Abstract
Sensing and adapting to the environment is one strategy by which bacteria attempt to maximize fitness in an unpredictable world; another is the stochastic generation of phenotypically distinct subgroups within a genetically clonal population. In culture, Salmonella Typhimurium populations are bistable for the expression of flagellin. We report that YdiV controls this expression pattern by preventing transcription of the sigma factor that recruits RNA polymerase to the flagellin promoter. Bistability ensues when the sigma factor is repressed in a subpopulation of cells, resulting in two phenotypes: flagellin expressors and flagellin nonexpressors. Although the ability to swim is presumably a critical survival trait, flagellin activates eukaryotic defense pathways, and Salmonella restrict the production of flagellin during systemic infection. Salmonella mutants lacking YdiV are unable to fully repress flagellin at systemic sites, rendering them vulnerable to caspase-1 mediated colonization restriction. Thus, a regulatory mechanism producing bistability also impacts Salmonella virulence.
Keywords: nongenetic variation, pyroptosis, bet-hedging, fliC, pathogenesis
Deterministic adaptation in response to environmental signals is a common and well-characterized strategy by which bacteria endure changing external conditions. Another strategy is bet-hedging, in the form of stochastically generated, phenotypically distinct subgroups within a genetically clonal population (1). Diversity may improve the chance that a clone will thrive in a fluctuating environment. In some cases, deterministic adaptation and stochastic survival strategies are integrated. For example, as a population of Bacillus subtilis enters the stationary phase, the probability that a subgroup of cells will become competent increases in response to environmental cues (2). The presence of two stable phenotypes within a genetically clonal population, under homogenous conditions, is termed bistability (1).
When the pathogen Salmonella enterica serovar Typhimurium is grown under homogenous conditions in rich media, transcription of the flagellin monomer fliC is bistable, with well-defined subpopulations of fliC-OFF and fliC-ON cells (3). In this organism, a three-class transcriptional cascade orders the production of flagellar proteins (4). Class I genes encode a transcriptional activation complex that is required for class II expression; class II proteins include the sigma factor (σ28, produced from the fliA gene) that recruits RNA polymerase to class III promoters, including the fliC promoter. Flagellar gene expression is controlled by environmental signals, and WT Salmonella strains capable of causing systemic infection regulate the production of flagellin in a host compartment-specific manner (3). During oral infection of mice, the pathogen breaches the mucosal layer of the gut, colonizes the lymphoid tissue (Peyer's patches) (5), and finally establishes a niche within phagocytic cells that ferry the organism to the spleen and other systemic tissues (6, 7). Salmonella transcribe fliC within the Peyer's patches; however, fliC is not expressed at systemic sites (3).
Salmonella reside within macrophages in vivo (6) and can translocate flagellin into the cytosol of host cells via the Salmonella pathogenicity island 1 (SPI-1) type III secretion system (8). Macrophages interpret intracytosolic flagellin as a danger signal, and initiate the proinflammatory cell death program pyroptosis in response (9, 10). Pyroptosis depends on expression of caspase-1 and is characterized by the maturation and release of the proinflammatory cytokines IL-1β and IL-18 and lysis of the macrophage. Mice deficient in caspase-1, IL-1β, or IL-18 are more susceptible to systemic Salmonella infection than WT mice, underscoring the importance of this pathway to host resistance (11, 12). Therefore, the down-regulation of flagellin in systemic tissues may prevent host cell death and inflammation.
In many bacterial species, motility is regulated by GGDEF and EAL domain proteins (13). We hypothesized that members of this protein family would regulate Salmonella flagellar genes in vivo. Here we show that an EAL-like protein, YdiV, represses fliC transcription in a subpopulation of cells in culture, producing bistability. YdiV also represses flagellar genes in systemic tissues, thereby protecting Salmonella from caspase-1–mediated bacterial clearance. We demonstrate that ydiV controls phenotypic heterogeneity in vitro and in vivo and modulates Salmonella virulence.
Results
EAL-Like Protein YdiV Suppresses the Inflammatory Capacity of Salmonella.
PFAM (14) and homology searches were used to identify the full complement of genes encoding GGDEF and EAL domain proteins in Salmonella (Fig. S1 and SI Materials and Methods). A targeted deletion was constructed in each gene, yielding a panel of 22 mutant strains. To identify GGDEF and EAL domain proteins that control Salmonella interactions with host phagocytes, murine bone marrow-derived macrophages were infected with individual mutants, and macrophage lysis was measured by lactate dehydrogenase release. We hypothesized that this screen would enable us to identify mutants with altered virulence.
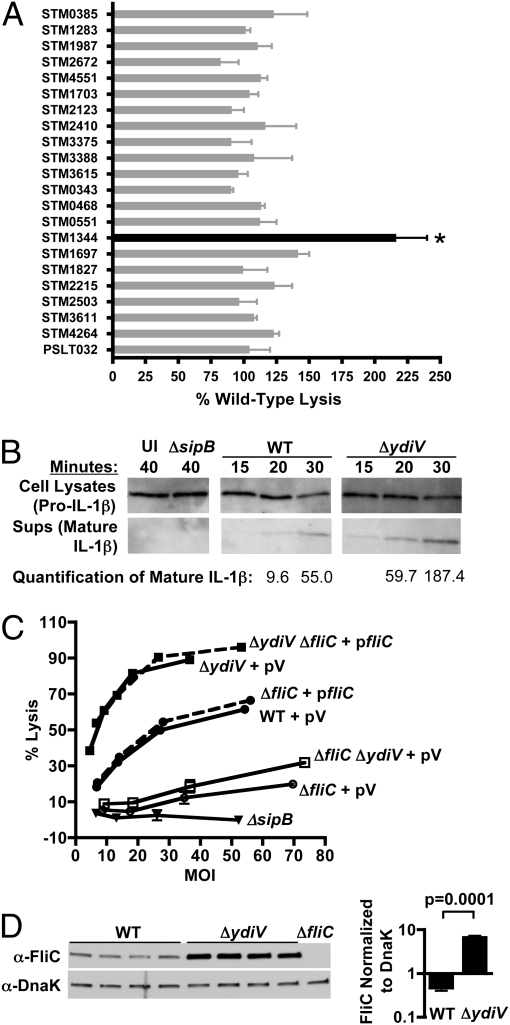
Twenty-one of the mutations had no effect on the ability of the bacteria to kill macrophages, but deletion of ydiV significantly increased the cytotoxicity of Salmonella (Fig. 1A). ΔydiV is on average 2.16 times more cytotoxic than WT, as noted by Hisert et al. (15). Enhanced killing by ΔydiV was accompanied by accelerated release of mature IL-1β, an endogenous substrate of caspase-1, into the supernatant (Fig. 1B). After 20 min, ΔydiV-infected macrophages released 6.2-fold more mature IL-1β into the supernatant than WT-infected macrophages as determined by densitometry (Fig. 1B). Release of mature IL-1β by macrophages was dependent on caspase-1 (Fig. S2).
Fig. 1.
An EAL domain protein modulates cytotoxicity by repressing fliC. (A) A panel of 22 GGDEF/EAL domain mutants was screened for the ability to trigger pyroptosis of bone marrow-derived macrophages. Macrophages were infected for 90 min at a multiplicity of infection (MOI) of 10. Only ΔydiV (STM1344) was significantly more cytotoxic than WT Salmonella (P < 0.05). Error bars represent the SD of two independent experiments. (B) Macrophages infected with ΔydiV (MOI = 12) release mature IL-1β into the supernatant more rapidly than macrophages infected with WT (MOI = 14). Values were determined using densitometry as described in Materials and Methods. Uninfected and ΔsipB (noncytotoxic control; MOI = 11) samples were collected at 40 min. (C) Hypercytotoxicity of ΔydiV is dependent on fliC. Dashed lines represent strains in which the ΔfliC mutation was complemented. pV, vector control. (D) ΔydiV overexpresses FliC. WT and ΔydiV cultures were grown to exponential phase in LB and evaluated by Western blot analysis. Four independent samples of each strain are shown. FliC expression was normalized to the DnaK loading control.
Overexpression of FliC Accounts for the Hypercytotoxicity of the ΔydiV Mutant.
Because FliC triggers macrophage pyroptosis (9, 10), excess FliC expression could account for the enhanced cytotoxicity of ΔydiV. Accordingly, fliC deletions were engineered into the ΔydiV and WT backgrounds. Both the ΔfliC and ΔydiV ΔfliC mutants killed poorly compared with the WT and ΔydiV parent strains, demonstrating the contribution of flagellin to cytotoxicity in both backgrounds (Fig. 1C). Reintroduction of FliC completely restored the hypercytotoxic phenotype characteristic of the ΔydiV parent strain. Western blot analysis confirmed that ΔydiV cultures contained 14.15 times more FliC protein than WT (average WT FliC:DnaK ratio, 0.465; average ΔydiV FliC:DnaK ratio, 6.578) (Fig. 1D). Taken together, these results demonstrate that YdiV represses fliC, and that the overproduction of flagellin by ΔydiV stimulates macrophage pyroptosis and cytokine production.
YdiV Controls the Heterogeneity of fliA Transcription.
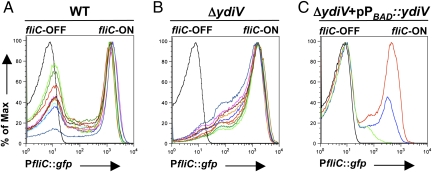
Transcription of fliC in WT Salmonella is bistable (3). To evaluate whether YdiV regulates bistability, fliC transcription was examined in single cells using a chromosomally integrated reporter construct in which expression of GFP was driven from the fliC promoter (PfliC::gfp). This test revealed that the overexpression of FliC observed on Western blot analysis was due to a dramatic increase in the percentage of the bacterial population transcribing fliC in ΔydiV cultures (Fig. 2 A and B). In contrast to WT, ΔydiV cultures were unimodally fliC-ON; the average percentage of fliC-ON was 61.24% for WT and 91.44% for ΔydiV (P = 7.8 × 10−10). Deletion of ydiV removed the persistently fliC-OFF subpopulation.
Fig. 2.
Bistability of fliC depends on ydiV. (A) Transcriptional fusion of the fliC promoter to gfp reveals distinct populations of fliC-OFF and fliC-ON cells in WT when cultures are interrogated by flow cytometry. (B) In contrast, ΔydiV demonstrates a unimodal distribution of predominantly fliC-ON cells. In A and B, 10 independent trials (colored traces) per strain are shown; the black trace represents GFP-negative control. MFIs of the fliC-ON populations are not significantly different between the two strains. fliC-ON is defined as all cells with fluorescence greater than that of the reporter-minus strain (average MFI, 1,084.7 for WT and 1,235.3 for ΔydiV:; P = 0.08). (C) When expression of ydiV is driven from the araBAD promoter, increasing concentrations of inducer correspondingly reduce the fliC-ON population (red trace, 0.0002% l-arabinose; blue trace, 0.002%; green trace, 0.2%). All experiments were performed using exponential-phase cultures.
To test whether complementation could reconstitute fliC bistability in the ΔydiV mutant, ydiV was cloned downstream of an arabinose-inducible promoter. Low concentrations of inducer restored a fliC-OFF population to the ΔydiV PfliC::gfp strain; at the highest concentration of inducer, fliC transcription was reduced below the level of detection in most cells (Fig. 2C). We conclude that YdiV represses fliC expression, and that a YdiV-directed process occurring in a subpopulation of cells produces bistability of fliC transcription.
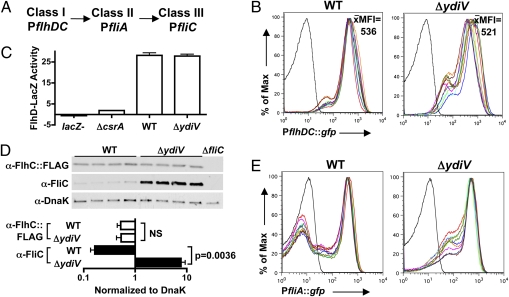
Products of the class I operon flhDC activate transcription of class II operons (Fig. 3A). The class II gene fliA encodes σ28, which activates transcription of fliC and other class III genes. PflhDC::gfp, a transcriptional reporter construct in which GFP expression is driven from the class I promoter, was used to investigate whether YdiV controls flagellar gene expression from the top of the regulatory cascade (Fig. 3A). The mean fluorescent intensities (MFIs) of WT and ΔydiV populations harboring this reporter were averaged over 10 trials per strain. These average MFIs did not differ significantly between the strains (Fig. 3B), indicating that YdiV does not regulate flhDC transcription. A flhD-lacZ translational reporter fusion revealed that the flhDC mRNA is translated equally by the WT and ΔydiV strains (Fig. 3C).
Fig. 3.
YdiV controls the heterogeneity of fliA (σ28) transcription. (A) The Salmonella flagellar gene regulatory cascade. (B) YdiV does not repress flhDC transcription. The average MFI of 10 independent trials per strain is reported. The black line represents GFP-negative control. (C) A lacZ translational reporter fusion to flhD reveals that YdiV does not repress the translation of class I proteins. csrA is required for efficient translation of the flhDC mRNA (32); thus, a ΔcsrA mutant serves as a negative control. (D) Western blot analysis demonstrates that WT and ΔydiV Salmonella contain similar amounts of FlhC protein. Four independent samples of each strain are shown. FlhC and FliC expression were normalized to the DnaK loading control for quantification. The ΔfliC mutant does not harbor the FlhC::3× FLAG construct. (E) YdiV represses fliA transcription. The black line represents GFP-negative control. All experiments were performed using exponential-phase cultures.
The FlhD4C2 complex is posttranslationally regulated by proteolytic degradation. To examine whether YdiV regulates this process, a 3× FLAG epitope tag fused to the C terminus of FlhC was used to compare the amount of a master flagellar regulatory protein in the ΔydiV and WT backgrounds (Fig. 3D). Western blot analysis revealed that ΔydiV cultures contained similar amounts of FlhC::3×FLAG as WT cultures (Fig. 3D), demonstrating that YdiV does not reduce fliC expression by degrading FlhD4C2.
Wada et al. (16) showed that YdiV can bind to FlhD and prevent FlhD4C2 from binding the fliA promoter, thereby inhibiting fliA transcription. Thus, we hypothesized that YdiV might initiate phenotypic heterogeneity in flagellar gene expression by directly repressing fliA; this heterogeneity could then be transmitted down through the flagellar gene cascade to fliC. To test this hypothesis, we used a transcriptional reporter construct in which GFP expression was driven from the fliA promoter (Fig. 3E). The fliA transcriptional distributions of WT populations were bistable, with distinct peaks of fliA-OFF and fliA-ON cells. However, in the ΔydiV strain, most cells were transcribing fliA; the average percentage of fliA-ON was 55.51% for WT and 87.03% for ΔydiV (P = 8.4 × 10−17). Thus, the transcriptional profiles of fliA in WT and ΔydiV closely mirrored those of fliC in the two backgrounds. We conclude that in WT Salmonella, YdiV prevents activation of fliA transcription in a subpopulation of cells. Because FliA is required for fliC expression, this repression causes bistability in the transcription of fliC.
YdiV Represses fliC Transcription in Vivo.
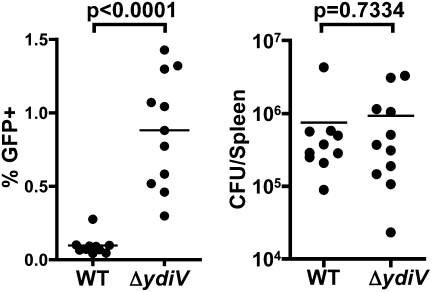
Salmonella living within macrophages require ClpXP protease, which degrades FlhD4C2, to suppress fliC transcription (3, 17). YdiV also posttranslationally regulates the flagellar master regulatory proteins to repress fliC (16). Thus, we hypothesized that YdiV might repress fliC as Salmonella adapts to life within a host cell. To examine fliC transcription in vivo, WT and ΔydiV strains harboring the PfliC::gfp reporter construct were orally inoculated into separate groups of C57BL/6 mice, and the percentage of bacteria transcribing fliC within splenocytes was determined. Indeed, 10-fold more ΔydiV bacteria than WT bacteria activated the fliC transcriptional reporter (mean value, 0.90% vs. 0.09%), demonstrating that YdiV represses class III flagellar genes in vivo (Fig. 4). Thus, YdiV regulates phenotypic heterogeneity in vitro and in vivo and plays a role in the maintenance of the fliC-OFF state during growth in the spleen.
Fig. 4.
ΔydiV Salmonella overexpress fliC in systemic tissues. C57BL/6 mice were orally infected with 106 ΔydiV or WT Salmonella harboring the PfliC::gfp reporter construct. Splenocytes were harvested from infected animals, and fliC expression by intracellular bacteria was quantified by flow cytometry as described in Materials and Methods. (Left) On average, 10 times more ΔydiV than WT transcribed fliC in the spleen. (Right) To facilitate direct comparison of gene expression from ΔydiV and WT bacteria, the two groups of mice were colonized to similar levels (Materials and Methods). Data from two independent experiments are combined.
ΔydiV Elicits an Enhanced Inflammatory Cytokine Response from the Host.
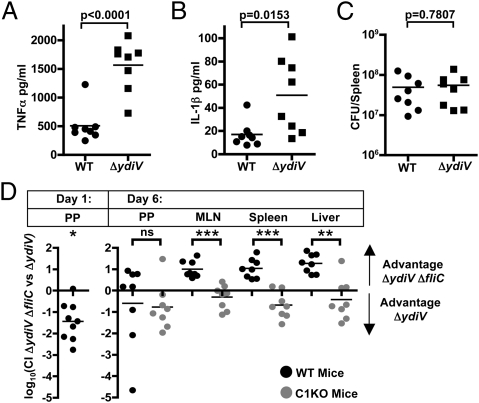
Concentrations of IL-1β and TNF-α were higher in the sera of ΔydiV-infected mice compared with WT-infected mice, indicating heightened activation of proinflammatory pathways and possibly pyroptotic cell death (Fig. 5 A–C). Activation of such mechanisms during colonization of systemic sites could promote bacterial clearance. If this were true, then we predicted that a ΔydiV ΔfliC mutant would outcompete the ΔydiV parent strain at systemic sites in the host.
Fig. 5.
YdiV is required to evade pyroptosis during infection. (A–C) Serum levels of TNFα and IL-1β were measured as described in Materials and Methods after oral infection with WT or ΔydiV. Concentrations of TNFα (A) and IL-1β (B) were higher in the sera after infection with ΔydiV. (C) To facilitate direct comparisons, the mice shown in A and B were colonized to similar levels with either WT or ΔydiV Salmonella (Materials and Methods). (D) Mice were orally gavaged with 106 cfu of ΔydiV (strain 2) and ΔydiV ΔfliC (strain 1), for a total of 2 × 106 cfu/mouse. Tissue colonization was measured at the indicated times, and the CIs were calculated as described in Materials and Methods. ΔydiV outcompetes ΔydiV ΔfliC in colonizing the Peyer's patches after 1 d of infection (n = 9 mice; data combined from two independent experiments). However, on day 6, ΔydiV ΔfliC outcompetes ΔydiV in the systemic tissues. This advantage disappears when the same experiment is performed in caspase-1 KO animals. *P < 0.01; **P < 0.001;***P < 0.0001.
YdiV Protects Salmonella from Pyroptosis.
We used a murine competitive infection model to assess the benefit of YdiV-mediated fliC repression within systemic tissues. Orally introduced Salmonella initially colonize the gastrointestinal tract, then infect the Peyer's patches (5) and mesenteric lymph nodes (MLN), and finally travel through the blood and lymph to the spleen, liver, and other systemic sites (6, 7). When WT Salmonella was coinfected with its ΔfliC derivative, the two strains colonized the Peyer's patches, MLNs, and spleen of C57BL/6 mice equally well (Fig. S3A). Next, the ΔydiV strain was competed against its ΔfliC derivative. Strikingly, on day 1 of the infection ΔydiV was significantly more likely than ΔydiV ΔfliC to be found in the Peyer's patches (P = 0.0013, one-sample t test with hypothetical mean of 0) (Fig. 5D). Despite this considerable advantage, on day 6, the ΔydiV ΔfliC strain consistently outcompeted the ΔydiV parent strain in the MLN, spleen, and liver (MLN, P = 0.0002; spleen, P = 0.0003; liver, P < 0.0001; one-sample t tests with hypothetical mean of 0) (Fig. 5D). No difference was observed in the Peyer's patches (P = 0.4040), where WT Salmonella normally transcribe flagellin late in infection (3). These data show that YdiV-dependent maintenance of the fliC-OFF state at systemic sites is critical for maximal colonization. When even a small percentage of Salmonella infecting systemic tissues are in the fliC-ON state (Fig. 4), a protective host response ensues (Fig. 5D).
The accelerated release of IL-1β by macrophages infected with ΔydiV compared with WT (Fig. 1B), along with the enhanced serum levels of both IL-1β and TNF-α in ΔydiV-infected mice (Fig. 5 A–C), suggest that YdiV-mediated fliC repression at systemic sites protects the pathogen against caspase-1–dependent proinflammatory pathways. To test this hypothesis, we competed ΔydiV against ΔydiV ΔfliC in caspase-1–deficient mice and measured bacterial colonization on day 6. In the MLN, spleen, and liver, the competitive indices (CIs) in caspase-1 deficient mice were significantly different from those observed during infection of WT animals (P values for comparisons between WT and caspase-1–deficient mice (Peyer's patches, P = 0.8245; MLN, P < 0.0001; spleen, P < 0.0001; liver, P = 0.0004; two-sample t tests) (Fig. 5D). The distributions of CIs were in fact skewed toward an advantage for ΔydiV in the caspase-1 deficient mice, although this effect was only statistically significant in the spleen (Peyer's patches, P = 0.0769, MLN, P = 0.1612, spleen: P = 0.0165, liver: P = 0.2405, one-sample t tests with hypothetical mean of 0) (Fig. 5D). These data show that YdiV-mediated fliC repression allows Salmonella to circumvent a specific, protective host response.
Discussion
YdiV bears no close homology to any currently characterized protein domain. Its nearest relatives are the EAL domain proteins, many of which are phosphodiesterases that degrade the bacterial second messenger c-di-GMP. However, YdiV is a very poor match to the EAL domain consensus sequence, and neither degrades nor binds c-di-GMP in vitro (18). Wada et al. (16) demonstrated that YdiV binds to FlhD and inhibits the transcription of the class II gene fliA. Intriguingly, Wada et al. observed that YdiV deterministically repressed flagellar genes in response to nutritional cues. Based on those findings and our present observations, we conclude that YdiV controls both stochastic and deterministic gene expression, tuning flagellar gene transcription in reponse to environmental signals.
The ΔydiV mutant is attenuated compared with WT in oral (average log10 CI, −0.7588; P = 0.0258) as well as i.p. and i.v. (15) infections (Fig. S3B). Intriguingly, the spread in our oral competition data demonstrates variation in the ability of ΔydiV to compete with WT in the deep tissues, which is consistent with the heterogeneity in fliC expression that we observed in ΔydiV populations ex vivo (Fig. 4) and the heterogeneity in the proinflammatory serum cytokine response that we found in mice infected with the ΔydiV mutant (Fig. 5 A–C). In fact, deletion of ydiV increased heterogeneity in vivo, in contrast to our in vitro results. The majority of bacteria isolated from both WT- and ΔydiV-infected mice were fliC-OFF (Fig. 4), most likely due to other regulatory mechanisms that repress fliC in systemic tissues. Indeed, we have previously shown that the protease ClpX represses fliC expression in macrophages (3). The presence of multiple mechanisms to ensure tight control of fliC reflects the importance of maintaining the fliC-OFF state in specific environments, including systemic tissues (Fig. 5D).
Genetic circuits that produce bistable patterns of gene expression often include positive feedback (1), and fliA is subject to two positive feedback loops (Fig. S4). The first loop increases fliA transcription by elevating the concentration of FlhD4C2 within the cell (19). In the second loop, FliA (σ28) directly recruits RNA polymerase to the fliA promoter (20). Because nonlinear gene expression dynamics are essential components of other bistable circuits (1, 21), one or both of these loops might produce an acceleration of fliA transcription that could potentially result in bistable expression of class III genes. In contrast, we discovered that bistability of fliC expression in Salmonella is regulated by YdiV, which controls a mechanism that operates upstream of both positive feedback loops (Fig. S4).
YdiV controls the proportion of cells in a population transcribing fliA and, consequently, the proportion transcribing fliC. This is likely accomplished by preventing the flagellar master regulatory complex from activating class II transcription, effectively locking a subpopulation of cells into fliA-OFF mode. Working with the laboratory Salmonella strain LT2, Saini et al. (22) demonstrated that flagellar class III transcription becomes unimodal when σ28 is unable to activate transcription at the fliA promoter. Therefore, we propose that ydiV controls bistability of fliA and fliC by ensuring that a subpopulation of cells is unable to activate fliA transcription (Fig. 3E), whereas the σ28 autoactivation loop likely controls the acceleration of fliA expression once fliA transcription begins (Fig. S4).
The ΔydiV mutant is hypercytotoxic (Fig. 1 A and C) (15). We have demonstrated that this phenotype requires fliC, and that many more cells in ΔydiV populations are transcribing fliC than in WT populations. An increase in the fliC-ON population could potentially have pleiotropic effects on cytotoxicity in this in vitro assay; expression of flagellar genes might increase both access to macrophages and the ability to deliver the toxic protein FliC. We used cytotoxicity as a screen to identify mutants that we hypothesized would have altered virulence, and thus focused subsequent experiments on the mechanism by which ydiV controls heterogeneity of fliC expression and the role of ydiV in maintaining the anatomical restriction of fliC expression in vivo.
Previous studies have demonstrated that Salmonella down-regulate fliC during growth in the spleen (3), that FliC triggers caspase-1–dependent death of macrophages (10), and that caspase-1–deficient mice are more susceptible to Salmonella infection (11, 12). Miao et al. (23) recently found that an i.p.-introduced strain of Salmonella, engineered to express fliC within a macrophage, induces pyroptosis of host cells and is outcompeted by isogenic WT bacteria at systemic sites. Here we show that depletion of YdiV naturally relieves fliC repression in a small percentage of systemic Salmonella, which is sufficient to trigger the caspase-1–dependent protective host response. This response is highly localized, since coinfecting fliC-OFF bacteria are not cleared to the same extent as the fliC-ON strain. We can eliminate the possibility that the production of FliC causes a metabolic burden that constrains colonization. If FliC production posed a disadvantage, we would not expect the fliC-expressing ΔydiV strain to able to colonize caspase-1 KO mice as as well or better than the ΔfliC ΔydiV strain (Fig. 5D).
Many pathogenic bacteria either repress flagellar genes throughout infection [e.g., enteric Yersinia (24), Listeria (25)] or have completely lost the ability to express flagellar genes [e.g., Shigella (26), Yersinia pestis (24)], thus avoiding the potent host defense response to flagellin. In contrast, Salmonella have evolved to regulate flagellar genes in a host compartment-specific manner (3). This allows the pathogen to benefit from flagellar gene expression in favorable host environments. Flagellin is a proinflammatory molecule, and motile Salmonella grow more quickly than nonmotile strains in the inflamed intestine, because they are able to migrate to the nutrient-rich zone close to the epithelium (27). However, our data demonstrate that tight repression of flagellin is required for Salmonella to thrive in systemic tissues. YdiV modulates phenotypic heterogeneity in Salmonella populations; given the costs and rewards involved in flagellar gene expression, this specialization appears to provide a mechanism by which the bacteria maximize fitness in the host. Thus, a mechanism controlling bistable gene expression significantly impacts the ability of an organism to cause infection.
Materials and Methods
Bacterial Strains, Plasmids and Culture Conditions.
The strains used in this study are listed in Table S1. As described previously, mutants were generated in the Salmonella enterica serovar Typhimurium 14028 background (28), and fliC and flhDC GFP reporters were constructed (3). The flhD-lacZ single-copy chromosomal translational fusion (29) has the reporter fused to the first 60 amino acids of FlhD. β-galactosidase activity was determined as described previously (30), and is reported as (activity units per A600 unit per mL of cell suspension) × 2,000, where activity units are μmol of ortho-nitrophenyl formed/min. The FlhC::3× FLAG tag fusion was a gift from the Rao laboratory (19). The fliA GFP reporter, pfliC, and pPBAD::ydiV are described in SI Materials and Methods. Strains were grown in LB at 37 °C with aeration unless noted otherwise. Carbenicillin, kanamycin, and gentamicin were added at 100, 50, and 20 μg/mL, respectively.
Mice.
C57BL/6 WT mice (Jackson Laboratories) and Casp1−/− mice (a gift from R. Flavell, Yale University, New Haven, CT) were housed under specific pathogen-free conditions in accordance with the University of Washington's Institutional Animal Care and Use Committee guidelines.
Competitive Infections.
Bacteria were grown overnight in LB without aeration. Fasting mice received 106 of each strain orally in 200 μL of 5% sodium bicarbonate/PBS. Organs were harvested at indicated time points. CIs were calculated as the output ratio of strain 1 to strain 2 divided by the input ratio of strain 1 to strain 2 (31). All statistical tests were applied to the log10 of the CIs. A one-sample, two-tailed t test with a hypothetical mean of 0 was used to determine whether either strain demonstrated a significant colonization advantage within the individual tissues. A two-sample, two-tailed t test was used to compare the results obtained in WT mice with those obtained in caspase-1 KO mice.
GFP Expression ex Vivo.
Fasting mice were orally gavaged with 106 bacteria in 200 μL of 5% sodium bicarbonate/PBS. The mice received 2 mg/mL of ampicillin in their drinking water during the fast and throughout the course of infection to maintain reporter constructs (3). Because the ability of ΔydiV to compete with WT is variable (Fig. S3B), we harvested bacteria from mice at approximately the same stage of infection to evaluate gene expression by ΔydiV and WT bacteria that had replicated to similar numbers within the host. Mice were killed on days 4 and 5, and bacteria from the spleens were analyzed as described previously (3).
Flow Cytometry.
Exponential-phase bacteria were analyzed using a FACScan flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (TreeStar). Cytokines in processed blood (see SI Materials and Methods for processing methods) were measured on a FACSCanto flow cytometer (BD Biosciences) using cytometric bead arrays (BD 552364 and 560232) in accordance with the manufacturer's instructions. Data were analyzed using FCAP Array software (BD Biosciences). Given the variable ability of ΔydiV to compete with WT, we collected blood from infected mice on days 5 and 6 to evaluate host cytokine production in mice with similar levels of bacterial colonization.
Macrophages.
Bone marrow macrophages derived from C57BL/6 mice were isolated and cultured as described previously (9). For macrophage infections, overnight bacterial cultures were diluted 1:15 and grown for 3 h, washed, and then resuspended in PBS. Bacteria were spun down onto macrophages at 200 × g for 10 min. Lactate dehydrogenase release was measured after a total of 90 min using the Promega CytoTox 96 Kit (G1780). Macrophages were seeded 2 × 104 per well in 96-well plates. Cytotoxicity was calculated as described previously (9).
Immunoblot Analysis.
Western blot analyses were performed on exponential-phase bacterial cultures using standard techniques (3) and developed with Amersham ECL Western Blotting Detection Reagents. Anti-3× FLAG antibody (F3165; Sigma-Aldrich) and anti-mouse HRP-antibody (NA931; Amersham) were used to detect the FlhC::3× FLAG fusion. IL-1β detection was performed using 2 × 105 macrophages per well in 24-well plates (SI Materials and Methods). Macrophages were treated overnight with 100 ng/mL of LPS and infected in media containing 5 mM glycine (9). Bacteria were prepared as for the cytotoxicity experiments. Processed cytokine was detected using anti–IL-1β antibody (AF-401-NA; R&D Systems) and HRP-conjugated anti-goat antibody (SC2350; Santa Cruz Biotechnology). National Institutes of Health ImageJ version 1.63 was used to quantitate protein expression.
Supplementary Material
Acknowledgments
We thank Caroline S. Harwood for helpful discussions. This work was supported by a University of Washington Cellular and Molecular Biology Training Grant, National Institute of General Medical Sciences Public Health Service National Research Service Award Grant T32 GM07270, and National Institutes of Health Grants P50 HG 02360 and U19 AI 090882A.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108963108/-/DCSupplemental.
References
- 1.Smits WK, Kuipers OP, Veening JW. Phenotypic variation in bacteria: The role of feedback regulation. Nat Rev Microbiol. 2006;4:259–271. doi: 10.1038/nrmicro1381. [DOI] [PubMed] [Google Scholar]
- 2.Magnuson R, Solomon J, Grossman AD. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell. 1994;77:207–216. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 3.Cummings LA, Wilkerson WD, Bergsbaken T, Cookson BT. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol Microbiol. 2006;61:795–809. doi: 10.1111/j.1365-2958.2006.05271.x. [DOI] [PubMed] [Google Scholar]
- 4.Chevance FF, Hughes KT. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol. 2008;6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jepson MA, Clark MA. The role of M cells in Salmonella infection. Microbes Infect. 2001;3:1183–1190. doi: 10.1016/s1286-4579(01)01478-2. [DOI] [PubMed] [Google Scholar]
- 6.Salcedo SP, Noursadeghi M, Cohen J, Holden DW. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell Microbiol. 2001;3:587–597. doi: 10.1046/j.1462-5822.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- 7.Vazquez-Torres A, et al. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–808. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- 8.Sun YH, Rolán HG, Tsolis RM. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J Biol Chem. 2007;282:33897–33901. doi: 10.1074/jbc.C700181200. [DOI] [PubMed] [Google Scholar]
- 9.Fink SL, Cookson BT. Caspase-1–dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 10.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin-1β via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 11.Lara-Tejero M, et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raupach B, Peuschel SK, Monack DM, Zychlinsky A. Caspase-1–mediated activation of interleukin-1β (IL-1β) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006;74:4922–4926. doi: 10.1128/IAI.00417-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfe AJ, Visick KL. Get the message out: Cyclic-Di-GMP regulates multiple levels of flagellum-based motility. J Bacteriol. 2008;190:463–475. doi: 10.1128/JB.01418-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finn RD, et al. The Pfam protein families database. Nucleic Acids Res. 2008;36(Database issue):D281–D288. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hisert KB, et al. A glutamate-alanine-leucine (EAL) domain protein of Salmonella controls bacterial survival in mice, antioxidant defence and killing of macrophages: Role of cyclic diGMP. Mol Microbiol. 2005;56:1234–1245. doi: 10.1111/j.1365-2958.2005.04632.x. [DOI] [PubMed] [Google Scholar]
- 16.Wada T, et al. EAL domain protein YdiV acts as an anti-FlhD4C2 factor responsible for nutritional control of the flagellar regulon in Salmonella enterica serovar Typhimurium. J Bacteriol. 2011;193:1600–1611. doi: 10.1128/JB.01494-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomoyasu T, Takaya A, Isogai E, Yamamoto T. Turnover of FlhD and FlhC, master regulator proteins for Salmonella flagellum biogenesis, by the ATP-dependent ClpXP protease. Mol Microbiol. 2003;48:443–452. doi: 10.1046/j.1365-2958.2003.03437.x. [DOI] [PubMed] [Google Scholar]
- 18.Simm R, Remminghorst U, Ahmad I, Zakikhany K, Römling U. A role for the EAL-like protein STM1344 in regulation of CsgD expression and motility in Salmonella enterica serovar Typhimurium. J Bacteriol. 2009;191:3928–3937. doi: 10.1128/JB.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saini S, Brown JD, Aldridge PD, Rao CV. FliZ Is a posttranslational activator of FlhD4C2-dependent flagellar gene expression. J Bacteriol. 2008;190:4979–4988. doi: 10.1128/JB.01996-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikebe T, Iyoda S, Kutsukake K. Structure and expression of the fliA operon of Salmonella typhimurium. Microbiology. 1999;145:1389–1396. doi: 10.1099/13500872-145-6-1389. [DOI] [PubMed] [Google Scholar]
- 21.Dubnau D, Losick R. Bistability in bacteria. Mol Microbiol. 2006;61:564–572. doi: 10.1111/j.1365-2958.2006.05249.x. [DOI] [PubMed] [Google Scholar]
- 22.Saini S, et al. FliZ induces a kinetic switch in flagellar gene expression. J Bacteriol. 2010;192:6477–6481. doi: 10.1128/JB.00751-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miao EA, et al. Caspase-1–induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapatral V, Minnich SA. Co-ordinate, temperature-sensitive regulation of the three Yersinia enterocolitica flagellin genes. Mol Microbiol. 1995;17:49–56. doi: 10.1111/j.1365-2958.1995.mmi_17010049.x. [DOI] [PubMed] [Google Scholar]
- 25.Way SS, et al. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell Microbiol. 2004;6:235–242. doi: 10.1046/j.1462-5822.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 26.Tominaga A, Mahmoud MA, Mukaihara T, Enomoto M. Molecular characterization of intact, but cryptic, flagellin genes in the genus Shigella. Mol Microbiol. 1994;12:277–285. doi: 10.1111/j.1365-2958.1994.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 27.Stecher B, et al. Motility allows S Typhimurium to benefit from the mucosal defence. Cell Microbiol. 2008;10:1166–1180. doi: 10.1111/j.1462-5822.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 28.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellermeier CD, Janakiraman A, Slauch JM. Construction of targeted single copy lac fusions using λ Red and FLP-mediated site-specific recombination in bacteria. Gene. 2002;290:153–161. doi: 10.1016/s0378-1119(02)00551-6. [DOI] [PubMed] [Google Scholar]
- 30.Slauch JM, Silhavy TJ. cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J Bacteriol. 1991;173:4039–4048. doi: 10.1128/jb.173.13.4039-4048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beuzón CR, Holden DW. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 2001;3:1345–1352. doi: 10.1016/s1286-4579(01)01496-4. [DOI] [PubMed] [Google Scholar]
- 32.Lawhon SD, et al. Global regulation by CsrA in Salmonella typhimurium. Mol Microbiol. 2003;48:1633–1645. doi: 10.1046/j.1365-2958.2003.03535.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.