stem-A-labeled constructs were separated into two groups. FRET distributions of the group that resulted in primer extension by one nucleotide (last nucleotide of the first repeat) are depicted as solid bars. FRET distributions of the group that resulted in processive repeat addition are shown as black outlines. (B) Same as A, with stem-B-labeled constructs. (C) Representative FRET trajectory of a single primer extended processively by wild-type, stem-A-labeled telomerase. (D) Same as C, with wild-type, stem-B-labeled telomerase. Insets show expanded view of the enzyme binding events (dashed box).
Discussion
In this work, we applied single-molecule FRET-based assays to the structural and functional characterization of the pseudoknot region of the telomerase RNA from Tetrahymena thermophila. The wealth of biochemical and genetic data and a robust reconstitution strategy make Tetrahymena an ideal model system for in vitro studies of the telomerase structure–function relationship.
Previous studies have shown that the pseudoknot region assumes different conformations in the protein-free and protein-bound states and suggested that the observed change may arise from folding of the pseudoknot (12, 29–31). Our single-molecule FRET data clearly demonstrate that the pseudoknot is not properly formed within the full-length, protein-free telomerase RNA, but is properly and stably folded upon assembly with Tetrahymena holoenzyme proteins TERT and p65. Given that the isolated pseudoknot sequence is folded when excised from the rest of the RNA, misfolding of the pseudoknot in the full-length RNA is likely due to competing interactions with other regions of the RNA, and the holoenzyme proteins promote pseudoknot formation by disrupting these nonproductive folding traps.
Using a single-molecule structure–function assay, we demonstrate that proper formation of the pseudoknot is not required for primer binding by telomerase, but is essential for the primer extension activity. The finding that pseudoknot formation is unnecessary for primer binding is not entirely surprising given that TERT has an RNA-independent DNA-binding activity (40). On the other hand, previous studies showed varying levels of activity defect in pseudoknot substitution and deletion mutants, complicating unambiguous determination of the functional importance of the pseudoknot structure (12, 13, 26–28). This discrepancy likely arises from incomplete disruption of the pseudoknot by the substitution mutants. Indeed, as demonstrated by our single-molecule data (Fig. 4D), pseudoknot can transiently form in mutant enzymes with nucleotide substitutions that disrupt pseudoknot formation. Although these mutations gave rise to reduced, but nonzero, levels of activity and processivity, examination of single-molecule FRET traces revealed that primer extension only occurred in the subset of binding events with pseudoknot properly folded (at least transiently). In an ensemble activity assay, such transient formation of the active conformation could mask the activity defect of pseudoknot disruption, especially when the pseudoknot folding and the catalytic steps are not rate limiting such that each binding event is sufficiently long to allow primer extension. Intriguingly, mutants in which the pseudoknot region is deleted completely often retained greater activity than nucleotide substitution mutants (12, 13), suggesting that the presence of an unfolded or misfolded pseudoknot has a greater deleterious effect on enzyme function than the complete absence of the pseudoknot. Based on the above evidence, we conclude that, when present in the telomerase enzyme, the pseudoknot sequence must fold into a pseudoknot structure to support catalytic activity.
It has been hypothesized that pseudoknot functions as a molecular switch, converting between different conformations during telomeric repeat synthesis (17, 29, 32, 33). Previous NMR studies suggested that an isolated pseudoknot sequence from the human telomerase RNA slowly interconverts between a hairpin state and the pseudoknot conformation on the timescale that is longer than seconds (22, 33), whereas mutational analysis of human telomerase supports a static pseudoknot structure (25). In the single-molecule time traces shown here, neither stem A nor stem B were observed to undergo conformational rearrangements in the wild-type pseudoknot during primer extension or enzyme translocation. These data suggest that the Tetrahymena pseudoknot remains stably folded and that it unlikely functions as a molecular switch during repeat addition, although we cannot formally rule out the possibility that transient conformational changes occur on a timescale faster than the time resolution (200 ms) used here.
The methods used here to investigate the conformation and functional role of Tetrahymena pseudoknot within catalytically active telomerase RNPs should be applicable to the structure–function study of other regions of the RNP complex, as well as telomerase RNPs from other species, helping to build a broad, cross-species understanding of the telomerase structure. Such direct investigations into the structural foundation of telomerase activity may also lead to the development of diagnostic tools for the detection of diseases involving telomerase malfunction.
Materials and Methods
Sample Preparation.
All RNAs used in this study were labeled and purified as described (16). Briefly, site-specifically modified RNA oligos containing 5NU (Dharmacon, refer to Table S1 for oligo sequence) were labeled with Cy3 or Cy5 monoreactive dyes (GE Health Science). After PAGE and HPLC purification, the labeled RNA oligos were ligated to form the full-length RNA through splinted ligation. Telomerase RNPs were reconstituted with labeled full-length RNA, immunopurified, and assayed for activity in the bulk as described before (39, 41). Purified FRET-labeled telomerase RNPs were used in subsequent FRET studies. More detailed description of sample preparation can be found in SI Materials and Methods.
Single-Molecule FRET Imaging and Data Analysis.
The FRET-labeled pseudoknot in isolation, the full-length RNA, and reconstituted RNPs were anchored onto quartz slides as described (SI Materials and Methods) and imaged at room temperature on a prism-type TIRF microscope using an alternating excitation scheme that switches between 532 and 635-nm laser illuminations (42). This scheme allowed us to monitor both FRET between Cy3 and Cy5 when excited by the 532-nm laser and emission of Cy5 when directly excited by the 635-nm laser. Only time trajectories collected from molecules that experienced single-step Cy3 and Cy5 bleaching events were used in subsequent analyses. FRET is defined as the ratio of Cy5 emission to the sum of Cy3 and Cy5 emissions. FRET histograms were constructed from FRET time trajectories of individual RNA or RNP complexes normalized over the trajectory lengths such that each complex or binding event contribute equally to the overall histogram.
Single-Molecule Structure–Function Assay for Telomerase.
Alexa Fluor-488-labeled and 5′ biotinylated (TG)8T2G4T2 primers were anchored to the streptavidin-coated quartz slides as described in SI Materials and Methods. Fluorescence emission was recorded at 5 Hz during the first 60 frames under 460-nm excitation, which allowed detection of the Alexa Fluor-488-labeled primers. Subsequently, the Cy3-Cy5-labeled telomerase RNPs were diluted in imaging buffer containing 10 μM dGTP and 100 μM chain-terminating ddTTP and flowed onto the slide to initiate the enzyme binding phase of the experiment. Data were collected with alternating four frames of 532-nm excitation and one frame of 635-nm excitation at 0.2 s per frame for 20 min. At the end of the binding phase, the enzyme solution was exchanged with the labeling solution [imaging buffer containing 20 nM Cy3-conjugated LO: Cy3-(CA)8] that simultaneously washed out the enzymes and labeled the primers with a Cy3 donor dye. Subsequently, a detection solution (imaging buffer containing 300 nM Cy5-conjugated DO: Cy5-C2A2C3) was immediately flowed onto the slide to wash out the excess LO and to initiate the activity detection phase of the experiment. Data were acquired at 30 Hz during the detection phase with continuous 532-nm excitation. Enzyme binding events were identified and classified according to their catalytic outcomes, which were determined by comparison to hybridization kinetics of synthetic primers corresponding to the no-extension, single-nucleotide, and processive repeat addition products (39) (Fig. S4). Histograms of FRET from individual telomerase binding events were constructed, excluding data derived from bleached or blinked Cy5 (as verified by direct excitation with 635-nm laser).
Supplementary Material
Acknowledgments.
We thank Dr. Kathy Collins and Dr. Tracy Bryan for materials and protocols for telomerase reconstitution, and Dr. Michael D. Stone for helpful discussions. X.Z. is a Howard Hughes Medical Institute investigator. J.Y.W. is a National Science Foundation Graduate Research Fellow.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Telomerase and Retrotransposons: Reverse Transcriptases that Shaped Genomes,” held September 29–30, 2010, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/telomerase_and_retrotransposons.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.F.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017686108/-/DCSupplemental.
References
- 1.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–952. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greider CW, Blackburn EH. Tracking telomerase. Cell. 2004;116:S83–86. doi: 10.1016/s0092-8674(04)00053-4. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 5.Chen JL, Greider CW. Telomerase RNA structure and function: Implications for dyskeratosis congenita. Trends Biochem Sci. 2004;29:183–192. doi: 10.1016/j.tibs.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Armanios MY, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 7.Collins K. The biogenesis and regulation of telomerase holoenzymes. Nat Rev Mol Cell Biol. 2006;7:484–494. doi: 10.1038/nrm1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero DP, Blackburn EH. A conserved secondary structure for telomerase RNA. Cell. 1991;67:343–353. doi: 10.1016/0092-8674(91)90186-3. [DOI] [PubMed] [Google Scholar]
- 9.ten Dam E, van Belkum A, Pleij K. A conserved pseudoknot in telomerase RNA. Nucleic Acids Res. 1991;19:6951. doi: 10.1093/nar/19.24.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai CK, Miller MC, Collins K. Template boundary definition in Tetrahymena telomerase. Genes Dev. 2002;16:415–420. doi: 10.1101/gad.962602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingner J, et al. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 12.Sperger JM, Cech TR. A stem-loop of Tetrahymena telomerase RNA distant from the template potentiates RNA folding and telomerase activity. Biochemistry. 2001;40:7005–7016. doi: 10.1021/bi0103359. [DOI] [PubMed] [Google Scholar]
- 13.Lai CK, Miller MC, Collins K. Roles for RNA in telomerase nucleotide and repeat addition processivity. Mol Cell. 2003;11:1673–1683. doi: 10.1016/s1097-2765(03)00232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witkin KL, Collins K. Holoenzyme proteins required for the physiological assembly and activity of telomerase. Genes Dev. 2004;18:1107–1118. doi: 10.1101/gad.1201704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connor CM, Collins K. A novel RNA binding domain in tetrahymena telomerase p65 initiates hierarchical assembly of telomerase holoenzyme. Mol Cell Biol. 2006;26:2029–2036. doi: 10.1128/MCB.26.6.2029-2036.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone MD, et al. Stepwise protein-mediated RNA folding directs assembly of telomerase ribonucleoprotein. Nature. 2007;446:458–461. doi: 10.1038/nature05600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingner J, Hendrick LL, Cech TR. Telomerase RNAs of different ciliates have a common secondary structure and a permuted template. Genes Dev. 1994;8:1984–1998. doi: 10.1101/gad.8.16.1984. [DOI] [PubMed] [Google Scholar]
- 18.Tzfati Y, Knight Z, Roy J, Blackburn EH. A novel pseudoknot element is essential for the action of a yeast telomerase. Genes Dev. 2003;17:1779–1788. doi: 10.1101/gad.1099403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dandjinou AT, et al. A phylogenetically based secondary structure for the yeast telomerase RNA. Curr Biol. 2004;14:1148–1158. doi: 10.1016/j.cub.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 20.Zappulla DC, Cech TR. Yeast telomerase RNA: A flexible scaffold for protein subunits. Proc Natl Acad Sci USA. 2004;101:10024–10029. doi: 10.1073/pnas.0403641101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JL, Blasco MA, Greider CW. Secondary structure of vertebrate telomerase RNA. Cell. 2000;100:503–514. doi: 10.1016/s0092-8674(00)80687-x. [DOI] [PubMed] [Google Scholar]
- 22.Kim NK, et al. Solution structure and dynamics of the wild-type pseudoknot of human telomerase RNA. J Mol Biol. 2008;384:1249–1261. doi: 10.1016/j.jmb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen G, Wen JD, Tinoco I., Jr Single-molecule mechanical unfolding and folding of a pseudoknot in human telomerase RNA. RNA. 2007;13:2175–2188. doi: 10.1261/rna.676707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilley D, Blackburn EH. The telomerase RNA pseudoknot is critical for the stable assembly of a catalytically active ribonucleoprotein. Proc Natl Acad Sci USA. 1999;96:6621–6625. doi: 10.1073/pnas.96.12.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen JL, Greider CW. Functional analysis of the pseudoknot structure in human telomerase RNA. Proc Natl Acad Sci USA. 2005;102:8080–8085. doi: 10.1073/pnas.0502259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Autexier C, Greider CW. Mutational analysis of the Tetrahymena telomerase RNA: Identification of residues affecting telomerase activity in vitro. Nucleic Acids Res. 1998;26:787–795. doi: 10.1093/nar/26.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Licht JD, Collins K. Telomerase RNA function in recombinant Tetrahymena telomerase. Genes Dev. 1999;13:1116–1125. doi: 10.1101/gad.13.9.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berman AJ, Gooding AR, Cech TR. Tetrahymena telomerase protein p65 induces conformational changes throughout telomerase RNA (TER) and rescues telomerase reverse transcriptase and TER assembly mutants. Mol Cell Biol. 2010;30:4965–4976. doi: 10.1128/MCB.00827-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharyya A, Blackburn EH. Architecture of telomerase RNA. EMBO J. 1994;13:5721–5731. doi: 10.1002/j.1460-2075.1994.tb06910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaug AJ, Cech TR. Analysis of the structure of Tetrahymena nuclear RNAs in vivo: Telomerase RNA, the self-splicing rRNA intron, and U2 snRNA. RNA. 1995;1:363–374. [PMC free article] [PubMed] [Google Scholar]
- 31.Yeoman JA, Orte A, Ashbridge B, Klenerman D, Balasubramanian S. RNA conformation in catalytically active human telomerase. J Am Chem Soc. 2010;132:2852–2853. doi: 10.1021/ja909383n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comolli LR, Smirnov I, Xu L, Blackburn EH, James TL. A molecular switch underlies a human telomerase disease. Proc Natl Acad Sci USA. 2002;99:16998–17003. doi: 10.1073/pnas.262663599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theimer CA, Finger LD, Trantirek L, Feigon J. Mutations linked to dyskeratosis congenita cause changes in the structural equilibrium in telomerase RNA. Proc Natl Acad Sci USA. 2003;100:449–454. doi: 10.1073/pnas.242720799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stryer L, Haugland RP. Energy transfer: A spectroscopic ruler. Proc Natl Acad Sci USA. 1967;58:719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ha T, et al. Probing the interaction between two single molecules: Fluorescence resonance energy transfer between a single donor and a single acceptor. Proc Natl Acad Sci USA. 1996;93:6264–6268. doi: 10.1073/pnas.93.13.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang X, et al. A single molecule study of RNA catalysis and folding. Science. 2000;288:2048–2051. doi: 10.1126/science.288.5473.2048. [DOI] [PubMed] [Google Scholar]
- 37.Theimer CA, Blois CA, Feigon J. Structure of the human telomerase RNA pseudoknot reveals conserved tertiary interactions essential for function. Mol Cell. 2005;17:671–682. doi: 10.1016/j.molcel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Ulyanov NB, Shefer K, James TL, Tzfati Y. Pseudoknot structures with conserved base triples in telomerase RNAs of ciliates. Nucleic Acids Res. 2007;35:6150–6160. doi: 10.1093/nar/gkm660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu JY, Stone MD, Zhuang X. A single-molecule assay for telomerase structure–function analysis. Nucleic Acids Res. 2010;38:e16. doi: 10.1093/nar/gkp1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finger SN, Bryan TM. Multiple DNA-binding sites in Tetrahymena telomerase. Nucleic Acids Res. 2008;36:1260–1272. doi: 10.1093/nar/gkm866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bryan TM, Goodrich KJ, Cech TR. Tetrahymena telomerase is active as a monomer. Mol Biol Cell. 2003;14:4794–4804. doi: 10.1091/mbc.E03-07-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapanidis AN, et al. Fluorescence-aided molecule sorting: Analysis of structure and interactions by alternating-laser excitation of single molecules. Proc Natl Acad Sci USA. 2004;101:8936–8941. doi: 10.1073/pnas.0401690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.